Abstract
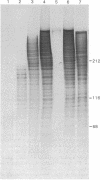
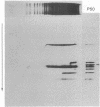
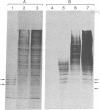
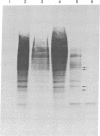
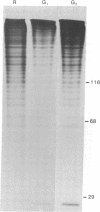
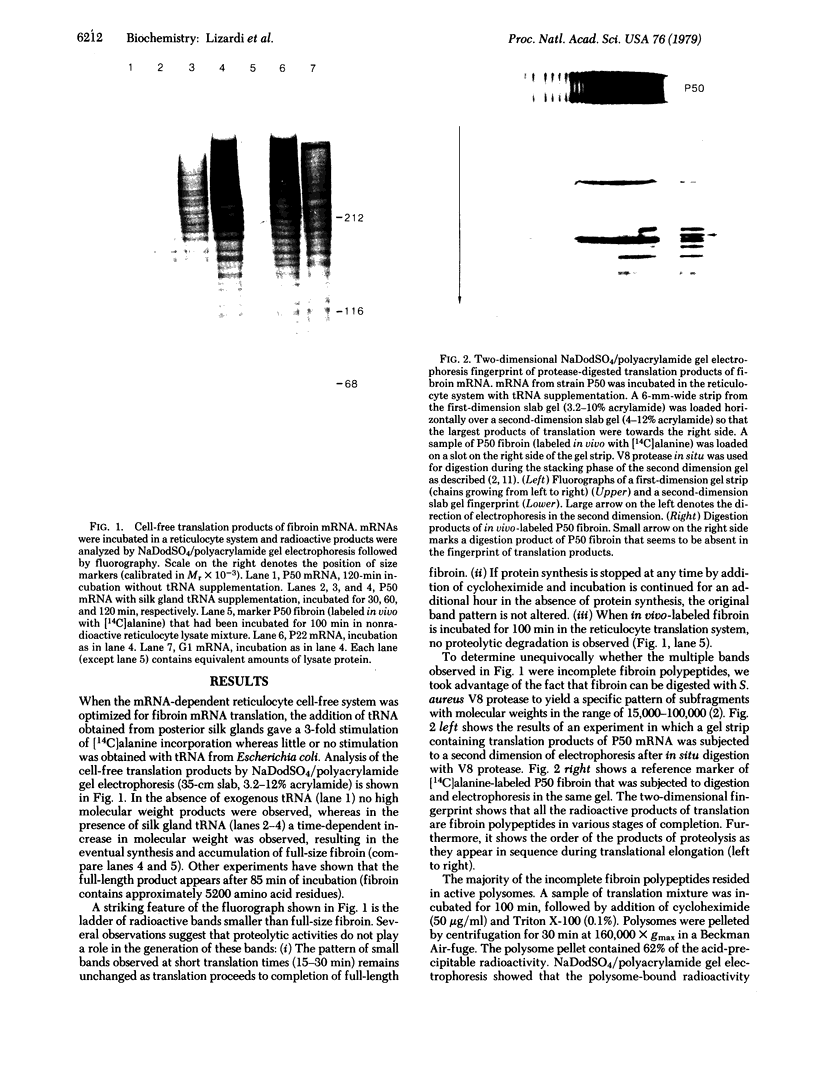
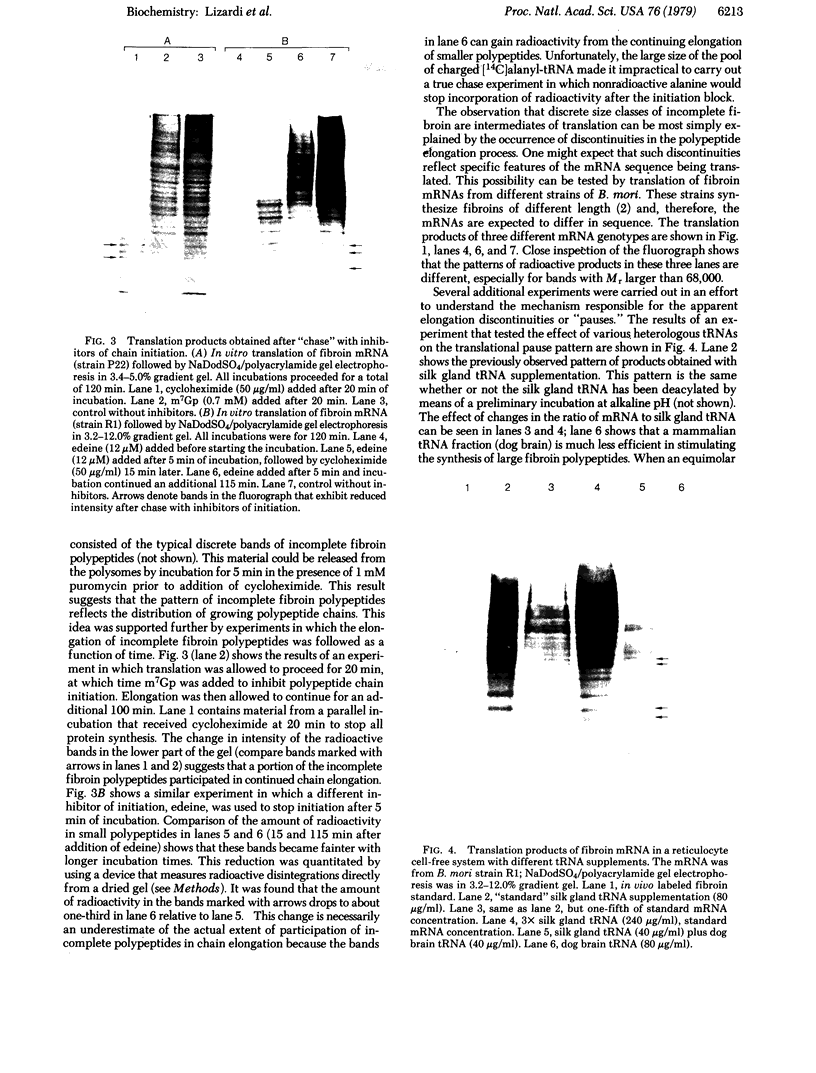
Silk fibroin mRNA was translated in a rabbit reticulocyte cell-free system. Addition of tRNA from silk glands was essential for complete translation of the fibroin polypeptide. (Mr approximately 400,000). Synthesis of full-sized product took at least 85 min. In addition to full-size product, a large number of smaller polypeptides were observed upon analysis by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Evidence is presented that these smaller polypeptides are growing fibroin chains that transiently accumulate as discrete size classes due to discontinuities in the translation process. These discontinuities, or pauses, occur at specific sites in the fibroin mRNA template. The relative duration of the pauses can be experimentally modulated by changing the source of the supplementary tRNA added to the in vitro system. Silk glands were incubated in organ culture under conditions where essentially exclusive labeling of newly synthesized fibroins was attained. Analysis in sodium dodecyl sulfate gels showed that the labeling pattern of nascent silk fibroins is similar to the pattern observed in the reticulocyte cell-free system. This result suggests that discontinuities or pauses in polypeptide chain elongation also occur in vivo under conditions of organ culture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chavancy G., Daillie J., Garel J. P. Adaptation fonctionnelle des t-RNA à la biosynthèse protéique dans un système cellulaire hautement différencié. IV. Evolution des t-RNA dans la glande séricigène de Bombyx mori L. au cours du dernier âge larvaire. Biochimie. 1971;53(11):1187–1194. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Delaney P., Siddiqui M. A. Changes in the in vivo levels of charged transfer RNA species during development of the posterior silkgland of Bombyx mori. Dev Biol. 1975 May;44(1):54–62. doi: 10.1016/0012-1606(75)90376-0. [DOI] [PubMed] [Google Scholar]
- Garel J. P., Garber R. L., Siddiqui M. A. Transfer RNA in posterior silk gland of Bombyx mori: polyacrylamide gel mapping of mature transfer RNA, identification and partial structural characterization of major isoacceptor species. Biochemistry. 1977 Aug 9;16(16):3618–3624. doi: 10.1021/bi00635a018. [DOI] [PubMed] [Google Scholar]
- Garel J. P., Mandel P., Chavancy G., Daillie J. Functional adaptation of tRNAs to protein biosynthesis in a highly differentiated cell system. III. Induction of isoacceptor tRNAs during the secretion of fibroin in the silkgland of Bombyx mori L. FEBS Lett. 1971 Jan 30;12(5):249–252. doi: 10.1016/0014-5793(71)80189-8. [DOI] [PubMed] [Google Scholar]
- Greene R. A., Morgan M., Shatkin A. J., Gage L. P. Translation of silk fibroin messenger RNA in an Ehrlich ascites cell-free extract. J Biol Chem. 1975 Jul 10;250(13):5114–5121. [PubMed] [Google Scholar]
- Lizardi P. M., Engelberg A. Rapid isolation of RNA using proteinase K and sodium perchlorate. Anal Biochem. 1979 Sep 15;98(1):116–122. doi: 10.1016/0003-2697(79)90714-0. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M. Genetic polymorphism of silk fibroin studied by two-dimensional translation pause fingerprints. Cell. 1979 Oct;18(2):581–589. doi: 10.1016/0092-8674(79)90074-6. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Manning R. F., Gage L. P. Physical map of the Bombyx mori DNA containing the gene for silk fibroin. J Biol Chem. 1978 Mar 25;253(6):2044–2052. [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza L., Araya A., Leon G., Krauskopf M. Specific alanine-tRNA species associated with fibroin biosynthesis in the posterior sild-gland of Bombyx mori L. FEBS Lett. 1977 May 15;77(2):255–260. doi: 10.1016/0014-5793(77)80246-9. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Dobkin C., Kramer F. R. Template-determined, variable rate of RNA chain elongation. Cell. 1978 Oct;15(2):541–550. doi: 10.1016/0092-8674(78)90022-3. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Protzel A., Morris A. J. Gel chromatographic analysis of nascent globin chains. Evidence of nonuniform size distribution. J Biol Chem. 1974 Jul 25;249(14):4594–4600. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Roth M. B., Manning R. F., Gage L. P. Alleles of the fibroin gene coding for proteins of different lengths. Cell. 1979 Jun;17(2):407–413. doi: 10.1016/0092-8674(79)90167-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Brown D. D. Isolation and identification of the messenger RNA for silk fibroin from Bombyx mori. J Mol Biol. 1972 Feb 14;63(3):409–429. doi: 10.1016/0022-2836(72)90437-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Fibroin messenger RNA and its genes. Adv Biophys. 1976;8:83–114. [PubMed] [Google Scholar]
- Suzuki Y., Suzuki E. Quantitative measurements of fibroin messenger RNA synthesis in the posterior silk gland of normal and mutant Bombyx mori. J Mol Biol. 1974 Sep 15;88(2):393–407. doi: 10.1016/0022-2836(74)90490-2. [DOI] [PubMed] [Google Scholar]
- Tashiro Y., Morimoto T., Matsuura S., Nagata S. Studies on the posterior silk gland of the silkworm, Bombyx mori. I. Growth of posterior silk gland cells and biosynthesis of fibroin during the fifth larval instar. J Cell Biol. 1968 Sep;38(3):574–588. doi: 10.1083/jcb.38.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Suzuki Y. Structural analysis of the fibroin gene at the 5' end and its surrounding regions. Cell. 1979 Feb;16(2):425–436. doi: 10.1016/0092-8674(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]