Abstract
Background
The gut-derived hormone ghrelin, especially its acylated form, plays a major role in the regulation of systemic metabolism and exerts also relevant cardioprotective effects, hence it has been proposed for the treatment of heart failure (HF). We tested the hypothesis that ghrelin can directly modulate cardiac energy substrate metabolism.
Methods and Results
We used chronically instrumented dogs, 8 with pacing-induced HF and 6 normal controls. 1.2 nmol/kg/hour of human des-acyl ghrelin was infused intravenously for 15 min, followed by washout (re-baseline) and infusion of acyl ghrelin at the same dose. 3H-oleate and 14C-glucose were co-infused and arterial and coronary sinus blood sampled to measure cardiac free fatty acids (FFA) and glucose oxidation and lactate uptake. As expected, cardiac substrate metabolism was profoundly altered in HF, since baseline FFA and glucose oxidation were, respectively, >70% lower and >160% higher compared to control. Neither des-acyl ghrelin nor acyl ghrelin affected significantly function and metabolism in normal hearts. However, in HF, des-acyl and acyl ghrelin enhanced MVO2 by 10.2±3.5 and 9.9±3.7%, respectively, (P<0.05), while cardiac mechanical efficiency was not significantly altered. This was associated, respectively, with a 41.3±6.7 and 32.5±10.9% increase in FFA oxidation and a 31.3±9.2 and 41.4±8.9% decrease in glucose oxidation (all P<0.05).
Conclusions
Acute increases in des-acyl ghrelin or acyl ghrelin do not interfere with cardiac metabolism in normal, while they enhance FFA oxidation and reduce glucose oxidation in HF, thus partially correcting its metabolic alterations. This novel mechanism might contribute to the cardioprotective effects of ghrelin in HF.
Keywords: heat failure, energy metabolism, hormones
The extrinsic regulation of cardiac energy substrate metabolism is effected by neural mediators and peptide hormones (1–3). Most studies on hormonal regulators have explored the effects of insulin and glucagon-like peptide, which are particularly interesting for their therapeutic use (4–5). Surprisingly, little attention has been paid to the potential control of cardiac metabolism by ghrelin, a gut-derived 28- aminoacid peptide known as a major stimulator of growth hormone release and food intake (6). Ghrelin is mainly produced by X/A-like cells of the gastric oxyntic glands, although several other tissues can synthesize it (7). Since the report of its discovery in 1999 (8), this hormone has been intensively investigated not only for its role in the regulation of appetite, but also for its diverse, direct actions on organs and systems, including heart and blood vessels (9). Associations between ghrelin or ghrelin receptors single nucleotide polymorphisms and cardiovascular diseases have been described (10). Prompted by initial findings in a rat model of myocardial infarction (11), some authors tested the therapeutic effects of ghrelin infusion in chronic heart failure patients: three weeks of treatment improved left ventricular function, exercise capacity and muscle mass (12). Ensuing experimental studies confirmed the potential curative action of this hormone in rodents subjected to myocardial infarction (13–15). The beneficial effects of ghrelin on the failing heart might be due to multiple mechanisms, thus far characterized mainly in vitro, encompassing anti-apoptotic protection and direct inotropic action (16–18). However, since the foremost role of this hormone is the control of systemic metabolism, one possible, important mechanism might be the normalization of cardiac energy substrate consumption. At rest, the healthy heart obtains most of the energy from the oxidation of free fatty acids (FFA) and lactate. In failing hearts, FFA oxidation falls concomitantly with an abnormal elevation in glucose oxidation (19–21). The pathophysiological significance of this alteration is still debated, nonetheless several investigators have proposed the use of modulators of cardiac metabolism for the treatment of heart failure (22). Ghrelin is an endogenously produced peptide, hence it might prove a very biocompatible candidate for the correction of cardiac metabolic alterations. To date, however, no studies have determined the direct effects of ghrelin on cardiac oxygen consumption and substrate oxidation, in vivo. The present study was aimed at filling this gap of knowledge. Ghrelin, an orexigenic peptide, promotes systemic anabolism, therefore we tested the hypothesis that high circulating levels of it can directly lower energy turnover both in normal and failing hearts, with possible differential effects on FFA and carbohydrates oxidation. We also evaluated potential repercussions of metabolic regulation by ghrelin on cardiac mechanical efficiency.
A further level of complexity is given by the presence of two distinct forms of circulating ghrelin. In order to exert its main functions via the GSH-R1a receptor, ghrelin must first undergo acylation with octanoic acid at its third serine residue (6). However, solid evidence indicates that also the non-acylated form, named des-acyl ghrelin, is active at many levels, in some cases sharing the functions of the acylated form and in other cases antagonizing them (6,9). Des-acyl ghrelin receptors have been postulated, but not identified yet. Therefore, our hypothesis was tested using des-acyl ghrelin and acyl ghrelin, alternatively. The study was performed by simultaneously measuring hemodynamics, cardiac function and rate of myocardial oxygen, FFA, glucose, and lactate consumption in conscious dogs. Large animal models are particularly advantageous for this type of studies, in that they allow withdrawal of blood samples from coronary sinus to measure cardiac substrate metabolism, in vivo.
Methods
Surgical instrumentation and hemodynamic measurements
Nineteen adult, male, mongrel dogs (25–27 kg) were chronically instrumented as previously described (23). Briefly, anesthesia was induced with propofol (6 mg/kg i.v.) and maintained with 1.5–2% isofluorane during 40% oxygen/60% air ventilation, a thoracotomy was performed in the left fifth intercostal space, a catheter was placed in the descending thoracic aorta, a solid-state pressure gauge (P6.5; Konigsberg Instruments) was inserted into the left ventricle through the apex, a Doppler flow transducer (Craig Hartley) was placed around the left circumflex coronary artery and a pair of pacing leads was fixed on the left ventricular (LV) free wall. Wires and catheters were run subcutaneously to the intrascapular region, the chest was closed in layers and the pneumothorax was reduced. Antibiotics were given after surgery and the dogs were allowed to fully recover. After 7–10 days of recovery from surgery, dogs were trained to lie quietly on the laboratory table. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Temple University and conform to the guiding principles for the care and use of laboratory animals published by the National Institutes of Health.
Experimental protocol
HF was induced in 13 dogs by pacing the LV at 210 beats/min for 3 weeks and at 240 beats/min for an additional week (23). Dogs were considered in congestive HF when LV end-diastolic pressure was ≥ 25 mmHg, reflected by clinical signs such as dyspnea and ascites. Six chronically instrumented dogs were used as normal control. The experiments were conducted in intact, conscious animals placed on the laboratory table following overnight fasting. HF dogs were studied at spontaneous heart rate, with the pacemaker turned off. Control hearts were paced at 140 beats/min to match the highest predictable spontaneous heart rate occurring in our HF dog model. A catheter was inserted into the coronary sinus, through a peripheral vein, under X-ray fluoroscopic guidance. Therefore we could withdraw paired blood samples from aorta and coronary sinus. Measurements and blood samples were taken at spontaneous heart rate, with the pacemaker turned off. After baseline hemodynamic measurements, the radioisotopic tracers [9,10-3H]-oleate (0.7 μCi/min) and [U-14C]-glucose (20 μCi as a bolus, followed by 0.3 μCi/min) were infused through a peripheral vein to track, respectively, the metabolic fate of FFA and glucose utilized by cardiac muscle as source of energy (23–24). In 8 HF and 6 control dogs, human des-acyl ghrelin (Bachem) was then infused at the dose of 4 μg/kg/hour (equivalent to 1.2 nmol/kg/hour) for 15 minutes. The infusion was then stopped for 60 minutes to re-establish the baseline condition, followed by 15 minutes infusion of 4 μg/kg/hour human acyl ghrelin (Bachem). Sequence of infusion, doses and infusion times were selected to 1) avoid acyl ghrelin-induced increases in growth hormone during the experiment (thus the acylated form was infused later) and 2) to obtain measureable effects while remaining within the time window that precedes increases in growth hormone. Given the unavailability of previous similar dog studies in literature, we used as a reference a protocol of acute ghrelin infusion in humans (25) at a dose unable to elicit major growth hormone changes within the first 15 minutes.
Once we determined the functional and metabolic response to ghrelin, we tested its potential analogies or differences with changes occurring during a standard stress such as beta-adrenergic stimulation: in 5 HF dogs, isotope labeled substrates where infused and hemodynamic measurements and blood samples taken at baseline and after 5 minutes of dobutamine infusion at 5 and 10 μg/kg/min.
At the end of the protocol, the HF dogs were euthanized with 100 mg/kg of sodium pentobarbital.
Hemodynamic recordings, blood gas analysis and calculated parameters
The aortic catheter was attached to a strain-gauge transducer to measure aortic pressure. LV pressure was measured using the solid-state pressure gauge. Blood flow in the left circumflex coronary artery was measured with a pulsed Doppler flowmeter (model 100, Triton Technology). LV diameter was measured by connecting the implanted piezoelectric crystals to an ultrasonic dimension gauge. All signals were digitally stored via an analog-digital interface (National Instruments) at a sampling rate of 250 Hz. Digitized data were analyzed off-line by commercially available software (Notocord hem evolution, Notocord). The parameters were determined during one respiratory cycle and comprised heart rate, mean aortic pressure, LV end-diastolic, peak systolic and end-systolic pressure, mean blood flow in the left circumflex coronary artery, the maximum and minimum of the first derivative of LV pressure. The difference between end-diastolic and end-systolic LV internal diameters was used as a surrogate of stroke volume and multiplied by heart rate to obtain a surrogate of cardiac output. The inverse of this latter was then multiplied by mean arterial pressure to calculate total peripheral resistance. Finally, the area of LV pressure-diameter loops (PDA) was calculated to obtain an index of stroke work (26–28).
Blood gases tension was determined using a blood gas analyzer (ABL 800 Flex, Radiometer ) and oxygen concentration was measured using a hemoglobin analyzer (OSM3, Rdiometer). LV myocardial oxygen consumption (MVO2) per beat was calculated by multiplying the arterial- coronary sinus difference in oxygen content by total mean coronary blood flow, assumed to be twice the mean blood flow in the left circumflex coronary artery, and divided by heart rate (23). Finally, LV external mechanical efficiency was calculated as the ratio PDA/MVO2/beat (26–28)
Circulating hormones
Hormones were measured in arterial plasma previously collected and stored at -80 °C. The concentration of the two forms of ghrelin was measured in duplicate by Enzyme Immunoassay (EIA) kit (SPI-Bio, Bertin Pharma, France), using a monoclonal antibody specific to the C-terminal part of either acylated ghrelin or des-acyl ghrelin (Des-Octanoyl-Ser3), respectively. Measurements were performed in peptides in plasma enriched with HCl immediately after separation from blood cells in order to preserve acylated ghrelin levels, according to the manufacturer’s instructions.
Plasma GH concentrations were measured in duplicate by Dog Growth Hormone Enzyme-Linked ImmunoSorbent Assay (ELISA) Kit, (CUSABIO, China).
Plasma insulin was assessed by Insulin (Porcine/Canine) ELISA (ALPCO Diagnostics, Salem, NH, USA), according to the manufacturer’s instructions.
Total and labeled metabolites
The concentrations of total and labeled FFA, glucose and lactate, which are three main cardiac energy substrates (3,20), were determined in arterial and coronary sinus blood samples, as previously described (23). Rapid measurements of total glucose and lactate were obtained from the same blood samples used for blood gas analysis tested with multi-purpose cartridges (ABL 800 Flex, Radiometer). These measurements were repeated 2–3 times per sample to verify their consistency. Total FFA concentration in plasma was determined spectrophotometrically. 3H-oleate activity was measured in plasma, whereas 14C-glucose activity was determined in blood deproteinized with ice-cold 1M perchloric acid (1:2 vol/vol). 3H2O and 14CO2 activities were also measured in plasma and whole blood, respectively.
Mean coronary blood flow and the specific activities of 3H-oleate and 14C-glucose were multiplied, respectively, by the arterial-coronary sinus difference of 3H2O and 14CO2 blood content and by mean coronary blood flow and then divided by heart rate to calculate the rate of FFA and glucose oxidation per beat. Arterial and coronary sinus concentration of lactate were multiplied by mean coronary blood flow and then divided by heart rate to calculate net chemical lactate uptake per beat.
Fractional shortening and Ca2+ transient measurements in isolated cardiomyocytes
Canine left ventricular myocytes were isolated from 4 failing and 4 normal dog hearts and fractional shortening and Ca2+ transients were measured as previously described by us (29–30) (see Data Supplements). Myocytes were chosen on the basis of their morphology (rod shaped) and absence of spontaneous contractions. The maximal magnitude of contraction was normalized to resting cell length and expressed as a percent shortening. For [Ca2+]i fluorescence measurements, the F0 (or F unstimulated) was measured as the average fluorescence of the cell 50 msec prior to stimulation. The maximal Fluo-4 fluorescence (F) was measured at peak amplitude and background fluorescence was subtracted from both parameters. Human des-acyl ghrelin or acyl ghrelin (Bachem) were added to the perfusate at the concentration of 50 nM. Using the same selected cell, the measurements were performed following this sequence: baseline, des-acyl ghrelin, rebaseline (after ~3 minutes washout time) and acyl ghrelin. At least 15 contraction cycles were analyzed in each condition. Data were acquired and analyzed using Clampex 10.2 electrophysiology software.
Real-time PCR and Western blot to measure the expression of cardiac GHS-R1a
RNA extraction from dog heart tissue and reverse transcription were performed as described by us (31) (see Data Supplements). The following primer sequences were used: dog GHSR-1a, Fwd 5′-CAGCCAATACTGCAACCTGG-3′ and Rev 5′-CACCCGGTACTTCTTGGACA-3′ (accession no. NM_001099945.1); 18s rRNA; Fwd 5′-CCCATTCGAACGTCTGCCCTATC-3′ and Rev 5′-TGCTGCCTTCCTTGGATGTGGTA-3′ (accession no. NR_003278.3). Real-time PCR was performed with 50 ng cDNA, 150 nmol/L of each primer and the IQ-SYBR-green mastermix (BioRad, Milan, Italy) using the ABI-Prism 7300 (Applied Biosystems), as previously described by us (32).
Proteins (60 μg) were extracted from cardiac tissue of normal and HF dogs, resolved in 10% SDS-PAGE and incubated with specific GHSR1a antibody (Santa Cruz, 1:500). Blots were reprobed with actin antibody (Santa Cruz, 1:500) for normalization.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed by employing commercially available software (SPSS Statistics, IBM). Hemodynamic and metabolic changes at different time points were compared by one-way ANOVA for repeated measures and comparisons between groups of two-way ANOVA, in both cases followed by Student-Newman post-hoc test. When samples were not normally distributed, a nonparametric test was used and data presented as dot plots. The sample size used for statistical analysis of PDA, coronary flow and cardiac metabolism was in some cases lower than the total number of dogs per group due to random technical problems, including distorted pressure-diameter loops, defective coronary flow probes and difficult blood sampling from the coronary sinus. For all the statistical analyses, significance was accepted at P<0.05.
Results
Circulating ghrelin, growth hormone and insulin
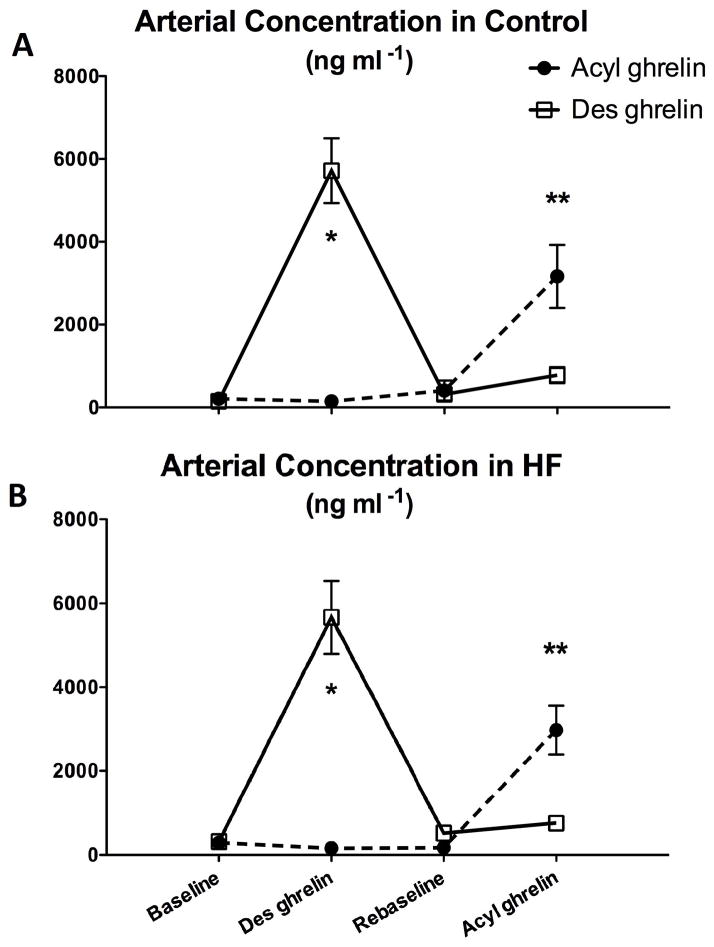
As shown in Figure 1, circulating des-acyl ghrelin was markedly increased after 15 minutes of infusion, both in normal and HF dogs. The washout period following the first infusion stop was able to establish re-baseline levels not significantly different from baseline. The ensuing 15 min infusion of acyl ghrelin produced a similar effect, although less pronounced compared to des-ghrelin infusion.
Figure 1.
Changes in arterial concentration of des-acyl or acyl ghrelin after 15 min of 4 μg/kg/hour infusion in control (panel A) and HF dogs (panel B). N=4 per group. *P<0.05 vs baseline; **P<0.05 vs re-baseline.
Since acyl ghrelin has a secretagogue function, it was important to rule out possible increases in growth hormone. In fact, growth hormone was not significantly changed throughout the experimental protocol in both groups (Table 1). We also evaluated possible insulin changes, as they could be a confounding factor in a study on cardiac metabolism. Also in this case, we did not observe significant changes (Table 1).
Table 1.
Arterial concentrations of growth hormone, insulin, glucose, free fatty acids and lactate, after 15 min of 4μg/kg/hour des-acyl or acyl ghrelin infusion in control and HF dogs.
| Group | Baseline | Des-acyl ghrelin | Rebaseline | Acyl ghrelin | |
|---|---|---|---|---|---|
| GH (ng ml) | HF (N=4) | 70.15 ± 12.95 | 71.77 ± 13.24 | 68.54 ± 11.32 | 75.98 ± 18.24 |
| Control (N=4) | 67.49 ±19.12 | 68.47 ± 21.88 | 76.30 ± 20.52 | 68.80 ± 21.66 | |
| Insulin (ng/ml) | HF (N=4) | 0.20 ± 0.003 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.004 |
| Control (N=4) | 0.20 ± 0.002 | 0.22 ± 0.02 | 0.20 ± 0.01 | 0.21 ± 0.01 | |
| Glucose (mg/dL) | HF (N=7) | 92.29 ± 1.35 | 91.44 ± 2.42 | 85.21 ± 3.43 | 85.29 ± 3.31 |
| Control (N=5) | 90.30 ± 1.64 | 91.30 ± 2.75 | 88.70 ± 1.66 | 89.60 ± 3.03 | |
| Free Fatty Acids (mM) | HF (N=7) | 0.62 ± 0.04 * | 0.65 ± 0.06 * | 0.68 ± 0.05 | 0.67 ± 0.07 |
| Control (N=5) | 0.83 ± 0.04 | 0.85 ± 0.06 | 0.76 ± 0.10 | 0.85 ± 0.11 | |
| Lactate (mM) | HF (N=7) | 0.91 ± 0.08 | 0.88 ± 0.09 | 0.97 ± 0.07 | 0.86 ± 0.10 |
| Control (N=5) | 1.02 ± 0.16 | 1.09 ± 0.17 | 1.15 ± 0.19 | 1.05 ± 0.15 |
Data are presented as mean ± SEM. GH, growth hormone; HF, heart failure.
P<0.05 HF vs Control.
Hemodynamics and cardiac function
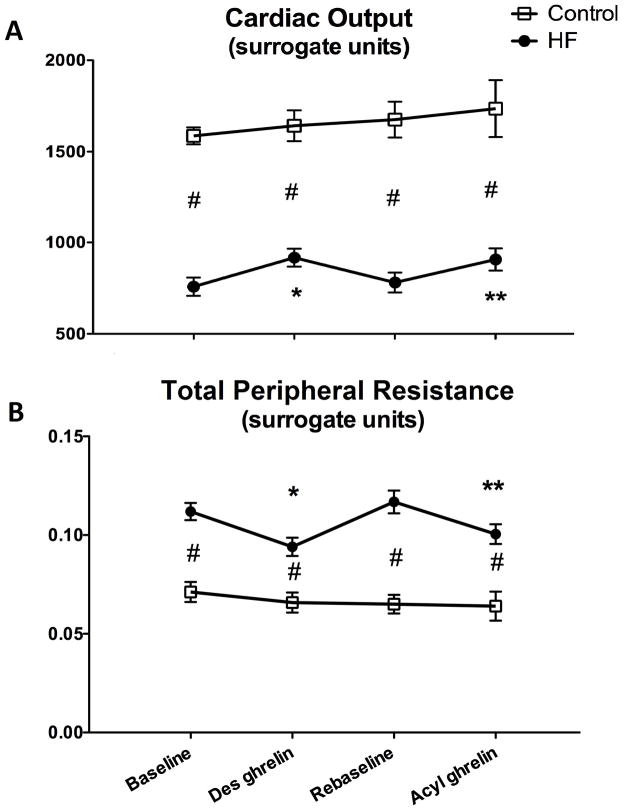
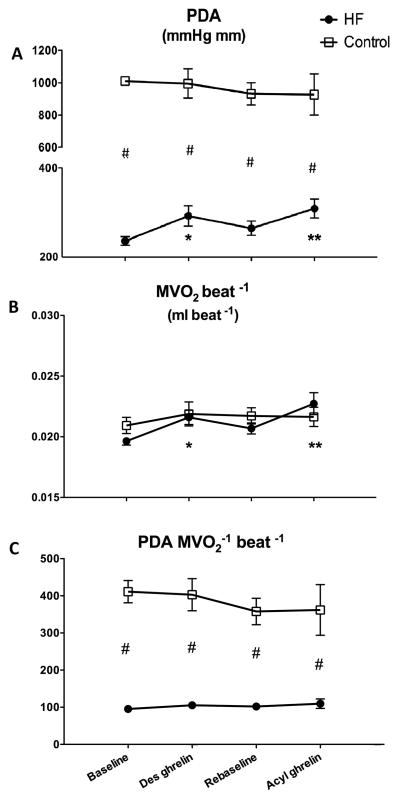
The degree of hemodynamic and cardiac function impairment in HF compared to control was consistent with previous studies in this dog model (23–24). Values of heart rate, LV systolic pressure, dP/dtmax, LV end-diastolic pressure, mean aortic pressure and left circumflex coronary artery blood flow in the in normal and HF are reported in Table 2. Neither des-acyl nor acyl ghrelin caused any significant changes. As regards cardiac function, LV end-diastolic diameter and dP/dtmax were not significantly affected by the two forms of ghrelin (Table 2). Cardiac output and total peripheral resistance in normal dogs did not display significant changes during des-acyl and acyl ghrelin administration (Figure 2). However, in HF dogs, cardiac output significantly increased by approximately 20%, while, consistent with stable values of mean aortic pressure, total peripheral resistance significantly decreased by approximately 20% in response to both of des-acyl and acyl ghrelin (Figure 2). Also PDA did not change significantly in normal dogs, while, in HF, it significantly increased by approximately 24% in response to both of des-acyl and acyl ghrelin (Figure 3A). Since LV end-diastolic diameter and pressure were not significantly altered by des-acyl or acyl ghrelin, (Table 2) the changes in PDA could not be attributed to increased preload.
Table 2.
Changes in hemodynamics after 15 min of 4μg/kg/hour des-acyl or acyl ghrelin infusion in control and HF dogs.
| Group | Baseline | Des-acyl ghrelin | Rebaseline | Acyl ghrelin | |
|---|---|---|---|---|---|
| HR (beat min) | HF (N=8) | 129.07 ± 3.61 | 130.27 ± 4.90 | 129.49 ± 3.31 | 130.12 ± 4.03 |
| Control (N=6) | 134.17 ± 3.46 | 136.84 ± 4.17 | 144.83 ± 4.32 | 145.00 ± 4.89 | |
| LVSP (mm Hg) | HF (N=8) | 100.59 ± 2.37 * | 101.95 ± 2.51 * | 106.34 ± 3.27 * | 106.67 ± 3.81 * |
| Control (N=6) | 133.91 ± 4.81 | 126.81 ± 4.21 | 127.33 ± 3.21 | 127.34 ± 3.65 | |
| dP/dt max (mmHg sec −1) | HF (N=8) | 1589.69 ± 96.60 * | 1659.44 ± 106.62 * | 1745.58 ± 92.79*† | 1769.71 ± 94.43 * |
| Control (N=6) | 3102.86 ± 212.86 | 2807.57 ± 157.14 | 2789.46 ± 171.53 | 2797.40 ± 196.75 | |
| LVEDP (mm Hg) | HF (N=8) | 25.22 ± 1.46 * | 25.15 ± 1.61 * | 23.70 ± 2.07 * | 23.01 ± 2.18 * |
| Control (N=6) | 4.89 ± 0.67 | 3.89 ± 1.84 | 4.79 ± 1.24 | 3.42 ± 1.35 | |
| MAP (mm Hg) | HF (N=8) | 85.07 ± 2.60 * | 86.17 ± 3.10 * | 89.34 ± 3.52 * | 90.23 ± 4.00 * |
| Control (N=6) | 111.60 ± 3.52 | 107.99 ± 4.09 | 107.83 ± 2.78 | 106.39 ± 4.00 | |
| MCBF (ml min) | HF (N=7) | 25.09 ± 1.07 | 26.44 ± 1.68 | 25.41 ± 1.29 | 26.86 ± 1.49 |
| Control (N=5) | 27.38 ± 0.67 | 27.59 ± 1.12 | 27.54 ± 0.98 | 27.72 ± 1.19 | |
| LVEDD (mm) | HF (N=6) | 43.85 ± 0.99 * | 44.50 ± 0.83 * | 44.36 ± 1.011 * | 45.02 ± 1.18 * |
| Control (N=4) | 38.54 ± 0.56 | 38.48 ± 0.26 | 38.37 ± 0.85 | 38.10 ± 1.00 |
Data are presented as mean ± S.E.M. HR heart rate; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; MAP, mean aortic pressure; MCBF, mean blood flow in the circumflex coronary artery; LVEDD, left ventricular end-diastolic diameter; HF, heart failure.
P<0.05 HF vs Control;
P<0.05 Rebaseline vs Baseline.
Figure 2.
Changes in a surrogate index of cardiac output (panel A) and of total peripheral resistance (panel B) after 15 min of 4 μg/kg/hour des-acyl or acyl ghrelin infusion in control (n=4) and HF (n=6) dogs. *P<0.05 vs baseline; **P<0.05 vs re-baseline; #P<0.05 HF vs control.
Figure 3.
Changes in pressure-diameter area (PDA, panel A), MVO2 (panel B) and mechanical efficiency indexed by the ratio PDA/MVO2 (panel C) after 15 min of 4 μg/kg/hour des-acyl or acyl ghrelin infusion in control (n=4 in panels A and C, n=6 in panel B) and HF (n=6 in panel A and C, n=7 in panel B) dogs. *P<0.05 vs baseline; **P<0.05 vs re-baseline; #P<0.05 HF vs control.
MVO2, mechanical efficiency and energy substrate oxidation
MVO2 increased significantly by approximately 10% in response to both of des-acyl and acyl ghrelin, but only in the HF group, with no significant differences at any time point between the two groups (Figure 3B). Consequently, the ratio PDA/MVO2, an index of mechanical efficiency, did not display significant changes during the experiment (Figure 3C).
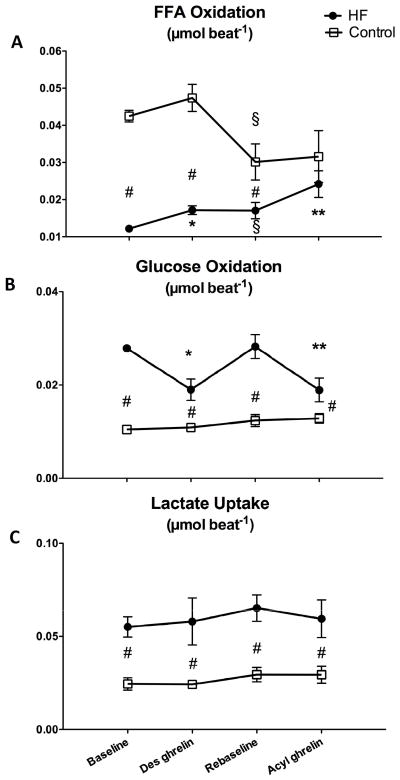
Des-acyl and acyl ghrelin infusion did not affect significantly the arterial concentration of FFA, lactate and glucose (Table 1). Nonetheless, cardiac FFA oxidation significantly increased by approximately 40 and 30% in response to des-acyl and acyl ghrelin, respectively, but only in the HF group (Figure 4A). After acyl ghrelin cardiac FFA oxidation was no longer significantly different compared to the control group. However this was only an apparent normalization of FFA metabolism, simply due to the significant drop of re-baseline values in control, which narrowed the differences between the two groups. Interestingly, in HF the FFA oxidation at re-baseline displayed the opposite change, i.e. it remained significantly higher compared to baseline. In contrast with FFA, cardiac glucose oxidation was significantly decreased by approximately 30 and 40% in response to des-acyl and acyl ghrelin, respectively, but only in the HF group, and remained significantly higher compared to the control group (Figure 4B). Net cardiac lactate uptake was significantly higher in HF compared to control and was not affected by ghrelin in any of the two groups (Figure 4C).
Figure 4.
Changes in cardiac free fatty acids (FFA, panel A) and glucose (panel B) oxidation and in lactate uptake (panel C) after 15 min of 4 μg/kg/hour des-acyl or acyl ghrelin infusion in control (n=5) and HF (n=7) dogs. *P<0.05 vs baseline; **P<0.05 vs re-baseline; #P<0.05 HF vs control; § P<0.05 re-baseline vs baseline.
Comparison with metabolic and functional changes induced by beta-adrenergic stimulation
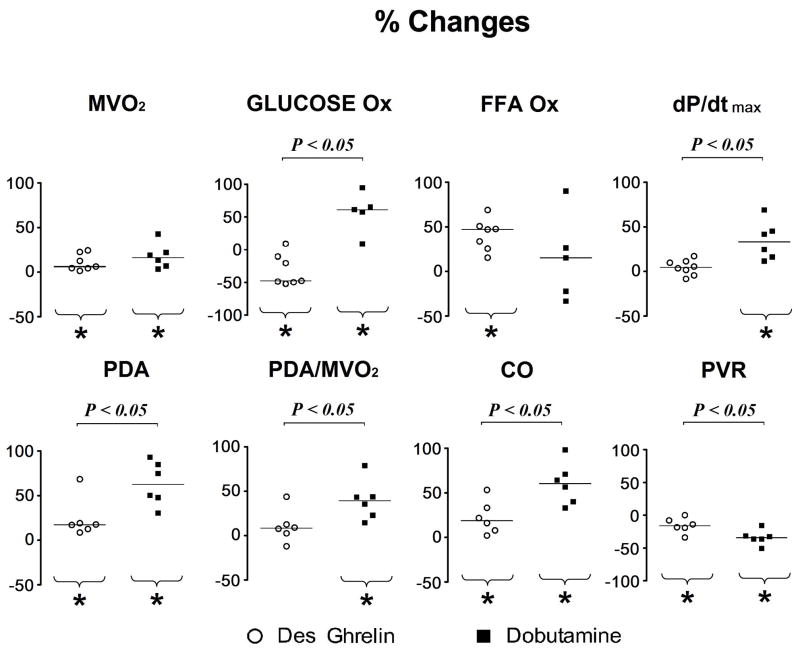
To test whether the partial normalization of energy substrate oxidation in response to ghrelin was simply the consequence of enhanced PDA and MVO2, we used beta-adrenergic stimulation as a term of comparison. Dobutamine, a widely used, selective beta-receptor agonist was infused at two different doses and we found that 10 μg/kg/min best matched the effect of des-acyl and acyl ghrelin on MVO2. The comparison is presented in Figure 5 as percent changes in metabolic and functional parameters. While the percent increase in MVO2 was not significantly different in failing hearts stimulated with des-acyl ghrelin compared to dobutamine, glucose oxidation in the two groups changed in opposite directions. Moreover, PDA was more enhanced by dobutamine group, due to a combination of more pronounced drop in peripheral resistance and to an increase in contractility as indicated by the positive percent change in dP/dtmax. This led to a higher cardiac output and mechanical efficiency after beta-adrenergic stimulation compared to des-acyl ghrelin.
Figure 5.
Percent changes versus baseline of metabolic and functional parameters after 15 min of 4 μg/kg/hour des-acyl ghrelin (sample sizes as described in the previous figures) or 5 min of 10 μg/kg/min dobutamine (n=5–6) infusion in HF dogs. FFA Ox, FFA oxidation; Gluc Ox, glucose oxidation; PDA, pressure diameter area; CO, cardiac output (surrogate index); PVR, peripheral vascular resistance (surrogate index). Data were compared with Mann-Whitney Rank Sum Test and the horizontal bars indicate the median. *P<0.05 vs baseline,
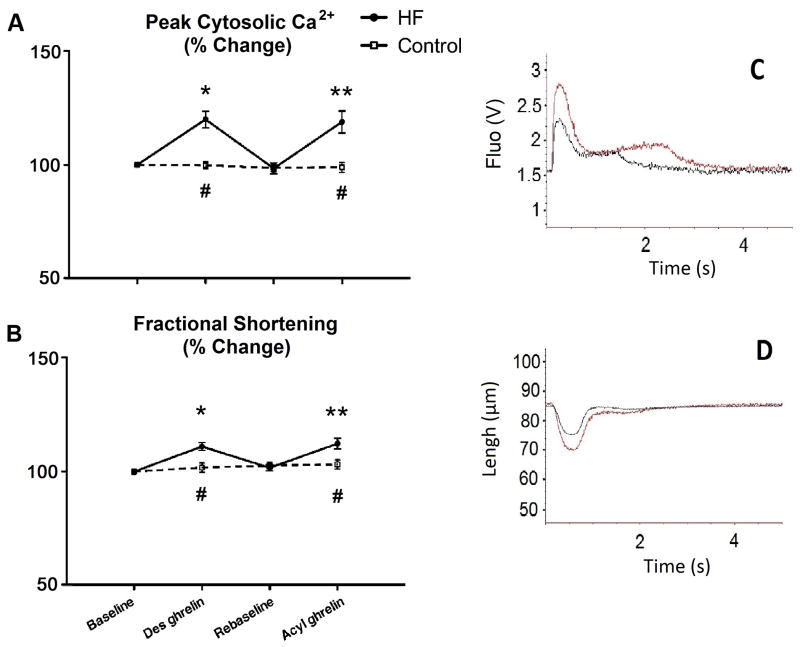
Fractional Shortening and Ca2+ transients in isolated cardiomyocytes
Ghrelin has been shown to exert positive inotropic actions in isolated rodent cardiomyocytes (18). Although we did not observe an increase in dP/dtmax in conscious dogs, we tested the sensitivity of canine cardiomyocytes to ghrelin in vitro and the potential contractile response to it. As show in Figure 6, HF cardiomyocytes were characteristically elongated. Fractional shortening increased significantly in response to both of des-acyl ghrelin and acyl ghrelin in HF, but not in control cardiomyocytes, which was mirrored by changes in peak cytosolic calcium.
Figure 6.
Changes in peak cytosolic Ca2+ (panel A) and in fractional shortening (panel B) in contracting cardiomyocytes isolated from control (n=4) and HF (n=4) and exposed to 50 nM of des-acyl or acyl ghrelin. Contraction was induced by electrical stimulation at 0.2 Hz. Representative tracings of Ca2+ and shortening are presented in panels C and D, respectively. *P<0.05 vs baseline; **P<0.05 vs re-baseline; #P<0.05 HF vs control.
GHS-R1a gene and protein expression in cardiac tissue
The gene expression of ghrelin receptor GHS-R1a has been found to be upregulated in human failing hearts (33). We tested a possible analogy between the human disease and our HF model. In dogs, GHS-R1a mRNA increased by 85.7±16.7% compared to normal hearts (P<0.05, n=5 per group), while protein expression was increased by 31±4.8% (P<0.05, n=3 per group, see also Figure 1 in Data Supplements)
Discussion
The novel finding of the present study is that acute increases of circulating des-acyl or acyl ghrelin partially reverse energy substrate metabolic alterations in failing hearts by lowering glucose oxidation, while stimulating FFA oxidation. This metabolic change occurred in response to higher myocardial need of oxygen consequent to increased stroke work and cardiac output. Interestingly, despite its systemic anabolic effects, at the organ level ghrelin promoted myocardial oxidation of fat, a less energetically efficient substrate. Nonetheless, oxygen demand matched cardiac performance, therefore mechanical efficiency, i.e. the ratio between LV stroke work and oxygen consumption, did not display significant changes. Acyl and des-acyl ghrelin bind different receptors while sharing some peripheral actions. We now provide evidence that one of the shared activities is the modulation of myocardial metabolism. On the other hand, neither form of ghrelin produced significant effects on the metabolism of normal hearts.
Other investigators have explored at biochemical level the control exerted by acyl and des-acyl ghrelin on glucose and medium chain fatty acids uptake in cultured HL-1 and primary cardiomyocytes: only des-acyl ghrelin stimulated fatty acids uptake, whereas acyl ghrelin, but not des-acyl ghrelin, prevented insulin-induced glucose uptake (34). The discordance between their results and ours, the latter rather indicating identical metabolic effects of the two forms of ghrelin, might be due to phenotypic disparities and/or to the peptide concentrations they found to be effective in vitro (1–3 μM) compared to the blood levels achieved during infusions in dogs (0.9–1.8 pM). Based on the present data and on the known competition between energy substrates, we can only generically speculate that, in failing hearts, acyl and des-acyl ghrelin possibly blocked or, vice versa, potentiated one of the key limiting steps of, respectively, the carbohydrate and the FFA oxidative pathway.
The causes of the different sensitivity of healthy versus diseased hearts are likely various and complex. One of them is perhaps the overexpression of acyl ghrelin receptor GSH-R1a that we found in canine failing hearts, consistent with previous reports in humans (33). Conceivably, heart failure might upregulate also the still unidentified des-acyl ghrelin receptor(s). The biological significance of augmented responsiveness to ghrelin warrants further investigations that might lead to important insights in the pathophysiology of HF. Of note, ghrelin is also synthesized by cardiomyocytes (35), where it may exert autocrine/paracrine functions, and is downregulated in failing hearts (33), therefore GSH-R1a upregulation is likely a compensatory mechanism perhaps triggered in part by intracellular ligand-receptor cross-talk.
Moderate improvements in stroke work and, consequently, in cardiac output, were the only significant functional changes observed in response to acyl and des-acyl ghrelin and occurred only in dogs with HF. Consistent with previous findings in humans infused with ghrelin (36–37), larger stroke volumes were not associated to changes in heart rate and were possibly due to decreased afterload rather than to increased cardiac contractility. The absence of significant changes in dP/dtmax during ghrelin infusion further supports this interpretation. Since other authors have documented a direct positive inotropic effect of ghrelin in cardiomyocytes isolated from normal rodent hearts (18), we repeated similar experiments in dog cardiac cells to rule out the possible lack of sensitivity due to species differences. Both acyl and des-acyl ghrelin enhanced cytosolic calcium release and contractility during electrical stimulation; however, this effect was present only in pathological cardiomyocytes. Our findings confirm in canine cardiomyocytes that ghrelin is a positive inotrope, in vitro, and provide additional evidence that the failing heart displays an augmented sensitivity to it. Prior ghrelin functional studies ex vivo have not compared normal with failing hearts. It is also important to consider that, in order to obtain measurable changes, the in vitro concentrations of acyl and des-acyl ghrelin used by us and by others were 10- to 25-fold higher than those achieved during the infusion in vivo, prompting questions about their pathophysiological or even therapeutic relevance.
A critically important problem for our experiments was to avoid the confounding interference of changes in circulating hormones or metabolic substrates concentration. In fact, any of those can potentially alter cardiac metabolism. We therefore designed our protocol based on a previous study in humans that tested different doses of ghrelin up to 1.5 nmol/kg/min (25). Those authors found no significant changes in growth hormone after 15 min of infusion. In addition, we first infused des-acyl ghrelin, which lacks the growth hormone secretagogue function and, as shown by blood analysis, was not converted into circulating acyl ghrelin. Our approach proved successful, in that we did not observe significant changes in circulating growth hormone as well as insulin and FFA, glucose and lactate, hence we can safely conclude that the effects of acyl and des-acyl ghrelin on cardiac metabolism were direct. Of note, the metabolic changes occurred in response to peaks of ghrelin concentration 7- to 8-fold higher than physiological, pre-prandial peaks (38). The functional and metabolic response of the failing heart to this gut hormone is peculiar, since it differs substantially from the one triggered by beta-adrenergic or cholinergic receptor stimulation, i.e. by the activation of the sympathetic or parasympathetic system. In fact, compared to ghrelin infusion, a moderate adrenergic stress with 10 μg/kg/min of dobutamine increased MVO2 by a similar extent, while functional performance was more markedly enhanced and myocardial glucose oxidation was increased rather than decreased. On the other hand, we previously showed that the stimulation of vagal cardiac efferents lowers, via muscarinic receptors, both of myocardial glucose consumption and MVO2 (28).
We did not test long-term effects of high ghrelin levels on cardiac metabolism, which is an obvious limitation of our study. Previous literature provides solid data documenting that chronic administration of ghrelin attenuates the symptoms and signs of HF (12–15), and we cannot exclude that the modulation of cardiac metabolism contributed to the therapeutic benefits. Patients who benefited from acyl ghrelin administration were injected intravenously with 2 μg/kg twice a day for 3 weeks (12), therefore their heart likely experienced repeated phases of metabolic changes similar to those described by us.
In conclusion, our study revealed a new cardiovascular component of the multifaceted functions accomplished by the gut hormone ghrelin, namely the direct modulation of myocardial FFA and glucose metabolism associated to MVO2 enhancement under pathological conditions. Such metabolic modifications might contribute to the therapeutic effects of ghrelin in HF.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by he NIH grants P01 HL-74237 and R01 HL-108213 (F.A.R.), HL-33921 and P01 HL-100806 (S.H.) and by the Ministero dell’Istruzione, Università e della Ricerca Scientifica e Tecnologica [Italian Ministry of Instruction and Research (MIUR: PRIN 2008EFHJ5H_02)] and Compagnia di San Paolo 2011 (R.G.).
Footnotes
Disclosures
None.
References
- 1.Goodwin GW, Ahmad F, Doenst T, Taegtmeyer H. Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am J Physiol. 1998;274:H1239–H1247. doi: 10.1152/ajpheart.1998.274.4.H1239. [DOI] [PubMed] [Google Scholar]
- 2.McConville P, Lakatta EG, Spencer RG. Greater glycogen utilization during 1- than 2-adrenergic receptor stimulation in the isolated perfused rat heart. Am J Physiol Endocrinol Metab. 2007;293:E1828–1835. doi: 10.1152/ajpendo.00288.2007. [DOI] [PubMed] [Google Scholar]
- 3.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 4.Grossman AN, Opie LH, Beshansky JR, Ingwall JS, Rackley CE, Selker HP. Glucose-insulin-potassium revived: current status in acute coronary syndromes and the energy-depleted heart. Circulation. 2013;127:1040–1048. doi: 10.1161/CIRCULATIONAHA.112.130625. [DOI] [PubMed] [Google Scholar]
- 5.Scheen AJ. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10:73–84. doi: 10.1038/nrcardio.2012.183. [DOI] [PubMed] [Google Scholar]
- 6.Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61:430–481. doi: 10.1124/pr.109.001958. [DOI] [PubMed] [Google Scholar]
- 7.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 8.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 9.Granata R, Isgaard J, Alloatti G, Ghigo E. Cardiovascular actions of the ghrelin gene-derived peptides and growth hormone-releasing hormone. Exp Biol Med (Maywood) 2011;236:505–514. doi: 10.1258/ebm.2011.010365. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Garcia EA, Korbonits M. Genetic studies on the ghrelin, growth hormone secretagogue receptor (GHSR) and ghrelin O-acyl transferase (GOAT) genes. Peptides. 2011;32:2191–2207. doi: 10.1016/j.peptides.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H, Kangawa K. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 12.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 13.Soeki T, Kishimoto I, Schwenke DO, Tokudome T, Horio T, Yoshida M, Hosoda H, Kangawa K. Ghrelin suppresses cardiac sympathetic activity and prevents early left ventricular remodeling in rats with myocardial infarction. Am J Pysiol Heart Circ Physiol. 2008;294:H426–432. doi: 10.1152/ajpheart.00643.2007. [DOI] [PubMed] [Google Scholar]
- 14.Huang CX, Yuan MJ, Huang H, Wu G, Liu Y, Yu SB, Li HT, Wang T. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides. 2009;30:2286–2291. doi: 10.1016/j.peptides.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Palus S, von Haehling S, Doehner W, Datta R, Zhang J, Dong JZ, Culler MD, Anker SD, Springer J. Effect of application route of the ghrelin analog BIM-28131 (RM-131) on body weight and body composition in a rat heart failure model. Int J Cardiol. 2013;168:2369–2374. doi: 10.1016/j.ijcard.2013.01.263. [DOI] [PubMed] [Google Scholar]
- 16.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–1037. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XB, Cao JM, Pang JJ, Xu RK, Ni C, Zhu WL, Asotra K, Chen MC, Chen C. The positive inotropic and calcium-mobilizing effects of growth hormone-releasing peptides on rat heart. Endocrinology. 2003;144:5050–5057. doi: 10.1210/en.2003-0025. [DOI] [PubMed] [Google Scholar]
- 18.Sun Q, Ma Y, Zhang L, Zhao YF, Zang WJ, Chen C. Effects of GH secretagogues on contractility and Ca2+ homeostasis of isolated adult rat ventricular myocytes. Endocrinology. 2010;151:4446–4454. doi: 10.1210/en.2009-1432. [DOI] [PubMed] [Google Scholar]
- 19.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 20.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res. 2011;90:202–209. doi: 10.1093/cvr/cvr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R, Menichetti L, L'Abbate A, Stanley WC, Recchia FA. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;293:H3270–3278. doi: 10.1152/ajpheart.00887.2007. [DOI] [PubMed] [Google Scholar]
- 22.Kolwicz SC, Jr, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 24.Qanud K, Mamdani M, Pepe M, Khairallah RJ, Gravel J, Lei B, Gupte SA, Sharov VG, Sabbah HN, Stanley WC, Recchia FA. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2098–2105. doi: 10.1152/ajpheart.00471.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D'Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Post H, d'Agostino C, Lionetti V, Castellari M, Kang EY, Altarejos M, Xu X, Hintze TH, Recchia FA. Reduced left ventricular compliance and mechanical efficiency after prolonged inhibition of NO synthesis in conscious dogs. J Physiol. 2003;552:233–239. doi: 10.1113/jphysiol.2003.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 28.Vimercati C, Qanud K, Ilsar I, Mitacchione G, Sarnari R, Mania D, Faulk R, Stanley WC, Sabbah HN, Recchia FA. Acute vagal stimulation attenuates cardiac metabolic response to β-adrenergic stress. J Physiol. 2012;590:6065–6074. doi: 10.1113/jphysiol.2012.241943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGinley JC, Berretta RM, Chaudhary K, Rossman E, Bratinov GD, Gaughan JP, Houser S, Margulies KB. Impaired contractile reserve in severe mitral valve regurgitation with a preserved ejection fraction. Eur J Heart Fail. 2007;9:857–864. doi: 10.1016/j.ejheart.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR. A caveolae-targeted l-type Ca2+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granata R, Settanni F, Gallo D, Trovato L, Biancone L, Cantaluppi V, Nano R, Annunziata M, Campiglia P, Arnoletti E, Ghè C, Volante M, Papotti M, Muccioli G, Ghigo E. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes. 2008;57:967–979. doi: 10.2337/db07-1104. [DOI] [PubMed] [Google Scholar]
- 32.Baragli L, Grande C, Gesmundo I, Settanni F, Taliano M, Gallo D, Gargantini E, Ghigo E, Granata R. Obestatin enhances in vitro generation of pancreatic islets through regulation of developmental pathways. PLoS One. 2013;8:e64374. doi: 10.1371/journal.pone.0064374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beiras-Fernandez A, Kreth S, Weis F, Ledderose C, Pöttinger T, Dieguez C, Beiras A, Reichart B. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides. 2010;31:2222–2228. doi: 10.1016/j.peptides.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Lear PV, Iglesias MJ, Feijóo-Bandín S, Rodríguez-Penas D, Mosquera-Leal A, García-Rúa V, Gualillo O, Ghè C, Arnoletti E, Muccioli G, Diéguez C, González-Juanatey JR, Lago F. Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology. 2010;151:3286–3298. doi: 10.1210/en.2009-1205. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias MJ, Piñeiro R, Blanco M, Gallego R, Diéguez C, Gualillo O, González-Juanatey JR, Lago F. Growth hormone releasing peptide (ghrelin) is synthesized and secreted by cardiomyocytes. Cardiovasc Res. 2004;62:481–488. doi: 10.1016/j.cardiores.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1483–1487. doi: 10.1152/ajpregu.2001.280.5.R1483. [DOI] [PubMed] [Google Scholar]
- 37.Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, Kojima M, Nakanishi N, Mori H, Kangawa K. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001;86:5854–5859. doi: 10.1210/jcem.86.12.8115. [DOI] [PubMed] [Google Scholar]
- 38.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.