Abstract
Background
Various cell types, including podocytes and parietal epithelial cells (PECs), play important roles in the development and progression of glomerular kidney diseases, albuminuria and glomerulosclerosis. Besides their role in renal pathologies, glomerular cells have emerging new functions in endogenous repair mechanisms. Better understanding of the dynamics of the glomerular environment and cellular composition in the intact living kidney is critically important for the development of new regenerative therapeutic strategies for kidney diseases. However, progress in this field has been hampered by the lack of in vivo research tools.
Summary
This review summarizes the current state-of-the-art in the application of the unique intravital imaging technology of multiphoton fluorescence microscopy for the dynamic visualization of glomerular structure and function over time in the intact, living kidney. Recently, this imaging approach in combination with transgenic mouse models allowed to track the fate of individual glomerular cells in vivo over several days and depicted the highly dynamic nature of the glomerular environment, particularly in disease conditions.
Key Messages
The technology is ready and available for future intravital imaging studies investigating new glomerular regenerative approaches in animal models.
The recent development and application of intravital fluorescence imaging approaches using multiphoton microscopy (MPM) solved a critical technical barrier in glomerular biology research. Until recently, most morphological and functional observations were based on cell culture models [1] and fixed tissue sections [2]. However, during the past few years, tremendous advances in the field of live imaging helped to modernize kidney research. The ability of MPM to directly visualize the changes in the structure and function of the same glomerulus in the intact living kidney over time with unprecedented, subcellular detail is an important technological breakthrough. In this brief review, first we summarize the most exciting new developments in fluorescence imaging technology for glomerular studies, and then highlight the key points of the new insights in the glomerular environment using MPM imaging, and the future directions in research and technology.
New developments in fluorescence imaging technology for studying the glomerulus
MPM is a powerful minimally-invasive imaging technique for the deep optical sectioning of living tissues [3,4]. The basic principles, applications, advantages, and limitations of this imaging technology for the study of the living intact kidney have been recently described in detail [5]. During the last decade, improved applications of intavital MPM have been developed and applied for the quantitative imaging of basic functions in renal (patho)physiology in the intact whole kidney [6,7] including the measurement of the magnitude and temporal oscillations in single nephron filtration rate, changes in blood flow and tubular flow, vascular resistance and permeability, renin granule content, release, and tissue renin activity [3,4,7]. MPM imaging also allowed the studying of intracellular variables in cells in the intact living kidney, such as intracellular calcium levels [3,8] and pH [5,9].
Importantly, confocal fluorescence imaging of the cellular and subcellular elements of the intact glomerulus and the glomerular filtration barrier (GFB) became possible not only in zebrafish [10] but also in the few surface glomeruli of most mouse strains [11]. In fact, the feasibility of routinely performing MPM imaging of glomeruli in the intact mouse kidney of the commonly used C57BL6 strain has been demonstrated in our previous publications [4,5,7], and it has been also confirmed by at least three independent laboratories [11-13]. The permeability of the GFB to various macromolecules, including the leakage of the clinically relevant albumin from glomerular capillaries to the Bowman's space, has been measured in the healthy mouse kidney and through the course of disease [14,15]. Also, the interactions between glomerular endothelium (including its glycocalyx), basement membrane, and podocytes have been visualized [4,15]. In addition to the analysis of glomerular and GFB functions, non-specific negative labeling techniques as shown in Fig. 1A allowed the visualization of migrating single cells within intact glomeruli in vivo [4].
Figure 1. Intravital MPM imaging of the structure and function of the glomerulus and the glomerular filtration barrier in the intact living kidney.
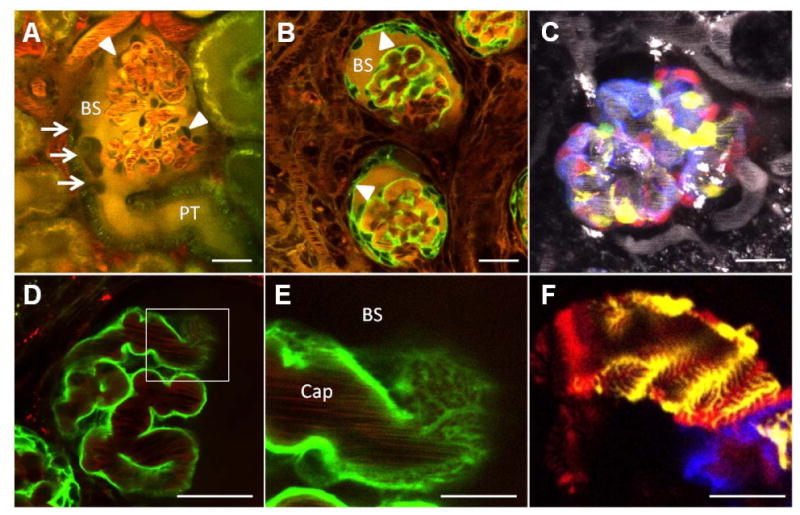
A: A dye exclusion technique negatively identifies podocytes around glomerular capillaries (arrowheads) in the Munich-Wistar-Fromter rat kidney, while the Bowman's space (BS) is filled with systemically injected and filtered Lucifer Yellow (yellow). In addition to podocytes, several other dark cells can be found attached to the parietal layer of the Bowman's capsule at the tubulo-glomerular junction (arrows). PT, proximal tubule. Plasma was labeled with Alexa594-albumin (red). B: Podocyte-specific expression of GFP (green) in Pod-GFP mice positively identifies visceral epithelial cells around glomerular capillaries. However, after unilateral ureter obstruction (UUO), podocytes migrate from the visceral to the parietal layer of the Bowman's capsule (arrowheads) leading to the appearance of GFP-positive cells on both layers. The glomerular permeability of systemically injected, various molecular weight markers through either the visceral or parietal layers of the Bowman's capsule can be quantitatively visualized. Shown here is the accumulation of the iv injected, freely filterable Lucifer Yellow (yellow) in the Bowman's space (BS). C: Multi-color labeling of podocytes in In Pod-Confetti mice. Individual podocytes are labeled by one of four different colors due to CFP (blue), GFP (green), YFP (yellow), or RFP (red) expression. Plasma dye is shown in grayscale. D-F: Optical imaging of the ultrastructural details of podocyte primary, secondary, and foot processes. Podocyte-specific expression of GFP in Pod-GFP mice shows podocytes in green in great detail (D). The area indicated by the rectangle is magnified in (E) and illustrates that optical sectioning horizontally through the surface of the capillary wall is able to spatially resolve the cell processes of podocytes. Dark objects in glomerular capillaries (Cap) are streaming red blood cells. Plasma was labeled with Alexa594-albumin (red). F: Interdigitating foot processes of three adjacent podocytes are shown in Pod-Confetti mice, each cell labeled by a different fluorescent protein (CFP/YFP/RFP). Bars are 20 μm each except in panels E-F (5μm).
Another technical innovation and new milestone for glomerular imaging in vivo was the combination of widely available mouse genetic strategies with MPM imaging. The development of new transgenic mouse models in which the lineage of various glomerular cells has been fluorescently tagged helped to properly identify and track individual cells within the glomerulus over time and to study the dynamics of cell motility as an addition to traditional genetic cell fate mapping [16]. For example, Fig. 1B-C demonstrate the use of the Tomato-GFP, and the Confetti (the combination of membrane-targeted CFP (blue), nuclear GFP (green), and cytosolic YFP (yellow) and RFP (red)) fluorescent reporters for labeling podocytes and cells of podocyte origin. Most recently, serial MPM imaging was performed on the same glomerulus over time in the intact Pod-GFP and Pod-Confetti mouse kidney in vivo, once in every 24 hours. This new approach was instrumental in depicting the dynamics and the dramatic changes in the morphology and cellular composition of the injured glomerulus [16]. Also, podocyte-specific expression of fluorescent reporters allowed to study the ultrastructural changes to primary, secondary and foot processes after podocyte injury using conventional fluorescence (optical) microscopy in both fixed kidneys and ex vivo [17,18]. This approach is exemplified in Fig. 1D-F. This is a significant technical advance which will ease podocyte research, since fluorescence microscopy is generally available in contrast to the cumbersome electron microscopy, which used to be the standard technique of podocyte foot process imaging.
Intravital imaging of glomerular remodeling – What did we learn so far?
Time-lapse MPM imaging provided new details and important in vivo confirmation of podocyte shedding, replacement, and the role of PECs in the pathology of glomerulosclerosis [4]. Direct visual evidence was shown in the puromycin (PAN)-model of focal segmental glomerulosclerosis (FSGS) for the rather rapid processes of podocyte detachment (possibly due to cell necrosis) and exit either downstream the tubular fluid or by breaking through the highly permeable PEC layer into the periglomerular interstitium [4]. Remarkably, MPM visualized the replacement of a detached podocyte by a new, negatively labeled cell which appeared to originate from the parietal Bowman's capsule [4]. These findings suggested the presence of dynamic regenerative mechanisms within the injured glomerulus consistent with the recent identification of a subset of highly proliferative PECs at the urinary pole of the Bowman's capsule within the rodent and human kidney functioning as renal progenitors [19-25]. These progenitor cells were proposed to proliferate, differentiate, and generate new podocytes by progressively migrating from the urinary pole to the vascular stalk [20,21,23]. Facilitating podocyte regeneration and therefore promoting functional repair of the injured GFB is a promising new therapeutic strategy, however several questions and mechanistic details remain to be clarified and understood in this process. Using a negative labeling technique due to dye exclusion, MPM imaging was able to visualize a few cells localized at the glomerulo-tubular junction which appeared to be loosely attached to the PEC layer (Fig. 1A). However, the exact identity and function of these cells are unclear and require further study.
Most recently, tracking cell fate using serial MPM imaging of the same glomerulus over time in vivo in novel transgenic mouse models provided new visual clues on cell migration, remodeling and the highly dynamic glomerular environment. In podocin-GFP mice, podocytes formed sporadic multicellular clusters after glomerular injury, caused by either unilateral ureteral ligation (UUO) or adriamycin administration, and migrated into the parietal Bowman's capsule [16] (Fig. 1B). Podocytes migrated to the PEC layer either via continuous transition at the vascular pole or by forming bridges across the Bowman's space close to the urinary pole or anywhere around the glomerular tuft (summarized in Fig. 2). The tracking of single cells in podocin-confetti mice featuring cell-specific expression of CFP, GFP, YFP or RFP (Fig. 1C) revealed the simultaneous migration of multiple podocytes [16]. Importantly, podocyte-to-PEC migration appeared to be a continuously ongoing phenomenon between 2-12 weeks after injury which progressively increased the coverage of the parietal Bowman's capsule by podocytes [16]. Although this is an established pathological process in the model of UUO [26], the robust cell migration and likely the proliferation of a yet to be identified precursor cell type may provide new clues for future regenerative approaches. Also, MPM imaging visualized nanotubes between visceral (podocytes) and parietal (PEC) layers of the Bowman's capsule which may function in cell migration or cell-to-cell communication [16].
Figure 2. Schematic illustration of the migration of podocytes and PECs in the intact kidney as observed with intravital MPM imaging.
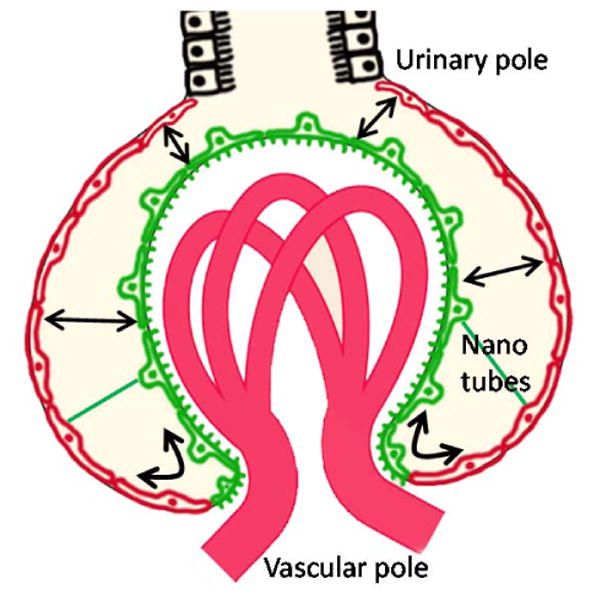
In addition to PEC-to-podocyte transition and cell replacement as established in the literature, MPM imaging observed the clustering and migration of podocytes (green) to the parietal layer of the Bowman's capsule (red), which was most robust after glomerular injury. Podocytes migrated to the PEC layer either via continuous transition at the vascular pole or by forming bridges across the Bowman's space close to the urinary pole or anywhere around the glomerular tuft (arrows). Nanotubes exist between visceral (podocytes) and parietal (PEC) layers of the Bowman's capsule (green line) which may function in cell migration or cell-to-cell communication.
Perhaps the most important MPM imaging finding, an in vivo visual clue suggesting podocyte regeneration, was the first time visualization of de novo podocyte formation. Using serial MPM imaging of podocin-confetti mice in the UUO model of renal fibrosis, we were able to observe the appearance of a new podocyte within 24 hours of the previous imaging session [16]. Altogether, these findings have further challenged the previous static view of podocytes and demonstrated that at least some podocytes have a highly dynamic, motile, and migratory phenotype.
What are the future needs and research directions?
Although the technology is in place to directly visualize the process of glomerular regeneration in the living kidney, and we have gained substantial new insights into this process, there are more questions than answers. How can intravital imaging help in the future to better understand the molecular and cellular mechanisms of glomerular regeneration? Positive identification and fluorescent tagging (lineage tracing) of various types of systemic and/or renal progenitor cell populations combined with serial MPM imaging will help to determine the origin of glomerular podocytes and other cell types and the dynamics of cell replacement. Future work may visualize podocyte replacement by migrating PECs as proposed and shown recently [20,27] or by other cells of extraglomerular origin, e.g. by the renin cell as suggested by the Shankland group [28]. In addition to tracking cells, MPM imaging can quantitatively visualize the dynamics of not only morphological but also functional regeneration of the same glomerulus and GFB over time. For example, the permeability of the GFB to various molecular tracers can be evaluated simultaneously with following the process of glomerular remodeling (Fig. 1B). Also, the use of new transgenic mouse models with multi-color podocyte labeling [17] allows the optical resolution of podocyte foot processes (Fig. 1D-F) for further studies of GFB ultrastructure changes during glomerular regeneration.
The use of future, ever-developing imaging techniques and approaches (e.g. long wavelength infrared lasers, extremely sensitive detectors, super-resolution nanoscopy) are expected to further push the limits of intravital, functional podocyte imaging. We anticipate that serial MPM of novel podocyte mouse models with fluorescent lineage tags as a novel, unique, state-of-the-art imaging approach will be used in the near future to directly and quantitatively visualize novel mechanisms of glomerular regeneration in vivo.
Acknowledgments
This work was supported in part by the US National Institutes of Health grant DK64324 to J.P.-P.
References
- 1.Mundel P, Reiser J, Kriz W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol. 1997;8:697–705. doi: 10.1681/ASN.V85697. [DOI] [PubMed] [Google Scholar]
- 2.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int. 1998;54:687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 3.Peti-Peterdi J, Toma I, Sipos A, Vargas SL. Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 2009;24:88–96. doi: 10.1152/physiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835–1841. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peti-Peterdi J, Burford JL, Hackl MJ. The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol. 2012;302:F227–F233. doi: 10.1152/ajprenal.00561.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–C916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 7.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol. 2006;291:F495–F502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- 8.Svenningsen P, Burford JL, Peti-Peterdi J. Atp releasing connexin 30 hemichannels mediate flow-induced calcium signaling in the collecting duct. Front Physiol. 2013;4:292. doi: 10.3389/fphys.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sipos A, Toma I, Kang JJ, Rosivall L, Peti-Peterdi J. Advances in renal (patho)physiology using multiphoton microscopy. Kidney Int. 2007;72:1188–1191. doi: 10.1038/sj.ki.5002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiessl IM, Bardehle S, Castrop H. Superficial nephrons in balb/c and c57bl/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One. 2013;8:e52499. doi: 10.1371/journal.pone.0052499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR, Hickey MJ. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 13.Khoury CC, Khayat MF, Yeo TK, Pyagay PE, Wang A, Asuncion AM, Sharma K, Yu W, Chen S. Visualizing the mouse podocyte with multiphoton microscopy. Biochem Biophys Res Commun. 2012;427:525–530. doi: 10.1016/j.bbrc.2012.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–1856. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–1350. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackl MJ, Burford J, Villanueva K, Lam L, Susztak K, Schermer B, Benzing T, Peti-Peterdi J. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in novel mouse models with fluorescent lineage tags. [10.1038/nm.3405];Nat Med. 2013 doi: 10.1038/nm.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grgic I, Brooks CR, Hofmeister AF, Bijol V, Bonventre JV, Humphreys BD. Imaging of podocyte foot processes by fluorescence microscopy. J Am Soc Nephrol. 2012;23:785–791. doi: 10.1681/ASN.2011100988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Höhne M, Ising C, Hagmann H, Völker LA, Brähler S, Schermer B, Brinkkoetter PT, Benzing T. Light microscopic visualization of podocyte ultrastructure demonstrates oscillating glomerular contractions. Am J Pathol. 2013;182:332–338. doi: 10.1016/j.ajpath.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasagni L, Romagnani P. Glomerular epithelial stem cells: The good, the bad, and the ugly. J Am Soc Nephrol. 2010;21:1612–1619. doi: 10.1681/ASN.2010010048. [DOI] [PubMed] [Google Scholar]
- 21.Romagnani P. Parietal epithelial cells: Their role in health and disease. Contrib Nephrol. 2011;169:23–36. doi: 10.1159/000313943. [DOI] [PubMed] [Google Scholar]
- 22.Romagnani P, Kalluri R. Possible mechanisms of kidney repair. Fibrogenesis Tissue Repair. 2009;2:3. doi: 10.1186/1755-1536-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol. 2009;20:2593–2603. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smeets B, Uhlig S, Fuss A, Mooren F, Wetzels JF, Floege J, Moeller MJ. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol. 2009;20:2604–2615. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes MS, Thornhill BA, Chevalier RL. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol. 2011;301:F110–117. doi: 10.1152/ajprenal.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: An evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 28.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol. 2013;183:542–557. doi: 10.1016/j.ajpath.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


