Abstract
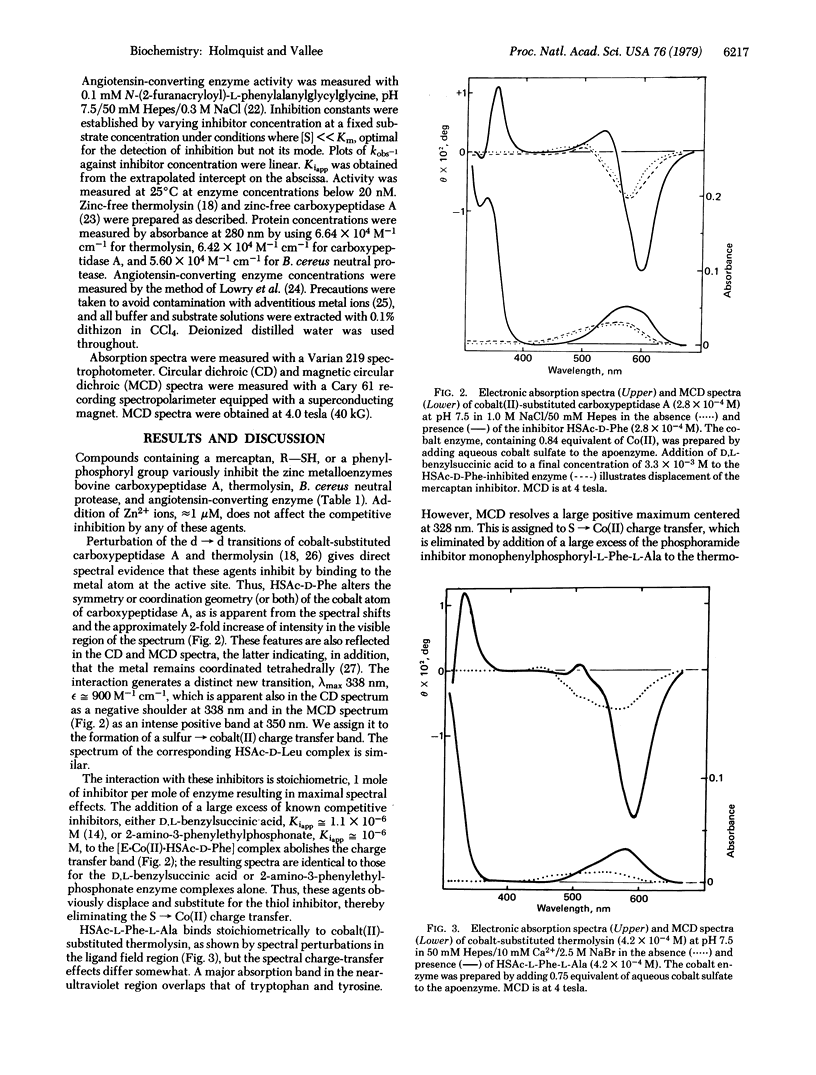
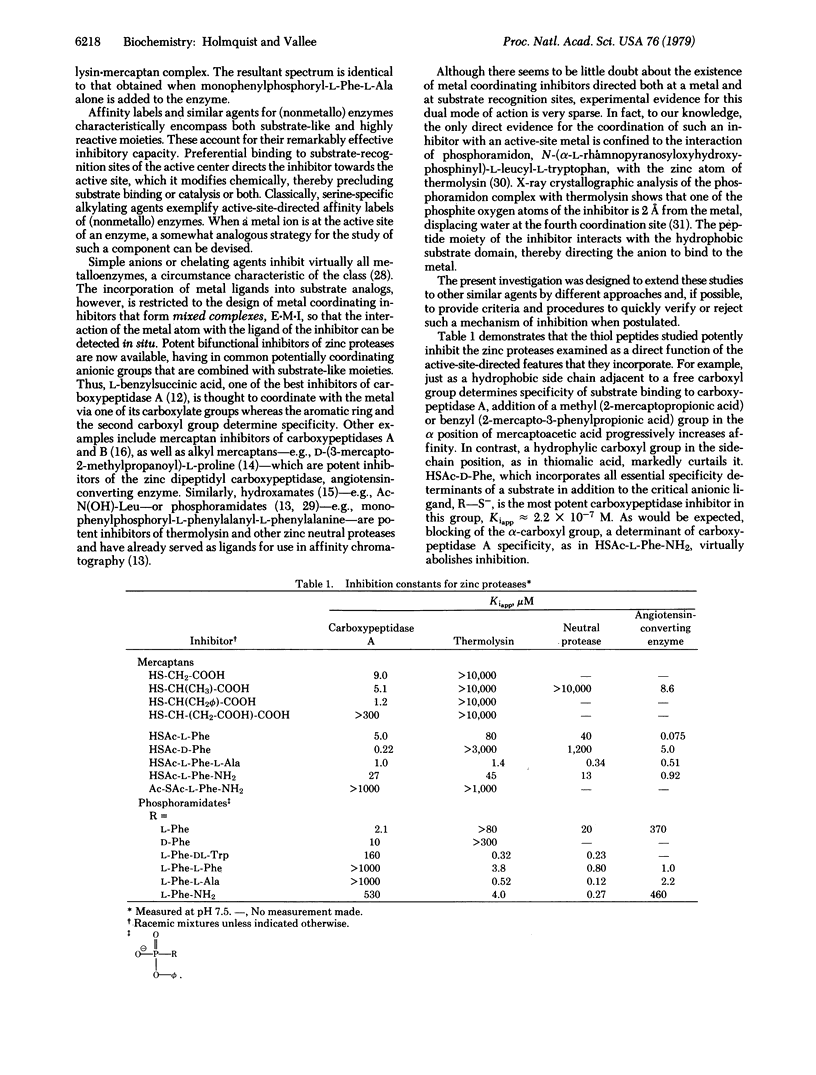
A group of active-site metal coordinating inhibitors of zinc proteases (carboxypeptidase A, thermolysin, Bacillus cereus neutral protease, and angiotensin-converting enzyme) have been synthesized and their properties investigated. Their general structures are R-SH and R-NH-PO2(O phi)H, where-S- or -O- serve as metal ligands and R refers to an amino acid or peptide group designed to interact with substrate recognition sites. These inhibitors can be extremely potent; thus, N-(2-mercaptoacetyl)-D-phenylalanine, e.g., inhibits carboxypeptidase A with a Kiapp of 2.2 x 10(-7) M. The spectral response of cobalt(II)-substituted thermolysin or carboxypeptidase A to the sulfur-containing inhibitors signals the direct interaction of the mercaptan with the metal. An S leads to Co(II) charge transfer band is generated near 340 nm and is detected by absorption, circular dichroism, and magnetic circular dichroism. The cobalt(II) spectra indicate both inner sphere coordination with sulfur and 4-coordination in the enzyme-inhibitor complex. Thus, the metal undergoes a simple substitution reaction, the inhibitor most likely displacing water at the fourth coordination site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. II. Inhibitors of the hydrolysis of oligopeptides. Biochemistry. 1970 Feb 3;9(3):602–609. doi: 10.1021/bi00805a022. [DOI] [PubMed] [Google Scholar]
- Breddam K., Bazzone T. J., Holmquist B., Vallee B. L. Carboxypeptidase of Streptomyces griseus. Implications of its characteristics. Biochemistry. 1979 Apr 17;18(8):1563–1570. doi: 10.1021/bi00575a028. [DOI] [PubMed] [Google Scholar]
- Byers L. D., Wolfenden R. Binding of the by-product analog benzylsuccinic acid by carboxypeptidase A. Biochemistry. 1973 May 22;12(11):2070–2078. doi: 10.1021/bi00735a008. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Cheung H. S., Sabo E. F., Ondetti M. A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry. 1977 Dec 13;16(25):5484–5491. doi: 10.1021/bi00644a014. [DOI] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B., Bünning P., Riordan J. F. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem. 1979 Jun;95(2):540–548. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- Holmquist B. Characterization of the "microprotease" from Bacillus cereus. A zinc neutral endoprotease. Biochemistry. 1977 Oct 18;16(21):4591–4594. doi: 10.1021/bi00640a009. [DOI] [PubMed] [Google Scholar]
- Holmquist B., Kaden T. A., Vallee B. L. Magnetic circular dichroic spectra of cobalt(II) substituted metalloenzymes. Biochemistry. 1975 Apr 8;14(7):1454–1461. doi: 10.1021/bi00678a016. [DOI] [PubMed] [Google Scholar]
- Holmquist B., Vallee B. L. Metal substitutions and inhibition of thermolysin: spectra of the cobalt enzyme. J Biol Chem. 1974 Jul 25;249(14):4601–4607. [PubMed] [Google Scholar]
- Kam C. M., Nishino N., Powers J. C. Inhibition of thermolysin and carboxypeptidase A by phosphoramidates. Biochemistry. 1979 Jul 10;18(14):3032–3038. doi: 10.1021/bi00581a019. [DOI] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Crystallographic study of the binding of dipeptide inhibitors to thermolysin: implications for the mechanism of catalysis. Biochemistry. 1977 May 31;16(11):2506–2516. doi: 10.1021/bi00630a030. [DOI] [PubMed] [Google Scholar]
- Koehler K. A., Lienhard G. E. 2-phenylethaneboronic acid, a possible transition-state analog for chymotrypsin. Biochemistry. 1971 Jun 22;10(13):2477–2483. doi: 10.1021/bi00789a008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane R. W., Ibers J. A., Frankel R. B., Papaefthymiou G. C., Holm R. H. Synthetic analogues of the active sites of iron-sulfur proteins. 14. Synthesis, properties, and structures of bis(o-xylyl-alpha,alpha'-dithiolato)ferrate(II, III) anions, analogues of oxidized and reduced rubredoxin sites. J Am Chem Soc. 1977 Jan 5;99(1):84–98. doi: 10.1021/ja00443a017. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Vallee B. L. Spectral properties of cobalt carboxypeptidase. The effects of substrates and inhibitors. Biochemistry. 1971 Nov;10(23):4263–4270. doi: 10.1021/bi00799a017. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I., Koehler K. A., Lindquist R. N. Enzymatic catalysis and the transition state theory of reaction rates: transition state analogs. Cold Spring Harb Symp Quant Biol. 1972;36:45–51. doi: 10.1101/sqb.1972.036.01.009. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- May S. W., Kuo J. Y. Preparation and properties of cobalt(II) rubredoxin. Biochemistry. 1978 Aug 8;17(16):3333–3338. doi: 10.1021/bi00609a025. [DOI] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Peptide hydroxamic acids as inhibitors of thermolysin. Biochemistry. 1978 Jul 11;17(14):2846–2850. doi: 10.1021/bi00607a023. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Condon M. E., Reid J., Sabo E. F., Cheung H. S., Cushman D. W. Design of potent and specific inhibitors of carboxypeptidases A and B. Biochemistry. 1979 Apr 17;18(8):1427–1430. doi: 10.1021/bi00575a006. [DOI] [PubMed] [Google Scholar]
- Oshima G., Gecse A., Erdös E. G. Angiotensin I-converting enzyme of the kidney cortex. Biochim Biophys Acta. 1974 May 20;350(1):26–37. doi: 10.1016/0005-2744(74)90199-5. [DOI] [PubMed] [Google Scholar]
- Peterson L. M., Sokolovsky M., Vallee B. L. Purification and crystallization of human carboxypeptidase A. Biochemistry. 1976 Jun 15;15(12):2501–2508. doi: 10.1021/bi00657a001. [DOI] [PubMed] [Google Scholar]
- Sampson E. J., Fedor J., Benkovic P. A., Benkovic S. J. Intramolecular and divalent metal ion catalysis. The hydrolytic mechanism of O-phenyl N-(glycyl)phosphoramidate. J Org Chem. 1973 Apr 6;38(7):1301–1306. doi: 10.1021/jo00947a011. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Rawlings J., McMillin D. R., Stephens P. J., Gray H. B. Infrared and visible circular dichroism and magnetic circular dichroism studies on cobalt (II)-substituted blue copper proteins. J Am Chem Soc. 1976 Dec 8;98(25):8046–8048. doi: 10.1021/ja00441a028. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Takeuchi T., Umezawa H. Letter: A thermolysin inhibitor produced by Actinomycetes: phospholamidon. J Antibiot (Tokyo) 1973 Oct;26(10):621–623. doi: 10.7164/antibiotics.26.621. [DOI] [PubMed] [Google Scholar]
- Sugiura Y. Electronic properties of sulfhydryl- and imidazole-containing peptide-cobalt(II) complexes: their relationship to cobalt(II)-substituted "blue" copper proteins. Bioinorg Chem. 1978;8(5):453–460. doi: 10.1016/s0006-3061(00)80280-x. [DOI] [PubMed] [Google Scholar]
- Sytkowski A. J. Metal stoichiometry, coenzyme binding, and zinc and cobalt enchange in highly purified yeast alcohol dehydrogenase. Arch Biochem Biophys. 1977 Dec;184(2):505–517. doi: 10.1016/0003-9861(77)90460-x. [DOI] [PubMed] [Google Scholar]
- Sytkowski A. J., Vallee B. L. Cobalt exchange in horse liver alcohol dehydrogenase. Biochemistry. 1978 Jul 11;17(14):2850–2857. doi: 10.1021/bi00607a024. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Bauer C. A. Reaction of peptide aldehydes with serine proteases. Implications for the entropy changes associated with enzymatic catalysis. Biochemistry. 1979 Apr 17;18(8):1552–1558. doi: 10.1021/bi00575a026. [DOI] [PubMed] [Google Scholar]
- Thompson R. C. Use of peptide aldehydes to generate transition-state analogs of elastase. Biochemistry. 1973 Jan 2;12(1):47–51. doi: 10.1021/bi00725a009. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. Transition state analog inhibitors and enzyme catalysis. Annu Rev Biophys Bioeng. 1976;5:271–306. doi: 10.1146/annurev.bb.05.060176.001415. [DOI] [PubMed] [Google Scholar]


