Abstract
Two different repair mechanisms of mesenchymal stem cells (MSCs) are suggested to participate in the repair of acute lung injury (ALI): (i) Cell engraftment mechanism, (ii) Paracrine/endocrine mechanism. However, the exact roles they play in the repair remain unclear. The aim of the study was to evaluate the role of paracrine/endocrine mechanism using a novel intrapleural delivery method of MSCs. Either 1 × 106 MSCs in 300 μl of PBS or 300 μl PBS alone were intrapleurally injected into rats with endotoxin-induced ALI. On days 1, 3 or 7 after injections, samples of lung tissues and bronchoalveolar lavage fluid (BALF) were collected from each rat for assessment of lung injury, biochemical analysis and histology. The distribution of MSCs was also traced by labelling the cells with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). MSCs intrapleural injection significantly improved LPS-induced lung histopathology compared with PBS-treated group at day 3. There was also a significant decrease in total cell counts and protein concentration in BALF at day 7 in the MSCs -treated rats compared to PBS control group. Tracking the DAPI-marked MSCs showed that there were no exotic MSCs in the lung parenchyma. MSCs administration resulted in a down-regulation of pro-inflammatory response to endotoxin by reducing TNF-α both in the BALF and in the lung, while up-regulating the anti-inflammatory cytokine IL-10 in the lung. In conclusion, treatment with intrapleural MSCs administration markedly attenuates the severity of endotoxin-induced ALI. This role is mediated by paracrine/endocrine repair mechanism of MSCs rather than by the cell engraftment mechanism.
Keywords: mesenchymal stem cells, acute lung injury, pleural cavity, lipopolysaccharides, cell therapy
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are major causes of acute respiratory failure with high morbidity and mortality in critically ill patients [1–4]. Despite improvements in supportive care, the mortality rate is still at 43% [2]. Our previous studies and other reports have demonstrated that bone marrow-derived mesenchymal stem cells (MSCs) participate in the repair of experimental ALI [5–13]. Although the mechanisms for MSCs on ALI were various in different studies, two main mechanisms of MSCs were suggested to be involved in it: (i) Cell engraftment mechanism, (ii) Paracrine/endocrine mechanism. However, the exact roles they play in the repair of ALI remain largely unknown. Several studies have demonstrated that the actual levels of MSC incorporation into the lung after intravenous injection were found very low in vivo. Therefore, the protective role of MSCs does not match with the rare account of them in the organ, indicating that paracrine/endocrine mechanism repair mechanism might play a more important role in the lung repair [5–8,14–22]. This hypothesis remains unproved because either classical intravenous (IV) or intratracheal (IT) delivery methods used in these studies cannot separate apart one mechanism from another.
Recently we have developed a novel intrapleural delivery method [23]. The MSCs delivered by this method can survive at least 1 month in vivo and their distribution was found to be limited to the surface of pleurae and in the pleural cavity, forming a ‘MSCs repository’ in vivo. As no MSCs were detected in the lung parenchyma, the cell repair mechanism by MSCs engraftment into the lung tissue cannot be supported.
For ALI model, we hypothesized that once we observed protective role of intrapleural injected MSCs to ALI, that role should be the result of the paracrine/endocrine repair mechanism. On the basis of this hypothesis, we delivered MSCs into pleural cavity in rats with endotoxin-induced ALI. The distribution of MSCs, the lung injury and histopathology with or without MSCs injection were compared. The aim of this study was to confirm that paracrine/endocrine mechanism of MSCs plays a role alone in the repair of ALI model.
Materials and methods
Animal care
The animals used in this study were specific pathogen-free male Sprague–Dawley rats (6–8 weeks old; Slac Laboratory Animal Co Ltd, Shanghai, China). The rats received humane care in compliance with the NIH principles of laboratory animal care and the Chinese national regulations for experimental animal care. All protocols were approved by the Animal Care and Use Committee of Fudan University.
Experimental design
Specific pathogen-free male Sprague–Dawley rats (250 ± 30 g) were first anaesthetized with diethyl ether inhalation combined with pentobarbital sodium (60 mg/kg) intraperitoneally (i.p.) ALI was then induced by the instillation of LPS from Escherichia coli O55:B5 (Sigma-Aldrich, St. Louis, MO, USA) at 7 mg/kg intratracheally (i.t.). Immediately after LPS administration, rats were given either MSCs (1 × 106 cells in 300 μl of PBS, MSCs treatment group) or 300 μl of PBS (PBS treatment group) intrapleurally as described below. The animals were anaesthetized and killed by incision of the abdominal aorta on days 1, 3 and 7 after transplantation (n = 6 rats per time-point per group) to harvest lung tissue samples and bronchoalveolar lavage fluid (BALF), which were stored at −80°C prior to analyses. Samples from naïve rats were also collected (n = 6 rats, normal control group) in the same way.
Additional four rats with ALI were intrapleurally injected with DAPI-marked MSCs [23]. They were used for tracking the DAPI-marked cells. Samples of pleurae and lungs were collected from them at day 3 and observed under a fluorescent microscope [23]. Frozen sections of lung tissues were prepared to see if there were DAPI-marked MSCs penetrated into the lung parenchyma.
Rat MSCs culture
Mesenchymal stem cells were isolated from rat bone marrow as described in our previous studies [10,23]. In brief, whole marrow was flushed from the tibias and femurs of Sprague–Dawley rats (4 weeks old, male) with ice-cold Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Gibco, Carlsbad, CA, USA). The cells were washed and plated in plastic flasks at 2 × 106 cells/mL in DMEM/F12 containing 10% foetal bovine serum, 100 units/mL penicillin and 100 μg/ml streptomycin (Gibco). The cell suspension was incubated at 37°C with 5% CO2; non-adherent cells were discarded 48 hrs later. For routine maintenance, the medium was replaced twice weekly and cells were subcultured at a one-to-three split ratio by trypsinization (0.25% trypsin/1 mmol/L EDTA; Gibco). More purified MSCs were obtained after passages.
ALI model and intropleural delivery of MSCs
For establishment of ALI model, rats were anaesthetized with diethyl ether inhalation and pentobarbital podium. The trachea was exposed after a 1 cm midline cervical incision and then the rat was fixed at a 60° angle. A dose of 7 mg/kg body weight of LPS in 0.3 ml of LPS-free saline (0.9% NaCl) was intratracheally injected slowly using a 1 ml syringe. The needle was bent at a 90° angle so as to be injected into and fixed in the trachea easily and safely. LPS administration was immediately followed by rotating the animals to attempt to homogeneously distribute LPS in the lungs, and then the incision was closed with sutures [24].
For intropleural delivery, a 5-mm incision was made on the right chest through the skin and the subcutaneous soft tissues. PBS with or without MSCs was then injected into the pleural cavity using a 20 μl plastic pipette tip, instead of a needle, connected to a 1 ml syringe, so as to avoid possible pneumothorax or lung injury that could have been caused by a needle. After injection, the incision was closed with sutures [23].
Lung wet/dry ratio and myeloperoxidase (MPO) activity
The superior and middle lobes of right lung were collected from each rat for assessment of the lung W/D ratio. Samples were weighed immediately after collection and then placed in a drying oven at 60°C for 48 hrs, and the dry weights were subsequently determined. The lung W/D ratio was calculated by dividing the wet weight by the dry weight.
Myeloperoxidase activity was measured and normalized by wet lung weight according to the manufacturers’ instructions (Nanjing Jiancheng Biological Engineering Institute, Jiangsu, China). One MPO activity unit is named as 1 μmol hydrogen peroxide (H2O2) decomposed by 1 g lung tissue in the reaction system at 37°C.
Total cell and neutrophil cell counts and protein concentration in BALF
Rats were anaesthetized with diethyl ether inhalation and pentobarbital podium (i.p.) and then killed by incision of the abdominal aorta. The trachea was cannulated and the right lung bronchus was tied. The left lung was lavaged three times with 3 ml cold saline each time (total volume was 9 ml). BALF was collected for total cell and neutrophil counts and centrifuged at 4°C and 1000 × g, the supernatant was collected in tubes and stored at −80°C for further analysis of total protein and cytokine levels. Total cell count was determined with the use of a counter. A cell smear was made using cytospin (Thermo Shandon, Inc., Pittsburgh, PA, USA), and the cells were visualized using Wright-Giemsa staining. A differential of the white blood cells was then obtained by counting 200 cells from a representative portion of the slide. The concentration of total protein in BALF was determined using BCA™ Protein Assay Kit (Thermo Scientific, Waltham, MA, USA).
Cytokine protein and mRNA measurements in BALF and/or lung homogenates
TNF-α and IL-10 were measured in both the BALF and lung homogenate samples with ELISA kits (rat TNF-a, DAKEWE; rat IL-10, Shanghai ExCell Biology, Inc., Shanghai, China). A quantity of 100 mg of tissue from the inferior lobe of right lung per sample was homogenated. The supernatant was collected and the cytokine and protein measurements were performed according to the manufacturers’ instructions. The cytokine levels in lung homogenates were normalized to individual protein concentrations measured as described above. TNF-α and IL-10 mRNA levels in lung homogenates were measured using quantitative real-time SYBR Green RT-PCR using ABI-7500 (ABI, Carlsbad, CA, USA). Primers used were: TNF-α, sense 5′-AGCGGAGGAGCAGCTGGAGT -3′; and antisense 5′-CGGGGCAGCCTTGTCCCTTG -3′; IL-10, sense 5′-AGCCAGACCCACATGCTCCGA-3′; and antisense 5′-AGGCTTGGCAACCCAAGTAACCCT-3′; GAPDH, sense 5′-AGAACATCATCCCTGCATCC-3′; and antisense 5′-TGGATACATTGGGGGTAGGA-3′. The PCR conditions for amplification of TNF-α, IL-10 and GAPDH were initially denatured at 95°C for 5 min., then subjected to 40 cycles at 94°C for 15 sec., 60°C for 45 sec. Reactions were performed in duplicate for each sample.
Lung morphology
Lung tissues from right lower lobe were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 5 μm thick sections. Sections were stained with haematoxylin and eosin. Lung pathology was evaluated blindly by a pathologist using a light microscope, according to four criteria: alveolar congestion, haemorrhage, infiltration or aggregation of neutrophils in airspaces or vessel walls and thickness of alveolar wall/hyaline membrane formation. Each criterion was graded according to a 5-point scale, as previously reported [25]. A total lung injury score was calculated as the sum of the four criteria.
Immunohistochemical staining
Sections were deparaffinized in xylene and rehydrated in alcohol. Endogenous peroxidase activity was blocked by 3% H2O2 for 10 min. incubation. Antigen retrieval was achieved by microwave heating twice in a citrate acid buffer (pH 6.0), and sections were blocked in 3% goat serum (Boster, Wuhan, China) at 37°C for 15 min. Slices were incubated with a primary antibody (anti- TNF-α and anti-IL-4 both at 1:100 dilution, Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) at 4°C overnight, and washed three times in PBS, then the slices were incubated with a biotin-labelled secondary antibody (goat anti-rabbit IgG at 1:100 dilution) for 30 min., washed three times in PBS, and incubated with an SABC (streptavidin–biotinylated complex) complex for 25 min. Immunostaining was achieved using 3,3′-diaminobenzidine tetra hydrochloride (DAB). All slides were counterstained with Haematoxylin. Immunostaining was evaluated using a light microscope according to staining levels. The brown colour of staining was graded according to a 5-point scale: 0 = non-staining, 1 = weak, 2 = moderate, 3 = strong, 4 = very strong. Both staining levels of bronchioles and small vessels were scored for TNF-α, the total score was calculated as the sum of these two items. For IL-4, only bronchioles were selected for scoring.
Statistical analysis
Data are shown as mean ± SD. Differences between groups were assessed using one-way analysis of variance, followed by SNK-q post hoc tests. Non-paired t-test was used to compare the means of lung injury scorings between MSCs treatment group and PBS control group. A value of P < 0.05 was considered statistically significant. Analyses were performed using spss 11.5 software (SPSS Inc., Chicago, IL, USA).
Results
Intrapleural administrated MSCs were limited to pleural cavity in rats with ALI
The distribution of intrapleural administrated DAPI-marked MSCs in rats with ALI was found to be limited to the surface of pleurae and in the pleural cavity, no fluorescent cells were found in the lung parenchyma (Fig. 1), similar to the results observed with healthy rats in our previous study [23].
Fig. 1.
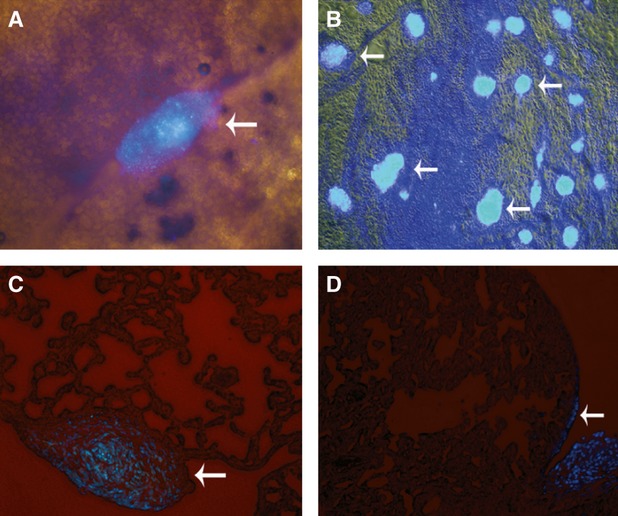
No MSCs were found in the lung parenchyma of rats with LPS-induced ALI after cells were injected intrapleurally. (A) Surface of right lung. (B) Surface of mediastinal pleura. (C, D) Frozen sections of lung tissue. DAPI-marked MSCs masses were found on the surfaces of pleurae and the lung, whereas no fluorescent cells were found in the lung parenchyma (white arrows). All images, ×200 magnification.
Intrapleural MSCs treatment improved LPS-induced lung injury
In the PBS treatment group, LPS induced marked inflammatory reaction and lung injury in lung tissue, which appeared as inflammatory infiltration, interalveolar septal thickening and alveolar congestion and haemorrhage in lung. The most severe lung injury induced by LPS was observed at day 3 after treatment with PBS (Fig. 2). Intrapleural administration of MSCs significantly improved the lung injury caused by LPS (Fig. 3). Compared with the PBS treatment group, the total severity scores of lung injury were significantly reduced at day 3 because of the intrapleural MSCs treatment (P < 0.01) (Fig. 2), suggesting that intrapleural MSCs therapy has a beneficial role in alleviating LPS-induced lung injury. Consistent with the improvement in the lung histopathology, macroscopic observation of the whole right lung specimens at day 3 also showed less severe lung injury in the MSCs treatment group (Fig. 4).
Fig. 2.
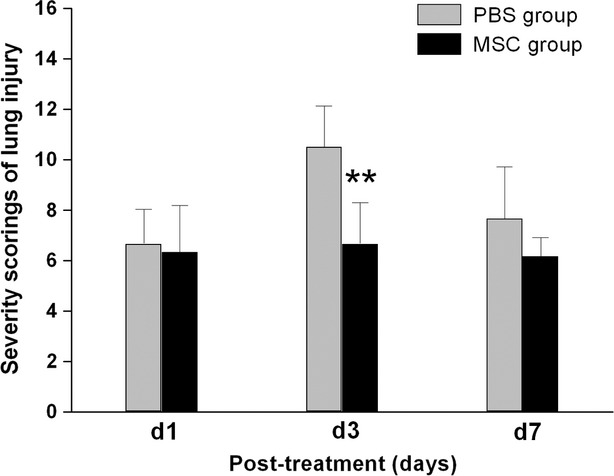
Intrapleural transplantation of MSCs improved lung injury as assessed by histological method. LPS-induced increase in lung injury score was significantly reduced at day 3 in the MSCs treatment group, and the peak of lung injury found at this time-point in the PBS treatment control group disappeared. Data are expressed as mean ± SD, n = 6 per group per time-point. **P < 0.01 versus the PBS treatment group at the same time-point. Data were analysed using non-paired t-test.
Fig. 3.
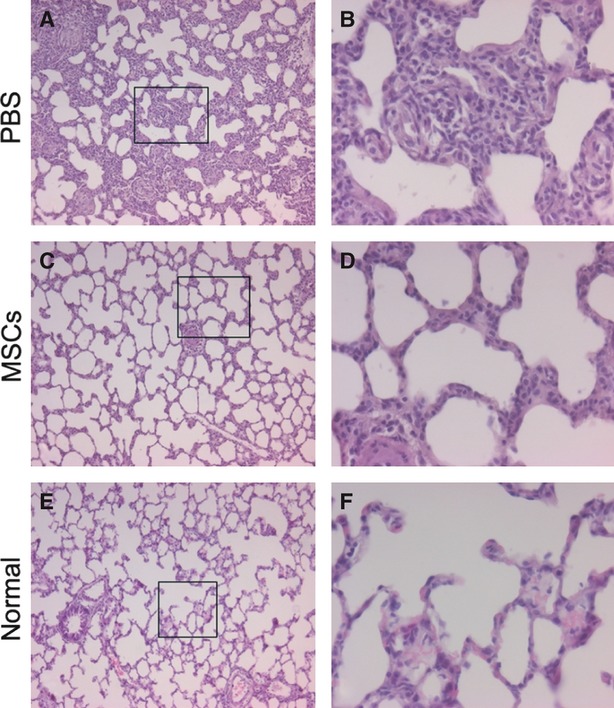
Haematoxylin and eosin staining of lung sections demonstrated attenuated lung injury in the MSCs treatment group at day 3 after instillation of endotoxin. (A, B) PBS-treated group. (C, D) MSCs-treated group. (E, F) Normal control. Histopathological changes in the lungs are shown at low (×100 for A, C, E) and high (×400 for B, D, F) magnification of the black boxed area on the left.
Fig. 4.
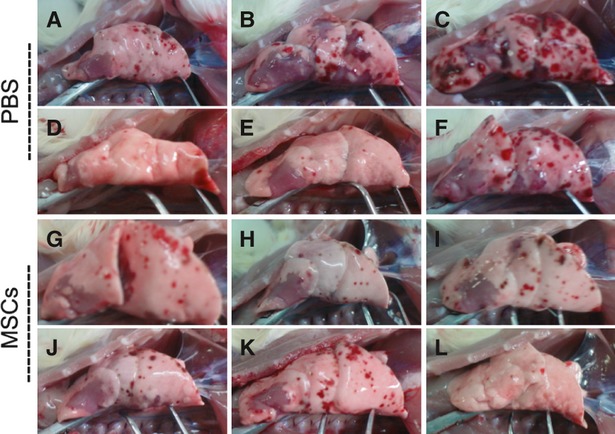
Macroscopic observation of the whole right lung specimens showed a decreased LPS-induced lung injury at day 3 in the MSCs treatment group. (A–F) Six specimens collected from PBS treatment group at day 3. (G–L) Six specimens collected from MSCs treatment group at day 3. The degree of haemorrhage showed on the surfaces of three lung specimens (B, C and F) collected in the PBS control group were more severe than that of any six specimens collected in MSCs treatment group.
Intrapleural administrated MSCs decreased protein concentration and cell count in BALF
Total protein concentration in the BALF, which reflects the extent of endothelial/epithelial permeability of the lung, was significantly increased in all rats even 7 days after LPS challenge. A significant decrease was observed at day 7 in MSCs treatment group compared with PBS treatment group (P < 0.05) (Fig. 5A). LPS challenge also significantly increased total cell count in BALF, including large number of red cells due to alveolar haemorrhage and neutrophils in the early day 1 and day 3 time-points. Total cell number in BALF was decreased due to MSCs treatment at day 7, compared with PBS treatment (P < 0.01) (Fig. 5B). The neutrophil counts between both groups were not significantly different at all three time-points (Fig. 5C). Macroscopic observation of the BALF at day 3 also suggested an attenuated haemorrhage induced by LPS in the MSCs treatment group (Fig. 6).
Fig. 5.
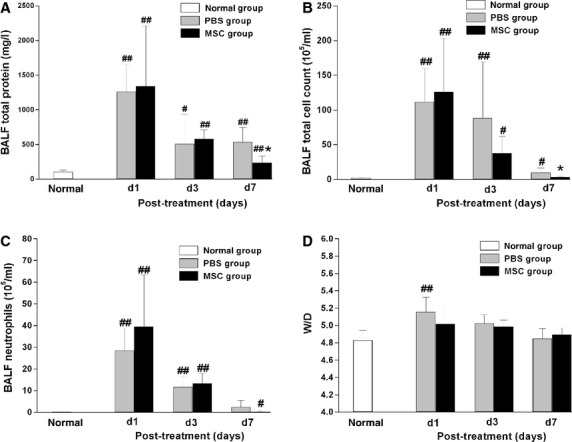
MSCs significantly improved endothelial/epithelial permeability of the lung. (A) BALF protein was significantly reduced in the MSCs treatment group compared with the PBS treatment group at day 7. (B) Similarly, total cell count in BALF was also significantly reduced in the MSCs treatment group at day 7.(C, D) Both neutrophil count in BALF and W/D of lung were not significantly different between MSCs- and PBS- treatment groups at the three time-points. Data are expressed as mean ± SD, n = 6 per group per time-point. *P < 0.05 versus the PBS treatment group at the same time-point, #P < 0.05 versus normal group, ##P < 0.01 versus normal group. Data were assessed using one-way analysis of variance with SNK-q post hoc tests.
Fig. 6.
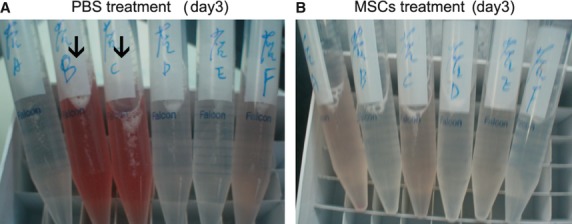
Macroscopic observation of the BALF suggested an attenuated haemorrhage induced by LPS at day 3 in the MSCs treatment group. (A) Six specimens collected from PBS treatment group at day 3. (B) Six specimens collected from MSCs treatment group at day 3. Obviously, the two reddest BALF samples (black arrows) were from the PBS control group rather than from the MSCs treatment group.
Effect of intrapleural administrated MSCs on lung W/D ratio and lung MPO activity
LPS challenge caused significant lung oedema at day 1, as shown by the increased lung W/D ratio compared with normal rats (P < 0.01) (Fig. 5D). Then the W/D ratio gradually decreased to nearly normal at day 7, suggesting lung oedema could recover spontaneously within 7 days. MSCs treatment showed a trend towards reduction in lung W/D ratio at day 1 and day 3 compared with PBS treatment group, although the difference was not significant between the two groups. Lung MPO activity was determined as an index of neutrophil sequestration. However, the difference of MPO activity was not significant between the MSCs- and PBS- treatment groups at all three time-points (Fig. 7).
Fig. 7.
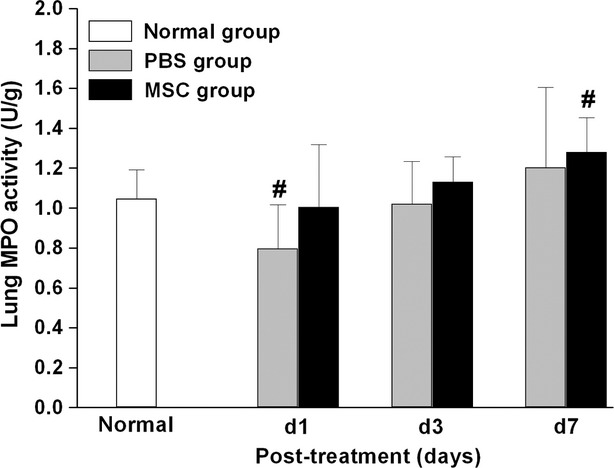
Intrapleural administration of MSCs did not affect the lung MPO activity after endotoxin-induced ALI, which is an index of neutrophil sequestration. No significant difference was detected between MSCs- and PBS- treatment groups at day 1, 3 or 7 after injury (n = 6 per group per time-point). Data are expressed as mean ± SD. #P < 0.05 versus normal group. Data were assessed using one-way analysis of variance with SNK-q post hoc tests.
Effect on pro-inflammatory and anti-inflammatory cytokines in lung tissue and BALF following endotoxin injury
We detected the levels of pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine IL-10 in lung tissue and BALF by ELISA. The mRNA levels of these cytokines in lung tissue were also examined by real-time RT-PCR. At day 3, TNF-α in BALF was significantly reduced in the MSCs treatment group compared with that in PBS treatment group (P < 0.05) (Fig. 8). Similar results of TNF-α protein were observed in lung tissue. TNF-αmRNA expression in lung tissue increased 3 days after LPS exposure, although no significant difference between the MSCs- and PBS- treatment groups was observed at all time-points. At day 1, the level of IL-10 in lung tissue was significantly higher in the MSCs treatment group than that in the PBS treatment group (P < 0.05) (Fig. 8), same with the results of IL-10 mRNA expression (P < 0.05) (Fig. 8). LPS induced a significant increase of IL-10 in BALF at day 1, then gradually decreased to the normal levels at day 7. The levels of IL-10 protein in BALF showed no difference between the MSCs group and the PBS group.
Fig. 8.
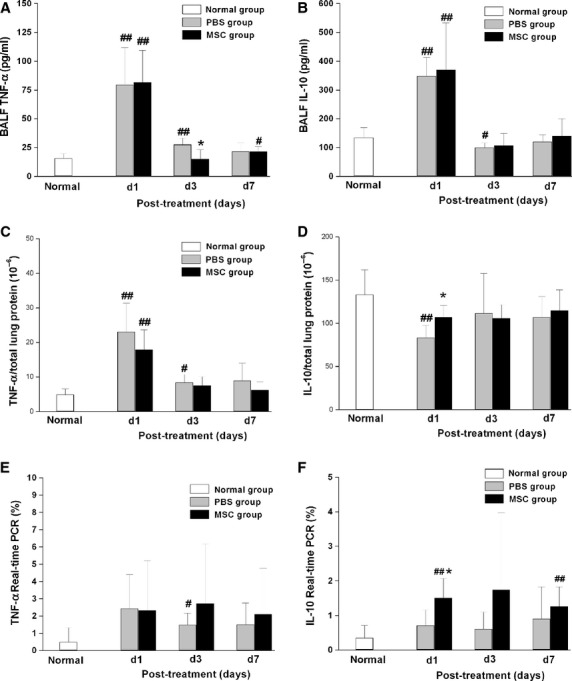
Levels of pro- and anti-inflammatory cytokines. LPS-induced increase of TNF-α in BALF was significantly reduced in the MSCs treatment group at day 3, whereas IL-10 in lung tissue was significantly increased at day 1 at both protein and at mRNA levels with MSCs therapy. (A, C, E) Levels of TNF-α in BALF, lung tissue and in mRNA respectively. (B, D, F) Levels of IL-10 in BALF, lung tissue and in mRNA respectively. Data are expressed as mean ± SD, n = 6 per group per time-point. *P < 0.05 versus the PBS treatment group at the same time-point, #P < 0.05 versus normal group, ##P < 0.01 versus normal group. Data were assessed using one-way analysis of variance with SNK-q post hoc tests.
The levels of TNF-α and another anti-inflammatory cytokine IL-4 in lung tissue were also analysed using immunostaining method. Only weak TNF-α staining was detected in bronchial ciliated columnar epithelial cells in normal lung (Fig. 9A). LPS induced a high level of TNF-α staining in bronchial ciliated columnar epithelial cells at day 1, which gradually decreased but did not reach normal level even at day 7 (Fig. 9A). Furthermore, strong staining of TNF-α located at the tunica adventitia of small vessels was also observed at day 1. However, the staining disappeared at day 7 (Fig. 9A). This finding indicated that the recovery of small vessel injury may be faster than that of bronchioles. MSCs treatment showed a trend towards reduction in TNF-α staining at day 3 and day 7 compared with PBS treatment group, although the difference was not significant between the two groups (Fig. 9C). Moderate staining of IL-4 located at bronchial ciliated columnar epithelial cells was detected in normal lung (Fig. 9B). After LPS challenge, the staining level of IL-4 did not change at day 1 (Fig. 9B). However, it increased rapidly from day 3 to day 7 (Fig. 9B). This result indicated that IL-4 may play a role in the repair of lung injury in a late phase rather than in an early phase. The difference of IL-4 was not significant between the MSCs- and PBS- treatment groups at all three time-points (Fig. 9D).
Fig. 9.
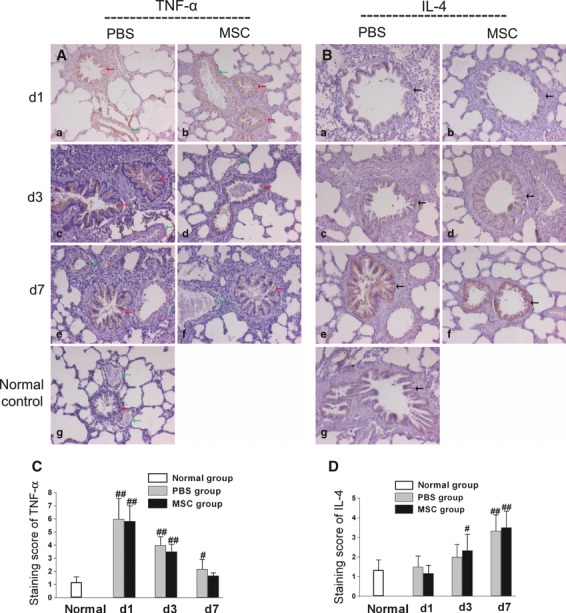
Immunostaining levels of TNF-α and IL-4. (A) and (C) LPS induced high levels of TNF-α staining in bronchial ciliated columnar epithelial cells (red arrows) as well as in the tunica adventitia of small vessels (green arrows). MSCs treatment showed a trend towards reduction in TNF-α staining at day 3 and day 7 compared with PBS treatment group, though the difference was not significant between the two groups. (B) and (D) LPS challenge did not change the staining level of IL-4 in bronchial ciliated columnar epithelial cells (black arrows) at day 1. However, it increased significantly from day 3 to day 7. The levels of IL-4 showed no difference between the MSCs group and the PBS group. All images, ×200 magnification. Data are expressed as mean ± SD, n = 6 per group per time-point. #P < 0.05 versus normal group, ##P < 0.01 versus normal group. Data were assessed using one-way analysis of variance with SNK-q post hoc tests.
Discussion
Recently we reported that when MSCs, labelled with either DAPI or GFP using a lentiviral vector, were delivered to the pleural cavity, they were found to present only on the surface of pleurae and in the pleural cavity. Also none of the MSCs was found to migrate into the lung parenchyma by intrapleural delivery method in normal rats. Both DAPI and GFP labelling methods showed similar results. Although DAPI decreased the proliferation rate of MSCs and may have the possibility of diffusion from the death cells, this labelling method is simple and relatively reliable [23]. In this study, the results of MSCs distribution in ALI model were similar to that of healthy rats previously described [23]. We found that intrapleural delivery of MSCs demonstrated both functional and histological benefit in the rat model of LPS-induced ALI, and there was no evidence of MSCs engraftment to the lung tissue. MSCs significantly alleviated LPS-induced lung histopathology, protein concentration and cell count in BALF. MSCs intrapleural delivery also shifts the increase of pro-inflammatory cytokines to anti-inflammatory cytokines response to LPS. This phenomenon showed that MSCs intrapleural delivery likely contributes to lung protection and repair via paracrine/endocrine mechanisms.
Acute lung injury is characterized by extensive damage to the barrier of the lung epithelium and endothelium, neutrophil influx into the lung and an imbalance between pro-inflammatory and anti-inflammatory mediators. Previous studies showed that MSCs, which were delivered either by IV or IT method, ameliorate the severity of lung injury despite the low engraftment levels been seen [5–8,14–18]. This beneficial effect was primarily demonstrated by significant survival advantage, improvement in histology and down-regulation of the acute inflammatory response to endotoxin [7].
In this study, experimental ALI was induced by LPS, which is a glycolipid of the outermost membrane of gram-negative bacteria. The optimal dose of LPS was determined as 7 mg/kg by our preliminary study. The dosage we selected in this study could lead to enough severe lung injury for the study although not causing the death of rats.
The first major finding of this study was that intrapleural MSCs administration led to a significant histological improvement at day 3, when the peak of the lung injury observed in the PBS treatment group. The improvement in histology was assessed quantitatively by the use of a lung injury score described previously [25]. Consistent with the improvement in histology, macroscopic observation of lung specimens and BALF at day 3 also showed a decreased LPS-induced lung injury and an attenuated haemorrhage in the MSCs treatment group. In addition, BALF protein, a marker of endothelial and epithelial permeability was significantly reduced in the rats treated with MSCs at day 7 time-points. The total cell count in BALF also displayed a tendency similar to BALF protein. However, in accordance with the report of Gupta et al. [7], the absolute neutrophil counts in BALF and the MPO activity in the lung tissue were not different between the PBS- and MSCs- treatment groups at any time-point. This suggests that the protection provided by MSCs is not dependent on the reduction of neutrophil recruitment to the lung. The exact effects of MSCs on neutrophil function and activity remain to be described.
The second major finding of this study was that the beneficial effects with MSCs appear to be mediated by a down-regulation of the acute inflammatory response to endotoxin. The pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine IL-10 were assessed both in BALF and lung tissues. The data demonstrated that MSCs therapy significantly reduced the LPS-induced increase of TNF-α levels in BALF at day 3, similar results were observed in lung tissue. On the other hand, the levels of IL-10 were significantly higher in the lung tissue at day 1 in MSCs treatment group and consistent with up-regulation of IL-10 mRNA expression. The levels of another important anti-inflammatory cytokine IL-4 in lung tissue were also analysed using immunostaining method. LPS challenge did not change the staining of IL-4 at day 1. However, the staining level increased rapidly from day 3 to day 7, suggesting that IL-4 may play a protective role at late time. These results are consistent with previous study [7] which indicated that the beneficial effects of MSCs treatment contributed to the shift from a pro-inflammatory to an anti-inflammatory response to endotoxin.
According to previous reports, two different mechanisms of MSCs participate in the repair of lung injury. Firstly, the implanted MSCs can engraft as type I and II epithelial cells, endothelial cells and fibroblasts [13,26–28], thereby leading regeneration to repair the damaged tissue (i.e., cell engraftment mechanism). Secondly, transplanted MSCs secrete large quantities of various soluble mediators such as anti-inflammatory factors and growth factors by paracrine/endocrine manner [29–36]. These bioactive factors have both immunomodulatory and trophic properties, and modulate the repair process through either protecting the undamaged cells or activating resident stem cells to form new tissues [31]. As more and more reports demonstrated that the levels of MSCs engraftment in the target organs were too low to explain the entire beneficial effects [5–7,14–18], recently some scholars suggest that paracrine/endocrine mechanism might play a more important role in the lung repair. However, this concept remains unproved. Previous studies could not analyse the two repair mechanisms of MSCs, respectively, neither by IV nor by IT as delivery method. In this study, intrapleurally delivered MSCs attenuate endotoxin-induced ALI, which could only attribute to the paracrine/endocrine mechanism of MSCs, without the evidence of cell engraftment.
Of note, the dose of MSCs used in this study was 1 × 106 cells per rat. The advantage of pleural cavity for MSCs delivery is that it is a large potential compartment that can receive larger dose of MSCs without restriction. Whether larger dose would lead to more effectiveness or which is the optimal dose still needs to be explored.
In summary, in this study we demonstrated that intrapleural delivery of MSCs attenuates endotoxin-induced ALI in the rat model of LPS-induced ALI, without the evidence of MSCs engraftment to the lung tissue. MSCs intrapleural delivery shifts the increase of pro-inflammatory cytokines to anti-inflammatory cytokines response to LPS. MSCs intrapleural delivery contributes to lung injury repairment via paracrine/endocrine mechanisms rather than the cell engraftment mechanism.
Acknowledgments
This study was supported by the National Natural Sciences Foundation of China (No 30971314 and 81170003), the Shanghai Elite Medical Talent Project (No. XYQ2011006), the Postdoctoral Science Foundation (No 20090460588) and the Shanghai Leading Talent Projects (No 036, 2010).
Author contributions
Zhao-hui Qin: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing. Jin-fu Xu: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing. Jie-ming Qu: Conception and design, administrative support, manuscript writing, final approval of manuscript. Jing Zhang: Manuscript writing. Yin Sai: Technical support. Chun-mei Chen: Administrative support. Lian Wu: Manuscript writing. Long Yu: Administrative support.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 3.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthay MA. Treatment of acute lung injury: clinical and experimental studies. Proc Am Thorac Soc. 2008;5:297–9. doi: 10.1513/pats.200708-141DR. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 6.Song L, Xu JF, Qu JM, et al. A therapeutic role for mesenchymal stem cells in acute lung injury independent of hypoxia-induced mitogenic factor. J Cell Mol Med. 2012;16:376–85. doi: 10.1111/j.1582-4934.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 9.Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao F, Zhang YF, Liu YG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. 2008;40:1700–5. doi: 10.1016/j.transproceed.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 11.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckett T, Loi R, Prenovitz R, et al. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12:680–6. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang JC, Summer R, Sun X, et al. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–42. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi R, Beckett T, Goncz KK, et al. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–9. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serikov VB, Popov B, Mikhailov VM, et al. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290:1033–45. doi: 10.1002/ar.20574. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Fang X, Krasnodembskaya A, et al. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–9. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. PNAS. 2009;106:16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthay MA, Thompson BT, Read EJ, et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest. 2010;138:965–72. doi: 10.1378/chest.10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang KY, Shih HC, How CK, et al. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest. 2011;140:1243–53. doi: 10.1378/chest.11-0539. [DOI] [PubMed] [Google Scholar]
- 23.Qin ZH, Qu J, Xu JF, et al. Intrapleural delivery of mesenchymal stem cells: a novel potential treatment for pleural diseases. Acta Pharmacol Sin. 2011;32:581–90. doi: 10.1038/aps.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HS, Kim HJ, Moon CS, et al. Inhibition of c-Jun NH2-terminal kinase or extracellular signal-regulated kinase improves lung injury. Respir Res. 2004;5:23. doi: 10.1186/1465-9921-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takao Y, Mikawa K, Nishina K, et al. Attenuation of acute lung injury with propofol in endotoxemia. Anesth Analg. 2005;100:810–6. doi: 10.1213/01.ANE.0000144775.19385.8C. [DOI] [PubMed] [Google Scholar]
- 26.Rojas M, Xu J, Woods CR, et al. Bone marrow derived mesenchymal stem cells in repair of the injured lung. Am J Resp Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 28.Kotton DN, Ma BY, Cardoso MW, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 29.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz EM, Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: expanding the spectrum of cell therapy. Isr Med Assoc J. 2009;11:209–11. [PubMed] [Google Scholar]
- 31.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss DJ, Kolls JK, Ortiz LA, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–67. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc. 2008;5:323–7. doi: 10.1513/pats.200712-169DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51:5–16. [PubMed] [Google Scholar]
- 35.Krause DS. Bone marrow-derived lung epithelial cells. Proc Am Thorac Soc. 2008;5:699–702. doi: 10.1513/pats.200803-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitkovsky D, Hescheler J. Adult mesenchymal stromal stem cells for therapeutic applications. Minim Invasive Ther Allied Technol. 2008;17:79–90. doi: 10.1080/13645700801969758. [DOI] [PubMed] [Google Scholar]


