SUMMARY
Background
Although irritable bowel syndrome (IBS) is a multisymptom disorder, abdominal pain drives illness severity more than other symptoms. Despite consensus that IBS trials should measure pain to define study entry and determine efficacy, the optimal method of measuring pain remains uncertain.
Aim
To determine whether combining information from multiple pain dimensions may capture the IBS illness experience more effectively than the approach of measuring `pain predominance' or pain intensity alone.
Methods
Irritable bowel syndrome patients rated dimensions of pain, including intensity, frequency, constancy, predominance, predictability, duration, speed of onset and relationship to bowel movements. We evaluated the impact of each dimension on illness severity using multivariable regression techniques.
Results
Among the pain dimensions, intensity, frequency, constancy and predictability were strongly and independently associated with illness severity; the other dimensions had weaker associations. The clinical definition of `pain predominance', in which patients define pain as their most bothersome symptom, was insufficient to categorize patients by illness severity.
Conclusions
Irritable bowel disease pain is multifaceted; some pain dimensions drive illness more than others. IBS trials should measure various pain dimensions, including intensity, constancy, frequency and predictability; this may improve upon the customary use of measuring pain as a unidimensional symptom in IBS.
INTRODUCTION
Although irritable bowel syndrome (IBS) is a multisymptom disorder, abdominal pain is a defining characteristic1 and a driver of healthcare resource utilization.2–4 Unlike most other IBS symptoms, such as bloating or abnormalities in stool frequency or form, abdominal pain independently drives health-related quality of life (HRQOL) decrements in IBS5 and is the principal driver of patient-reported symptom severity.4, 6, 7 In short, IBS is partly defined by pain, and pain is the cornerstone of the IBS illness experience for many patients.
However, as with other types of chronic pain, the pain of IBS is complex and multifaceted. As some dimensions of IBS pain may drive illness severity more than others, it is simplistic to treat pain as a unidimensional symptom. Data indicate that pain intensity, as measured by a numeric rating scale (NRS), is highly predictive of HRQOL and other severity measures in IBS,7 but less is known about the incremental value of other IBS pain dimensions, including frequency, constancy, duration, bothersomeness, predictability, speed of onset and relationship to bowel movements. In other chronic pain conditions, pain is typically assessed in terms of its affective impact, sensory intensity and pain descriptors (e.g. cramping, throbbing and aching).8, 9 It is important to understand the predictive value of different pain dimensions in IBS, not only to guide patient-reported outcome (PRO) measurement for future clinical trials but also to define better the inclusion criteria for these trials in the first place. Similarly, it is important to define clearly `pain predominance' in IBS, as future clinical trials of visceral analgesics may aim to recruit patients who describe pain as their predominant symptom. As pain has many dimensions, it remains unclear which dimensions of pain should be employed to define `pain predominance' in IBS.
In this study, we performed analyses using a well-defined IBS cohort to measure the impact of individual pain dimensions on illness severity. We hypothesized that different pain dimensions have varying abilities to predict illness severity. We further hypothesized that combining information from multiple dimensions may capture the IBS illness experience more effectively than measuring individual dimensions alone. Finally, we hypothesized that the clinical definition of `pain predominance', in which patients define pain as their most bothersome symptom,10 may be necessary, but is insufficient to categorize optimally patients by illness severity; it may be more useful to define pain predominance by combining multiple symptom dimensions.
METHODS
Patients
We prospectively evaluated patients aged 18 years or older with Rome III positive IBS (including IBS-C, IBS-D and IBS-M) enrolled in the IBS Patient Reported Observed Outcomes and Function (PROOF) cohort. The current study presents data obtained from a new survey of this cohort. An overview of the PROOF methodology can be found in previous publications.7, 11 PROOF is an internet-based, longitudinal, observational registry of IBS patients from a network of eight geographically diverse U.S. centres. PROOF does not mandate specified treatments or protocols; patients receive the usual care of their healthcare providers. Each PROOF investigator is an experienced gastroenterologist with knowledge of the appropriate application of the Rome III criteria. The study was approved by the University of California at Los Angeles Institutional Review Board and was conducted in accordance with the institutional guidelines regulating human subject research.
IBS pain dimensions
Pain can be measured with several dimensions. In this study, we identified and prospectively measured two sets of IBS pain dimensions: one set pertaining to the overall pain experience of IBS, and one set related specifically to IBS acute pain episodes, defined as discrete periods when IBS pain starts or worsens. Acute pain episodes are variably described as `flare-ups',5 `attacks'12 and `breakthrough' pain episodes, and are experienced by many patients with IBS symptoms.
Dimensions of overall pain experience
We measured the following dimensions of the overall IBS pain experience:
Intensity: Data from the chronic pain literature indicate that pain intensity is a key attribute to monitor for both study entry and outcome measurement.13 We therefore measured IBS pain intensity with a 10-point abdominal pain NRS with the following question: `On a scale from 1 (no pain) to 10 (worst possible pain), how bad has your abdominal pain been, on average, over the last 10 days?' This is a modification of the 11-point NRS supported by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) for the non-IBS pain literature.13, 14 We have found that the 10-point NRS behaves in a nearly identical psychometric manner as the 11-point NRS.7
Frequency: In addition to pain intensity, it is important to understand the frequency by which pain occurs, independent of intensity. We asked patients to rate the frequency of their abdominal pain over a typical 10-day period using an item derived from the IBS Symptom Severity Scale (IBS-SSS) instrument.15 Patients were instructed to `enter the number of days that you get pain in every 10 days. For example, if you enter 4, it means that you get pain four out of every 10 days. If you get pain every day, enter 10'.
Constancy: Clinicians recognize that some patients with IBS always have pain, whereas others describe cycles of pain periodicity. We posed the following question derived from the Functional Bowel Disease Severity Index (FBDSI) Instrument:16 `Is your abdominal pain constant? (i.e. present all of the time and everyday?)'.
Relationship with bowel movements: Many patients with IBS obtain relief of their pain upon stool passage. Although pain relief with defecation is part of the Rome III diagnostic criteria for IBS, its presence is not mandatory to diagnose the syndrome.1 As defecation is partly under voluntary control, it is possible that patients with pain relieved by defecation maintain better control over their abdominal pain and, perhaps, are better able to cope with their illness. However, this hypothesis has not been formally tested. We asked patients to rate the frequency that abdominal pain improves or stops after a bowel movement using a five-point Likert scale from the Rome III battery,1 as follows: 1 – `never or rarely'; 2 – `sometimes'; 3 – `often'; `4 – `most of the time'; 5 – `always'.
Pain predominance: The Rome III IBS guidelines suggest that clinicians should identify and focus treatment efforts on the patients' primary or `most bothersome' symptom.1 Although IBS is a multisymptom disorder, it is often helpful to understand which symptom is predominant in each patient's illness experience, and to ensure that the treatment plan addresses that symptom. However, it remains unclear whether this clinical definition of pain predominance is a reliable predictor of global illness severity. We therefore posed the following question, which has been previously used as a measure of symptom `predominance'10: `If you could get rid of the single most bothersome IBS symptom, which one would you choose?' Patients could select one from a list of nine cardinal IBS symptoms, including `belly pain'. We stratified patients into those who endorsed pain as their most bothersome symptom vs. those who did not – a previously employed measure of pain predominance.10
Dimensions of IBS acute pain episodes
In addition to measuring dimensions of the overall pain experience, we measured several dimensions of IBS acute pain episodes. Recent literature has focused on pain episodes as a potentially important part of the overall pain experience in IBS. These pain episodes have been the target of at least one clinical drug trial,12 and have also been recently described and characterized in various cohorts of IBS patients outside the context of clinical trials.17–19 We first asked patients whether they experience acute painful episodes (as opposed to `discomfort' alone), and limited our subsequent analyses only to those patients who reported acute pain episodes. As different patients use different words to describe a pain episode, we next asked patients to select among a group of descriptors for their acute pain episodes, including `pain attack', `pain breakthrough', `pain flare-up', `pain bout' and `pain episode' itself. The descriptors were displayed in random order to minimize the risk of order effect. In addition, patients could provide their own descriptors using an open-ended field. The online survey automatically incorporated the patients' own language into the questions that followed (e.g. if a patient described his/her pain episodes as `attacks', then the survey referred to `attacks' to ensure that the language was concordant with the patients' personal semantics). For simplicity, we will refer to these periods as `pain episodes' throughout the manuscript. Patients endorsing the presence of acute pain episodes were asked to rate the following dimensions of their episodes:
Intensity: We asked patients: `During a typical IBS pain episode, how severe does your pain get on a scale from 0 (no pain) to 10 (worst imaginable pain)?' Patient responded using the 11-point NRS supported by IMM-PACT14 and the FDA.20
Frequency: We asked patients to estimate the frequency of their acute pain episodes over a defined 30-day period. We selected this time period based on recall periods for previous clinical trial work measuring acute pain episodes in IBS.12 Patients were instructed to `indicate about how many days you have pain episodes over a typical 30-day period. For example, if you select four, it means that you have pain episodes four out of every 30 days. If you have episodes every day, select 30'. In addition, we sought to measure the average number of episodes experienced per day. Patients were asked: `On a typical day when you do have an IBS pain episode, how many episodes do you have during the day? For example, if you select 4, that means you experience 4 pain episodes during a typical day (even if your pain episodes varies from day to day, please give us your best estimate)'.
Duration: The duration of acute pain episodes may impact illness severity independent of frequency and intensity. We asked patients: `When you do have a pain episode, about how long does your episode typically last?' Patient selected among the following options: `less than 10 min', `10–30 min', `30 min to 1 h', `1–4 h, `all day long', `2 days long' and `more than 2 days long'.
Speed of onset: Some patients describe pain episodes that come on rapidly over seconds or minutes. Others describe pain that builds and crescendos over a longer period. As it is possible that speed of onset may be an independent dimension of pain episodes, we asked patients: `When you have an IBS pain episode, about how quickly does the episode usually come on?'. Patients selected among the following options: `seconds to a minute', `1–5 min', `5–10 min', `10–30 min', `30 min to an hour', `over 1–2 h' and `several hours'.
Predictability: The predictability of pain has important clinical implications. In migraine headache, patients who can detect a preceding aura may reach for timely therapeutic interventions in anticipation of the inevitable headache to follow, whereas those without an aura may be less likely to initiate timely therapy. The same may apply to IBS; some patients describe situational, physical or psychosocial cues that reliably predict an oncoming pain episode, whereas others lack this predictive ability and suffer pain episodes without detectable warning. We posed the following question: `Some people with IBS can predict when a pain episode is about to come on while others cannot. In thinking about your IBS pain episodes, how reliably can you predict, in advance, that an episode is about to happen on a scale from 0 (IBS episodes are totally unpredictable) to 10 (IBS episodes are totally predictable)?'
Analyses
Predictive value of `pain predominance'
We first evaluated the clinical definition of pain predominance, measured using the definition described above and suggested by previous authors10 and the Rome III guidance.1 We performed a series of bivariate analyses to compare the pain-predominant vs. nonpain-predominant patients across a range of metrics. Specifically, we measured IBS symptom severity with the Irritable Bowel Severity Scoring System,15 FBDSI16 and BEST score,21 disease-targeted HRQOL with the IBS-QOL instrument,22 generic HRQOL with the EQ5D11, 23 and CDC-4, worker productivity with the IBS version of the Work Productivity Activity Index (WPAI:IBS),24 gastrointestinal-specific anxiety with the visceral sensitivity index (VSI),25, 26 generic psychological function with the Hospital Anxiety and Depression (HAD) scale and symptom coping using a five-point Likert scale. Finally, we measured resource utilization, including self-reported physician visits and current number of IBS therapies. We used t-tests to compare continuous variables between groups and chi-squared tests for categorical variables. We expressed the bivariate relationship between pain predominance and each index using a T-value, P-value and Pearson's correlation coefficient, and employed a P-value of <0.05 as evidence for statistical significance. As we evaluated multiple comparisons, we calculated a Bonferroni-corrected P-value for each bivariate analysis.
Incremental value of individual pain dimensions
We next conducted a series of multivariable regression analyses to measure the independent contribution of each pain dimension stratified by IBS illness severity metrics. We first conducted models to measure the five dimensions of the overall pain experience, and then conducted a second set of models to evaluate the five dimensions of acute pain episodes. We calculated the proportion of variance for each illness severity metric explained by the models, expressed with the R2-statistic, and measured the P-value for each attribute's beta coefficient. In addition, we calculated the squared semi-partial correlations of each pain attribute to measure the unique proportion of variance in each illness severity metric accounted for by each pain dimension after removing the effects of shared variance. We depict the semi-partial correlations with tiered bar grafts demonstrating the relative influence of competing pain dimensions, along with the absolute R2 explained by each model.
RESULTS
Patient characteristics
Table 1 provides characteristics of the 258 patients in the analyses. The patient profiles are consistent with previous studies in IBS. Namely, the patients were primarily middle aged (mean age = 43 ± 15 years) and women (82%). The population was varied across demographic characteristics, including race, education and income. Eighteen per cent of the cohort had IBS-C, 29% IBS-D and 53% IBS-M using Rome III subclassification criteria.1 Using IBS-SSS criteria for symptom severity, 17%, 46% and 37% of patients had mild, moderate and severe IBS symptoms.
Table 1.
Patient characteristics
| Variable | Mean (n = 258) |
|---|---|
| Age (mean years ± s.d.) | 42 ± 15 |
| Gender (% female) | 82 |
| Race (%) | |
| White | 82 |
| Black | 6 |
| Other | 12 |
| Education (%) | |
| Graduated high school | 78 |
| Graduated college | 65 |
| Postgraduate education | 27 |
| Income (%) | |
| <$50 000 annual | 49 |
| $50 000 to $100 000 annual | 29 |
| >$100 000 annual | 22 |
| Marital status (% married) | 48 |
| IBS subtype (%) | |
| IBS with constipation (IBS-C) | 18 |
| IBS with diarrhoea (IBS-D) | 29 |
| Mixed IBS (IBS-M) | 53 |
| IBS duration (%) | |
| 6 months to 1 year | 4.0 |
| 1–2 years | 7.4 |
| 2–5 years | 18.1 |
| 5–10 years | 23.5 |
| 10–20 years | 30.9 |
| More than 20 years | 16.1 |
| IBS pain severity (10-point numeric rating scale) | |
| In all patients at baseline | 4.5 ± 2.5 |
| In patients with ≥3 out of 10 points at baseline | 5.6 ± 2.0 |
| Global IBS severity (0–20 rating scale) | 11 ± 5 |
| IBS-SSS trichotomized severity (%) | |
| Mild (score of 75–175) | 17 |
| Moderate (score of 175–300) | 46 |
| Severe (>300) | 37 |
| IBS-QOL overall score (mean ± s.d.) | 62.7 ± 22 |
| Worker Productivity Activity Index (WPAI:IBS) (%) | |
| Work week absent from IBS (absenteeism) | 3.6 |
| Work week impaired from IBS (presenteeism) | 34.4 |
Predictive value of `pain predominance'
Eighty four per cent of the patients in PROOF reported experiencing abdominal pain within the previous 10 days of the survey. Of this group, 19% had `pain predominant IBS', defined as pain being the most bothersome symptom.10 Table 2 provides the bivariate relationships between patients with vs. without pain predominance. There were no significant differences between groups for all but five of the 17 metrics – that is, the clinical definition of pain predominance (pain as `most bothersome' symptom) was not generally predictive of illness severity. There were no significant differences between groups when applying a Bonferroni correction requiring a P ≤ 0.003.
Table 2.
`Pain predominant' vs. `nonpain predominant' groups
| Metric | Nonpain predominant mean (n = 190) | Pain predominant mean (n = 45) | T-value | r-Value | P-value |
|---|---|---|---|---|---|
| General health (1–4; lower = worse) | 2.35 | 2.42 | −0.42 | −0.164 | 0.68 |
| Symptom coping (1–5; lower = worse) | 4.12 | 3.91 | 1.06 | −0.608 | 0.29 |
| HAD Anxiety Scale Score (0–30; higher = worse) | 3.31 | 3.42 | −0.35 | 0.269 | 0.72 |
| HAD Depression Scale Score (0–30; higher = worse) | 2.19 | 2.22 | −0.13 | 0.425 | 0.90 |
| VSI Scale Score (0–75; higher = worse) | 39.80 | 48.27 | −2.91 | 0.456 | 0.004 |
| Severity of symptoms past week (0–20; higher = worse) | 10.31 | 10.58 | −0.34 | 0.575 | 0.73 |
| CDC4 Score (0–30; higher = worse) | 25.27 | 24.20 | 0.29 | 0.197 | 0.77 |
| IBS QOL Score (0–100; lower = worse) | 63.29 | 60.58 | 0.73 | −0.523 | 0.47 |
| FBDSI Scale Score | 80.89 | 102.73 | −2.05 | 0.307 | 0.04 |
| EuroQual Utility Score (0–1; lower = worse) | 0.69 | 0.64 | 1.19 | −0.393 | 0.24 |
| BEST Scale Score (0–100; higher = worse) | 37.93 | 39.80 | −0.79 | 0.352 | 0.43 |
| IBS-SSS Scale Score (0–500; higher = worse) | 267.17 | 304.09 | −2.28 | 0.644 | 0.02 |
| WPAI absenteeism (% week absent from IBS) | 3.06 | 5.86 | −1.15 | 0.391 | 0.25 |
| WPAI presenteeism (% week impaired at work from IBS) | 32.11 | 44.41 | −2.58 | 0.609 | 0.01 |
| No. IBS therapies currently receiving (out of 16) | 1.48 | 1.33 | 0.57 | 0.100 | 0.57 |
| No. physician visits in previous year | 2.49 | 4.20 | −2.64 | 0.124 | 0.009 |
Patients with pain predominant IBS state that pain is their `most bothersome' symptom. The data reveal only minimal differences in illness severity between groups using this definition. When using a Bonferroni correction for multiple testing (requiring a significance threshold of P ≤ 0.003), none of the relationships was significant.
Incremental value of individual pain dimensions
Dimensions of overall pain experience
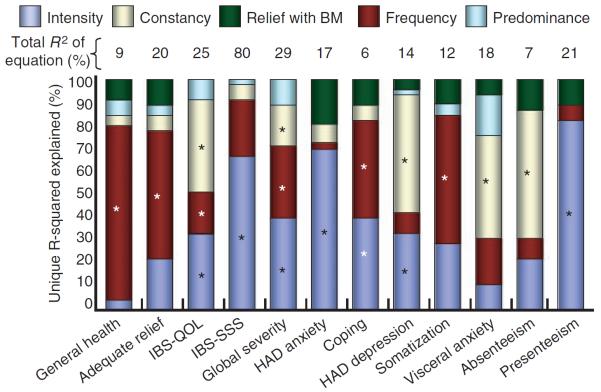
Table 3 displays the results of regression analyses stratified by IBS severity metrics. The P-values in Table 3 present the significance level beta-coefficients for individual pain dimensions derived from regression models, and therefore measure significance while adjusting for simultaneously measured pain dimensions. Figure 1 depicts the relative contribution of each pain dimension towards explaining the variance in each index. Among the various pain dimensions, the `predominance' and `relation to bowel movement' dimensions were least predictive across metrics, whereas intensity, frequency and constancy were most predictive. When analysed as a group, the pain dimensions explained the largest proportion of variance for overall symptom severity (R2 = 80% for IBS-SSS; R2 = 29% for severity NRS), IBS-QOL scores (R2 = 25%) and presenteeism (R2 = 21%). The pain dimensions explained the lowest proportion of variance for generalized anxiety (6%) as measured by HAD.
Table 3.
Multivariable regression analyses including five dimensions of overall pain experience, stratified by illness severity metrics
|
P-value |
|||||||
|---|---|---|---|---|---|---|---|
| Metric | Intercept | Predominance | Severity | Constancy | Frequency | Relief with BM | R2 from equation |
| General health | 0.0000 | 0.52 | 0.74 | 0.41 | 0.01 | 0.38 | 0.07 |
| Symptom coping | 0.0000 | 0.38 | 0.0005 | 0.0002 | 0.90 | 0.09 | 0.17 |
| HAD Anxiety Scale Score | 0.0000 | 0.93 | 0.44 | 0.75 | 0.39 | 0.67 | 0.02 |
| HAD Depression Scale Score | 0.0003 | 0.42 | 0.04 | 0.006 | 0.22 | 0.71 | 0.12 |
| VSI Scale Score | 0.0000 | 0.11 | 0.16 | 0.001 | 0.07 | 0.70 | 0.16 |
| Severity of symptoms past week | 0.0000 | 0.03 | 0.0001 | 0.006 | 0.0008 | 0.97 | 0.29 |
| Considerable relief? | 0.0000 | 0.62 | 0.12 | 0.32 | 0.007 | 0.25 | 0.20 |
| CDC4 Score | 0.0000 | 0.12 | 0.58 | 0.001 | 0.01 | 0.04 | 0.16 |
| IBS-QOL Score | 0.0000 | 0.15 | 0.001 | 0.0002 | 0.02 | 0.71 | 0.24 |
| FBDSI severity | 0.0000 | 0.92 | 0.05 | 0.26 | 0.11 | 0.02 | 0.13 |
| EuroQual Utility Score | 0.0000 | 0.44 | 0.0006 | 0.001 | 0.02 | 0.47 | 0.24 |
| BEST Scale Score | 0.0000 | 0.45 | 0.007 | 0.16 | 0.05 | 0.32 | 0.14 |
| Absenteeism | 0.2503 | 0.96 | 0.16 | 0.02 | 0.38 | 0.26 | 0.07 |
| Presenteeism | 0.0003 | 0.74 | 0.0003 | 0.67 | 0.48 | 0.26 | 0.21 |
| No. therapies | 0.0000 | 0.23 | 0.12 | 0.71 | 0.59 | 0.005 | 0.07 |
| No. physician visits | 0.0090 | 0.15 | 0.13 | 0.77 | 0.64 | 0.23 | 0.05 |
Each cell provides the P-value for the beta-coefficient of each pain attribute in the model. Bold values indicate statistical significance. The data reveal that pain `predominance' and `relief with bowel movements (BM)' were not predictive of most indices. In contrast, pain `severity', `frequency' and `constancy' were highly predictive of most indices. Taken together, the pain dimensions explained the largest proportion of variance (R2) for weekly symptom severity ratings (29%) and IBS-QOL (24%).
Figure 1.
Independent contribution of individual dimensions of overall pain experience to illness severity metrics. Pain intensity captured 5–85% of the explained variance across metrics. Pain frequency and constancy accounted for most of the remaining explained variance across metrics. Pain bothersomeness and relief with bowel movements (BM) contributed small amounts to understanding the various metrics. Bars with asterisks indicate statistically significant associations. The models explained between 6% and 80% of the overall variance of each outcome; the lowest and highest explained variance were for generalized anxiety (overall R2 = 6%) and IBS-SSS severity scores (overall R2 = 80%) respectively. For ease of interpretation, the bars compartmentalize the relative proportion of each metric explained by the pain dimensions; for reference, the total absolute explained variance is provided above each bar.
Dimensions of IBS acute pain episodes
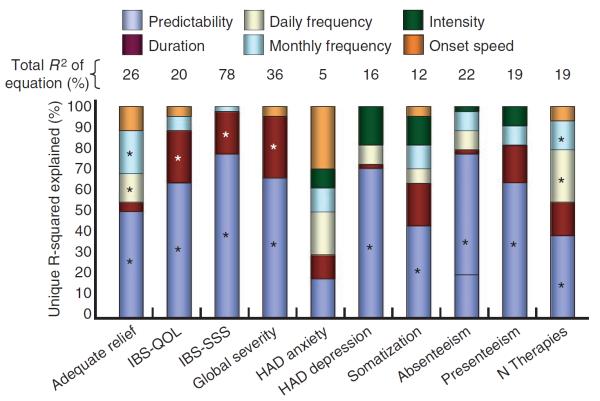
We analysed data from 146 patients who reported experiencing episodes of acute pain. These patients most frequently referred to the episodes as `pain flare-ups' (34%), followed by `pain episodes' (28%), `pain attacks' (19%), `pain bouts' (6%) and `pain breakthroughs' (1%). Thirteen per cent of respondents selected an alternative to the available categories, such as `cramp attack', `stomach bother' and `IBS cycle', among others. Table 4 and Figure 2 displays the results of regression analyses using the pain episode dimensions as predictors of illness severity. Among the acute pain dimensions, predictability (i.e. ability to predict an episode is coming) was most strongly associated with the IBS illness severity metrics. Figure 3 presents the distribution of patient ability to predict acute pain attacks. In contrast, the intensity of acute episodes was not predictive across metrics (unlike the predictive ability of overall pain intensity; Table 3). Similarly, the frequency of acute pain episodes had minimal predictive value. When analysed as a group, the pain episode dimensions explained the largest proportion of variance (R2) for IBS-SSS (78%), weekly symptom severity ratings (36%) and assessment of `adequate relief' (26%). As with the pain dimensions for the overall pain experience, the pain attack dimensions also explained the lowest proportion of variance for generalized anxiety (5%).
Table 4.
Multivariable regression analyses including six dimensions of acute pain episodes, stratified by illness severity metrics
|
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|
| Metric | Intercept | Daily frequency | Monthly frequency | Duration | Intensity | Speed of onset | Predictability | R2 from equation |
| No. therapies | 0.04 | 0.43 | 0.01 | 0.06 | 0.89 | 0.72 | 0.006 | 0.19 |
| IBS-QOL Score | <0.001 | 0.09 | 0.71 | 0.004 | 0.74 | 0.78 | <0.001 | 0.20 |
| HAD Anxiety Scale Score | 0.005 | 0.58 | 0.32 | 0.51 | 0.62 | 0.31 | 0.37 | 0.05 |
| HAD Depression Scale Score | 0.24 | 0.92 | 0.15 | 0.78 | 0.10 | 0.49 | 0.0007 | 0.16 |
| IBS-SSS severity | 0.50 | 0.25 | 0.57 | 0.0001 | 0.65 | 0.61 | <0.001 | 0.49 |
| WPAI absenteeism | 0.05 | 0.47 | 0.17 | 0.35 | 0.58 | 0.85 | 0.002 | 0.22 |
| WPAI presenteeism | 0.56 | 0.24 | 0.84 | 0.15 | 0.33 | 0.84 | 0.01 | 0.19 |
| Somatization Score (PHQ-15) | 0.0001 | 0.37 | 0.29 | 0.08 | 0.28 | 0.18 | 0.02 | 0.12 |
| Considerable relief? | 0.82 | 0.03 | 0.04 | 0.41 | 0.77 | 0.13 | 0.0007 | 0.26 |
| Severity of symptoms past week | 0.71 | 0.89 | 0.93 | 0.0001 | 0.53 | 0.59 | <0.001 | 0.36 |
| Perceived control of pain | 0.0005 | 0.04 | 0.98 | 0.78 | 0.72 | 0.91 | 0.27 | 0.06 |
Each cell provides the P-value for the beta-coefficient of each pain attribute in the model. Bold values indicate statistical significance. The data reveal that pain episode `predictability' (i.e. ability to predict an episode is coming) was the strongest predictor of IBS-related health across metrics. In contrast to overall intensity of pain (Table 3), intensity of pain episodes was not predictive across metrics. Similarly, the frequency of acute pain episodes revealed minimal predictive value. The duration of acute pain episodes revealed intermediate predictive value. Taken together, the pain dimensions explained the largest proportion of variance (R2) for IBS-SSS (49%), weekly symptom severity ratings (36%) and assessment of `considerable relief' (26%).
Figure 2.
Independent contribution of individual dimensions of acute pain episodes to illness severity metrics. The data indicated that pain episode predictability captured at least one-third of the explained variance for all metrics except HAD anxiety. Episode duration and frequency added an additional 25%, on average, across metrics (with exception of perceived control index, in which frequency was predominant). Pain episode severity and onset speed captured only small amount of the explained variance across metrics. Bars with asterisks indicate statistically significant associations. The absolute proportion of variance explained by each model is listed atop each bar.
Figure 3.
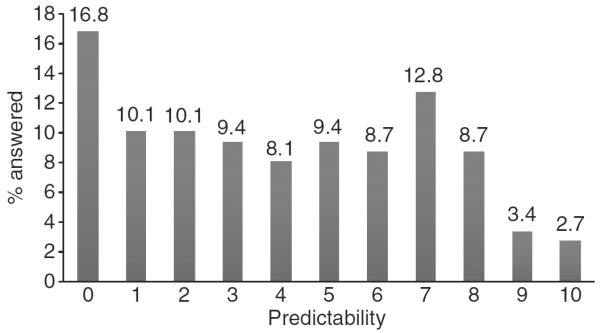
Distribution of Patient Ability to Predict Pain Episodes. Patients were posed the following question: `Some people with IBS can predict when a pain episode is about to come on while others cannot. In thinking about your IBS pain episodes, how reliably can you predict, in advance, that an episode is about to happen on a scale from 0 (IBS episodes are totally unpredictable) to 10 (IBS episodes are totally predictable)?' The data reveal a right skewed distribution with <3% able to `totally' predict their pain episodes. In contrast, the most common response was that pain episodes were `totally unpredictable'.
DISCUSSION
Whereas the Rome III criteria for IBS allow either abdominal pain or discomfort,1 earlier diagnostic criteria, such as the Kruis et al.,27 Manning et al.28 and Rome I,29 specified pain as the hallmark symptom of IBS. Although IBS is a multisymptom disorder, most patients report at least some abdominal pain attributable to their IBS. Moreover, abdominal pain is the principal driver of illness severity in IBS, and drives HRQOL more than any other bowel symptom.4–6 In short, IBS can be reasonably classified as a persistent pain syndrome in many patients; PRO measures for IBS clinical trials must capture the pain experience in a reliable and valid manner.
In this study, we explored the various dimensions of pain in IBS to help guide PRO measurement for future clinical trials, and also to define better the inclusion criteria for trials that seek to measure and treat abdominal pain in IBS. This approach is consistent with PRO guidance in other chronic pain disorders that emphasize the multidimensionality of pain. For example, the NIH-sponsored Patient Reported Outcomes Measurement Information System (PROMIS) includes a pain instrument that specifies intensity, duration and frequency of pain.30 Although the multidimensionality of pain is well accepted in PROMIS, there has been relatively little work performed to explore this concept in IBS.
Our study has four key findings: first, although we confirmed previous data that measuring pain intensity is important in IBS,4, 6 we found that this is necessary, but not sufficient to understand fully the global pain experience in IBS. Instead, future IBS pain measures should also evaluate the frequency and constancy of pain, as these dimensions each provide incremental explanatory value over and above pain intensity alone. In addition, measuring the predictability of pain may be important for understanding the acute pain experience in IBS. These findings should be borne in mind as investigators develop and refine conceptual frameworks for future PROs in IBS. Additional research in other IBS cohorts should further explore the dimensionality of pain in IBS to evaluate whether similar findings emerge.
Second, we found that the clinical definition of pain predominance, in which patients describe pain as their most bothersome symptom,1, 10 is inadequate to gauge fully the overall illness severity in IBS; however, measuring pain predominance does correlate with total physician visits, visceral anxiety and work productivity (Table 2). The suboptimal performance of pain predominance is similar to previous data that patients reporting pain as their most bothersome symptom have a similar psychological and symptom burden as those who do not.10 This has clinical implications as physicians often ask patients to identify their most bothersome symptom to determine the course of questioning and subsequent treatment. Our data suggest that although measuring pain predominance may provide some sense of overall distress, it is not sufficient to understand the overall pain experience of IBS. That is, fully understanding IBS pain will require sampling multiple dimensions of pain – not merely whether it is the most bothersome symptom.
Third, from the viewpoint of a clinical trial design, our data suggest that trials aimed at treating the pain of IBS should not employ inclusion criteria on the basis of a single pain dimension (e.g. only enrolling patients with a pain NRS score of ≥320, 31). Instead, trials may be better served to consider multiple dimensions of pain when determining study eligibility. Future research should develop and validate multidimensional criteria to determining study eligibility; measuring one pain dimension alone may be inadequate to craft a study population.
Fourth, we found that among patients who experience acute pain episodes, the predictability of the episodes is the most important determinant of overall illness severity. This is consistent with the finding that anticipatory concerns are common in patients with IBS, particularly when symptoms wax and wane with limited predictability. A recent study found that few IBS patients know where, when, or what triggers a symptom flare.32 Patients often engage in advanced planning to pursue normal activities, such as knowing the locations of bathrooms out of the home, planning out meals, and reducing participation in daily activities. Anticipatory anxiety of pain, in particular, plays an important role in central pain amplification in chronic pain states. Compared with healthy controls, IBS patients demonstrate a greater startle response after receiving an aversive abdominal stimulation.33 This suggests that anticipatory fear of pain may amplify the intensity of pain and therefore contribute to overall illness severity. These observations are not limited to IBS; other digestive disorders, notably inflammatory bowel disease, lead to anticipatory concerns of impending disease flares.34 In this study, we found a wide variation in patients' ability to predict acute pain episodes in IBS (Figure 3), with the most prevalent response that pain episodes are `totally unpredictable'; <3% of patients reported episodes that were `totally predictable'. Moreover, we found that patients who are better at predicting pain in advance of an acute episode have higher overall HRQOL, lower symptom severity, improved work productivity, less depression and better symptom control compared with those who cannot reliably predict when an episode will occur. Future research should further explore this concept of pain predictability and also determine whether and how to incorporate predictability into both PROs and everyday clinical care.
Our findings regarding pain predictability have potential clinical and academic implications. Clinically, providers should routinely ask patients to assess their ability to predict painful attacks. Patients who cannot reliably predict pain onset might benefit from maintaining a journal to identify contextual cues that are associated with their painful episodes, such as dietary, social, psychological, temporal or other predictive factors. This might empower patients to help manage their disease and minimize the cycle of anticipatory anxiety. For clinical trials aimed at treating painful episodes in IBS, both inclusion criteria and outcomes measurement might benefit from capturing information about pain predictability. Finally, this finding suggests that rapid acting visceral analgesics may play an important role in IBS for patients able to predict the onset of an acute pain episode with sufficient time to spare – similar to the paradigm for acute migraine headache heralded by an aura.
Our study is limited because it is an observational cohort of patients, not a controlled clinical trial. However, we believe that there are important benefits of monitoring IBS patients outside of a clinical trial. Moreover, an observational cohort is well suited for the purpose of psychometric validation of PROs. In addition, our results cannot be generalized to all IBS patients. Nonetheless, our cohort is reflective of other IBS populations as the patients are primarily middle aged and women, are varied across demographic characteristics and have distributions across severity strata that are similar to other clinic patient populations.6 Nonetheless, it will be useful to continue this line of inquiry in other IBS population as PRO development activities continue to evolve.
Another limitation is that some illness severity domains are more apt to cross pain dimensions than others, and some of this may be driven by circularity. For example, the IBS-SSS includes a pain intensity scale and therefore it is not surprising that IBS-SSS scores are highly related to pain intensity. In contrast, other domains are purely psychological (e.g. HAD anxiety, HAD depression, etc.) and have no direct overlap with pain dimensions and therefore they may be less likely to cut across dimensions. Other domains fully avoid circularity, yet still cut across many dimensions simultaneously. It is likely that some of the relationships are partly driven by the structure of the scale, some by the content of the scale and some by both. Although many of the relationships emerging from Tables 2 and 3 may reflect the underlying meaning beyond what we have discussed here, we are reluctant to over-interpret potentially the data given the multiple comparisons we have tested in this exploratory study. However, we do believe that the findings suggest that any new PRO in IBS should be multidimensional to cut across concepts, as some (but not all) of the tested domains accomplish in this study.
In conclusion, we found that abdominal pain in IBS has several dimensions. Although measuring pain intensity is important to understand the illness experience in IBS,4 it is necessary but not sufficient to capture symptom burden and impact adequately. Future PROs in IBS should collect information about various dimensions of pain, including intensity, frequency, constancy, and predictability; pain should not be considered unidimensional. In addition, we have developed an online calculator to help define inclusion criteria for IBS clinical trials aimed at abdominal pain – this may be an improvement over the use of arbitrary, non-empirically based thresholds that are customarily employed. Finally, clinicians should de-emphasize using the pain as the `most bothersome' symptom to drive treatment decisions primarily, and should consider focusing on other aspects of pain to predict illness severity, such as the ability to predict pain episodes reliably, the frequency of pain and the presence of pain as a constant feature.
ACKNOWLEDGEMENTS
Declaration of personal interests: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veteran Affairs. The Principal Investigator, Dr Spiegel, maintained full control over all aspects of the study design, implementation, data collection, data analysis, data interpretation and manuscript preparation. Dr Spiegel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Spiegel has served as a consultant and advisory board member for AstraZeneca, Ironwood, Prometheus, Takeda and Procter & Gamble, and has received research funding from Prometheus, Rose Pharma A/G, Takeda and Shire Pharmaceuticals. Declaration of funding interests: Drs Spiegel, Chang and Bolus are supported by U01 NIH/NIAMS AR057936A. Dr Chang is supported by NIH Grant No. P50 DK64539. This study was supported by an investigator-initiated research grant by Rose Pharma A/S.
REFERENCES
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead WE, Crowell MD, Bosmajian L, et al. Existence of irritable bowel syndrome supported by factor analysis of symptoms in two community samples. Gastroenterology. 1990;98:336–40. doi: 10.1016/0016-5085(90)90822-i. [DOI] [PubMed] [Google Scholar]
- 3.Taub E, Cuevas JL, Cook EW, III, Crowell M, Whitehead WE. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Dig Dis Sci. 1995;40:2647–55. doi: 10.1007/BF02220455. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103:2536–43. doi: 10.1111/j.1572-0241.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel BM, Gralnek IM, Bolus R, et al. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Arch Intern Med. 2004;164:1773–80. doi: 10.1001/archinte.164.16.1773. [DOI] [PubMed] [Google Scholar]
- 6.Lembo A, Ameen VZ, Drossman DA. Irritable bowel syndrome: toward an understanding of severity. Clin Gastroenterol Hepatol. 2005;3:717–25. doi: 10.1016/s1542-3565(05)00157-6. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel B, Bolus R, Harris LA, et al. Measuring IBS patient reported outcomes with an abdominal pain numeric rating scale: results from the proof cohort. Aliment Pharmacol Ther. 2009;30:1159–70. doi: 10.1111/j.1365-2036.2009.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55:195–203. doi: 10.1016/0304-3959(93)90148-I. [DOI] [PubMed] [Google Scholar]
- 10.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–6. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel BMRHL, Lucak S, Mayer E, et al. Developing valid and reliable health utilities in irritable bowel syndrome: results from the IBS PROOF cohort. Am J Gastroenterol. 2009;104:1984–91. doi: 10.1038/ajg.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellstrom PM, Hein J, Bytzer P, Bjornsson E, Kristensen J, Schambye H. Clinical trial: the GLP-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomised, placebo-controlled, double-blind study. Aliment Pharmacol Ther. 2009;29:198–206. doi: 10.1111/j.1365-2036.2008.03870.x. [DOI] [PubMed] [Google Scholar]
- 13.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Li Z, Toner BB, et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986–95. doi: 10.1007/BF02064187. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel BBR, Talley J, Chang L. Patient Willingness to Use ROSE-010: A Subcutaneous (SC) GLP-1 Analogue for IBS Pain Episodes. New Orleans, LA: 2010. Digestive Disease Week. Volume Abstract S1316. [Google Scholar]
- 18.Chang LSY, Bytzer P, TAck J, Mueller-Lissner S, Schambye H, Hellstrom PM. Impact of pain attacks on behavior and health-related outcomes in patients with irritable bowel syndrome meeting Rome III criteria. Gastroenterology. 2010;138:S111–2. [Google Scholar]
- 19.Hellström PMSY, Bytzer P, Tack J, Mueller-Lissner S, Schambye H, Chang L. Characteristics of acute pain attacks in patients with irritable bowel syndrome (IBS) meeting Rome III criteria. Gastroenterology. 2010;138:S–100. doi: 10.1038/ajg.2011.78. [DOI] [PubMed] [Google Scholar]
- 20.Trentacosti AHR, Burke L, Griebel D, Kennedy D. Evolution of clinical trials for irritable bowel syndrome: issues in endpoints and study design. Am J Gastroenterol. 2010;105:731–5. doi: 10.1038/ajg.2010.12. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel BMRN, Mayer E, Bolus R, Chang L. Development and initial validation of a concise point-of-care IBS severity index: the 4-item “BEST” questionnaire. Gastroenterology. 2006;130:S1040. [Google Scholar]
- 22.Patrick DLDD, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 23.Lackner JMGG, Zack MM, Katz LA, et al. Measuring health-related quality of life in patients with irritable bowel syndrome: can less be more? Psychosom Med. 2006;68:312–20. doi: 10.1097/01.psy.0000204897.25745.7c. [DOI] [PubMed] [Google Scholar]
- 24.Reilly MCBA, Ricci JF, Santoro J, Stevens T. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire – irritable bowel syndrome version (WPAI:IBS) Aliment Pharmacol Ther. 2004;20:459–67. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 25.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 26.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 27.Kruis W, Thieme C, Weinzierl M, Schussler P, Holl J, Paulus W. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology. 1984;87:1–7. [PubMed] [Google Scholar]
- 28.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–4. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drossman DA, Thompson WG, Talley NJ. Identification of sub-groups of functional gastrointestinal disorders. Gastroenterology Intl. 1990;3:159–72. [Google Scholar]
- 30.Revicki DA, Chen WH, Harnam N, Cook KF, Amtmann D, Callahan LF, Jensen MP, Keefe FJ. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–69. doi: 10.1016/j.pain.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L, Drossman DA. Rome Foundation Endpoints and Outcomes Conference 2009: optimizing clinical trials in FGID. Am J Gastroenterol. 2010;105:722–30. doi: 10.1038/ajg.2010.49. [DOI] [PubMed] [Google Scholar]
- 32.Drossman DA, Chang L, Schneck S, Blackman C, Norton WF, Norton NJ. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig Dis Sci. 2009;54:1532–41. doi: 10.1007/s10620-009-0792-6. [DOI] [PubMed] [Google Scholar]
- 33.Naliboff BD, Waters AM, Labus JS, et al. Increased acoustic startle responses in IBS patients during abdominal and non-abdominal threat. Psychosom Med. 2008;70:920–7. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irvine EJ. Review article: patients' fears and unmet needs in inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20(Suppl. 4):54–9. doi: 10.1111/j.1365-2036.2004.02053.x. [DOI] [PubMed] [Google Scholar]