Abstract
Endoplasmic reticulum (ER) stress activates an adaptive unfolded protein response (UPR) that facilitates cellular repair, however, under prolonged ER stress, the UPR can ultimately trigger apoptosis thereby terminating damaged cells. The molecular mechanisms responsible for execution of the cell death program are relatively well characterized, but the metabolic events taking place during the adaptive phase of ER stress remain largely undefined. Here we discuss emerging evidence regarding the metabolic changes that occur during the onset of ER stress and how ER influences mitochondrial function through mechanisms involving calcium transfer, thereby facilitating cellular adaptation. Finally, we highlight how dysregulation of ER–mitochondrial calcium homeostasis during prolonged ER stress is emerging as a novel mechanism implicated in the onset of metabolic disorders.
Keywords: Endoplasmic reticulum–mitochondria axis, ER stress, Mitochondrial bioenergetics, Calcium
1. Introduction
The ER is a membranous organelle found in all eukaryotic cells that is crucial for normal cell function and development (Schröder, 2008; Giorgi et al., 2009). It plays a fundamental role in synthesis, folding, sorting, and delivery of proteins to the appropriate cellular destination. Several ER chaperones such as GRP78, GRP94, calnexin and calreticulin bind to the hydrophobic domains of newly synthesized unfolded proteins, to facilitate proper protein folding and inhibit protein–protein aggregation. ATPase activities of ER-localized chaperones provide energetic-influx essential to process thermodynamically unfavorable folding intermediates (Craven et al., 1996; Saris et al., 1997; Schröder, 2008). Disulfide bond formation and N-linked glycosylation also play significant roles in protein folding and are favored by the unique oxidative environment of the ER (Schröder, 2008).
The ER is the main intracellular reservoir for calcium (Ca2+). Many ER chaperones are Ca2+ dependent. Under basal conditions, the luminal Ca2+ concentration is maintained at ~1–2 mM, while prevailing concentrations in the cytosol are around 0.1 μM (Giorgi et al., 2009; Decuypere et al., 2011). ER Ca2+ homeostasis is maintained by controlling efflux via both RyR and IP3R and influx via the SERCA (Giorgi et al., 2009; Decuypere et al., 2011).
ER regulation of Ca2+ homeostasis is a key component of cellular signaling, adaptation, and survival (Schröder, 2008). An elaborate communication system has developed between ER and other organelles, including the Golgi apparatus, plasma membrane, nucleus, and mitochondria (Schröder, 2008; Giorgi et al., 2009; Decuypere et al., 2011). This review will focus on communication between ER and mitochondria, particularly the ability of ER Ca2+ signals to modulate mitochondrial bioenergetics, thus, influencing cellular metabolism and survival.
2. Organelle function
2.1. A platform for sensing ER stress
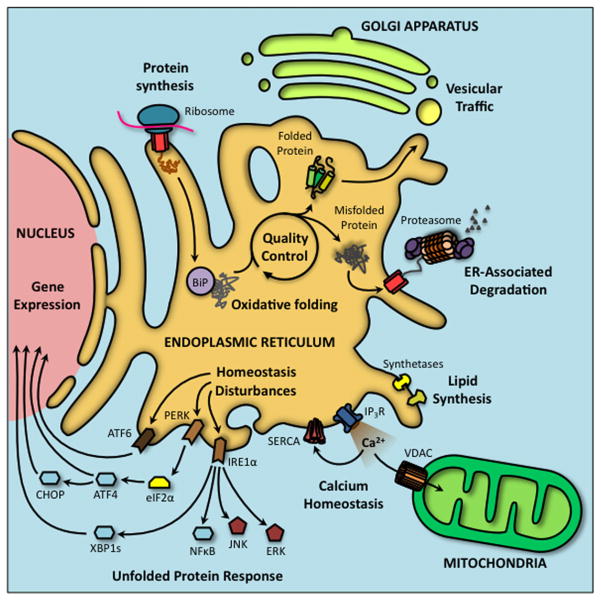
Compromising normal ER function leads to the accumulation of misfolded proteins, which triggers an adaptive ER response referred to as the unfolded protein response (UPR). The UPR restores organelle homeostasis by increasing chaperone abundance, inhibiting general protein synthesis, enhancing degradation of misfolded proteins, and increasing total ER volume (Schröder, 2008). ER stress is sensed predominantly by three ER transmembrane proteins; PERK, IRE1α and ATF6, each of which activates its own a unique cascade of down-stream events. When misfolded proteins accumulate in the lumen of the ER, GRP78 binds to the exposed hydrophobic domains of these unfolded proteins and is competed away from the luminal side of each of the three sensors, relieving inhibition and triggering activation (Kohno et al., 1993; Bertolotti et al., 2000). Although all three-stress sensors participate in the UPR, IRE1α-and PERK-mediated events are most directly relevant to changes in metabolism (Fig. 1).
Fig. 1.
Major physiological functions of the endoplasmic reticulum and their perturbations cause ER stress and activate the unfolded protein response. The endoplasmic reticulum (ER) fulfills several important processes within the cell. Proteins of the secretory pathway are synthesized and folded in the oxidative environment of the ER lumen. Properly folded proteins can be distributed to other subcellular compartments through vesicular traffic. Terminally misfolded proteins are exported to the cytoplasm where they are degraded by the proteasome. The ER plays a major role in lipid biosynthesis and calcium handling, the latter being a primary messenger for communication between ER and mitochondria. The ER serves as a homeostatic stress sensor, activating the adaptive unfolded protein response (UPR) to restore the folding capacity of the ER.
IRE1α is a dual kinase/endonuclease that undergoes oligomerization and activation upon release by GRP78. IRE1α is involved in cytoplasmic splicing of several mRNAs and is necessary for the translation of the fully active isoform of the transcription factor XBP1 (XBP1s). XBP1s, in turn, promotes the transcription of several target genes associated with the UPR, including GRP78 (Lee et al., 2003). Activated IRE1α can also form a multiprotein complex with TRAF2, ASK1 and BAX/BAK, activate JNK, NFkB and MAPK pathways, and tip the balance between inhibition and activation of apoptosis depending upon the duration of ER stress (Hetz et al., 2006; Schröder, 2008).
PERK is a kinase that phosphorylates the translation factor eIF2α, leading to inhibition of general protein translation and promoting preferential translation of the transcription factor ATF4 (Harding et al., 2000a). ATF4 translocates to the nucleus and induces the transcription of additional UPR target genes. ATF4, XBP1s, and ATF6 all converge on the promoter of the gene encoding the transcription factor CHOP, which is associated with apoptotic cell death (Harding et al., 2000b). This is one mechanism through which prolonged activation of all three arms of the UPR can promote cell death, although, experimental evidence demonstrates that CHOP is not essential for cells to die in response to ER. Rather, it appears that prolonged perturbation of ER–mitochondrial calcium homeostasis mobilizes diverse calcium-induced pathways contributing to cell death.
2.2. ER and organelle connections: the mitochondria-associated membrane (MAM)
Dynamic interactions with the ER have been described for most every cellular compartment. As an example, depletion of ER calcium stores activates store-operated Ca2+ entry (SOCE) which then facilitates influx of external calcium through ER–plasma membrane contact sites as a means of replenish internal calcium stores (Wu et al., 2006). Physical interaction between ER and late endosomes (Rocha et al., 2009) and between ER and peroxisomes (Rosenberger et al., 2009) have also been reported, and they seem to be important for lipid metabolism. Among all of the ER partners, mitochondria are arguably the most prominent with regard to regulating metabolism and cell survival. The two organelles physically interact, forming specialized contacts referred to as mitochondrial associated membranes, or MAM, in which membrane and luminal components can intermix and exchange (Vance, 1990). MAM composition adapts in response to multiple internal and external stimuli (Myhill et al., 2008; Bui et al., 2010). Formation and dissolution of ER–mitochondrial contacts is dependent on changes in organelle dynamics. Many proteins involved in ER tubule fusion (Myhill et al., 2008; Bui et al., 2010), mitochondrial distribution (Misko et al., 2010) and organelle morphology (de Brito and Scorrano, 2008) are either integral components of MAM or interact with it.
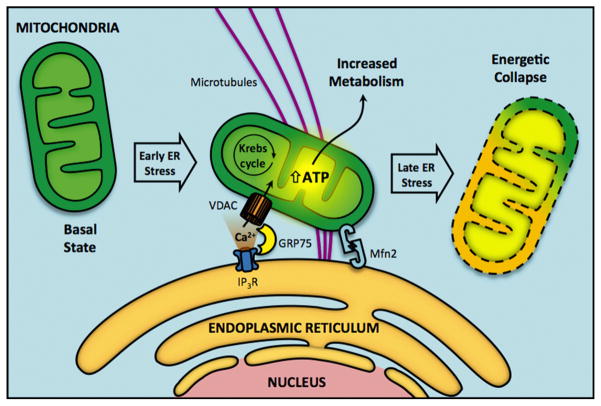
MAM architecture is complex and involves a large number of proteins with widely varying functions (Giorgi et al., 2009). The main ER–mitochondria Ca2+ transfer channels, IP3R and VDAC, located in the ER and mitochondrial sides of MAM, respectively, form a complex with the chaperone GRP75, thus physically connecting the two organelles (Szabadkai et al., 2006). MAM also contain several phospholipid synthesizing enzymes, directing proper sequential lipid synthesis and transfer (Stone and Vance, 2000). Among proteins at the ER–mitochondrial interface, the dynamin-related mitochondrial fusion protein Mfn2 plays an important anchoring role. It is present at both the ER and mitochondrial surface (de Brito and Scorrano, 2008) and directs ER–mitochondrial tethering in addition to its well characterized role regulating intermitochondrial contacts (Chen et al., 2003; Koshiba et al., 2004). In summary, the core protein components of MAM suggest that this structure facilitates a two-way supply of fundamental metabolites (e.g. lipids) or messengers (e.g. Ca2+) that collectively maintain and control mitochondrial function determining the bioenergetic fate of the cell (Stone and Vance, 2000; Giorgi et al., 2009) (Fig. 2).
Fig. 2.
Early phases of endoplasmic reticulum (ER) stress trigger an increase in mitochondrial metabolism which depends critically upon organelle coupling and Ca2+ transfer. The onset of ER stress is characterized by a redistribution of the reticular and mitochondrial networks towards the perinuclear region and a microtubule-dependent increase in their points of connection. Physical coupling is achieved by anchoring proteins, such as mitofusin 2 (Mfn2). This interaction allows an increased Ca2+ transfer from ER to mitochondria, which enhances mitochondrial bioenergetics and ATP production. However, if stress persists, this response promotes mitochondrial collapse and triggers apoptotic cell death.
3. ER and cell physiology: ER stress and the regulation of mitochondrial metabolism
Among the extensive physical and functional ER–mitochondria interactions, Ca2+ exchange is perhaps the best-established mode of communication (Giorgi et al., 2009; Decuypere et al., 2011). Mitochondrial uptake of Ca2+ released through ER-localized IP3R stimulates mitochondrial respiration and ATP production. The activities of several Krebs cycle dehydrogenases are stimulated by Ca2+ either directly (isocitrate and α-ketoglutarate dehydrogenases) or indirectly (pyruvate dehydrogenase) (Decuypere et al., 2011). Basal IP3R Ca2+ release provides essential physiological control over mitochondrial bioenergetics. IP3R knockdown or pharmacological inhibition compromises mitochondrial function, lowering ATP production and triggering autophagy (Cárdenas et al., 2010). Thus, on-going constitutive IP3R Ca2+ release from ER is required for efficient mitochondrial respiration and maintenance of cellular bioenergetics.
As previously discussed, the URP is activated when the capacity of the ER to cope adequately with protein folding is overwhelmed due to an increase in protein load and/or a disruption in the conditions required for appropriate folding. Quality control proteins and molecular pathways induced in response to ER stress are well characterized (Schröder, 2008). However, the metabolic tuning required for cell survival during this process is still poorly understood. Two recent studies have focused on the metabolic events that occur during different stages of ER stress, prior to induction of cell death. Our group showed that during early stages of ER stress, interactions between the mitochondrial and reticular networks increase in a microtubule-dependent manner. As a consequence, Ca2+ transfer to mitochondria is facilitated, thereby enhancing mitochondrial respiration, reductive power and ATP production (Bravo et al., 2011). Wang et al. (2011) showed that prolonged treatment of interleukin 3-dependent Bak−/− Bax−/− hematopoietic cells with tunicamycin for 24 h, resulting in sustained activation of the UPR, caused a profound decrease in mitochondrial metabolism, suggesting metabolic insufficiency as a potential eventual cause of ER stress-associated cell death. Together these studies suggest that during stress, local mitochondria–ER interactions provide the structural framework for an appropriate bioenergetic response that permits cell adaptation (Fig. 2). However whether the initial adaptive metabolic response is necessary for activation of the nuclear branch of the UPR transcriptional program remains to be studied.
4. Organelle pathology: ER and metabolic control in disease
A variety of pathologies such as type-2 diabetes mellitus, insulin resistance, Alzheimer’s disease and cardiovascular disease are characterized by a decrease in cell metabolism. However, the molecular mechanisms responsible for this dysfunction are not fully understood. Alterations in cellular Ca2+, signs of ER stress, decreases in mitochondrial membrane potential and reduced ATP levels are recurrent molecular events associated with these pathologies (Kelley et al., 2002; Lim et al., 2009). As discussed, alterations in the contacts between ER and mitochondria are critical events in the regulation of cellular metabolism (Decuypere et al., 2011) and breakdown of productive ER–mitochondrial communication could underlie mitochondrial dysfunction, metabolic imbalance, and ultimately lead to cell death.
Luciani et al. (2009) have recently demonstrated that the activity of ER Ca2+ channels determines the susceptibility of β-cells to ER stress caused by impaired SERCA function. Their studies implicate mitochondria in apoptotic death of β-cells when ER Ca2+ homeostasis is disrupted. The relevance of these alterations to human diseases has yet to been demonstrated. A causal link between XBP-1, ER stress and development of metabolic pathologies, like insulin resistance and diabetes, was recently described (Ozcan et al., 2004). XBP-1 interacts directly with the transcription factor FoxO1, a regulator of gluconeogenesis, promoting its proteasomal degradation. Hepatic overexpression of XBP-1 results in a markedly reduced blood glucose and increased glucose tolerance in mouse models of insulin resistance and diabetes types 1 and 2. These changes are accompanied by a reduction in FoxO1 in liver, demonstrating that XBP-1 can regulate glucose homeostasis in response to ER stress (Zhou et al., 2010).
Finally, recent reports have suggested that mitochondrial stress can trigger an ER stress response through the induction of gluconeogenic enzymes, as occurs during conditions that lead to insulin resistance (Lim et al., 2009). In summary, the presence of prolonged ER stress and mitochondrial dysfunction in some pathologies, suggests that disruption of ER–mitochondria interactions may be responsible for metabolic alterations detected in a broad number of diseases. Future studies should not only focus on clarifying the metabolic regulation exerted by the ER on mitochondria, but also recognize the importance of mitochondria metabolism in the regulation of ER function and how this two-way “street” is involved in the regulation of cellular metabolism.
Acknowledgments
This research was funded in part by Comision Nacional de Ciencia y Tecnologia (CONICYT), Chile (FONDECYT 1080436 to S.L.; FONDECYT 1110180 to M.C., FONDECYT 3110039 to Z.P.; FONDAP 15010006 to S.L., A.Q., and M.C.), the National Institutes of Health (HL072016, and HL097768 to B.A.R.) and the American Heart Association (0655202Y to B.A.R.). R.B., F.P., A.E.R. and V.P hold CONICYT PhD fellowships.
Abbreviations
- ASK1
apoptosis signal-regulating kinase 1
- ATF4
activating transcription factor 4
- ATF6
activating transcription factor 6
- BAX
Bcl2 associated X protein
- BAK
BCL2-antagonist/killer
- CHOP
C/EBP-homologous protein
- eIF2α
eukaryotic initiation factor alpha subunit
- ER
endoplasmic reticulum
- FoxO1
forkhead box O1 transcription factor
- GRP78
glucose-regulated protein 78
- IP3R
inositol triphosphate receptor
- IRE1α
inositol-requiring enzyme 1 alpha
- JNK
c-Jun N-terminal kinase
- MAM
mitochondria-associated membrane
- MAPK
mitogen activated protein kinase
- Mfn2
mitofusin2
- NFkB
nuclear factor kB
- RyR
ryanodine receptor
- SERCA
sarco/endoplasmic reticulum Ca2+ ATPase
- SOCE
store-operated calcium entry
- TRAF2
TNF receptor-associated factor 2
- UPR
unfolded protein response, XBP1
- VDAC
voltage-dependent anion channel
- X-box
binding protein 1
Footnotes
Conflict of interest
None.
References
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, et al. Increased ER–mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124:2143–52. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui M, Gilady SY, Fitzsimmons REB, Benson MD, Lynes EM, Gesson K, et al. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–83. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RA, Egerton M, Stirling CJ. A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J. 1996;15:2640–50. [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB. The IP(3) receptor–mitochondria connection in apoptosis and autophagy. Biochim Bio-phys Acta. 2011;1813:1003–13. doi: 10.1016/j.bbamcr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–27. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000a;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000b;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–6. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- Kelley D, He J, Menshikova E, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kohno K, Normington K, Sambrook J, Gething MJ, Mori K. The promoter region of the yeast KAR2 (BiP) gene contains a regulatory domain that responds to the presence of unfolded proteins in the endoplasmic reticulum. Mol Cell Biol. 1993;13:877–90. doi: 10.1128/mcb.13.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–62. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009;21:169–77. doi: 10.1016/j.cellsig.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Luciani DS, Gwiazda KS, Yang TLB, Kalynyak TB, Bychkivska Y, Frey MHZ, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422–32. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–40. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19:2777–88. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–25. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger S, Connerth M, Zellnig G, Daum G. Phosphatidylethanolamine synthesized by three different pathways is supplied to peroxisomes of the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2009;1791:379–87. doi: 10.1016/j.bbalip.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Saris N, Holkeri H, Craven RA, Stirling CJ, Makarow M. The Hsp70 homologue Lhs1p is involved in a novel function of the yeast endoplasmic reticulum, refolding and stabilization of heat-denatured protein aggregates. J Cell Biol. 1997;137:813–24. doi: 10.1083/jcb.137.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–94. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–40. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–11. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–56. [PubMed] [Google Scholar]
- Wang X, Eno CO, Altman BJ, Zhu Y, Zhao G, Olberding KE, et al. ER stress modulates cellular metabolism. Biochem J. 2011;435:285–96. doi: 10.1042/BJ20101864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chen KH, Cao W, Zeng J, Liao H, Zhao L, et al. Mutation of the protein kinase A phosphorylation site influences the anti-proliferative activity of mitofusin 2. Atherosclerosis. 2010;211:216–23. doi: 10.1016/j.atherosclerosis.2010.02.012. [DOI] [PubMed] [Google Scholar]