Abstract
Chorioamnionitis is a frequent cause of preterm birth and is associated with an increased risk for injury responses in the lung, GI tract, brain and other fetal organs. Chorioamnionitis is a polymicrobial non-traditional infectious disease because the organisms causing chorioamnionitis are generally of low virulence and colonize the amniotic fluid often for extended periods, and the host (mother and the fetus) does not have typical infection related symptoms such as fever. In this review, we discuss the effects of chorioamnionitis in experimental animal models that mimic the human disease. Our focus is on the immune changes in multiple fetal organs and the pathogenesis of chorioamnionitis induced injury in different fetal compartments. Since chorioamnionitis disproportionately affects preterm infants, we discuss the relevant developmental context for the immune system. We also provide a clinical context for the fetal responses.
Clinical Context for Chorioamnionitis
This review will emphasize primarily the effects of chorioamnionitis on fetal inflammation and immune modulation in animal models because information on human fetal and newborn responses are limited and compromised by the complex nature of chorioamnionitis syndromes. An assumption has been that the normal fetus is in a sterile intrauterine environment, but this assumption is being challenged by more analytical and sensitive PCR and deep sequencing techniques [1]. These studies are presently in their infancy, but our hunch is that the layers of the endometrium/decidua/chorion/amnion are a highly active innate host defense system that normally is protecting the fetus from pathogens but perhaps not from low grade and benign commensal organisms. It is clear that early preterm deliveries following preterm labor with or without rupture of membranes are highly associated with chorioamnionitis [2]. More recent studies using culture or PCR demonstrate that chorioamnionitis is caused by polymicrobial infection with organisms not traditionally considered as pathogens [1, 3, 4]. However, it is also well established that presence of bacteria in the amniotic fluid does not always result in preterm delivery, nor does it always induce chorioamnionitis. Thus, studies of immunologic outcomes in preterm newborns following exposure to chorioamnionitis are confounded by the imprecision of the histologic diagnoses. A diagnosis of chorioamnionitis provides no information about the organisms, the duration of the exposures, and very little about the intensity of the fetal exposure. Clinical outcomes are further confounded by the frequent use of antibiotics and the appropriate use of antenatal corticosteroids, which will modulate immune responses. A further difficulty with the interpretation of clinical studies is the lack of a control group for comparison with infants exposed to chorioamnionitis. Infants from indicated deliveries, often for growth restriction or pre-eclampsia, are not normal. Therefore, we will emphasize the experimental studies that can demonstrate the fetal responses to chorioamnionitis can occur. What does occur is no doubt much more complex and will be the subject for research for years to come.
Animal models of chorioamnionitis
Chorioamnionitis in humans is an ascending infection, where the organisms in the upper genital area ascend in to the chorio-decidual space or the chorioamnion space through the cervix [5]. Organisms are thought to spread diffusely through the chorio-decidual or the chorioamnion plane and then invade in to the amniotic cavity. However, a recent study using molecular microbiologic techniques in human placentae demonstrated that the initial event is a localized chorio-decidual infection, which then invades into the amniotic cavity and thereby infecting amniotic fluid and the fetus prior to diffuse chorio-decidual inflammation [6]. This sequence is consistent with experiments in the Rhesus macaque demonstrating that localized chorio-decidual infection with live Group B streptococci did not trigger preterm labor until the amniotic fluid was colonized [7]. However, a transient chorio-decidual infection can induce cytokine production in the amniotic fluid, which resulted in fetal lung inflammation without overt infection of amniotic fluid, or preterm labor [8]. Therefore animal models of chorioamnionitis resulting from injection of inflammatory agents or organisms into the amniotic fluid reproduce the pathology of chorioamnionitis. In this paper we will review experiments in which the sheep, non-human primates, mouse, rabbits were given intra-amniotic or intrauterine injection of agonists/organisms. We will not review experiments with intraperitoneal or intravascular injection of agonists since these models reflect maternal septicemia or bacteremia, which is a rare event in human chorioamnionitis.
Models of chorioamnionitis have been described with intrauterine injection of agonists or live bacteria in the mouse [9, 10], and the rabbit [11–13]. Chorioamnionitis can also be induced by intra-amniotic injection in the sheep using different agonists including IL-1ß [14], IL-1α [14], LPS (ligand for TLR4) [15], and live Ureaplasma parvum [16]. In the Rhesus macaque, intra-amniotic injection of Group B streptococci [17], Ureaplasma parvum [18], IL-1ß [19, 20] or TNF [19] causes chorioamnionitis. In the sheep, intra-amniotic injection of PamCysK4 (ligand for TLR2) induced weak fetal lung inflammation, but poly I:C (TLR3 ligand) did not cause inflammation [21]. Interestingly intraamniotic injection of TNF α [22], IL-6 or IL-8 [23] did not induce lung inflammation in fetal sheep and intra-amniotic injection of IL-6 or IL-8 did not induce preterm labor in the rhesus macaque [19]. These experiments demonstrate relative specificity or potency of responses to different inflammatory agents.
Ureaplasma spp. are among the smallest free-living, self-replicating microorganisms organisms and are the most frequently isolated from women with chorioamnionitis [24, 25]. The strongest evidence that Ureaplasma can cause preterm labor is from experiments in Rhesus macaques. Intra-amniotic injection of Ureaplasma parvum or the related organism Mycoplasma hominis induced chorioamnionitis, fetal inflammation, and preterm labor [18]. In the sheep, Ureaplasma parvum is poorly cleared from the amniotic compartment after IA injection but does not cause preterm labor [26]. In the amniotic fluid of sheep, Ureaplasmas have antigenic variations with changes in the multi-banded antigen and extensive horizontal gene transfer resulting in resulting in hybrid forms of Ureaplasma serovars, implying unstable genotypes during the course of infection [25, 27, 28]. These antigenic changes perhaps evolved as a mechanism to evade host immune system. Curiously, chorioamnionitis caused by Ureaplasma is of variable intensity in fetal sheep – ranging from essentially none to severe, but without differences in the titer of the organism in amniotic fluid [26, 27]. Similarly in the human, Ureaplasma can be recovered without severe chorioamnionitis or progressive preterm labor [29, 30].
Chorioamnionitis - inflammation/injury and maturation of the fetal lung
One of the striking and counter intuitive effects of intra-amniotic injection of LPS or IL-1 in the fetal sheep is the increase in pulmonary surfactant lipids (Figure 1A) and surfactant associated proteins A, B, C [14, 16, 20, 31, 32]. The increase in airway surfactant and changes in lung structure results in improved lung mechanics in the preterm resulting in `clinical lung maturation”. This effect of chorioamnionitis induced lung maturation is more potent than that resulting from maternal administration of betamethasone in the sheep [33]. IA injection of LPS with tracheal ligation prevented the lung maturation [34, 35], but fetal intratracheal infusion of LPS or IL-1 resulted in increased lung maturation, demonstrating the sufficiency of direct airway contact with LPS or IL-1 for lung maturation [36, 37]. The precise signaling that results in fetal lung maturation is not known. However, intramuscular injection of an anti-CD18 antibody largely decreased inflammatory cell influx in the fetal sheep and significantly decreased the lung maturation [38]. Similarly, human IL-1 receptor antagonist decreased the IA LPS induced lung maturation [39]. These experiments in the fetal sheep demonstrate that IL-1 signaling is central to the lung maturation induced by LPS. The numbers of inflammatory cells recruited to the lung correlated with the surfactant lipid pool size, suggesting that it will be difficult to separate the inflammatory effects of these agonists from the beneficial lung maturation effects [14].
Figure 1. IA LPS induces lung maturation but interrupts normal alveolar development in preterm lambs.
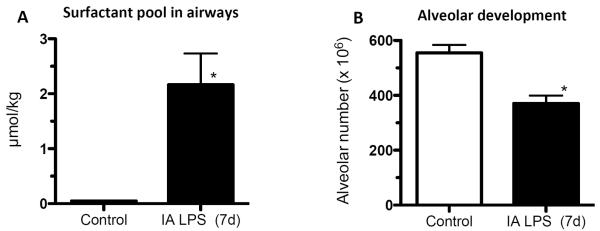
Sheep were given IA LPS (E. coli lipopolysaccharide) and delivered 7d later at 125d gestational age (80% gestation). (A) IA LPS increased surfactant saturated phosphatidyl choline (the largest component of pulmonary surfactant) in the airways. (B) Lung morphometry revealed ~30% fewer alveoli in the IA LPS group. (*p<0.05 vs. control, Data from [39] and [40]).
Despite the beneficial lung maturation, IA injection of pro-inflammatory agonists interferes with normal lung development. In the sheep, IA LPS decreased alveolar numbers, caused thinning of the alveolar septae and increased the size of the alveoli (Figure 1B) [40]. An important feature of alveolarization is the expression of elastin that identifies sites of secondary septation. IA LPS induced an aberrant and decreased expression of elastin [41]. This inhibition of formation of terminal respiratory units was also reproduced in fetal mouse lung explants treated with LPS [42]. In this explant model, LPS inhibited FGF-10 expression, which required macrophage NF-κB signaling [43]. IA LPS in the preterm fetal sheep also inhibited several genes critical for vascular development including VEGF-A, VEGFR2, and NOSIII [44]. IA LPS caused smooth muscle hypertrophy in the resistance arterioles and adventitial fibroblast proliferation of the fetal sheep lung [44]. These changes of vascular remodeling resulted in increased pulmonary vascular resistance [45].
In the sheep, IA injection of U. parvum increased lung surfactant pools and induced a clinical lung maturation, although less consistently than LPS or IL-1 [16, 46, 47]. IA U. parvum also induced mild lung inflammation with mixed neutrophil and monocytic infiltration and low cytokine expression in the fetal lung. However, Ureaplasma caused a mild decrease in elastic foci in secondary alveolar septae, impaired alveolar development, and increased smooth muscle around bronchioles and pulmonary artery/arterioles [48], similar to the changes caused by LPS. In contrast to what happens in the sheep, IA Ureaplasma parvum (and the related organism Mycoplasma hominis) caused severe fetal inflammation in the fetal Rhesus macaque lung, characterized by increased neutrophils and macrophages and alveolar type II cell proliferation [18]. These results suggest species differences in susceptibility to Ureaplasma.
In experiments simulating the effects of intrapartum exposure to inflammation, fetal lung inflammation induced changes similar to bronchopulmonary dysplasia in transgenic mice. Human IL-1b expressed in airway epithelial cells during the latter part of mouse gestation caused neutrophil and monocyte influx into the lung, respiratory insufficiency with increased postnatal mortality [49]. IL-1b disrupted alveolar septation with aberrant alpha-smooth muscle actin and elastin deposition in the septa of distal airspaces. IL-1beta also disrupted pulmonary vascular development.
Chorioamnionitis and Downstream Inflammatory Signaling in the Fetal Lung
Intra-amniotic injection of IL-1 induced a robust expression of IL-1ß, IL-8, GM-CSF, MCP-1, and the acute phase reactant serum amyloid A3 in the lungs of fetal sheep and fetal monkeys [20, 50–52]. Interestingly, the Th1 cytokines IFNγ, IL-12, type I interferon inducible genes CXCL9 (MIG) and CXCL10 (IP-10) and the Th2 cytokines IL-4, IL-13 were not induced [20]. IA LPS increased expression of additional genes inducible by the type I interferon signaling, including CXCL9 (MIG) and CXCL10 (IP-10) [53]. In the fetal sheep, IA LPS increased lung expression of TLR4 and TLR2 mRNA expression [21] but decreased TLR4 expression in the gut [54] demonstrating organ specific responses. Nether IA IL-1 nor LPS significantly increased expression of TNFα in the lung, and IA TNF did not induce chorioamnionitis or fetal lung inflammation [22]. The counter regulatory cytokine IL-10 and IL-1ra are only modestly increased in the fetal lung exposed to IA IL-1 or LPS [39].
Expression of candidate genes associated with lung injury was assessed. Caveolins (Cavs) are implicated as major modulators of lung injury and remodeling by multiple signaling pathways by virtue of their strategic positioning in the lipid rafts of plasma membranes. IA LPS decreased the expression of Cav-1 in the preterm fetal lung [55]. The decreased expression of Cav-1 was associated with the activation of the Smad2/3, Stat 3, a-SMase/ceramide pathways, and increased expression of HO-1 [55]. Consistent with activation of Smads, IA LPS increased lung TGF-ß1 mRNA and protein expression [56]. However the lung expression of CTGF – a key pro-fibrotic protein induced by TGF-ß, decreased upon exposure to IA LPS [56]. Metallo-proteinases regulate the breakdown of extracellular matrix in the lung. The expression of MMP-9 was increased in sheep given IA LPS [57] and in transgenic mice that over express IL-1ß in the lung [58]. Sonic Hedgehog (Shh) signaling is a major pathway directing lung development. IA LPS also decreased Shh mRNA levels and Gli1 protein expression in the fetal sheep [41]. Clearly, chorioamnionitis directly and indirectly changes multiple pathways that have been linked to lung growth and development.
Modulation of cellular innate immunity in the fetus after chorioamnionitis
The sentinel immune cell of the lung is alveolar macrophage. In adult humans and animals, macrophages are located in the airspaces directly in contact with the alveolar hypo-phase. Fetuses do not normally have alveolar macrophages. In mice, macrophages can be detected in the lung interstitium from early gestation [43], while in other species including non-human primates and sheep, very few mature macrophages are found in the fetal lung [50]. In all species, mature alveolar macrophages begin populating the lung in large numbers postnatally with the onset of air breathing. Immature lung monocytes from preterm sheep have a minimal response (IL6 secretion) to an in vitro challenge to LPS and do not respond to TNFα [50]. However, IA LPS matures the lung monocytes by stimulating GMCSF and PU.1 expression in the fetal lung. These monocytes migrate into the fetal alveolar spaces and respond vigorously to both LPS and TNFα in vitro [50].
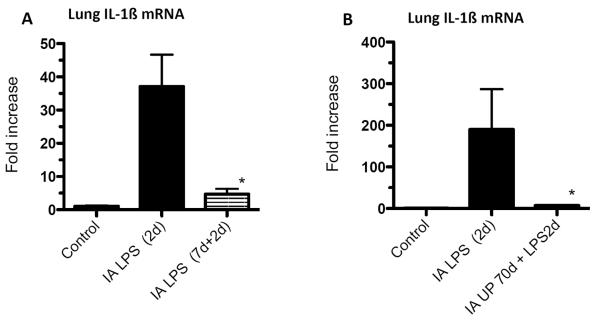
IA LPS also can cause an innate immune tolerance in the fetus. In adult animals and humans, endotoxin tolerance is the suppression of LPS signaling caused by a complex re-programming of inflammatory responses [59]. Pro-inflammatory cytokine expression is down regulated, while there is no change or an increase in the expression of anti-inflammatory genes, antimicrobial genes, genes mediating phagocytosis [59]. In the preterm fetal sheep, exposure to IA LPS 2d before delivery induces a robust expression to cytokines in the fetal lung [52]. However, if the fetus is exposed to two IA LPS injections of the same dose 7d and 2d prior to delivery, the fetal lung is refractory to the 2nd LPS injection (Figure 2A) [60]. Interestingly, both lung and blood monocytes are refractory to an in vitro challenge with LPS [60, 61]. Although the precise mechanisms are not known, the expression of negative regulator of Toll/IL-1 signaling, IRAK-M is increased in both the lung and blood monocytes [60]. Unlike the adult monocytes with endotoxin tolerance in which there is sparing of IL-10, IL-1RA, TGF-ß1 expression [62], there was a near global down-regulation of expression of these cytokines in the fetal lung with endotoxin tolerance. The lung and blood monocytes from fetal sheep exposed to two injections of IA LPS were also refractory to stimulation by a host of other TLR agonists including PamCysK4 (TLR2), flagellin (TLR5), and CpG-DNA (TLR9) [63].
Figure 2. Repeated exposure to IA LPS or a chronic exposure to IA U. parvum induces endotoxin tolerance in preterm lambs.
IL-1ß mRNA expression was measured in the lung obtained at autopsy from sheep delivered at 125d gestational age (80% gestation). (A) Compared to a single exposure to IA LPS (E. coli lipopolysaccharide) 2d before delivery, a second exposure to IA LPS 7d before delivery decreased IL-1ß mRNA expression induced by IA LPS 2d prior to delivery B) A chronic exposure to live UP (U. parvum) given 70d before delivery decreased lung IL-1ß mRNA induced by IA LPS 2d prior to delivery. In frame A IL-1ß expression was measured by a RNAse protection assay while frame B used quantitative rt-PCR using Taqman probes accounting for the different values (*p<0.05 vs. IA LPS 2d, Data from [60] and [65]).
Since Ureaplasma colonization in the setting of polymicrobial flora is common in women with chorioamnionitis, we asked if Ureaplasma would modulate LPS responses. Intra-amniotic injection of U parvum in sheep profoundly suppressed LPS-induced responsiveness [64, 65]. While acute but not chronic exposure to U parvum suppressed LPS-induced chorioamnion inflammation [64], only chronic exposure suppressed fetal lung inflammation (Figure 2B) [65]. Thus, the innate immune tolerance induced by U parvum can have different temporal profiles in different fetal organs.
Very preterm infants frequently develop postnatal sepsis, and many of these infants have had exposure to chorioamnionitis. To simulate this scenario, we gave two IA LPS injections 7d and 2d prior to delivery at 85% term gestation. After delivery, the preterm lambs were given a low intravenous dose of LPS. Compared to control lambs exposed to IA saline, the lambs exposed to IA LPS had less hypotension suggesting a systemic tolerance for cardiovascular effects of LPS [66]. However, no fetal tolerance was seen for cytokine production in multiple fetal organs [66]. Other curious interactions between prenatal and postnatal exposure have also been reported. Rats exposed to IA LPS have impaired postnatal alveolar development [67]. Exposure to 95% oxygen (severe hyperoxia) postnatally further decreased alveolar septation and increased the pulmonary hypertension induced by IA LPS. However, moderate hyperoxia (80% oxygen) improved alveolar growth and ameliorated the development of postnatal pulmonary hypertension [67]. These experiments demonstrate that immune modulation caused by chorioamnionitis may have profound effects on postnatal immune responses to secondary injurious and infectious exposures.
Effects of maternal betamethasone on chorioamnionitis
Maternal betamethasone is the standard therapy for women with threatened preterm labor to decrease RDS and infant death [68]. The delivery of many women given corticosteroids often is delayed for several days to weeks [69], and histologic chorioamnionitis is clinically silent in a majority of women in early gestational preterm labor [70]. Therefore, the combined fetal exposures to antenatal corticosteroids and chorioamnionitis are common, but the order of exposures can vary. Betamethasone modulates LPS-induced chorioamnionitis in the sheep depending on the relative timing of betamethasone, IA LPS injection, and the preterm delivery. When betamethasone and LPS were administered simultaneously, LPS induced lung and chorioamnion inflammation was suppressed at 2d but amplified at 5 and 15d after the exposures [71, 72]. However maternal betamethasone administered before IA LPS, suppressed IA LPS induced inflammation, activation of thymic cells. However, betamethasone did not decrease LPS induced inflammation when administered after IA LPS. These experiments indicate time dependent anti-inflammatory effects of betamethasone [73–75]. Interestingly, the animals with inhibited LPS inflammation (maternal betamethasone given prior to IA LPS) had decreased lung maturation compared to those exposed to IA LPS followed by maternal betamethasone. These results suggest that lung maturation is driven by inflammation in this chorioamnionitis model [73, 74].
Inflammation and injury in the fetal skin
Fetal skin from preterm infants exposed to chorioamnionitis obtained at autopsy had mixed neutrophils, T-cells and histiocytic infiltrates into the superficial dermis. TLR2 but not TLR4 immunoreactivity in the skin was stronger in fetuses with chorioamnionitis than in those without chorioamnionitis. These data demonstrate that dermatitis can be a component of the fetal inflammatory response syndrome (FIRS) [76]. Consistent with the human data, IA LPS induced significant increases in IL-1ß, IL-6, IL8 and TNFα in the fetal sheep skin [77]. Furthermore, using fetal surgery in the sheep with isolation of skin/amnion from the lung and the gut, we demonstrated that fetal skin inflammation needed direct exposure of the skin to LPS, as opposed to being a secondary response to a systemic inflammation [77]. These changes of skin inflammation and upregulation of cytokine expression in the fetal sheep skin were also demonstrated 7 days after IA U. parvum exposure [78]. Although clinicians do not normally think of skin as a response organ, these data clearly demonstrate that skin is a component of fetal inflammatory response syndrome (FIRS) induced by chorioamnionitis.
Immune alteration in the thymus
In humans, thymus development occurs early in gestation with distinct cortical and medullary compartments formed by 16 weeks gestation with population by T-cell subsets including CD4 and CD8 single and double positive αβTCR+ and γδ TCR+ cells as well as CD4+CD25+ FOXP3+ cells [79–85]. These CD4+CD25+ FOXP3+, called regulatory T cells (Tregs), are a vital mechanism of negative regulation of immune-mediated inflammation via multiple mechanisms [86]. Other cell lineages including macrophages and myeloid and plasmacytoid dendritic cells are also present by 16 weeks gestation [81, 87, 88]. Between 14 and 16 wks of gestation, mature T-cells begin to seed the peripheral immune system [83, 85, 89]. The fetal T-cells are thought to be polarized towards a regulatory phenotype, to control fetus responses against maternal chimera [90].
Thymic involution has been widely reported to occur with chorioamnionitis, and particularly with funisitis in preterm or term infants [91–97]. Consistent with the clinical reports, experimental chorioamnionitis induced by IA LPS decreased thymus weight and cortico-medullary ratio beginning at 5h post exposure [98, 99], effects that lasted for several days (5–7d). However, another study did not find the same abnormal thymic architecture 7d after IA LPS [100]. Increased percentages of CD3+ cells, but decreased percentages of FOXP3+ cells, were reported, based on immunohistochemistry analyses, with relatively similar kinetics (starting early after IA exposure and lasting for up to a week) [98, 99]. LPS exposure also reduced the percentages of MHC Class II+ cells, in both the thymic cortex and the medulla, although the type(s) of cells that were diminished –antigen-presenting cells or thymic epithelial cells (TEC)- was not further characterized [100]. These observations could be important, since interactions between TECs and thymocytes are critical for thymic function (reviewed in [101]). At least part of the mechanism involved in acute thymic involution are increased expression of inflammatory cytokines, such as members of the IL-6 family and type I interferons, as well as glucocorticoids induced by activation of the hypothalamus-pituitary adrenal axis [99, 102–105].
Immune alteration in fetal spleen and lymph nodes
Preterm 20–25 wk fetuses exposed to chorioamnionitis have increased neutrophils in the spleen (5-fold) and in the bone marrow [106]. In contrast, the percentage of red and white pulp areas occupied by naïve T cells (CD45RA+), memory T cells (CD45RO+) and B cells (CD20+) decreased significantly in fetuses exposed to chorioamnionitis compared with control subjects (30 wks GA). These patients also exhibited diminished numbers of T cell foci whereas the number of B cell foci was normal [107]. A major limitation of the clinical studies is that the data are from fetuses that are either aborted, stillborn or from early postnatal death reflecting the most severe cases.
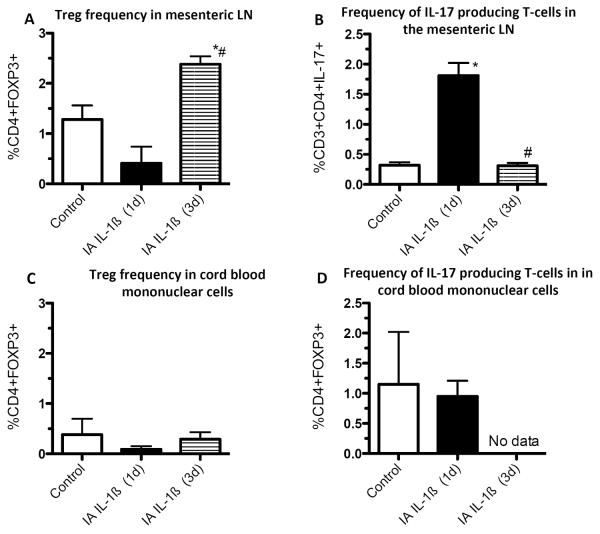
In the sheep, IA LPS or IA IL-1β increased the size of the posterior mediastinal lymph nodes (PMLN) and spleen [34, 48, 108]. In this model, LPS also increased the frequency of CD3, CD4, CD8 and γδ T cells 2–3 days post exposure [34, 48]. In addition, IA IL-1β modified the polarization of the immune response, notably decreasing by 75% the frequency of FOXP3+ cells 1 and 3 days post exposure, with a return to baseline 7d after exposure [108]. Similarly, in fetal rhesus macaques, a single IA-IL-1 exposure altered the balance between inflammatory and Treg cells in spleen and mediastinal and mesenteric lymph nodes: Treg (CD3+CD4+FOXP3+ cells) frequency decreased at 24h, while IL-17A-producing cells increased [20]. In this model of single acute exposure to a very potent inflammatory stimulus, Tregs rebounded and their frequency was increased at 72h compared to controls. IL-17 up regulation was also transient [20]. In addition to modifying T cell subsets, IA IL-1β increased the frequency of activated macrophages and neutrophils (iNOS positive) in the subcapsular and parafollicular regions of the fetal sheep PMLN [108]. Although not much information is available for the spleen, IA LPS exposure increased the expression of IL-1β, IL-6 and IL-8 mRNA in fetal lambs 2d after exposure [35].
Chorioamnionitis can be an indolent infection and repeated injections of intra-amniotic LPS induced endotoxin tolerance in the lung and blood monocytes of fetal sheep [60]. Therefore, we evaluated the changes in lymphoid organs in response to repeated IA LPS injections. In contrast to an acute exposure, repeated IA LPS administered from 90 to 110 days' gestation in the sheep did not change organ weights (PMLN, thymus, spleen) or the proportion of CD8 and γδ T cells [109]. However, repeated LPS exposure increased CD4+ T cell frequency in PMLN, as well as the proportion of CD4+CD25+ cells in this organ. These CD4+CD25+ cells were not further characterized, so they could be a sign of either persisting effector T cell activation, or on the contrary, an indication that chronic chorioamnionitis induced Treg expansion as a counter-regulatory mechanism.
Limited data on responses of lymphoid organs were also reported in Rhesus macaques infected with IA Ureaplasma parvum or Mycoplasma hominis, at ~80% gestation [18]. Fetal spleens had diffuse hyperplasia of reticulo-endothelial cells, accompanied by increases in neutrophils, T-cells, and plasma cells in the red pulp and reactive proliferation of lymphoid follicles 15d after infection. No changes in the lymphoid organs were apparent 6d after exposures [18]. Curiously, these changes of splenic hyperplasia are the opposite of splenic depletion reported in humans [107].
Fetal gut immune alterations, inflammation and injury after chorioamnionitis
Since chorioamnionitis is associated with an increased risk for necrotizing enterocolitis in preterm infants [70, 110], we explored the intestinal responses to experimental chorioamnionitis in the sheep. IA LPS, IL-1 or live U. parvum induced a fetal ileal inflammation, decreased epithelial barrier integrity and an enterocyte injury response [54, 111, 112]. IA IL-1a increased intestinal mRNAs for IL-4, IL-10, IFN-g, and TNF-a. Numbers of CD3+ and CD4+ T-cells and myeloperoxidase+ cells were increased whereas the anti-inflammatory FoxP3+ T-cells were decreased. Intra-amniotic Ureaplasma exposure caused inflammation within 7d with damaged villus epithelium and gut barrier loss. Proliferation, differentiation, and maturation of enterocytes were significantly reduced after 7d of Ureaplasma exposure, leading to severe villus atrophy at 14d. The Ureaplasma mediated impaired development and villus atrophy of the fetal gut was largely prevented by intra-uterine IL-1 receptor antagonist treatment, demonstrating again the importance of IL-1 signaling in this pathogenesis [112]. These experiments indicate that chorioamnionitis can induce intestinal pathology that could be a precursor to necrotizing enterocolitis.
Fetal brain inflammation and injury after chorioamnionitis
Intrauterine injection of live E. coli in rabbits at 70% gestation induced apoptosis and disorganization of the brain white matter 5 to 6 days after exposure [113] but no inflammation in the fetal brain was detected at 30 hours post exposures although a robust placenta and lung inflammation were noted [11]. These experiments demonstrate that fetal brain inflammation and injury was delayed relative to lung inflammation.
In the mice, intrauterine injection of a low dose LPS induced increased expression of IL-6 in the amniotic fluid but only caused preterm labor in 30% of the mice. However, this exposure to LPS significantly increased IL-1ß and IL-6 mRNA expression in the fetal brain [114], decreased dendritic counts in cortical cultures [10, 115]. Expression of glial fibrillary acidic protein, a marker of astrocyte development, and of PLP1/DM20, a pro-oligodendrocyte marker, did not change significantly [114].
In the fetal sheep at about 70–80% gestation, IA LPS induced apoptosis, disrupted axonal development and caused microglial (resident macrophages) activation in all regions of the brain 14–28d after exposure [116, 117]. Furthermore astrocytes were increased in the brain and cerebellum of LPS-exposed fetuses but not in the spinal cord. Mature oligodendrocytes (myelin producing cells) decreased in the cerebral and cerebellar white matter, the cerebral cortex, caudate putamen, and hippocampus 14 days after LPS. Neurons in the cerebral cortex, hippocampus, and substantia nigra were reduced 14 days after LPS [117]. This increase in microglial activation was also reported in rabbits exposed to intrauterine LPS [12]. Although the mechanisms of brain injury are not known, the studies suggest that systemic inflammation leads to microglia activation in the fetal brain causing an injury response. It is important to note that all animal models of chorioamnionitis caused only microscopic changes in the brain and no cystic changes were visible [116].
Immune Alterations in Blood
In a large biomarker study of 506 preterm and term infants out of the 27 markers measured in the blood, only IL-1β, IL-6, and IL-8 were selectively associated with chorioamnionitis and FIRS [118]. Similarly, the white blood count and CRP increased in the maternal and fetal blood, but these changes were modest and of no clinical value for the diagnosis of early onset sepsis [119, 120]. In infants exposed to chorioamnionitis defined by clinical criteria, cord blood T-cells appeared activated compared to T-cells from term infants as judged by the expression of CD25, HLA-DR or CD69 [121].
In the preterm fetal sheep, IA LPS increased cord blood IL-6 and IL-8 5h -7d post injection [122], and had significant effect on blood neutrophils. LPS or IL-1 injection first induced neutropenia 1-2d post stimulation, followed by neutrophilia and increased platelet counts 7d later [39, 108, 122]. Interestingly, in the fetal rhesus, alterations in the frequency of Tregs (Foxp3+) and IL-17 producing cells induced in lymphoid tissues by IA IL-1ß were not detected in the peripheral blood (Figure 3) [20]. This experiment demonstrates the important concept that immune responses occurring in tissues may not be visible in the blood. In contrast, blood monocytes from fetal sheep exposed to two injections of IA LPS have decreased responsiveness to LPS compared to blood monocytes from control sheep demonstrating immune modulation by chorioamnionitis.
Figure 3. IA IL-1ß induces changes in the Tregs and IL-17 producing cells in the mesenteric lymph node but not the blood in preterm rhesus macaques.
Changes in the frequency of Tregs (CD4+, FOXP3+) and IL-17 producing CD3+ CD4+ cells were measured by flow cytometry 1d or 3d after IA IL-1ß in rhesus macaques delivered at 80% gestation. Note the large dynamic changes with an initial reduction and a subsequent rebound in Tregs in the mesenteric LN (lymph node). The changes in IL-17 producing cells were reciprocal to the Treg frequency in the mesenteric LN. In contrast no changes in either Tregs or IL-17+ cells were detected in the blood mononuclear cells. (*p<0.05 vs. control, #p<0.05 vs. IA IL-1ß (1d), Data from [20]).
Compartmentalization of fetal inflammatory response syndrome (FIRS)
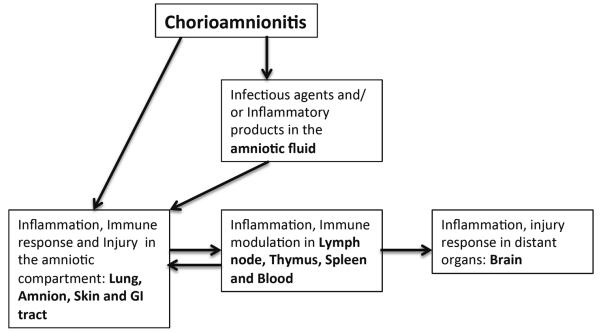
FIRS is the systemic response of the fetus to chorioamnionitis, generally diagnosed clinically as inflammatory cells in the umbilical cord or increased cytokines/acute phase reactants in the cord blood [123, 124]. FIRS has been associated with fetal organ injury responses [124]. The infection in chorioamnionitis is largely restricted to the amniotic compartment and is not systemically disseminated since only 5% of preterm infants with histologic chorioamnionitis have early onset sepsis [125]. Similarly, IA LPS is not detectable in the blood in fetal sheep [60]. Further, an intravascular injection of 6μg LPS causes profound hypotension in preterm sheep, but an IA dose of up to 100mg (10,000 fold excess of IV dose) is well tolerated without hypotension in the fetus [66, 122]. These data strongly argue for a strict compartmentalization of FIRS. Therefore, the adverse effects of chorioamnionitis on organs such as the fetal brain must be mediated by inflammation transduced in organs in immediate contact with the amniotic fluid, which are the lung, gastrointestinal tract, chorioamnion, and skin (Figure 4). To understand which of the organs in contact with the amniotic fluid mediates FIRS, we surgically isolated and infused LPS into lung, the gut, and the amniotic fluid (chorioamnion/skin) [34, 35]. Exposure of the fetal lung and fetal skin/amnion to LPS (but not gastro-intestinal tract) caused FIRS in the fetal sheep.
Figure 4. Schematic representation of the pathogenesis of FIRS after chorioamnionitis.
The initial event is the inflammation and immune response in the amniotic compartment consisting of the amniotic fluid and the organs in contact with the amniotic fluid – amnion/chorion, skin, lung and the GI tract. Immune modulation in the lymphoid organs (thymus, spleen and lymph nodes) and the blood likely occurs via signaling from the amniotic compartment. The injury response in the fetal organs is either due to direct contact (in the amniotic compartment) with the inflammatory products/microorganisms or in distant organs due to immune changes in the blood or lymphatic tissues.
The picture that emerges from animal experiments is that chorioamnionitis induces FIRS in distinct compartments (Table 1): a) in organs in direct contact with the amniotic fluid (lung, GI tract, skin and chorioamnion) and b) in organs not in direct contact with the amniotic fluid e.g. brain (Figure 4). Immune changes in lymphoid organs that may mediate organ injury response include polarization of T-cells in lymphoid organs to pro-inflammatory IL-17 producing cells with a decrease in anti-inflammatory Tregs [20]. Consistent with this notion, clinical studies demonstrate that chorioamnionitis can predispose the preterm infant to increased injury responses to post natal noxious stimuli such as post natal sepsis and mechanical ventilation [126, 127]. On the contrary, the immune modulation can also be anti-inflammatory e.g. endotoxin tolerance in blood monocytes [60]. Therefore the injury responses to chorioamnionitis can be quite variable.
Table 1.
Summary of immune, inflammatory and injury responses in fetus after chorioamnionitis
| Tissue | Stimulus and animal model | Structural alteration | Immune alteration |
|---|---|---|---|
|
| |||
| Lung | IA LPS, IL-1 or U. parvum in Sheep, mouse, rabbit or monkey | ↑ Surfactant lipids, surfactant associated proteins A,B,C [14, 16, 20, 31, 32]. | ↑ Recruited inflammatory cells Neutrophil and monocytic infiltration [52, 122] |
| ↑ Smooth muscle hypertrophy in the resistance arterioles and adventitial fibroblast proliferation [44] | ↑ IL-1β, IL-8, GM-CSF, MCP-1 production [50–52, 122] | ||
| ↓ Alveolar number, alveolar septae [40, 49] | ↑ TLR-2, TLR-4 expression [21] | ||
| ↓ Elastin and Caveolin expression [41, 55] | ↓ Monocyte/ Macrophage response (tolerance) [60, 63, 65] | ||
| ↑Alveolar type II cell proliferation [18]. | |||
|
| |||
| Skin | IA LPS or U. parvum in Sheep | Dermatitis [77, 78] | ↑ IL-1β, IL-6, IL-8, TNF-α production [77, 78]. |
|
| |||
| Thymus | IA LPS in Sheep | ↓ Weight and corticomedullary ratio [98, 99]. | ↑CD3 + T-cells [98, 99] |
| ↓% FOXP3+ T-cells, MHC-II+ cells [98, 99]. | |||
|
| |||
| Spleen / Lymph nodes | IA LPS, IL-1 or U. parvum in Sheep or monkey | ↑ Size and hyperplasia reticulo-endothelial cells [34, 48, 108]. | ↑ % CD3, CD4, CD8 and γδ-T-cells [34, 48]. Activation of macrophages and neutrophils (acute exposure) [18, 108]. |
| ↓%FOXP3+ T-cells / ↑ %Th17+ T-cells (early response) [20, 108]. | |||
| ↑ %FOXP3+ T-cells/ ↓ %Th17+ T-cells (late response) [20]. | |||
|
| |||
| Gut | IA LPS, IL-1 and U. parvum in sheep | ↓Epithelial barrier integrity, proliferation, maturation and differentiation of enterocytes [54, 111, 112] | ↑ %CD3, CD4 T-cells [111]. |
| ↑ Villus atrophy and ↓, villus development [112] | ↓%FOXP3+ T-cells [111]. | ||
|
| |||
| Brain | IU LPS in Mouse, rabbit or Sheep | ↑ microglia, astrocytes, disrupted axonal development and apoptosis [116, 117] | ↑IL-1-B, IL-6 mRNA [114]. |
| ↓Oligodendrocytes, neurons [117]. | ↑Microglial activation [12, 116, 117] | ||
| ↓ Dentritic cells [10, 115] | |||
|
| |||
| Blood | IA LPS or IL-1 in Sheep | ↑ IL-6, IL-8, serum amyloid A3 [122, 129]. | |
| ↓ Neutrophils (early respose), and ↑ Neutrophils, platelets (late response) [108, 122] | |||
Summary of fetal inflammation after chorioamnionitis
Unlike the cytokine storm described in adults with systemic inflammatory responses induced by infections, trauma, and burns [128], the preterm infant with chorioamnionitis has a subtle FIRS as measured by increases in the traditional clinical markers - white blood counts and CRP. The organ injury responses in multiple organs evident in animal models are microscopic in nature that would not be detectable by conventional imaging techniques such as X-rays or ultrasound. Despite the subtle changes in inflammation measurable in the blood, the injury response in the preterm fetus can be quite damaging, and is complex due to the superimposition of immune changes/inflammation upon developing organs (Table 1). Fetal surgical experiments suggest that chorioamnionitis-induced pulmonary inflammation is important in the pathogenesis of FIRS [35]. However what precisely cause the immune changes in lymphoid organs, which cells are responsible for FIRS, and which other organs are responsible for signaling remain to be studied.
Acknowledgments
Funded by NIH grant HD57869 (to SGK) and U01 HL101800 (to AHJ and CAC)
REFERENCES
- [1].DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gravett MG, Rubens CE, Nunes TM. Global report on preterm birth and stillbirth (2 of 7): discovery science. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S2. doi: 10.1186/1471-2393-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PloS one. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. American journal of obstetrics and gynecology. 2008;198:110, e111–117. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- [5].Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. New England Journal of Medicine. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- [6].Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89:924–936. doi: 10.1038/labinvest.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grigsby PL, Novy MJ, Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reproductive sciences. 2010;17:85–94. doi: 10.1177/1933719109348025. [DOI] [PubMed] [Google Scholar]
- [8].Adams Waldorf KM, Gravett MG, McAdams RM, Paolella LJ, Gough GM, Carl DJ, et al. Choriodecidual Group B Streptococcal Inoculation Induces Fetal Lung Injury without Intra-Amniotic Infection and Preterm Labor in Macaca nemestrina. PloS one. 2011;6:e28972. doi: 10.1371/journal.pone.0028972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. American journal of obstetrics and gynecology. 1995;172:1598–1603. doi: 10.1016/0002-9378(95)90503-0. [DOI] [PubMed] [Google Scholar]
- [10].Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davies JK, Shikes RH, Sze CI, Leslie KK, McDuffie RS, Jr., Romero R, et al. Histologic inflammation in the maternal and fetal compartments in a rabbit model of acute intra-amniotic infection. American journal of obstetrics and gynecology. 2000;183:1088–1093. doi: 10.1067/mob.2000.108888. [DOI] [PubMed] [Google Scholar]
- [12].Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, et al. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:946–954. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- [13].Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. American journal of obstetrics and gynecology. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- [14].Willet KE, Kramer BW, Kallapur SG, Ikegami M, Newnham JP, Moss TJ, et al. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. American Journal of Physiology Lung Molecular Cellular Physiology. 2002;282:L411–L420. doi: 10.1152/ajplung.00097.2001. [DOI] [PubMed] [Google Scholar]
- [15].Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. American journal of obstetrics and gynecology. 2000;182:401–408. doi: 10.1016/s0002-9378(00)70231-6. [DOI] [PubMed] [Google Scholar]
- [16].Moss TJ, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Intrauterine Ureaplasma infection accelerates fetal lung maturation and causes growth restriction in sheep. American journal of obstetrics and gynecology. 2005;192:1179–1186. doi: 10.1016/j.ajog.2004.11.063. [DOI] [PubMed] [Google Scholar]
- [17].Gravett MG, Haluska GJ, Cook MJ, Novy MJ. Fetal and maternal endocrine responses to experimental intrauterine infection in rhesus monkeys. American journal of obstetrics and gynecology. 1996;174:1725–1731. doi: 10.1016/s0002-9378(96)70203-x. discussion 1731–1723. [DOI] [PubMed] [Google Scholar]
- [18].Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reproductive sciences. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- [19].Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. American journal of obstetrics and gynecology. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- [20].Kallapur SG, Presicce P, Senthamaraikannan P, Alvarez M, Tarantal AF, Miller LM, et al. Intra-amniotic IL-1ß induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol. 2013 doi: 10.4049/jimmunol.1300270. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, et al. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatric research. 2008;63:388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- [22].Ikegami M, Moss TJ, Kallapur SG, Mulrooney N, Kramer BW, Nitsos I, et al. Minimal Lung and Systemic Responses to TNF{alpha} in Preterm Sheep. American journal of physiology Lung cellular and molecular physiology. 2003;285:L121–L129. doi: 10.1152/ajplung.00393.2002. [DOI] [PubMed] [Google Scholar]
- [23].Kallapur SG, Moss TJ, Auten RL, Jr., Nitsos I, Pillow JJ, Kramer BW, et al. IL-8 signaling does not mediate intra-amniotic LPS-induced inflammation and maturation in preterm fetal lamb lung. American journal of physiology Lung cellular and molecular physiology. 2009;297:L512–519. doi: 10.1152/ajplung.00105.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DiGiulio DB. Diversity of microbes in amniotic fluid. Seminars in fetal & neonatal medicine. 2012;17:2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- [25].Paralanov V, Lu J, Duffy LB, Crabb DM, Shrivastava S, Methe BA, et al. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 2012;12:88. doi: 10.1186/1471-2180-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dando SJ, Nitsos I, Kallapur SG, Newnham JP, Polglase GR, Pillow JJ, et al. The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy? PloS one. 2012;7:e29856. doi: 10.1371/journal.pone.0029856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Knox CL, Dando SJ, Nitsos I, Kallapur SG, Jobe AH, Payton D, et al. The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum. Biol Reprod. 2010;83:415–426. doi: 10.1095/biolreprod.109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xiao L, Paralanov V, Glass JI, Duffy LB, Robertson JA, Cassell GH, et al. Extensive horizontal gene transfer in ureaplasmas from humans questions the utility of serotyping for diagnostic purposes. J Clin Microbiol. 2011;49:2818–2826. doi: 10.1128/JCM.00637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- [30].Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. American journal of obstetrics and gynecology. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- [31].Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. JOURNAL OF CLINICAL INVESTIGATION. 1997;99:2992–2999. doi: 10.1172/JCI119494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2001;280:L279–L285. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- [33].Newnham JP, Moss TJ, Padbury JF, Willet KE, Ikegami M, Ervin MG, et al. The interactive effects of endotoxin with prenatal glucocorticoids on short-term lung function in sheep. American Journal of Obstetrics & Gynecology. 2001;185:190–197. doi: 10.1067/mob.2001.114500. [DOI] [PubMed] [Google Scholar]
- [34].Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Polglase GP, Newnham JP, et al. Modulation of fetal inflammatory response on exposure to lipopolysaccharide by chorioamnion, lung, or gut in sheep. American journal of obstetrics and gynecology. 2010;202:77, e71–79. doi: 10.1016/j.ajog.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kemp MW, Kannan PS, Saito M, Newnham JP, Cox T, Jobe AH, et al. Selective Exposure of the Fetal Lung and Skin/Amnion (but Not Gastro-Intestinal Tract) to LPS Elicits Acute Systemic Inflammation in Fetal Sheep. PloS one. 2013;8:e63355. doi: 10.1371/journal.pone.0063355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moss TJ, Nitsos I, Kramer BW, Ikegami M, Newnham JP, Jobe AH. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. American journal of obstetrics and gynecology. 2002;187:1059–1065. doi: 10.1067/mob.2002.126296. [DOI] [PubMed] [Google Scholar]
- [37].Sosenko IR, Kallapur SG, Nitsos I, Moss TJ, Newnham JP, Ikegami M, et al. IL-1alpha causes lung inflammation and maturation by direct effects on preterm fetal lamb lungs. Pediatric research. 2006;60:294–298. doi: 10.1203/01.pdr.0000233115.51309.d3. [DOI] [PubMed] [Google Scholar]
- [38].Kallapur SG, Moss TJM, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited Inflammatory Cells Mediate Endotoxin Induced Lung Maturation in Preterm Fetal Lambs. Am J Respir Crit Care Med. 2005;172:1315–1321. doi: 10.1164/rccm.200506-1007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179:955–961. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willet KE, Jobe AH, Ikegami M, Brennan S, Newnham J, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatric research. 2000;48:782–788. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- [41].Collins JJ, Kuypers E, Nitsos I, Jane Pillow J, Polglase GR, Kemp MW, et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. American journal of physiology Lung cellular and molecular physiology. 2012;303:L778–787. doi: 10.1152/ajplung.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev Dyn. 2005;233:553–561. doi: 10.1002/dvdy.20362. [DOI] [PubMed] [Google Scholar]
- [43].Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, et al. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. American journal of physiology Lung cellular and molecular physiology. 2004;287:L1178–1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- [45].Polglase GR, Hooper SB, Gill AW, Allison BJ, Crossley KJ, Moss TJ, et al. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. Journal of applied physiology. 2010;108:1757–1765. doi: 10.1152/japplphysiol.01336.2009. [DOI] [PubMed] [Google Scholar]
- [46].Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. American journal of obstetrics and gynecology. 2008;198:122, e121–128. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Moss TJ, Nitsos I, Knox CL, Polglase GR, Kallapur SG, Ikegami M, et al. Ureaplasma colonization of amniotic fluid and efficacy of antenatal corticosteroids for preterm lung maturation in sheep. American journal of obstetrics and gynecology. 2009;200:96, e91–96. doi: 10.1016/j.ajog.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. American journal of physiology Lung cellular and molecular physiology. 2010;299:L852–860. doi: 10.1152/ajplung.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol. 2007;36:32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, et al. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. American journal of physiology Lung cellular and molecular physiology. 2007;293:L345–353. doi: 10.1152/ajplung.00003.2007. [DOI] [PubMed] [Google Scholar]
- [51].Shah TA, Hillman NH, Nitsos I, Polglase GR, Pillow JJ, Newnham JP, et al. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatric research. 2010;68:210–215. doi: 10.1203/PDR.0b013e3181e9c556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- [53].Kallapur SG, Jobe AH, Ikegami M, Bachurski CJ. Increased IP-10 and MIG expression after intra-amniotic endotoxin in preterm lamb lung. Am J Respir Crit Care Med. 2003;167:779–786. doi: 10.1164/rccm.2203030. [DOI] [PubMed] [Google Scholar]
- [54].Wolfs TG, Buurman WA, Zoer B, Moonen RM, Derikx JP, Thuijls G, et al. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PloS one. 2009;4:e5837. doi: 10.1371/journal.pone.0005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kunzmann S, Collins JJ, Yang Y, Uhlig S, Kallapur S, Speer CP, et al. Antenatal Inflammation Reduces Cav-1 Expression and Influences Multiple Signaling Pathways in Preterm Fetal Lungs. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kunzmann S, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation induced TGF-beta1 but suppressed CTGF in preterm lungs. American journal of physiology Lung cellular and molecular physiology. 2007;292:L223–231. doi: 10.1152/ajplung.00159.2006. [DOI] [PubMed] [Google Scholar]
- [57].Sweet DG, Huggett MT, Warner JA, Moss TJ, Kloosterboer N, Halliday HL, et al. Maternal Betamethasone and Chorioamnionitis Induce Different Collagenases during Lung Maturation in Fetal Sheep. Neonatology. 2008;94:79–86. doi: 10.1159/000115949. [DOI] [PubMed] [Google Scholar]
- [58].Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- [59].Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends in immunology. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- [60].Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJ, Hillman NH, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- [61].Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- [62].Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- [63].Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate immunity. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- [64].Snyder CC, Wolfe KB, Gisslen T, Knox CL, Kemp MW, Kramer BW, et al. Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. American journal of obstetrics and gynecology. 2013;208:399, e391–398. doi: 10.1016/j.ajog.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, et al. Chronic Fetal Exposure to Ureaplasma parvum Suppresses Innate Immune Responses in Sheep. J Immunol. 2011;187:2688–2695. doi: 10.4049/jimmunol.1100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gisslen T, Hillman NH, Musk GC, Kemp MW, Kramer BW, Senthamaraikannan P, et al. Repeated exposure to intra-amniotic LPS partially protects against adverse effects of intravenous LPS in preterm lambs. Innate immunity. 2013 doi: 10.1177/1753425913488430. [DOI] [PubMed] [Google Scholar]
- [67].Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, Balasubramaniam V, et al. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. American journal of physiology Lung cellular and molecular physiology. 2010;299:L735–748. doi: 10.1152/ajplung.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- [69].Banks BA, Cnaan A, Morgan MA, Parer JT, Merrill JD, Ballard PL, et al. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. American journal of obstetrics and gynecology. 1999;181:709–717. doi: 10.1016/s0002-9378(99)70517-x. [DOI] [PubMed] [Google Scholar]
- [70].Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. American journal of obstetrics and gynecology. 2006;195:803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- [71].Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, et al. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. American journal of physiology Lung cellular and molecular physiology. 2003;284:L633–L642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- [72].Newnham JP, Kallapur SG, Kramer BW, Moss TJ, Nitsos I, Ikegami M, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. American journal of obstetrics and gynecology. 2003;189:1458–1466. doi: 10.1067/s0002-9378(03)00758-0. [DOI] [PubMed] [Google Scholar]
- [73].Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, et al. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. American journal of physiology Lung cellular and molecular physiology. 2012;302:L380–389. doi: 10.1152/ajplung.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wolfe KB, Snyder CC, Gisslen T, Kemp MW, Newnham JP, Kramer BW, et al. Modulation of Lipopolysaccharide-Induced Chorioamnionitis in Fetal Sheep by Maternal Betamethasone. Reproductive sciences. 2013 doi: 10.1177/1933719113488445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kuypers E, Collins JJ, Jellema RK, Wolfs TG, Kemp MW, Nitsos I, et al. Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PloS one. 2012;7:e38257. doi: 10.1371/journal.pone.0038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur S, Newnham JP. Exposure to In Utero Lipopolysaccharide Induces Inflammation in the Fetal Ovine Skin. Reproductive sciences. 2011;18:88–98. doi: 10.1177/1933719110380470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kemp MW, Saito M, Kallapur SG, Jobe AH, Keelan JA, Li S, et al. Inflammation of the Fetal Ovine Skin Following in utero Exposure to Ureaplasma parvum. Reproductive sciences. 2011;18:1128–1137. doi: 10.1177/1933719111408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. European journal of immunology. 2005;35:383–390. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- [80].Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- [81].Farley AM, Morris LX, Vroegindeweij E, Depreter ML, Vaidya H, Stenhouse FH, et al. Dynamics of thymus organogenesis and colonization in early human development. Development. 2013;140:2015–2026. doi: 10.1242/dev.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Galy A, Verma S, Barcena A, Spits H. Precursors of CD3+CD4+CD8+ cells in the human thymus are defined by expression of CD34. Delineation of early events in human thymic development. The Journal of experimental medicine. 1993;178:391–401. doi: 10.1084/jem.178.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lobach DF, Haynes BF. Ontogeny of the human thymus during fetal development. Journal of clinical immunology. 1987;7:81–97. doi: 10.1007/BF00916002. [DOI] [PubMed] [Google Scholar]
- [84].Lobach DF, Hensley LL, Ho W, Haynes BF. Human T cell antigen expression during the early stages of fetal thymic maturation. J Immunol. 1985;135:1752–1759. [PubMed] [Google Scholar]
- [85].Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- [86].Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Janossy G, Bofill M, Poulter LW, Rawlings E, Burford GD, Navarrete C, et al. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J Immunol. 1986;136:4354–4361. [PubMed] [Google Scholar]
- [88].Muller C, Ziegler A, Muller C, Hadam M, Waller HD, Wernet P, et al. Divergent expression of HLA-DC/MB, -DR, and -SB region products on normal and pathological tissues as detected by monoclonal antibodies. Immunobiology. 1985;169:228–249. doi: 10.1016/s0171-2985(85)80036-x. [DOI] [PubMed] [Google Scholar]
- [89].Blom B, Res PC, Spits H. T cell precursors in man and mice. Critical reviews in immunology. 1998;18:371–388. doi: 10.1615/critrevimmunol.v18.i4.50. [DOI] [PubMed] [Google Scholar]
- [90].Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].De Felice C, Latini G, Del Vecchio A, Toti P, Bagnoli F, Petraglia F. Small thymus at birth: a predictive radiographic sign of bronchopulmonary dysplasia. Pediatrics. 2002;110:386–388. doi: 10.1542/peds.110.2.386. [DOI] [PubMed] [Google Scholar]
- [92].De Felice C, Toti P, Santopietro R, Stumpo M, Pecciarini L, Bagnoli F. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. The Journal of pediatrics. 1999;135:384–386. doi: 10.1016/s0022-3476(99)70140-x. [DOI] [PubMed] [Google Scholar]
- [93].Toti P, De Felice C, Stumpo M, Schurfeld K, Di Leo L, Vatti R, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Human pathology. 2000;31:1121–1128. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- [94].Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D'Addario V, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. American journal of obstetrics and gynecology. 2006;194:153–159. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- [95].El-Haieg DO, Zidan AA, El-Nemr MM. The relationship between sonographic fetal thymus size and the components of the systemic fetal inflammatory response syndrome in women with preterm prelabour rupture of membranes. BJOG : an international journal of obstetrics and gynaecology. 2008;115:836–841. doi: 10.1111/j.1471-0528.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- [96].Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, et al. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;29:639–643. doi: 10.1002/uog.4022. [DOI] [PubMed] [Google Scholar]
- [97].Jeppesen DL. The size of the thymus: an important immunological diagnostic tool? Acta paediatrica. 2003;92:994–996. [PubMed] [Google Scholar]
- [98].Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, et al. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. American journal of obstetrics and gynecology. 2010;202:476, e471–479. doi: 10.1016/j.ajog.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kuypers E, Wolfs TG, Collins JJ, Jellema RK, Newnham JP, Kemp MW, et al. Intraamniotic Lipopolysaccharide Exposure Changes Cell Populations and Structure of the Ovine Fetal Thymus. Reproductive sciences. 2013 doi: 10.1177/1933719112472742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Melville JM, Bischof RJ, Meeusen EN, Westover AJ, Moss TJ. Changes in fetal thymic immune cell populations in a sheep model of intrauterine inflammation. Reproductive sciences. 2012;19:740–747. doi: 10.1177/1933719111432873. [DOI] [PubMed] [Google Scholar]
- [101].Irla M, Hollander G, Reith W. Control of central self-tolerance induction by autoreactive CD4+ thymocytes. Trends in immunology. 2010;31:71–79. doi: 10.1016/j.it.2009.11.002. [DOI] [PubMed] [Google Scholar]
- [102].Anz D, Thaler R, Stephan N, Waibler Z, Trauscheid MJ, Scholz C, et al. Activation of melanoma differentiation-associated gene 5 causes rapid involution of the thymus. J Immunol. 2009;182:6044–6050. doi: 10.4049/jimmunol.0803809. [DOI] [PubMed] [Google Scholar]
- [103].Demoulins T, Abdallah A, Kettaf N, Baron ML, Gerarduzzi C, Gauchat D, et al. Reversible blockade of thymic output: an inherent part of TLR ligand-mediated immune response. J Immunol. 2008;181:6757–6769. doi: 10.4049/jimmunol.181.10.6757. [DOI] [PubMed] [Google Scholar]
- [104].Sempowski GD, Rhein ME, Scearce RM, Haynes BF. Leukemia inhibitory factor is a mediator of Escherichia coli lipopolysaccharide-induced acute thymic atrophy. European journal of immunology. 2002;32:3066–3070. doi: 10.1002/1521-4141(200211)32:11<3066::AID-IMMU3066>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [105].Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. Journal of neuroimmunology. 1998;82:40–46. doi: 10.1016/S0165-5728(97)00186-0. [DOI] [PubMed] [Google Scholar]
- [106].Stallmach T, Karolyi L. Augmentation of fetal granulopoiesis with chorioamnionitis during the second trimester of gestation. Human pathology. 1994;25:244–247. doi: 10.1016/0046-8177(94)90195-3. [DOI] [PubMed] [Google Scholar]
- [107].Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato MC, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. American journal of clinical pathology. 2004;122:765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- [108].Kallapur SG, Kramer BW, Nitsos I, Pillow JJ, Collins JJ, Polglase GR, et al. Pulmonary and Systemic Inflammatory Responses to Intraamniotic IL-1 alpha in fetal sheep. American journal of physiology Lung cellular and molecular physiology. 2011;301:L285–L295. doi: 10.1152/ajplung.00446.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lee AJ, Lambermont VA, Pillow JJ, Polglase GR, Nitsos I, Newnham JP, et al. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. American journal of obstetrics and gynecology. 2011;204:364.e317–e324. doi: 10.1016/j.ajog.2010.11.015. [DOI] [PubMed] [Google Scholar]
- [110].Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. The Journal of pediatrics. 2013;162:236–242. e232. doi: 10.1016/j.jpeds.2012.07.012. [DOI] [PubMed] [Google Scholar]
- [111].Wolfs TG, Kallapur SG, Polglase GR, Pillow JJ, Nitsos I, Newnham JP, et al. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PloS one. 2011;6:e18355. doi: 10.1371/journal.pone.0018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wolfs TG, Kallapur SG, Knox CL, Thuijls G, Nitsos I, Polglase GR, et al. Antenatal ureaplasma infection impairs development of the fetal ovine gut in an IL-1-dependent manner. Mucosal Immunol. 2013;6:547–556. doi: 10.1038/mi.2012.97. [DOI] [PubMed] [Google Scholar]
- [113].Yoon B, Kim C, Romero R, Jun J, Park K, Choi S, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. American journal of obstetrics and gynecology. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- [114].Burd I, Brown A, Gonzalez JM, Chai J, Elovitz MA. A mouse model of term chorioamnionitis: unraveling causes of adverse neurological outcomes. Reproductive sciences. 2011;18:900–907. doi: 10.1177/1933719111398498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. Journal of neuroscience research. 2010;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nitsos I, Rees SM, Duncan J, Kramer BW, Harding R, Newnham JP, et al. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J Soc Gynecol Investig. 2006;13:239–247. doi: 10.1016/j.jsgi.2006.02.011. [DOI] [PubMed] [Google Scholar]
- [117].Gavilanes AW, Strackx E, Kramer BW, Gantert M, Van den Hove D, Steinbusch H, et al. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. American journal of obstetrics and gynecology. 2009;200:437, e431–438. doi: 10.1016/j.ajog.2008.12.003. [DOI] [PubMed] [Google Scholar]
- [118].Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, et al. Cord blood biomarkers of the fetal inflammatory response. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2009;22:379–387. doi: 10.1080/14767050802609759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zanardo V, Peruzzetto C, Trevisanuto D, Cavallin F, Vedovato S, Straface G, et al. Relationship between the neonatal white blood cell count and histologic chorioamnionitis in preterm newborns. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:2769–2772. doi: 10.3109/14767058.2012.712562. [DOI] [PubMed] [Google Scholar]
- [120].Howman RA, Charles AK, Jacques A, Doherty DA, Simmer K, Strunk T, et al. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PloS one. 2012;7:e51836. doi: 10.1371/journal.pone.0051836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PloS one. 2011;6:e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, et al. Dose and time response for inflammation and lung maturation after intra-amniotic endotoxin in preterm lambs. American Journal of Respiratory and Critical Care Medicine. 2001;164:982–988. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- [123].Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- [124].Gomez R, Romero R, Ghezzi F, Yoon B, Mazor M, Berry S. The fetal inflammatory response syndrome. American journal of obstetrics and gynecology. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- [125].Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012;129:e134–141. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- [126].Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatric research. 1999;46:566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- [127].Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. The Journal of pediatrics. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- [128].Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci. 2006;101:189–198. doi: 10.1254/jphs.crj06010x. [DOI] [PubMed] [Google Scholar]
- [129].Wilson TC, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Pulmonary and systemic induction of SAA3 after ventilation and endotoxin in preterm lambs. Pediatric research. 2005;58:1204–1209. doi: 10.1203/01.pdr.0000185269.93228.29. [DOI] [PubMed] [Google Scholar]