Abstract
Cerebrolysin is the only drug available for clinical use containing active fragments of some important neurotrophic factors obtained from purified porcine brain proteins, which has long been used for the treatment of dementia and stroke sequels. Cerebrolysin has growth factor-like activities and promotes neuronal survival and sprouting, however, its molecular mechanism still needs to be determined. It has been shown that Cerebrolysin may interact with proteolytic pathways linked to apoptosis. Administration of Cerebrolysin significantly reduces the number of apoptotic neurons after glutamate exposure. Furthermore, it has been reported that Cerebrolysin inhibits free radicals formation and lipid peroxidation. In vitro we evaluated the protective effects of Cerebrolysin towards spontaneous and induced apoptotic death in cells from healthy individuals. Peripheral blood lymphocytes (PBLs) from 10 individuals were used as cell model; 2-deoxy-D-ribose (dRib), a highly reducing sugar, was used as paradigm pro-apoptotic stimulus. Apoptosis was analysed using flow cytometry and fluorescence microscopy. Our results showed that Cerebrolysin significantly reduced the number of apoptotic PBLs after dRib treatment, although it had no significative effects on cells cultured in standard conditions. Our work showed a protective effect of Cerebrolysin on oxidative stress-induced apoptosis and suggested that PBLs can be used as an easy obtainable and handy cell model to verify Cerebrolysin effects in neurodegenerative pathologies.
Keywords: cerebrolysin, apoptosis, lymphocytes
The multimodal agent Cerebrolysin (Cere) (Ever Neuro Pharma GmbH, Oberburgau, Austria) consists of approximately 25% low molecular weight biologically active peptides, which are actually active fragments of some important neurotrophic factors (CNTF, GDNF, IGF-1, IGF-2) [1] and 75% free amino acids obtained by enzymatic breakdown of purified porcine brain proteins. Cere shows neuroprotective activity and neurotrophic function increasing neuronal survival after different types of lesions in vitro and in animals models of neurodegeneration [2,3], moreover, it promotes neuritic outgrowth and cholinergic fibres regeneration [4,5]. Cere has been used for the treatment of dementia and sequel of stroke and improves memory in patients with mild to moderate cognitive impairment [6,7]. The mechanism of action of neurotrophic factors is based on the modulation of four important endogenous neurobiological processes: neurotrophicity, neuroprotection, neuroplasticity and neurogenesis. Nowadays, Cerebrolysin is a multimodal drug with pleiotropic neuroprotective effects having the capacity to simultaneously regulate, in the post-lesional brain, all the four previously reported neurobiological processes. The pharmacological multimodal effect is based on the ability to link immediate acute neuroprotection with long-term reparatory processes (neurotrophicity, neuroplasticity, neurogenesis) in a similar way to the sequence of endogenous post-lesional regulation [8].
The aim of this research was to in vitro evaluate the protective effects of Cere towards spontaneous and induced apoptotic death in cells from healthy individuals. For this purpose peripheral blood lymphocytes (PBLs) were used as cell model; 2-deoxy-D-ribose (dRib), a highly reducing sugar, was used as paradigm pro-apoptotic stimulus [9]. We analysed PBLs from 15 healthy individuals (nine women and six men), aged between 41 and 63, non-affected by neurological, immunological, vascular or genetic diseases. PBLs were seeded in six-wells plates and cultured for 48 hrs with or without 10 mM dRib and with two different Cere concentrations: 0.8 and 1.6 mg/ml. Cells were collected after 1 and 48 hrs of culture and analysed using flow cytometry [10]. Cells treated with 0.8 mg/ml Cere were also seeded on microscope slides and analysed for alterations in mitochondrial membrane potential (ΔΨm) with JC1, for plasma membrane translocation of phosphatidylserine (PS) with Annexin V, and for caspase-3, -7, -8 and -9 activation with “Carboxyfluorescein FLICA Apoptosis Detection Kit Caspase Assay” (FLICA), as previously described [11]. Statistical analysis of the data obtained was performed using Student's t-test; P-values less than 0.05 were considered to be statistically significant.
Addition of different Cere concentrations to cells cultured without dRib did not alter the number of apoptotic cells (P > 0.05) (Fig. 1a). Addition of Cere to dRib-treated cells determined a reduction in the number of apoptotic cells compared to cells treated only with dRib. After 48 hrs of culture, the number of apoptotic cells in PBLs cultured with 0.8 and 1.6 mg/ml Cere was significantly lower than in PBLs cultured without Cere (P < 0.05) (Fig. 1b).
Fig. 1.
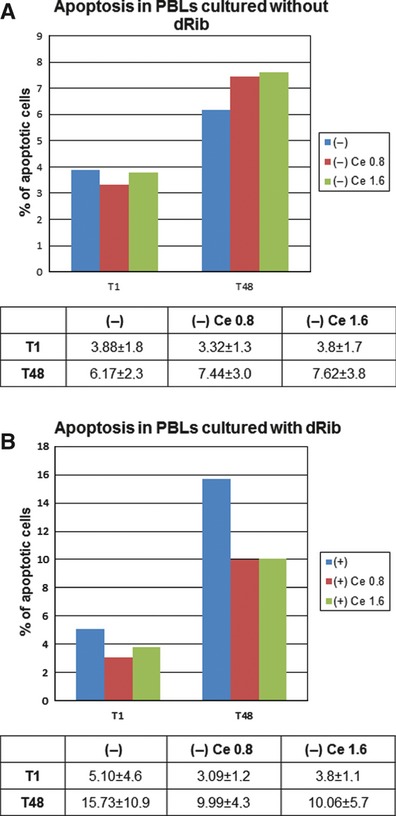
(a) Cytofluorimetric analysis of PBLs cultured in standard condition (−) plus 0.8 and 1.6 mg/ml Cere after 1 and 48 hrs of culture. (b) Cytofluorimetric analysis of dRib treated PBLs cultured without Cere (+) and with 0.8 and 1.6 mg/ml Cere after 1 (T1) and 48 (T48) h of culture. Values are expressed as mean percentage ±SD of apoptotic cells.
Analysis of ΔΨm in dRib-treated PBLs cultured with Cere, after 48 hrs of culture showed reduced green fluorescence respect to dRib-treated PBLs cultured without Cere (Fig. 2a and b), demonstrating a lower degree of ΔΨm. Analysis of PS externalization by Annexin V staining demonstrated that, after 48 hrs of incubation, the number of apoptotic cells was lower in dRib treated PBLs cultured with Cere than in dRib-treated PBLs cultured without Cere (Fig. 2c and d; Table 1). Analysis of caspase activation showed that after 48 hrs of incubation, the levels of activated caspase -3 and -7, -8 and -9 were greater in dRib treated PBLs cultured with Cere than in dRib-treated PBLs cultured without Cere (Fig. 2e, f, g, h, i and j respectively; Table 1).
Fig. 2.
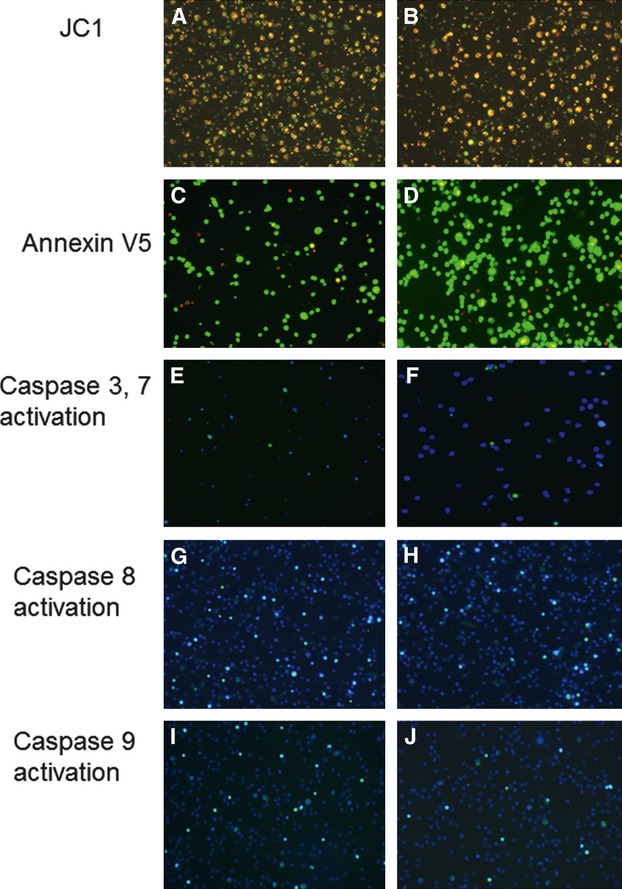
PBLs treated with dRib without Cere (left panel) and PBLs cultured with dRib plus Cere 0.8 mg/ml (right panel) after 48 hrs of culture. (a, b) Evaluation of mitochondrial membrane depolarization with JC1 staining. Green fluorescent mitochondria reflect a drop in ΔΨm. (c, d) Evaluation of externalization of PS and cell viability using AnnVCy3 (red fluorescence) and 6-CFDA (green fluorescence) staining respectively. Double stained (red-green) cells were apoptotic. (e, f, g, h, i, j) Evaluation of caspase-3 and -7, -8 and -9 activation by FLICA staining and simultaneous staining of nuclei with Hoechst 33258. Green fluorescence represents caspase activation.
Table 1.
Mean percentage (±SD) of cells showing PS externalization and caspase activation in dRib treated PBLs (+) and dRib treated PBLs cultured with Cere, after 1 (T1) and 48 (T48) h of incubation. Microscopy analysis was performed by two independent observers. For quantitative evaluation, mean percentage (±SD) of positive cells was calculated evaluating five fields for each slide
| Annexin V | Caspase 3 and 7 | Caspase 8 | Caspase 9 | |
|---|---|---|---|---|
| T1+ | 5.0 ± 1.3 | 4.0 ± 0.9 | 4.2 ± 1.2 | 3.3 ± 0.6 |
| T1 + Cere 0.8 | 2.3 ± 0.5 | 3.5 ± 0.7 | 3.2 ± 0.9 | 2.0 ± 0.7 |
| T48+ | 12.1 ± 3.2 | 11.3 ± 1.8 | 9.2 ± 3.1 | 7.8 ± 1.9 |
| T48 + Cere 0.8 | 7.1 ± 1.8 | 8.0 ± 1.4 | 7.2 ± 1.3 | 5.5 ± 1.1 |
Studies on primary cultures of embryonic chick cortical neurons have demonstrated that administration of Cere significantly reduces the number of apoptotic cells after several apoptotic stimuli [12,13]. It has also been demonstrated that the use of Cere prevents nuclear structural changes typical of apoptosis [14]. It has been suggested that the antiapoptotic effect of Cere may involve the inhibition of calpain [15] a calcium-dependent cysteine protease implicated in apoptosis [16]. Finally, it has been demonstrated that Cere is able to protect neurons against metal-catalysed oxidative damage even though it remains less obvious if it directly acts as a free radical scavenger or it induces expression of scavenging enzymes [17,18].
In this work we used for the first time human peripheral cells to investigate the antiapoptotic effects of Cere. These cells were used to establish a simple and reproducible method of investigating drug effect in easily obtainable cells from normal and pathological genetic human conditions, as previously reported [19]. Our data showed that administration of Cere to PBLs cultured in standard condition has no effect on the reduction of the percentage of apoptotic cells. On the other hand, in dRib-treated PBLs, Cere determines a decrease in the percentage of apoptotic cells, although without a dose-dependent pattern. Our data supported an obvious efficacy of Cere in reducing oxidative stress-induced apoptosis in PBLs. However, as Cere effects are evident only after dRib treatment, we can hypothesize that this molecule might play a direct role in the activation or enhancement of some protective cell mechanism in response to endogenous or exogenous toxic stimuli. Moreover, our data, showing a reduction in apoptosis after Cere administration in PBLs, suggest that the protective effects of Cere can be extended also to non-neuronal cells and that these cells may be used also to study pathological conditions in which drug effect on oxidative stress-induced apoptosis will be evaluated (Formichi et al., unpublished data).
Acknowledgments
This work was supported by Ever Neuro Pharma GmbH (Unterach, Austria) (grant to AF).
Author contributions
PF designed the study, performed the research, analysed the data and wrote the paper. E.R. performed the research, analysed the data and wrote the paper. G.D.M. acquired the data. C.B., D.M. and A.F. designed the research study, revised the paper and approved the submitted and final version.
Conflict of interest
None.
References
- 1.Chen H, Tung YC, Li B, et al. Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol Aging. 2007;28:1148–62. doi: 10.1016/j.neurobiolaging.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 2.HutterPaier B, Fruhwirth M, Grygar E, et al. Cerebrolysin(R) protects neurons from ischemia-induced loss of microtubule – associated protein 2. J Neural Transm Suppl. 1996;46:276. doi: 10.1007/978-3-7091-6892-9_21. [DOI] [PubMed] [Google Scholar]
- 3.Schwab M, Schaller R, Bauer R, et al. Morphofunctional effects of moderate forebrain ischemia combined with short-term hypoxia in rats – protective effects of Cerebrolysin. Exp Toxicol Pathol. 1997;49:29–37. doi: 10.1016/S0940-2993(97)80053-X. [DOI] [PubMed] [Google Scholar]
- 4.Tatebayashi Y, Lee MH, Li L, et al. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer's disease. Acta Neuropathol. 2003;105:225–32. doi: 10.1007/s00401-002-0636-3. [DOI] [PubMed] [Google Scholar]
- 5.Satou T, Itoh T, Tamai Y, et al. Neurotrophic effects of FPF-1070 (Cerebrolysin (R)) on cultured neurons from chicken embryo dorsal root ganglia, ciliary ganglia, and sympathetic trunks. J Neural Transm. 2000;107:1253–62. doi: 10.1007/s007020070015. [DOI] [PubMed] [Google Scholar]
- 6.FrancisTurner L, Valouskova V. Nerve growth factor and nootropic drug Cerebrolysin but not fibroblast growth factor can reduce spatial memory impairment elicited by fimbria-fornix transection: short-term study. Neurosci Lett. 1996;202:193–6. doi: 10.1016/0304-3940(95)12240-0. [DOI] [PubMed] [Google Scholar]
- 7.Ruther E, Ritter R, Apecechea M, et al. Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of the Alzheimer-type (SDAT) Pharmacopsychiatry. 1994;27:32–40. doi: 10.1055/s-2007-1014271. [DOI] [PubMed] [Google Scholar]
- 8.Wei ZH, He QB, Wang H, et al. Meta-analysis: the efficacy of nootropic agent Cerebrolysin in the treatment of Alzheimer's disease. J Neural Transm. 2007;114:629–34. doi: 10.1007/s00702-007-0630-y. [DOI] [PubMed] [Google Scholar]
- 9.Kletsas D, Barbieri D, Stathakos D, et al. The highly reducing sugar 2-deoxy-D-ribose induces apoptosis in human fibroblasts by reduced glutathione depletion and cytoskeletal disruption. Biochem Biophys Res Commun. 1998;243:416–25. doi: 10.1006/bbrc.1997.7975. [DOI] [PubMed] [Google Scholar]
- 10.Nicoletti I, Migliorati G, Pagliacci MC, et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow-cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 11.Formichi P, Battisti C, Tripodi SA, et al. Apoptotic response and cell cycle transition in ataxia telangiectasia cells exposed to oxidative stress. Life Sci. 2000;66:1893–903. doi: 10.1016/s0024-3205(00)00515-4. [DOI] [PubMed] [Google Scholar]
- 12.lHartbauer M, Hutter-Paier B, Skofitsch G, et al. Antiapoptotic effects of the peptidergic drug Cerebrolysin on primary cultures of embryonic chick cortical neurons. J Neural Transm. 2001;108:459–73. doi: 10.1007/s007020170067. [DOI] [PubMed] [Google Scholar]
- 13.Gutmann B, Hutter-Paier B, Windisch M, et al. Long-term effects of cerebrolysin on spatial navigation: comparison of different treatment-schedules. Neurobiol Aging. 2002;23:S86. [Google Scholar]
- 14.Onishchenko LS, Gaikova ON, Yanishevskii SN. Changes at the focus of experimental ischemic stroke treated with neuroprotective agents. Neurosci Behav Physiol. 2008;38:49–54. doi: 10.1007/s11055-008-0007-1. [DOI] [PubMed] [Google Scholar]
- 15.Wronski R, Kronawetter S, Hutter-Paier B, et al. A brain derived peptide preparation reduces the translation dependent loss of a cytoskeletal protein in primary cultured chicken neurons. J Neural Transm Suppl. 2000;59:263–72. doi: 10.1007/978-3-7091-6781-6_28. [DOI] [PubMed] [Google Scholar]
- 16.Squier MKT, Miller ACK, Malkinson AM, et al. Calpain activaction in apoptosis. J Cell Physiol. 1994;159:229–37. doi: 10.1002/jcp.1041590206. [DOI] [PubMed] [Google Scholar]
- 17.Hutter-Paier B, Grygar E, Fruhwirth M, et al. Further evidence that Cerebrolysin(R) protects cortical neurons from neurodegeneration in vitro. J Neural Transm Suppl. 1998;53:363–72. doi: 10.1007/978-3-7091-6467-9_32. [DOI] [PubMed] [Google Scholar]
- 18.Hutter-Paier B, Steiner E, Windisch M. Cerebrolysin(R) protects isolated cortical neurons from neurodegeneration after brief histotoxic hypoxia. J Neural Transm Suppl. 1998;53:351–61. doi: 10.1007/978-3-7091-6467-9_31. [DOI] [PubMed] [Google Scholar]
- 19.Formichi P, Radi E, Battisti C, et al. Apoptosis in CADASIL: an in vitro study of lymphocytes and fibroblasts from a cohort of Italian patients. J Cell Physiol. 2009;219:494–502. doi: 10.1002/jcp.21695. [DOI] [PubMed] [Google Scholar]


