Abstract
Physical activity modulates bone growth during adolescence, but an effective activity has not been identified for general use.
PURPOSE
To examine the effect of a school-based resistance-training program on skeletal growth in peri-menarcheal females.
METHODS
6th grade girls participated in a 7-month, resistance-training program (INT) embedded in physical education classes (PE). Age and maturity matched controls (CON) from a neighboring school participated in standard PE. INT dose defined high (HI) and low (LO) groups. At baseline (BL) and follow-up (FU), Non-INT organized activity (PA, h/wk) and maturity status were recorded; DXA scans assessed total-body, distal radius, proximal femur, and lumbar spine. Regression models analyzed growth in bone outcomes for HI v CON, accounting for age, Tanner stage, height, and PA.
RESULTS
44 girls (22 HI, 22 CON) were 11.7 ± 0.3 yrs at BL; all were ≤6 mo post-menarche and did not differ in bone growth over the course of the intervention (p>0.05). However, in a sub-analysis limited to subjects who were Tanner breast II (T2) or III (T3) at BL (n=21 CON, n=17 HI), T2 HI had greater gains in narrow neck (NN) width (p=0.01) compared to T2 CON, while T3 HI had greater gains in L3 BMD (p=0.03) compared to T3 CON.
CONCLUSIONS
In a group of T2 and T3 6th grade girls, a school-based resistance-training intervention produced maturity-specific differential gains for HI v CON at the hip and spine.
Keywords: bone growth, adolescents, exercise intervention, resistance training
Introduction
Adolescent bone accrual is closely tied to pubertal development. For females, menarche is pivotal, with approximately half of adult bone mass acquired in four circum-menarcheal years [1-3]. Although genetics are paramount, modifiable factors, especially physical activity and diet, are responsible for as much as 40% of the variation in peak bone mass [4]. Impact activity, such as gymnastics, is particularly osteogenic, demonstrating greater potency than cyclic loading activities such as distance running and non-weight bearing activities such as swimming [5-8]. Existing evidence suggests that elevated bone accrual in gymnasts is manifested through adaptations in both geometry and volumetric density, yielding elevated theoretical bone strength [9-12].
Resistance training presents an alternative, non-impact mechanism for osteogenic stimulation. However, the bulk of existing knowledge pertains to older age groups. In pre-menopausal women (20-39 yrs. of age), 18-24 mo. resistance training interventions increased lumbar spine and hip BMD by 1.9-2.5% over baseline [13, 14]. Retrospective and cross-sectional studies in both males and females correlated geometric and material skeletal properties with high doses of resistance training during adolescence, inferring a skeletal response [15, 16]. A comparison of active female weightlifters (age 30.6 + 6.7 yrs) who began their sport during adolescence versus untrained females (age 22.6 + 1.8 yrs), identified 26-38% advantages in cortical cross-sectional area at the distal radius and radial shaft and 41-43% advantages in bone strength index, providing compelling evidence of skeletal adaptation to resistance training [17].
We are aware of only four prospective studies that evaluate skeletal effects of resistance training in girls. One randomized, controlled 15-month intervention yielded a 3.7% increase in femoral neck aBMD in girls more than two years post-menarche (aged 14-17 yrs., n=67), but was plagued by an 89% drop-out rate from the resistance group [18]. In a 26 wk. resistance-training trial in girls who were greater than three years post-menarche (mean age 16 yrs., n=36), there was a trend toward increased lumbar spine BMC and aBMD in the intervention group relative to controls [19]. This intervention was limited by inadequate sample size and the use of hydraulic resistance machines in which intensity varies with subject motivation. A third study, evaluating a younger cohort, included a 10-week weight circuit component within a larger 10-month intervention of mixed osteogenic activities. These pre-menarcheal, early pubertal girls (aged 9-10 years, n=71) exhibited large relative gains in BMC (2.4-10.3%), but it is unclear whether resistance training was directly responsible [20]. A fourth study examined a similarly aged group of girls (911 yrs, n=257) completing a 44-week school-based intervention including skipping, dancing, playground circuits and simple resistance exercises with exercise bands. This cohort exhibited a trend towards increased femoral neck (FN) BMC and FN cross sectional area in the intervention group [21].
As the circum-menarcheal years are a pivotal time for bone growth, our primary aim was to examine the effects of a resistance intervention on bone acquisition in early peri-menarcheal girls. We chose to analyze a school-based, teacher-led intervention already incorporated into the current physical education curriculum. As the skeleton is highly responsive to mechanical loading during pubertal growth, we hypothesized that a resistance intervention in this age group would confer an advantage in skeletal growth.
Methods
Subjects and Study Design
45 peri-menarcheal 6th grade girls (INT), aged 11-12 years, were recruited from a single middle school for participation in a twice-weekly, seven-month, targeted resistance program embedded in required physical education classes. 23 age and maturity matched control subjects (CON) were recruited from a neighboring middle school in the same school district and participated in standard physical education classes (not including resistance training). School populations were similar for race composition and socioeconomic status. Exclusion criteria were as follows, though no subjects required exclusion): 1) maturity status greater than one-year post-menarche; 2) history of growth or eating disorder, metabolic bone disease, medical or orthopedic condition precluding exercise; 3) use of medication that inhibits bone accrual; 4) oral contraceptive/hormone use. Baseline (pre-intervention, BL) and follow-up (post-intervention, FU) assessments of regional and total body bone and lean mass (DXA), anthropometrics and maturational status were recorded. Organized, non-intervention physical activity participation (PA) and intervention dose were quantified via questionnaire and observation, respectively. Study protocol was approved by the Institutional Review Board for Human Subject Protection; subjects and parent(s)/guardian(s) provided written, informed assent and consent.
Intervention Protocol
This study examined the skeletal benefits of an established resistance-training program at a local middle school. This program commences as students enter the 6th grade; it includes floor and resistance exercises, utilizing body weight, hand-held weights and resistance bands to provide progressive overload. Exercises are performed in timed circuits for 8-12 minutes, 2-3 times per week on non-consecutive days. Following a 3-4 week series of introductory sessions in which form and technique are emphasized, exercise difficulty is gradually increased in 2-3 week increments. Variation in specific exercises for each muscle group is included to maintain interest. Participants are encouraged to exercise at an intensity level that would preclude the completion of an additional repetition at the conclusion of the allotted time for each specific exercise. The intervention activity began one month after physical education classes commenced in the fall and continued for the remaining 8 months of the school year. Subjects were measured during the last month of the school year; therefore, this analysis assesses an average of 7 months of intervention exposure. A single member of the research team observed and recorded daily minutes of intervention participation and effort for each subject. Effort was assessed on a scale of 1 to 3 representing low, average, and high effort, respectively. From these data, total effort-minutes of intervention were calculated as total sum [daily intervention minutes * daily effort], providing an intervention ‘dose’ for each subject.
Non-intervention Organized Physical Activity
Participation in non-intervention, non-physical education organized physical activity (PA, h/wk) was recorded at BL and FU (with parental assistance), using an interviewer-administered questionnaire, developed and validated in a similar cohort (comparison between retrospective subject PA questionnaires and prospective coach activity logs indicated R > 0.97, p< 0.001) [22]. Average PA h/wk was calculated for two periods: 1) six months prior to BL measurements (BLPA); 2) baseline to follow-up interval (FUPA). These values were used as covariates, controlling for variation in BL and FU bone outcomes related to non-intervention PA.
Maturational Variation
Tanner breast (TB) and pubic stages were self-assessed at baseline and follow-up (with parental assistance), using annotated line drawings [23]. Combined Tanner stage was calculated (Tanner breast + Tanner pubic) to obtain a composite picture of maturational stage. Menarcheal date (if post-menarche) was recorded and used to calculate gynecologic age as weeks pre or post menarche (measurement date – menarche date). Gynecologic age is reported for the few subjects who attained menarche, but could not be calculated for most, as they remained pre-menarcheal. Use of oral contraceptives/hormones was queried and recorded at baseline and follow-up; subjects using oral contraceptive were excluded from these analyses.
Bone Assessment
Total-body and regional DXA scans were performed by a single ISCD certified technologist using a GE Healthcare (Madison, WI) Lunar iDXA densitometer. Standard protocol was used to obtain bone mineral content (BMC), areal bone mineral density (aBMD), and projected area for distal radius, proximal femur, and lumbar spine (LS). Total-body scans (EnCore software version 13.31) assessed BMC, non-bone fat free mass (FFm) and fat mass. Ultra-distal (UD, 4%) and 1/3 (33%) radius were analyzed based upon a reference line placed at the ulnar aspect of the proximal border of the radial physis or physeal scar. Proximal femur outcomes included hip structural analysis parameters: narrow neck (NN) width (cm), NN CSMI (cm4), and NN section modulus (Z, cm3). Lumbar spine scans were performed in posteroanterior (PA) and lateral decubitus positions. Central trabecular aBMD (g/cm2) was obtained using a custom 1 cm2 box drawn in the center of the L3 vertebral body lateral scan.
Coefficients of variation (CVs) for DXA variables were calculated using duplicate scans of 30 post-menopausal females. Femoral neck BMD, NN width, and total body BMD and BMC demonstrated CVs <1%, while AP spine BMD, FN BMC, 1/3 radius BMC demonstrated CVs of < 2.0%. 1/3 radius BMD and UD radius BMC yielded CVs of <3% and <4% respectively. HAS parameters CSMI and Z CVs were 4.66% and 5.18%, respectively.
Anthropometry
Anthropometry was assessed at BL and FU, coincident with DXA scans. Height was measured via wall-mounted stadiometer to the nearest 0.1 cm and weight via electronic force plate to the nearest 0.01 kg. Forearm length was measured from the tip of the olecranon to the tip of the ulnar styloid using a ruler, to the nearest 0.1 cm.
Analysis
Sample size estimates were calculated for comparisons of resistance-trainers versus controls for two outcomes, forearm aBMD (g/cm2) and ultradistal radius (UD) Index of Bone Compressive Strength (IBS, g2/cm4). Independent samples were assumed, with a 2-sided alpha of 0.05 [24,25]. As there are no data available for skeletal adaptations to resistance training in early post-menarcheal girls, calculations are based upon gymnast vs. non-gymnast comparisons. Assuming intervention benefits of only 50% those noted for gymnasts over non-gymnasts [26,27], cell sizes of 25-31 would provide at least 80% power to detect a significant intervention benefit. With greater intervention benefits (75% of the observed gymnast advantage), cell sizes of 10-14 would be sufficient.
To minimize variation in maturational development during intervention exposure, subjects who attained menarche > 6 months prior to BL and subjects who did not attain at least TB 2 at FU were excluded from the present analyses (n=4; 3 INT, 1 CON, Figure 1). Based upon a distinct cut point in intervention ‘dose’, high dose (HI, n=22) and low dose (LO, n=20) intervention groups were established (mean effort minutes = 774 [LO] and 1561[HI]). As the LO group completed a much lower intervention dose (Mann Whitney U p < 0.001), they were excluded from the present analysis. Thus, a total of 44 girls, aged 11.0-12.1 years (22 HI, 22 CON), were included in the primary analyses.
Fig. 1.
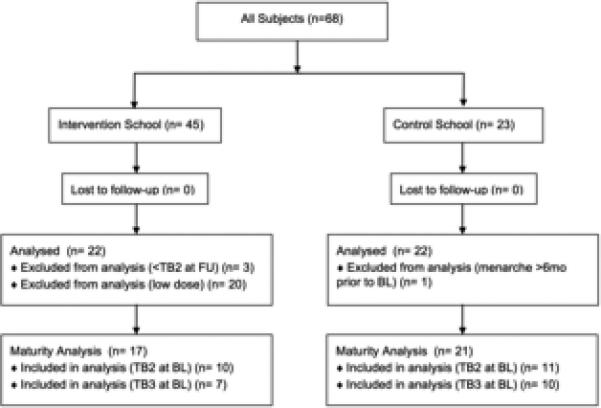
CONSORT Diagram
TB=Tanner breast, BL=baseline, FU=follow-up
Multilevel regression models assessed the effect of the intervention on bone acquisition at the radius, hip, lumbar spine, and sub-head regions, evaluating bone acquisition in the entire group (HI v CON). All outcomes and covariates were evaluated for normality using the Kolmogorov-Smirnov test; no transformations were necessary. Time-varying predictors at level 1 (within-subject) included: combined Tanner stage, chronologic age, PA, and height (forearm length was substituted for height in radius models). Non-time-varying predictors at level 2 (subject-level) included: intervention indicator, height velocity (maturational marker), and BL sub-head FFM, accounting for body size characteristics without removing the effects of the intervention (FFM gains are to be expected as a result of the intervention). To control for varying measurement intervals, an age*intervention interaction term was added to the model (using change in age to define measurement interval). Thus a positive age*intervention interaction indicates a larger intervention effect over a longer measurement interval, which must be true if we are to assume the intervention had a positive effect on bone parameters.
To examine the effects of the intervention in a more maturationally homogeneous cohort, a second regression analysis was performed, including only girls who were Tanner breast II (T2) or III (T3) at BL (n=17 HI, n=21 CON). All outcome measures were expressed as percent change and covariates included: measurement interval, FUPA, Tanner breast stage, and change in height (change in forearm length was substituted for forearm outcomes). An interaction term, T3*intervention, was added to evaluate for a maturity-specific intervention effect (T2 v T3). Effect sizes (Cohen's f were calculated with f ≥0.02, 0.15, and 0.35 signifying small, medium and large effects, respectively [28].
Results
Subject Characteristics
There were no differences in baseline or follow-up subject characteristics for the entire group (HI v CON), except for measurement interval (HI = 33.5 ± 3.1 wks, CON = 29.5 ± 2.4 wks, p<0.001). For the T2 and T3 analysis, group characteristics are presented in Table 1; group differences for T3 v T3 and within T2 and T3 (HI v CON) are noted.
Table 1.
Subject Characteristics
| Tanner 2 (n = 21) | Tanner 3 (n = 17) | T2 HI (n=10) | T2 CON (n=11) | T3 HI (n=7) | T3 CON (n=10) | |
|---|---|---|---|---|---|---|
| Measurement Interval (wks) | 31.71 ± 3.82 | 30.65 ± 2.35 | 34.39 ± 3.25b | 29.39 ± 2.50 | 31.49 ± 2.27 | 29.93 ± 2.30 |
| Intervention Dose (effort*min) | 1523.7 ± 276.67 | 1507.04 ± 219.08 | 1523.7 ± 276.67 | 1507.4 ± 219.08 | ||
| Baseline Characteristics: | ||||||
| Age (yr) | 11.60 ± 0.29 | 11.75 ± 0.31 | 11.53 ± 0.25c | 11.60 ± 0.35 | 11.83 ± 0.28 | 11.76 ± 0.28 |
| Gynecologic Age (wks) | −14.93 ± 14.38 (n=6) | −1.98 ± 16.66 (n=9) | −18.61 ± 9.02 (n=0) | −7.57 ± 25.05 (n=1) | −9.89 ± 17.53 (n=1) | 4.34 ± 14.55 (n=4) |
| Height (cm) | 151.02 ± 5.18a | 155.10 ± 4.79 | 150.41 ± 5.19 | 150.92 ± 5.51 | 153.89 ± 4.56 | 156.67 ± 3.87b |
| Weight (kg) | 41.88 ± 5.84 | 45.04 ± 5.21 | 43.82 ± 7.03 | 39.65 ± 3.73 | 44.09 ± 4.67 | 46.22 ± 5.34b |
| Sub-head FFM (kg) | 26.86 ± 3.04a | 29.17 ± 2.59 | 27.32 ± 3.03 | 26.17 ± 3.11 | 28.60 ± 2.54 | 29.87 ± 2.27b |
| Organized non-intervention Physical Activity (h/wk) | 3.04 ± 2.76 | 2.26 ± 1.48 | 2.22 ± 2.04 | 3.66 ± 3.19 | 1.60 ± 0.74 | 2.85 ± 1.83 |
| Follow-up Characteristics: | ||||||
| Age (yr) | 12.21 ± 0.28 | 12.34 ± 0.31 | 12.19 ± 0.24 | 12.16 ± 0.34 | 12.44 ± 0.28 | 12.33 ± 0.26 |
| Gynecologic Age (wks) | 18.07 ± 13.35 (n=6) | 28.54 ± 15.53 (n=9) | 17.21 ± 8.33 (n=4) | 19.79 ± 25.96 (n=2) | 22.21 ± 16.59 (n=4) | 33.60 ± 14.28 (n=5) |
| Height (cm) | 154.83 ± 5.06 | 158.12 ± 5.30 | 154.44 ± 5.07 | 154.35 ± 5.44 | 156.57 ± 5.56 | 160.13 ± 3.73b |
| Weight (kg) | 45.085 ± 5.51 | 48.21 ± 5.26 | 47.69 ± 6.29b | 42.45 ± 3.38 | 46.80 ± 4.96 | 49.49 ± 5.16b |
| Sub-head FFM (kg) | 29.97 ± 3.11 | 31.64 ± 2.84 | 30.88 ± 3.05 | 28.91 ± 3.07 | 30.93 ± 2.71 | 32.40 ± 2.61b |
| Organized non-intervention Physical Activity (h/wk) | 3.23 ± 3.08 | 2.75 ± 2.25 | 3.24 ± 3.19 | 3.11 ± 3.15 | 2.71 ± 2.30 | 2.88 ± 2.32 |
mean ± SD
Significant difference from Tanner 2 group (HI and CON) p<0.05
Significant difference from T2 CON p<0.05
Significant difference from T3 HI p<0.05
Multi-level Regression Entire Group (Hi v CON)
No significantly different intervention effect was found between the HI and CON groups for growth in any bone outcome over the course of the intervention (p>0.05).
T2, T3 Group Regression (% Change)
When T2 and T3 groups were evaluated together, there was no significant intervention effect for % change in any bone outcome. However, there was a trend toward a positive intervention effect at spine BMC (p=0.050,f=0.15, medium effect), arms BMC (p=0.074, f=0.12, small effect), L3 BMC (p=0.097, f=0.11, small effect), NN width (p=0.078, f=0.10, small effect), and sub-head BMC (p=0.116, f=0.08, small effect).
With the addition of an interaction term (T3*intervention), NN width and L3 BMD exhibited differential, maturity-specific, intervention effects (p<0.05) (Table 2). Specifically, a significant positive intervention effect emerged for T2 NN width (p=0.011) and T3 L3 BMD (p=0.032). The intervention effect was not significant at T3 NN width (p=0.851) or T2 L3 BMD (p=0.608). Adjusted % change for these bone outcomes were calculated and are depicted in Figure 2. Raw % change values are not reported due to disparate measurement intervals (Table 1).
Table 2.
Percent Change Regression Models with Interaction Term
| NN width | L3 BMD | |
|---|---|---|
| Measurement interval | −0.192 (0.002) | 0.360 (0.039) |
| FUPA | 0.016 (0.029) | −0.139 (0.055) |
| Change in height | 0.046 (0.759) | 0.174 (0.245) |
| Tanner breast III group | 0.262 (0.920) | −0.066 (0.652) |
| Intervention | 0.053 (0.011) | −0.403 (0.608) |
| T3 × Intervention | −0.027 (0.027) | 0.368 (0.049) |
| R2 | 0.355 | 0.372 |
Data are presented as Partial Correlation Coefficient (p-value)
Fig. 2.
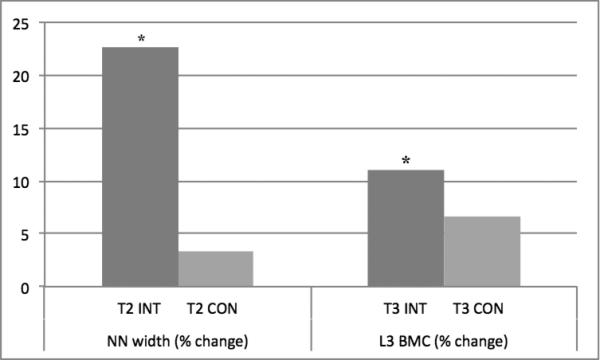
T2 and T3 Percent Change in NN width and L3 BMD
Outcomes adjusted for measurement interval, follow-up PA, Tanner breast stage, and change in height. NN=narrow neck, BMD=areal bone mineral density, T2=Tanner breast 2 at baseline, T3=Tanner breast 3 at baseline
Discussion
This school-based resistance intervention produced maturity- and region-specific bone gains in adolescent girls. Intervention participants who were Tanner 2 at baseline had significantly greater gains in bone parameters at the hip (NN width), while Tanner 3 participants had significantly greater gains at the spine (L3 BMD) compared to maturity-matched controls. Combined, T2 and T3 intervention subjects showed a trend towards increased bone acquisition at the spine (spine BMC, L3 BMC), arms (BMC), hip (NN width), and sub-head (BMC) regions. This 8-12 min resistance intervention administered by physical education instructors during standard PE class, 2-3 times per week, may serve as an important osteoporosis prevention strategy in adolescent girls.
The mechanostat model [44] suggests that the growing skeleton responds to mechanical strain by increasing periosteal apposition, resulting in wider bones. Estrogen exposure inhibits periosteal apposition [45] and thus growth in bone width, but augments trabecular bone acquisition [46, 47]. Therefore, increased bone loading during early maturation (low estrogen exposure) would be expected to increase bone width, whereas later loading (during increased estrogen exposure) would be expected to increase bone density and strength. This aligns with our findings that during early maturity (T2) intervention participation resulted in increased NN width, while intervention participation during later maturity (T3) was associated with increased trabecular (spine) bone acquisition.
Our findings are consistent with previous school-based intervention studies in girls of a similar maturational age. In a cohort of 4th-6th grade girls participating in a 7 month jumping intervention for 10-12 minutes per day, 2 days per week, prepubertal girls (Tanner breast 1 at baseline) demonstrated no increase in bone acquisition as a result of the intervention, whereas early pubertal (Tanner breast 2 or 3 at baseline) subjects demonstrated significant intervention benefits at the femoral neck and lumbar spine compared to maturity-matched controls (1.6-1.9% differential raw gains) [29, 30]. Similarly, in our T2 and T3 subjects we identified a significant, positive intervention effect, with more mature subjects acquiring more bone at the spine and less mature subjects improving hip geometry and strength. Our adjusted differential gains (4.4% for L3 BMD and 19.3% at NN width) were greater than the raw differential gains noted in this jumping program.
Applying an intervention protocol more similar to our own (resistance and high-impact aerobic exercises 30 minutes, 3 times per week), Morris et. al. found significantly increased raw bone acquisition at the spine, hip, leg, arm, and total-body (0.5% to 5.5% differential gains) in a cohort of 4th-5th grade girls [20]. However, after controlling for height and total body mass, no intervention benefit was seen for spine BMC or femoral neck BMD. At least half of these subjects were Tanner breast 1 at baseline. Previous work has shown girls with Tanner breast of 1 to be less responsive to bone loading [29, 30, 36], which may explain the loss of an intervention effect after controlling for height and body mass. The conclusions of this study must be interpreted with caution as no attempt was made to control for maturity level, nor activity completed outside the intervention protocol, both of which are known to modulate bone growth.
Our study is enhanced by rigorous quantification of the intervention and statistical adjustment for non-intervention factors that may have contributed to bone acquisition. As the rate of adolescent bone accrual is closely tied to maturation, change in bone parameters must be controlled for stage of pubertal development, differentiating bone acquisition due to maturationally-variable growth rates from that due to an intervention. Analyses centered at menarche or peak height velocity (PHV, peak linear growth rate) minimize the confounding effects of maturation in bone growth studies. As many subjects in the present study were pre-menarcheal at FU, and PHV could not be calculated with only 2 data points, Tanner breast stage was chosen as a surrogate marker for maturation and entered into the model. As prior studies have demonstrated intervention effects in TB2 and TB3 subjects but not TB1 subjects [29, 30], we limited our analysis to include only subjects who were TB2 or TB3 at baseline. To further investigate maturity effects, an interaction term was added to the model, identifying a significant differential effect of the intervention in TB2 vs. TB3 subjects that has not been reported in previous studies.
In addition to maturation, the effects of physical activity outside the intervention were carefully measured and accounted for. Whereas many pediatric studies utilize a physical activity questionnaire [29, 30, 37, 38] or accelerometers [39] assessing only 7 days of general activity, we quantified all organized activity that occurred in the 6 months prior to and during the intervention. Adjusting the bone growth data for a comprehensive measure of organized, outside PA diminishes the likelihood that observed bone gains are in fact attributable to PA completed outside the intervention.
An additional strength of this study is the nature of the resistance intervention, which was designed and driven by physical education instructors with the goal of improving adolescent musculoskeletal health. The intervention requires minimal equipment and is easily communicated to new instructors. Incorporating the intervention into the standard curriculum reaches students who may not seek out after school physical activities, and thus may stand to benefit most from the intervention. These advantages increase the value of our findings as skeletal health benefits resulted from an easily replicated and cost-effective intervention that may be implemented as a readily-generalizable public health program.
There are several limitations to our study. First, the disparate measurement interval between the intervention and control groups complicated the analysis of bone growth; control subjects had a longer period of time between DXA scans in which to accrue bone. To overcome this problem, we used percent change as our outcome measure and controlled for measurement interval duration in all of our analyses. The disparate interval suggests that the beneficial effect of the intervention is most likely conservative. Second, as HSA relies on a two-dimensional scan to produce three-dimensional geometric and strength measures, large output variability may occur with inconsistent subject positioning [29]. Although all scans were completed by the same trained technician, thereby removing some variability, subtle variations in subject position may still have occurred. Third, this intervention was performed in a relatively small, homogenous population of middle school girls; results may not be generalizable to the population as a whole. Finally, the small sample size of the T2, T3 sub-analysis may be slightly underpowered for skeletal sites at which the intervention advantages are only 50% of previously observed gymnast advantages. FN and L3 intervention advantages were approximately 75% of previously reported gymnast forearm aBMD advantages, and, thus, were appropriately powered. It will be illustrative to evaluate changes in a larger sample.
Conclusions
This easily-implemented, school-based, resistance intervention produced significant region- and maturity-specific bone acquisition advantages in peri-menarcheal girls. Less mature (TB2) girls benefited most at the hip, whereas more mature (TB3) subjects gained most at the spine. As increased peak bone mass is protective against osteoporosis development, and osteoporotic fracture occurs most often at the hip and spine, this program provides an attractive, easily-generalized, public health intervention to minimize the significant morbidity associated with osteoporosis. Previous work has demonstrated that increased bone acquisition in adolescence may be maintained into adulthood [40-43]. However, further evaluation and follow-up is necessary to determine whether the effects of this intervention are long lasting.
Acknowledgements
This research was supported by the UW Institute for Clinical and Translational Research, funded through a Clinical and Translational Science Award, 9U54TR000021 from NIH/NCATS.
Footnotes
Disclosures:
The author's declare no conflicts of interest.
References
- 1.Sabatier JP, Guaydier-Souquieres G, Benmalek A, Marcelli C. Evolution of lumbar bone mineral content during adolescence and adulthood: a longitudinal study in 395 healthy females 10-24 years of age and 206 premenopausal women. Osteoporos Int. 1999;9:476–82. doi: 10.1007/s001980050173. [DOI] [PubMed] [Google Scholar]
- 2.Cadogan J, Blumsohn A, Barker ME, Eastell R. A longitudinal study of bone gain in pubertal girls: anthropometric and biochemical correlates. J Bone Miner Res. 1998;13:1602–12. doi: 10.1359/jbmr.1998.13.10.1602. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18(Suppl 3):S191–4. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. United States. 2004:866–75. doi: 10.1086/420771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, Howman-Giles R. Bone mineral density in adolescent female athletes: relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002;34:286–94. doi: 10.1097/00005768-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL. Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. Osteoporos Int. 1998;8:152–8. doi: 10.1007/BF02672512. [DOI] [PubMed] [Google Scholar]
- 7.Cassell C, Benedict M, Specker B. Bone mineral density in elite 7- to 9-yr-old female gymnasts and swimmers. Med Sci Sports Exerc. 1996;28:1243–6. doi: 10.1097/00005768-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Grimston SK, Willows ND, Hanley DA. Mechanical loading regime and its relationship to bone mineral density in children. Med Sci Sports Exerc. 1993;25:1203–10. [PubMed] [Google Scholar]
- 9.Dowthwaite JN, Flowers PP, Spadaro JA, Scerpella TA. Bone geometry, density, and strength indices of the distal radius reflect loading via childhood gymnastic activity. J Clin Densitom. United States. 2007:65–75. doi: 10.1016/j.jocd.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowthwaite JN, Scerpella TA. Skeletal geometry and indices of bone strength in artistic gymnasts. J Musculoskelet Neuronal Interact. 2009;9:198–214. [PMC free article] [PubMed] [Google Scholar]
- 11.Ward KA, Roberts SA, Adams JE, Mughal MZ. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone. United States. 2005:1012–8. doi: 10.1016/j.bone.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Dyson K, Blimkie CJ, Davison KS, Webber CE, Adachi JD. Gymnastic training and bone density in pre-adolescent females. Med Sci Sports Exerc. 1997;29:443–50. doi: 10.1097/00005768-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lohman T, Going S, Pamenter R, Hall M, Boyden T, Houtkooper L, Ritenbaugh C, Bare L, Hill A, Aickin M. Effects of resistance training on regional and total bone mineral density in premenopausal women: a randomized prospective study. J Bone Miner Res. 1995;10:1015–24. doi: 10.1002/jbmr.5650100705. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Miner Res. 1995;10:574–85. doi: 10.1002/jbmr.5650100410. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson MK, Johnell O, Obrant KJ. Is bone mineral density advantage maintained long-term in previous weight lifters? Calcif Tissue Int. 1995;57:325–8. doi: 10.1007/BF00302066. [DOI] [PubMed] [Google Scholar]
- 16.Conroy BP, Kraemer WJ, Maresh CM, Fleck SJ, Stone MH, Fry AC, Miller PD, Dalsky GP. Bone mineral density in elite junior Olympic weightlifters. Med Sci Sports Exerc. 1993;25:1103–9. [PubMed] [Google Scholar]
- 17.Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int. 2002;70:469–74. doi: 10.1007/s00223-001-1019-9. [DOI] [PubMed] [Google Scholar]
- 18.Nichols DL, Sanborn CF, Love AM. Resistance training and bone mineral density in adolescent females. J Pediatr. United States. 2001:494–500. doi: 10.1067/mpd.2001.116698. [DOI] [PubMed] [Google Scholar]
- 19.Blimkie CJ, Rice S, Webber CE, Martin J, Levy D, Gordon CL. Effects of resistance training on bone mineral content and density in adolescent females. Can J Physiol Pharmacol. 1996;74:1025–33. [PubMed] [Google Scholar]
- 20.Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD. Prospective ten-month exercise intervention in premenarcheal girls: positive effects on bone and lean mass. J Bone Miner Res. 1997;12:1453–62. doi: 10.1359/jbmr.1997.12.9.1453. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald HM, Kontulainen SA, Petit MA, Beck TJ, Khan KM, McKay HA. Does a novel school-based physical activity model benefit femoral neck bone. Osteoporos Int. 2008;19:1445–56. doi: 10.1007/s00198-008-0589-z. [DOI] [PubMed] [Google Scholar]
- 22.Dowthwaite JN, Scerpella TA. Distal radius geometry and skeletal strength indices after peripubertal artistic. Osteoporos Int. 2011;22:207–16. doi: 10.1007/s00198-010-1233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 24.Borenstein M, Rothstein H, Cohen J. Sample Power V2.0. SPSS; Chicago, Ilinnois: 2001. [Google Scholar]
- 25.Length RV. Java Applets for Power and Sample Size, Calculated on 04/16/2009. 2009 [Google Scholar]
- 26.Dowthwaite JN, Flowers PP, Spadaro JA, Scerpella TA. Bone geometry, density, and strength indices of the distal radius reflect loading. J Clin Densitom. 2007;10:65–75. doi: 10.1016/j.jocd.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scerpella TA, Dowthwaite JN, Gero NM, Kanaley JA, Ploutz-Snyder RJ. Skeletal benefits of pre-menarcheal gymnastics are retained after activity. Pediatr Exerc Sci. 2010;22:21–33. doi: 10.1123/pes.22.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res. 2002;17:363–72. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 30.Mackelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J Pediatr. United States. 2001:501–8. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 31.Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and Girls: the POWER PE study. J Bone Miner Res. 2008;23:1002–11. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- 32.Heinonen A, Sievanen H, Kannus P, Oja P, Pasanen M, Vuori I. High-impact exercise and bones of growing girls: a 9-month controlled trial. Osteoporos Int. 2000;11:1010–7. doi: 10.1007/s001980070021. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22:434–46. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 34.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. Pediatrics. 2003;112:e447. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 35.Sundberg M, Gardsell P, Johnell O, Ornstein E, Karlsson MK, Sernbo I. Pubertal bone growth in the femoral neck is predominantly characterized by increased bone size and not by increased bone density--a 4-year longitudinal study. Osteoporos Int. 2003;14:548–58. doi: 10.1007/s00198-003-1406-3. [DOI] [PubMed] [Google Scholar]
- 36.Dowthwaite JN, DiStefano JG, Ploutz-Snyder RJ, Kanaley JA, Scerpella TA. Maturity and activity-related differences in bone mineral density: Tanner I vs. II and gymnasts vs. non-gymnasts. Bone. United States. 2006:895–900. doi: 10.1016/j.bone.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 37.McKay HA, Petit MA, Schutz RW, Prior JC, Barr SI, Khan KM. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. J Pediatr. United States. 2000:156–62. doi: 10.1016/s0022-3476(00)70095-3. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald HM, Kontulainen SA, Petit MA, Beck TJ, Khan KM, McKay HA. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos Int. 2008;19:1445–56. doi: 10.1007/s00198-008-0589-z. [DOI] [PubMed] [Google Scholar]
- 39.Meyer U, Romann M, Zahner L, Schindler C, Puder JJ, Kraenzlin M, Rizzoli R, Kriemler S. Bone. United States: 2010. Elsevier Inc; 2011. Effect of a general school-based physical activity intervention on bone mineral content and density: a cluster-randomized controlled trial. pp. 792–7. [DOI] [PubMed] [Google Scholar]
- 40.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int. 2011;22:2205–10. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs RK, Bauer JJ, Snow CM. Jumping improves hip and lumbar spine bone mass in prepubescent children: a randomized controlled trial. J Bone Miner Res. 2001;16:148–56. doi: 10.1359/jbmr.2001.16.1.148. [DOI] [PubMed] [Google Scholar]
- 42.Gunter K, Baxter-Jones AD, Mirwald RL, Almstedt H, Fuller A, Durski S, Snow C. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone. United States. 2008:710–8. doi: 10.1016/j.bone.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Janz KF, Letuchy EM, Eichenberger Gilmore JM, Burns TL, Torner JC, Willing MC, Levy SM. Early physical activity provides sustained bone health benefits later in childhood. Med Sci Sports Exerc. 2010;42:1072–8. doi: 10.1249/MSS.0b013e3181c619b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frost HM. The Utah paradigm of skeletal physiology: an overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18:305–16. doi: 10.1007/s007740070001. [DOI] [PubMed] [Google Scholar]
- 45.Seeman E. Periosteal bone formation--a neglected determinant of bone strength. In: N Engl J Med. United States. 2003:320–3. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 46.Mora S, Goodman WG, Loro ML, Roe TF, Sayre J, Gilsanz V. Age-related changes in cortical and cancellous vertebral bone density in girls: assessment with quantitative CT. AJR Am J Roentgenol. 1994;162:405–9. doi: 10.2214/ajr.162.2.8310936. [DOI] [PubMed] [Google Scholar]
- 47.Ausili E, Rigante D, Salvaggio E, Focarelli B, Rendeli C, Ansuini V, Paolucci V, Triarico S, Martini L, Caradonna P. Determinants of bone mineral density, bone mineral content, and body composition in a cohort of healthy children: influence of sex, age, puberty, and physical activity. Rheumatol Int. 2012;32:2737–43. doi: 10.1007/s00296-011-2059-8. [DOI] [PubMed] [Google Scholar]


