Abstract
Stem cell therapy is a new strategy for chronic ischaemic heart disease in patients. However, no consensus exists on the most optimal delivery strategy. This randomized study was designed to assess cell delivery efficiency of three clinically relevant strategies: intracoronary (IC) and transendocardial (TE) using electromechanical mapping guidance (NOGA) compared to surgical delivery in a chronic pig model of ischaemic cardiomyopathy. Twenty-four animals underwent delivery of 107 autologous Indium-oxine-labelled bone marrow-derived mesenchymal stem cells (MSC) 4 weeks after infarction and were randomized to one of three groups (n = 8 each group): IC, TE or surgical delivery (reference group). Primary endpoint was defined as percentage (%) of injected dose per organ and assessed by in vivo gamma-emission counting. In addition, troponin and coronary flow were assessed before and after MSC injection. Blinded endpoint analysis showed no significant difference in efficiency after surgical (16 ± 4%), IC (11 ± 1%) and TE (11 ± 3%) (P = 0.52) injections. IC showed less variability in efficiency compared with TE and surgical injection. Overall, TE injection showed less distribution of MSC to visceral organs compared with other modalities. Troponin rise and IC flow did not differ between the percutaneous groups. This randomized study showed no significant difference in cell delivery efficiency to the myocardium in a clinically relevant ischaemic large animal model between IC and TE delivery. In addition, no differences in safety profile were observed. These results are important in view of the choice of percutaneous cell delivery modality in future clinical stem cell trials.
Keywords: stem cells, catheters, chronic ischaemia, nuclear medicine
Introduction
After myocardial infarction (MI), chronically ischaemic myocardium may result in variable degrees of scar tissue. Native endogenous repair mechanisms are insufficient to prevent cardiac remodelling to occur, consequently infarct-related heart failure remains a major cause of morbidity and mortality [1]. Cell therapy emerged as an innovative and attractive therapeutic approach for patients with chronic myocardial ischaemia. The ultimate goal of this treatment is to support and enhance the endogenous repair mechanisms by replacing dysfunctional cardiomyocytes and inducing angiogenesis.
A modest beneficial effect was observed in clinical and pre-clinical studies [2,3]. One of the critical issues for the limited success of stem cell-based therapy for myocardial repair is an efficient method for cell delivery [4]. Currently, two percutaneous approaches (e.g. intracoronary (IC) and transendocardial (TE) delivery) have been applied for treatment with different cell populations in patients with chronic ischaemia [5–8].
However, a direct randomized comparison between IC and TE using the NOGA system with blinded endpoint analysis in a chronic MI model has not been performed. Surgical injection is considered as a reference strategy, because direct visualization of the area of interest, and direct monitoring of the injection is possible. A (pre-) clinically applicable method for accurate quantification of cell retention is direct cell radiolabelling by Indium-111 oxine (111In). This allows determination of cell transplantation efficiency, and thereby enables optimization of cell delivery. Therefore, our primary objective was to determine the most efficacious cell delivery technique in a randomized comparison between these clinically available transplantation modalities in a chronic ischaemic large animal model.
Materials and Methods
Animals
Twenty-four female Dutch Landrace pigs (±70 kg) received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals”, published by the National Institutes of Health (National Institutes of Health publication 85-23, revised 1985). The study protocol was approved by the Animal Experimentation Committee of the University of Utrecht.
Anaesthesia and euthanasia
Animals were anaesthetized in the supine position and intubated with an endotracheal tube. The animals were mechanically ventilated with the use of a positive-pressure ventilator with a mix of oxygen and air (FiO2 0.5). General anaesthesia/analgesia was maintained with midazolam (0.7 mg/kg/hr), sufentanyl citrate (2 μg/kg/hr) and pancuronium bromide (0.1 mg/kg/hr). Metoprolol was administered intravenously (5 mg) to reduce the mechanical stress of the heart. The day before operation, 160 mg acetylic salicylic acid and a bolus of 75 mg clopidogrel were administered. During surgery, animals were anticoagulated with heparin (ACT > 250 sec.). At the end of the experiment, the animals were killed by pentobarbital overdose.
Myocardial infarction procedure
During the entire procedure, electrocardiogram, arterial pressure and capnogram were continuously monitored. MI was created by temporary proximal ligation of the left circumflex artery (LCX) for 75 min., as previously described [9]. To prevent ventricular arrhythmias, 300 mg amiodarone was given intravenously.
Randomized comparison on delivery efficiency
In 24 healthy animals, MI was surgically induced after median sternotomy. Four weeks later, the animals received autologous cell transplantation and were killed after nuclear imaging (Fig. 1). After surgery, animals were randomly assigned to one of three groups (n = 8 per group): IC delivery, TE delivery or surgical delivery (reference group). The randomization scheme was stored in a sealed envelope and retrieved after induction of MI by a person not involved in the study. After recovery, the animals received daily an oral dose of 50 mg metoprolol, 400 mg amiodarone, 75 mg clopidogrel and 160 mg acetylic salicylic acid until termination to prevent thrombosis and arrhythmias. Primary blinded endpoint was defined as percentage (%) of injected dose per organ derived from whole-body images observed at 4 hrs after injection. To evaluate myocardial damage of percutaneous interventions, blood samples (2.5 ml) were collected after MI, before and 6 hrs after the intervention for the measurement of plasma concentration of cTnI.
Fig. 1.
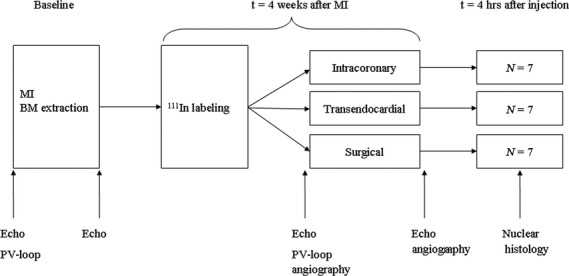
Study design. Intracoronary infusion was performed by stop-flow technique and transendocardial injection by using electromechanical mapping guidance (NOGA). Surgical injection was used as a reference group in this randomized study. BM: Bone marrow; MI: myocardial infarction; 111In: Indium; PV-loop: pressure-volume loop.
Cell culture and labelling
A total of 20–25 ml bone marrow was extracted from the sternum by a heparinized syringe before creating MI. Bone marrow-derived MSC were isolated by Ficoll density gradient centrifugation. Autologous MSC were isolated and cultured in M-199 (Lonza, Verviers, Belgium) supplemented with 10% FBS, heparin and 1% penicillin/streptomyocin. The cells were incubated at 37°C and medium was changed every 3 days. Cells were cultured in 75 cm2 flask and passaged when they reached confluence, till passage 5–7. MSC were frozen in 10% DMSO and 90% culture medium. MSC were characterized as previously described [10]. Seven days prior to transplantation, cells were thawed, plated in flasks and grown to confluency. At the day of cell delivery, before trypanization, cells were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Carlsbad, CA, USA) according to manufacturer's protocol.
Subsequently, MSC (107) were labelled with 30 MBq 111In at 37°C for 20 min. After incubation, cells were washed three times with HANKS buffer (Invitrogen) to remove unbound label. Radiolabel uptake efficiency was measured with a dose calibrator (Veenstra, Joure, The Netherlands). After labelling, cell viability was assessed via trypan-blue (Sigma-Aldrich, St. Louis, MO, USA) counting. Before injection, MSC were resuspended in 2 or 10 ml PBS, depending on the delivery technique.
Intracoronary delivery
Four weeks after MI, an over-the-wire balloon (Boston Scientific Corp, Natick, MA, USA) of equivalent size to the proximal LCX artery was placed. The balloon was inflated at low pressure (2 atm) at the same location and 3.3 ml of cell suspension was infused over 30–45 sec. The angioplasty balloon was deflated after 3 min. This procedure was repeated three times and a total of 10 ml (107 cells) was infused. After the procedure, coronary angiography was performed to confirm vessel patency using the Thrombolysis in Myocardial Infarction (TIMI) score [11]. Blinded image analysis was performed by an independent observer not involved in the study protocol.
Transendocardial delivery
After placement of an 8-F sheath into the femoral artery, a three-dimensional electromechanical map of the left ventricle (LV) was obtained using the NOGA system (Biosense Webster, Cordis, Johnson & Johnson, USA) as described previously [12,13]. First, an electromechanical map was obtained by retrograde passage of the catheter through the aortic valve into the cavity of the LV. Next, 10 TE injections of approximately 0.2 ml were slowly performed (30–40 sec.) using the MYOSTAR® injection catheter (Biosense Webster, Cordis, Johnson & Johnson, Diamond Bar, USA). Two injections were placed in the infarct zone and eight in the border zone. Injections were only given in areas with a unipolar voltage >6 mV [12,13].
Surgical delivery
Lateral thoracotomy was performed and the pericardium was opened to expose the lateral surface. A 1-ml syringe with a 27-gauge needle was used to inject 107 labelled cells. In total, 10 injections of 0.2 ml were performed across the lateral wall in the border and infarcted zone delineated by superficial stitches.
Imaging and analysis
The anaesthetized pigs were positioned in supine position and scanned using a dual-head gamma camera (Forte, Philips, Best, The Netherlands) within 4 hrs after injection to quantify in vivo MSC distribution. A whole-body scan was acquired using the following imaging parameters: medium-energy general-purpose collimator and 512 × 1024 projection matrix. After termination, whole organs (heart; lungs; liver; spleen; kidneys; bladder including urinary catheter) and catheter systems were scanned ex vivo as static anterior and posterior images for 5 min. with the following parameters: medium-energy general-purpose collimator and 256 × 256 projection matrix. Two energy windows were acquired at 174 and 247 keV. The retained activity in syringes was measured by the dose calibrator (Veenstra). After correction for half-life, background and attenuation reconstruction, regions of interest were placed over the major visceral organs and whole-body, using manufacturer's software (Pegasys; Philips, Best, The Netherlands). Post-mortem segmental analysis of the LV was performed in a subset of animals by cutting the LV into five slices from base to apex.
Echocardiography
Chamber dimensions were obtained from transthoracic ultrasound images (5-MHz probe, IE-33, Philips) in short-axis view at the mid-papillary level. All echocardiographic data were analysed using the same protocol. The LV internal diameter (LVID) was measured in longitudinal length and the internal area (LVIA) was obtained without including the papillary muscles in end-systole and end-diastole. The FAS was calculated as ((LVIAED-LVIAES)/LVIAED) × 100. Echocardiographic data were collected after stabilization of the haemodynamics at baseline, MI and before MSC injection. A short echocardiogram was performed after cell injection to exclude a tamponade. Analysis was performed in a blinded fashion.
Pressure-Volume loop protocol
A 7-F conductance catheter was inserted via the left carotid artery into the LV and connected with a signal processor (Leycom CFL, CD-Leycom, Zoetermeer, the Netherlands). The catheter was placed retrogradely along the long axis of the LV. The correct position of the conductance catheter was verified by echocardiography or angiography and by inspection of the segmental conductance signals. The conductance signals were calibrated by thermodilution and hypertonic saline dilution [14,15]. For thermodilution cardiac output measurements and hypertonic saline infusion, a 7-F Swan–Ganz catheter was placed via the right jugular vein into the right pulmonary artery. Data were collected during steady-state conditions with the respirator systems turned off. From these signals, haemodynamic indices were derived. Data analysis and calculations were performed with custom-made software (CD Leycom), as previously described [16]. Parameters of global systolic and diastolic function were calculated during steady-state conditions. Data were collected after stabilization of the haemodynamics at baseline and before MSC injection.
Post-mortem examination
After the pigs were killed, transverse slices of the heart were obtained. All major visceral organs were weighed. Heart samples were snap frozen using liquid nitrogen. Before cutting sections of 7 μm, samples were mounted in Tissue Tek OCT. To detect autologous MSC in histology sections, cells were pre-labelled with CFSE and nuclei were stained with Hoechst dye. Samples were analysed by fluorescence microscopy.
Statistical analysis
Values derived from echocardiography, nuclear imaging and cTnI were analysed in a blinded fashion. Statistical comparison of data between three delivery groups was carried out using one-way anova with Bonferroni post hoc correction, or in case of two groups with an independent t-test. cTnI was compared to baseline using a paired sample t-test. Accuracy of in vivo imaging was determined using a Pearson correlation and intraclass correlation coefficient (ICC). Data are presented as mean ± SE. P-values < 0.05 were considered statistically significant.
Results
Procedural and safety data
In total, 24 animals were included in the efficiency study. Three animals were excluded from the study because of cardiac tamponade demonstrated by obduction (Surgical group; day 1 after MI), sudden death probably because of a fatal arrhythmia as signs of heart failure were absent (IC group; day 28 after MI) and mechanical dysfunction of the MYOSTAR® injection catheter (TE group). After TE injections, no cardiac tamponade was observed by echocardiography. After TE and IC infusion, TIMI 3 flow of the LCX was established in all cases. In one animal (surgical group), pressure-volume loop measurements could not be performed because of instable catheter position. Four hours after MI induction, troponin levels increased to 27 ± 7 μg/l. Four weeks later, a slight increase in cTnI after cell injection was observed in both TE (n = 6) and IC (n = 5) groups from 0.12 ± 0.1 μg/l to 2.70 ± 1.0 μg/l (P = 0.052) and from 0.14 ± 0.04 μg/l to 1.47 ± 0.8 μg/l (P = 0.389) respectively. However, no statistical difference between groups before and after injection was observed (P = 0.789 and P = 0.377) respectively. No significant differences in haemodynamic and echocardiographic parameters between the delivery groups at baseline and 4 weeks after MI were observed, indicating a correct randomization during the study (Table 1). Overall labelling uptake was 61 ± 2% and cell viability was 69 ± 3% before injection. No significant differences were found in cell characteristics (e.g. label uptake, viability, number of cells, labelling time and time from injection till scan) between the three delivery modalities.
Table 1.
Haemodynamics, cardiac geometry and function at baseline and 4 weeks after myocardial infarction
| Parameter | Baseline | 4 weeks after MI | P-value between groups | |||||
|---|---|---|---|---|---|---|---|---|
| IC | TE | Surgical | IC | TE | Surgical | Baseline | Termination | |
| n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 6 | |||
| HR (beats/min.) | 53 ± 4 | 55 ± 3 | 52 ± 2 | 62 ± 3 | 63 ± 2 | 57 ± 4 | 0.864 | 0.456 |
| MAP (mmHg) | 100 ± 3 | 107 ± 4 | 100 ± 4 | 89 ± 7 | 99 ± 5 | 91 ± 6 | 0.308 | 0.455 |
| LVIDED(cm) | 4.8 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.2 | 0.400 | 0.943 |
| LVIDES(cm) | 3.3 ± 0.1 | 3.1 ± 0.2 | 3.5 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.2 | 3.9 ± 0.3 | 0.144 | 0.541 |
| FAS (%) | 49 ± 1 | 50 ± 2 | 47 ± 1 | 41 ± 2 | 36 ± 3 | 32 ± 3 | 0.333 | 0.107 |
| PV-loop derived | ||||||||
| ESP (mmHg) | 108 ± 6 | 103 ± 8 | 100 ± 6 | 95 ± 10 | 87 ± 8 | 76 ± 3 | 0.721 | 0.270 |
| EDP (mmHg) | 9.0 ± 0.5 | 8.0 ± 0.2 | 8.1 ± 0.3 | 13 ± 0.7 | 15 ± 0.7 | 15 ± 1.0 | 0.293 | 0.231 |
| dP/dtMAX (mmHg/sec.) | 1242 ± 34 | 1329 ± 86 | 1293 ± 54 | 1056 ± 128 | 1004 ± 87 | 843 ± 53 | 0.610 | 0.323 |
| dP/dtMIN (mmHg/sec.) | −1074 ± 120 | −933 ± 116 | −948 ± 84 | −960 ± 102 | −949 ± 79 | −833 ± 27 | 0.711 | 0.493 |
| EDV (ml) | 106 ± 3 | 99 ± 6 | 104 ± 5 | 131 ± 7 | 138 ± 13 | 147 ± 5 | 0.528 | 0.530 |
| ESV (ml) | 41 ± 3 | 45 ± 7 | 39 ± 3 | 62 ± 4 | 66 ± 7 | 69 ± 4 | 0.602 | 0.655 |
| EF (%) | 59 ± 3 | 61 ± 4 | 64 ± 3 | 52 ± 4 | 49 ± 6 | 52 ± 3 | 0.486 | 0.868 |
HR: heart rate; MAP: mean aortic pressure; LVIDED: left ventricular internal diameter end-diastolic; LVIDES: left ventricular internal diameter end-systolic; FAS: fractional area shortening; ESP: end-systolic pressure; EDP: end-diastolic pressure; dP/dtMAX: maximal rate of LV pressure increase; dP/dtMIN: maximal rate of LV pressure decrease; EDV: end-diastolic volume; ESV: end-systolic volume; EF: ejection fraction derived from dP/dt; PV-loop: pressure volume loop. Data are presented as mean ± SE.
No difference in delivery efficiency to the heart
Whole-body γ-scan revealed a trend towards higher retention of MSC after surgical delivery (16 ± 4%) compared with IC (11 ± 1%) and TE (11 ± 3%), but this difference was not statistically significant (P = 0.52). Variation in delivery efficiency was less in the IC group (Fig. 2). Qualitative analysis after TE delivery showed a higher local retention of cells at the mid-papillary level in the targeted area compared with widespread distribution of cells in the infarcted area after IC infusion (Fig. 3).
Fig. 2.
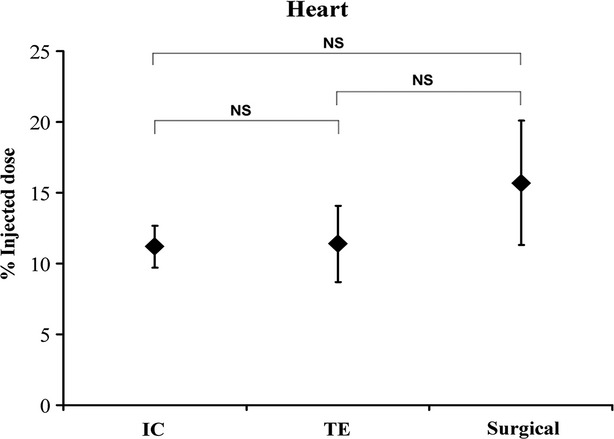
Myocardial retention of autologous MSC at 4 hrs after IC, TE and surgical delivery. No difference in myocardial retention between delivery techniques was detected (n = 7/group; P = 0.52). Variation in efficiency was higher after TE and surgical transplantation (reference group) compared with IC infusion. IC: intracoronary; NS: not significant; TE: transendocardial.
Fig. 3.
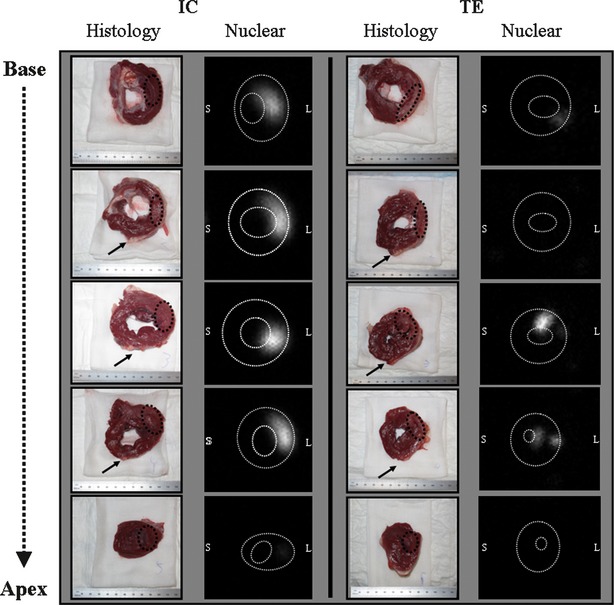
Segmental analysis of the LV after percutaneous delivery. To visualize cell retention in the LV, the heart was cut into five slices from apex to base (histological images). Static anterior images were obtained from all slices within 4 hrs after cell delivery. Note that after TE delivery, cells were mainly retained in the midlateral wall whereas IC infusion showed a more scattered distribution of MSC in the posterolateral wall. Representative histological and nuclear images were derived from the same animals for both groups. Black arrow: left anterior descending artery; IC: intracoronary; L: lateral wall; TE: transendocardial; White dotted lines: endocardial and epicardial border; S: septal wall; Black dotted line: area of interest.
Variation in biodistribution of injected MSC between delivery modalities
High accumulation of 111In-labelled cells in the lungs occurred in all injection groups (Fig. 4). However, TE administration led to significant less retention of cells in the pulmonary tract compared with surgical delivery (P < 0.05). No trend in the lungs was seen for IC (P = 0.52). Low numbers of MSC were detected in the kidneys, liver and spleen. Significantly more labelled cells were distributed to the kidneys after TE injection compared with the other techniques (TE versus IC P < 0.05; TE versus surgical P < 0.001). A minimal amount of labelled MSC was located in the musculoskeletal system and none in the brain. For each delivery modality, about 45% of radioactive cells accumulated in non-target organs.
Fig. 4.
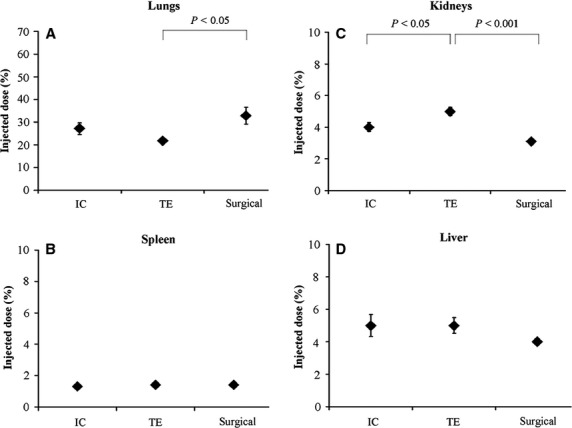
Biodistribution of 111In-labelled MSC to non-target organs. Distribution of 111In-labelled MSC in various organs after IC, TE and surgical (reference) injection (n = 7/group). IC: intracoronary; TE: transendocardial.
Accuracy of in vivo imaging
Figure 5 displays a high correlation between whole-body radiation data and ex vivo measurements (R2 = 0.827). These data support the translational potential of whole-body γ-scan to guide cell therapy approaches from pre-clinical to clinical applications. To describe how strongly measurements resemble each other within the same group, a reliability analysis was performed. The intraclass correlation coefficient (ICC) was above 0.79 in all cases, underlying a strong reliability within groups.
Fig. 5.
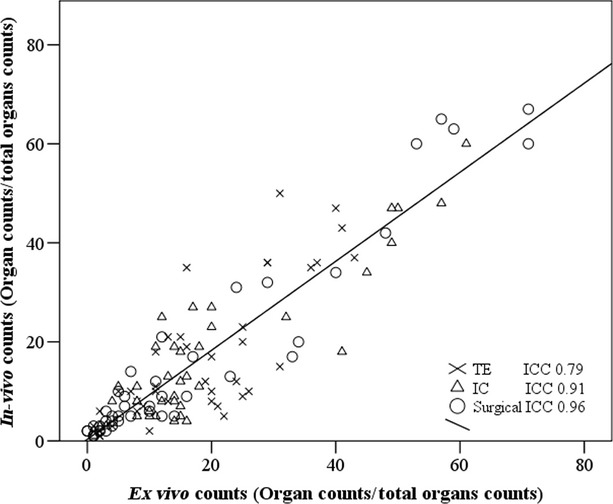
Correlation between in vivo and ex vivo gamma emission counts from major organs. Correlation between in vivo and ex vivo gamma emission counts from heart, lungs, liver, spleen and bladder including urine catheter. The R2 and the intraclass correlation coefficient (ICC) demonstrate a high agreement between quantitative whole-body imaging and post-mortem images.
Histological analysis
Fluorescent microscopy confirmed the presence of CFSE-labelled MSC in the heart, directly after IC, TE and surgical injection (Fig. 6). Clusters of cells were present within the infarcted area and border zone in the histological samples of TE and surgically injected animals, whereas after IC infusion MSC were observed scattered throughout the targeted myocardium. Control samples taken from the remote area showed no CFSE-labelled MSC (data not shown).
Fig. 6.
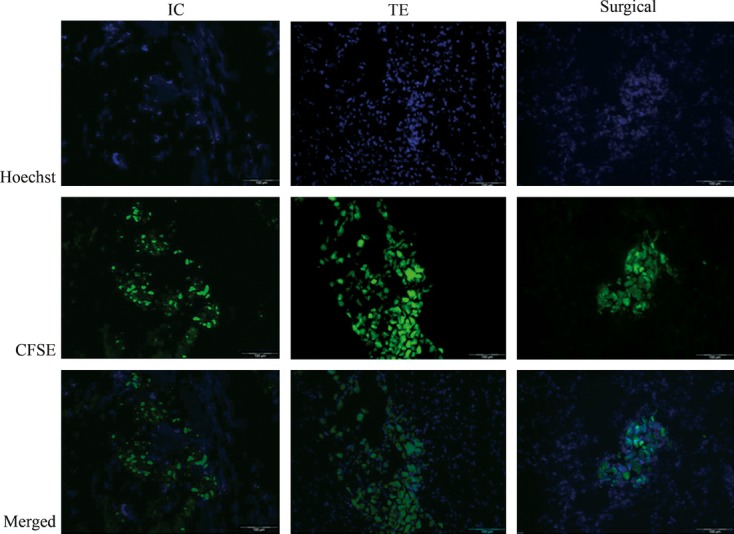
Fluoroscopic images of labelled MSC in the heart. Representative histological sections were stained with Hoechst for nuclei (blue). The green colour indicates presence of CFSE-labelled MSC after IC, TE or surgical delivery. Sections were derived from the same animals as shown in figure 3. Samples taken from remote area showed no CFSE-labelled MSC (data not shown). Scale bar 100 μm, 200× magnification.
Discussion
In this study, for the first time, a randomized comparison with blinded endpoint analysis between IC and TE using the NOGA system was performed and related to surgical injection (reference group). We used a stable porcine model of ischaemic cardiomyopathy and used state-of-the-art nuclear cell tracking to determine the most optimal stem cell delivery strategy in terms of delivery efficiency. The main novel findings are: (1) TE injection was not superior to IC in delivery efficiency; (2) Both techniques were safe, with no significant difference in myocardial damage and no mortality after cell injection.
We observed a slightly higher efficiency (11%) in chronic ischaemic myocardium at 4 hrs after percutaneous cell delivery, which is in contrast to previous studies [17–20] that reported lower retention of transplanted cells (<10%) in acute porcine MI models. The discrepancy between our observations and previous observations can be explained by the differences in study design, MI model, cell type and delivery method. We performed an adequately powered randomized study for one time-point (4 hrs after injection) with blinded endpoint analysis and standard medical care comparable with the clinical treatment of patients with chronic ischaemic cardiomyopathy. MSC retention was assessed in a large animal model of ischaemic cardiomyopathy (i.e. 4 weeks after MI) whereas others performed cell delivery within 7 days after acute MI. In addition, we used autologous MSC in contrast to allogeneic or human MSC, thereby preventing an immunological response or ectopic tissue formation [21–23]. Until now, no study determined MSC retention via nuclear imaging in an ischaemic cardiomyopathy model using the NOGA electronic mapping system, which allows accurate injection by identifying the area of MI and border zone without fluoroscopic guidance [24]. Previous studies on this topic used the Stiletto catheter (Boston Scientific Corporation) or performed surgical injection [17,19].
It was anticipated by others that TE would yield better results than IC. Surprisingly, we could not observe a significant difference in efficiency between percutaneous delivery groups. Moreover, IC infusion clearly demonstrated less variation in efficiency compared to TE. A possible explanation for this finding could be that TE is relatively hampered by (1) the occurrence of premature ventricular arrhythmias as a result of the myocardial injection, or (2) the presence of the posterolateral papillary muscle leading to less stable catheter and needle position during TE delivery. IC is an easy method and relatively operator independent. Our segmental analysis by ex vivo γ-scan revealed site-specific retention of MSC after TE in the ischaemic area compared with uniform distribution of cells after IC. These observations were also found in large acute MI models [20], but were until now not confirmed in an ischaemic cardiomyopathy model. Our findings are also in line with a recently published rodent study showing superiority of IC in terms of uniformity of cell distribution, but additionally also more myocyte regeneration and amount of viable tissue in the area at risk [25].
Commonly mentioned safety issues of cell delivery include (i) arrhythmias [26] and death, (ii) no reflow [17] and myocardial damage because of cell plugging [27], (iii) cardiac tamponade [28] and (iv) cell distribution to non-target organs. We did not observe decreased coronary flow after IC or TE delivery during coronary angiogram. This may be because of the anticoagulation protocol and lower injected cell number compared with other studies [17,29]. This study evaluated the effect of 107 MSC and no dose finding was performed. Regarding myocardial damage, we observed an increase in cTnI (<10%) after percutaneous delivery. Echocardiographic images obtained after TE showed no signs of myocardial perforation. In agreement with other groups [17,19], we noted a substantial redistribution of delivered MSC for all three techniques to non-targeted organs (about 45%). Cells were mainly retained in the lungs and to a lesser extent in the left-sided circulation indicating that most cells left the target area via the myocardial venous or lymphatic system. The possibility that less homing signals are present in chronic damaged myocardium and could lead to the higher redistribution remains to be elucidated. Although no acute adverse effects (e.g. respiratory failure) were observed due to extra-cardiac distribution, long-term side effects cannot be ruled out yet, as the follow-up was 4 hrs.
Recommendations for future stem cell studies
Based on our results (e.g. delivery efficiency and safety data) and others [3], the choice of delivery method could depend on medical indication and practical aspects, as TE and IC yield similar results.
As MSC were injected through an open coronary artery, we suggest the use of IC for patients with a patent coronary artery and TE for severe and untreatable obstruction and/or severe stenosis of epicardial native vessels when using similar amount of cells. In this scenario, IC delivery cannot reach the regions of interest. This is important in view of (large-scaled) clinical trials. As the majority of the delivered cells were retained in non-target organs, organ toxicity should be evaluated in future (pre-) clinical studies.
In our study, low efficiency (11%) to the heart was found using percutaneous delivery techniques. This finding should trigger researchers to develop new catheters or strategies (e.g. microtissues, image fusion or molecular approaches) to improve targeted cell retention.
Limitations
The design of our study did not include a functional evaluation of the different cell distribution, as the goal was to determine the most optimal delivery strategy by evaluating short-term MSC retention. In a pre-clinical meta-analysis (52 studies; 900 animals) we have previously shown that improvements in ejection fraction (EF) after cell therapy were observed [3]. Interestingly, no differences in EF between IC, TE and surgical injection could be noticed. In addition, multivariate analysis showed that the method of delivery was a non-significant predictor of EF improvement [3].
To date, numerous human and pre-clinical trials have already been conducted to assess the efficacy and safety aspects of cardiac stem cell therapy. Obviously, differences exist between large animal models and clinical practice. Healthy young large animals differ from older patients with long-standing coronary artery disease, and frequently co-morbidities (e.g. diabetes, hypertension, renal failure) are present. Despite these differences, a recently published pre-clinical meta-analysis showed that large animal models are valid to predict the outcome of clinical trials [3].
Relatively low cell viability after 111In labelling was observed; however, no difference between groups was found. Moreover, the overall viability was comparable to other groups (69% versus74%) [30].
Conclusions
This randomized study showed no significant difference in delivery efficiency to the myocardium in a clinically relevant ischaemic large animal model. Moreover, no differences in safety profile were observed. These results suggest that the choice of delivery modality could depend on medical indication and practical aspects (costs, side effects on non-target organs and operator experience), as TE and IC yield similar results.
Acknowledgments
This work was supported by the Netherlands Heart Foundation ‘[2003B07304 and 2010T025]’, BSIK program “Dutch Program for Tissue Engineering”, ‘[grant 6746]’, and a Bekalis price (PD). This research is part of the Project P1.04 SMARTCARE of the research programme of the BioMedical Materials institute, co-funded by the Dutch Ministry of Economic Affairs, Agriculture and Innovation. The final contribution of the Netherlands Heart Foundation is gratefully acknowledged.
We gratefully acknowledge Cees Verlaan, Merel Schurink, Marlijn Jansen, Maringa Emons, Ben van Middelaar and Joyce Visser for excellent technical assistance and animal care. In addition, the authors acknowledge the technical assistance provided by Fred van het Schip and Sridevi Jaksani. The authors would also like to thank John Bemelmans and Sieneke Heeres for their skilled technical assistance with the MSC labelling with 111In and with the pig in vivo and ex vivo scintigraphy studies. We thank P. Agostoni MD, PhD (interventional cardiologist) for grading the coronary angiographies.
Conflict of interest statement
None.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: a report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 3.van der Spoel TIG, Jansen of Lorkeers S, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy; systematic review and meta-analysis of large animal models of ischemic heart disease. Cardiovasc Res. 2011;91:649–58. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 4.Bartunek J, Dimmeler S, Drexler H, et al. The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. Eur Heart J. 2006;27:1338–40. doi: 10.1093/eurheartj/ehi793. [DOI] [PubMed] [Google Scholar]
- 5.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 6.Katritsis DG, Sotiropoulou PA, Karvouni E. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–9. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 7.Tse HF, Thambar S, Kwong YL, et al. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (PROTECT-CAD trial) Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 8.van Ramshorst J, Bax JJ, Beeres SLMA, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 9.Timmers L, Henriques JPS, de Kleijn DPV, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–10. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Noort WA, Oerlemans MIFJ, Rozemuller H, et al. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01455.x. doi: 10.1111/j.1582-4934.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count : a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–88. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Haim SA, Osadchy D, Schuster I, et al. Nonfluoroscopic, in vivo navigation and mapping technology. Nat Med. 1996;2:1393–5. doi: 10.1038/nm1296-1393. [DOI] [PubMed] [Google Scholar]
- 13.Gepstein L, Hayam G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart: in vitro and in vivo accuracy results. Circulation. 1997;95:1611–22. doi: 10.1161/01.cir.95.6.1611. [DOI] [PubMed] [Google Scholar]
- 14.Baan J, van der Velde ET, de Bruin HG, et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70:812–23. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]
- 15.Steendijk P, Baan J. Comparison of intravenous and pulmonary artery injections of hypertonic saline for the assessment of conductance catheter parallel conductance. Cardiovasc Res. 2000;46:82–9. doi: 10.1016/s0008-6363(00)00012-2. [DOI] [PubMed] [Google Scholar]
- 16.Steendijk P, Baan J, Jr, Van Der Velde ET, et al. Effects of critical coronary stenosis on global systolic left ventricular function quantified by pressure-volume relations during dobutamine stress in the canine heart. J Am Coll Cardiol. 1998;32:816–26. doi: 10.1016/s0735-1097(98)00313-1. [DOI] [PubMed] [Google Scholar]
- 17.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–22. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 18.Gyongyosi M, Hemetsberger R, Wolbank S, et al. Imaging the migration of therapeutically delivered cardiac stem cells. JACC: Cardiovasc Imaging. 2010;3:772–5. doi: 10.1016/j.jcmg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Hou D, Youssef EA-S, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I–150. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 20.Perin EC, Silva GV, Assad JA, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–95. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–9. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 22.Lyngbaek S, Ripa R, Haack-Sorensen M, et al. Serial in vivo imaging of the porcine heart after percutaneous, intramyocardially injected 111In-labeled human mesenchymal stromal cells. Int J Cardiovas Imaging. 2010;26:273–84. doi: 10.1007/s10554-009-9532-4. [DOI] [PubMed] [Google Scholar]
- 23.Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–29. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 24.Kornowski R, Hong MK, Gepstein L, et al. Preliminary animal and clinical experiences using an electromechanical endocardial mapping procedure to distinguish infarcted from healthy myocardium. Circulation. 1998;98:1116–24. doi: 10.1161/01.cir.98.11.1116. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Guo Y, Ou Q, et al. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–64. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dib N, Dinsmore J, Lababidi Z, et al. One-year follow-up of feasibility and safety of the first U.S., randomized, controlled study using 3-dimensional guided catheter-based delivery of autologous skeletal myoblasts for ischemic cardiomyopathy (CAuSMIC Study) J Am Col Cardiol Intv. 2009;2:9–16. doi: 10.1016/j.jcin.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Mansour S, Vanderheyden M, De Bruyne B, et al. Intracoronary delivery of hematopoietic bone marrow stem cells and luminal loss of the infarct-related artery in patients with recent myocardial infarction. J Am Coll Cardiol. 2006;47:1727–30. doi: 10.1016/j.jacc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 28.Gyongyosi M, Lang I, Dettke M, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med. 2009;6:70–81. doi: 10.1038/ncpcardio1388. [DOI] [PubMed] [Google Scholar]
- 29.Vulliet PR, Greeley M, Halloran SM, et al. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. The Lancet. 2004;363:783–4. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 30.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–61. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]


