Abstract
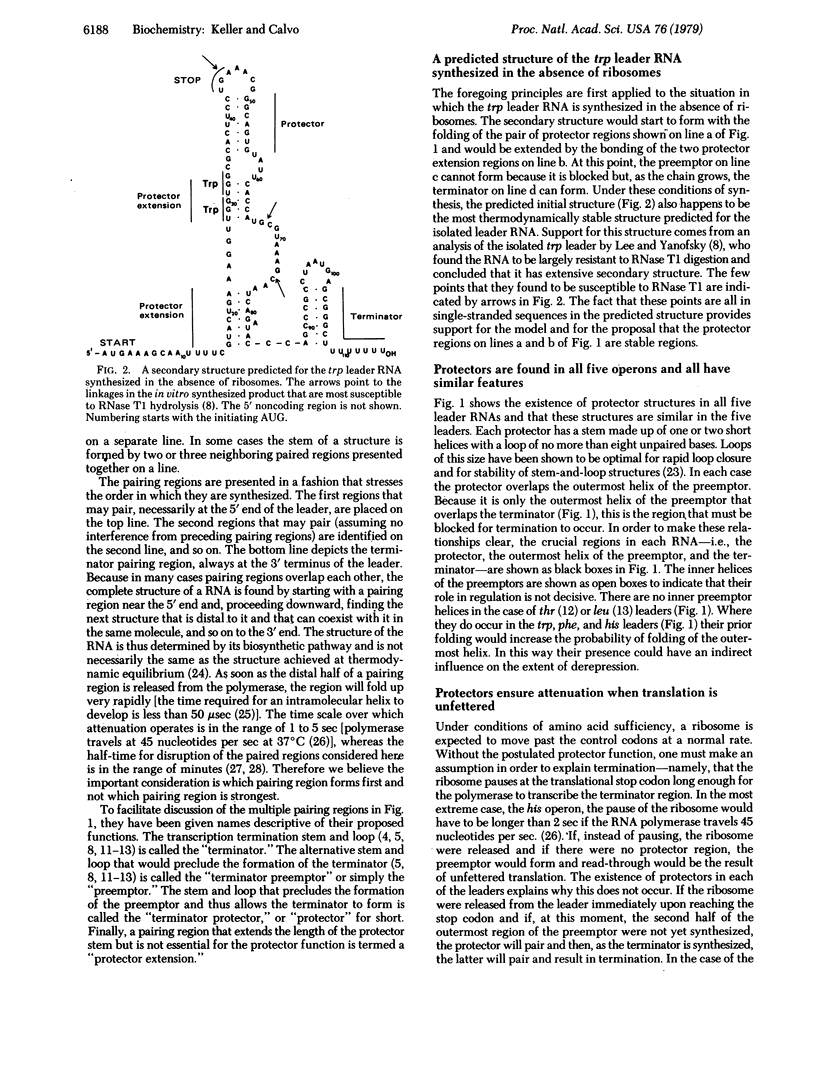
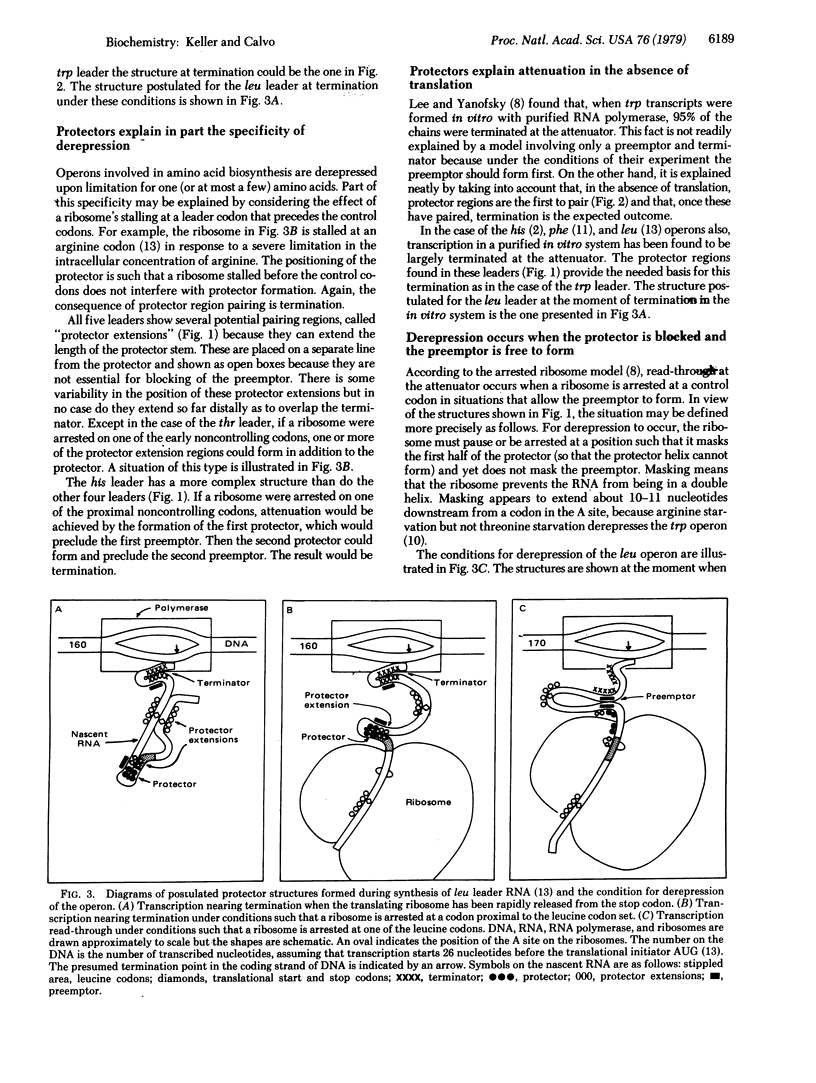
The trp, phe, his, thr, and leu operons of enteric bacteria are regulated by a transcriptional attenuation mechanism. Under conditions of amino acid sufficiency, transcription terminates at an attenuator site after a leader of about 150 nucleotides has been synthesized. Under conditions of limitation of a controlling amino acid, transcription continues past the attenuator into adjacent structural genes. As demonstrated by others, each of the five leader RNAs contains two regions of potential secondary structure which are partially overlapping. One of these regions occurs at the 3' terminus of the leader and is named the "terminator." The other region, which potentially can preclude the formation of the terminator, is named the "preemptor." Conditions that allow the preemptor to form result in derepression. We report here that the five published leader RNA sequences contain an additional potential region of secondary structure, which we call the "protector." The protector partially overlaps the preemptor in such a way that pairing of the former precludes pairing of the latter. For derepression to occur, a ribosome that is translating the leader must block the protector without blocking the preemptor, a condition that is met when the ribosome is arrested at the 3' end of a set of control codons. Including the protector in the model for attenuation explains why derepression of the operon does not result from the arrest of a ribosome at a codon preceding the control set. It also explains why termination is the outcome when transcription occurs in the absence of ribosomes. Finally, termination is the predicted outcome when unfettered translation of the leader RNA occurs, resulting in release of the ribosome at the translational stop signal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Blasi F., Di Lauro R., Frunzio R., Bruni C. B. Nucleotide sequence of the attenuator region of the histidine operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4276–4280. doi: 10.1073/pnas.75.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Sueoka N. Effect of ambient conditions on conformations of tryptophan transfer ribonucleic acid of Escherichia coli. J Biol Chem. 1968 Oct 25;243(20):5329–5336. [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Bertrand K., Bennett G., Yanofsky C. Comparison of the nucleotide sequences of the initial transcribed regions of the tryptophan operons of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):193–217. doi: 10.1016/s0022-2836(78)80005-9. [DOI] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Miozzari G. F., Yanofsky C. Translation of the leader region of the Escherichia coli tryptophan operon. J Bacteriol. 1978 Mar;133(3):1457–1466. doi: 10.1128/jb.133.3.1457-1466.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Brammar W. J., Pouwels P. H. Punctuation of transcription in vitro of the tryptophan operon of Escherichia coli. A novel type of control of transcription. Mol Gen Genet. 1975;136(3):199–214. doi: 10.1007/BF00334015. [DOI] [PubMed] [Google Scholar]
- Pouwels P. H., van Rotterdam J. In vitro synthesis of enzymes of the tryptophan operon of Escherichia coli. Evidence for positive control of transcription. Mol Gen Genet. 1975;136(3):215–226. doi: 10.1007/BF00334016. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER S., MAGASANIK B. EFFECT OF ALPHA-METHYLHISTIDINE ON THE CONTROL OF HISTIDINE SYNTHESIS. J Mol Biol. 1964 Sep;9:670–682. doi: 10.1016/s0022-2836(64)80174-1. [DOI] [PubMed] [Google Scholar]
- Sommer H., Schmitz A., Schmeissner U., Miller J. H. Genetic studies of the lac repressor. VI. The B116 repressor: an altered lac repressor containing amino acid specified by both the trp and lacI leader regions. J Mol Biol. 1978 Aug 15;123(3):457–469. [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck R. E., Zachau H. G. Characterization of the native and denatured conformations of tRNA Ser and tRNA Phe from yeast. Eur J Biochem. 1972 Oct;30(2):382–391. doi: 10.1111/j.1432-1033.1972.tb02109.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Weidner H., Crothers D. M. Pathway-dependent refolding of E. coli 5S RNA. Nucleic Acids Res. 1977 Oct;4(10):3401–3414. doi: 10.1093/nar/4.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]


