Abstract
OBJECTIVE
Patients with oral cavity squamous cell carcinoma (OCSCC) undergo adjuvant radiation for pathologically high risk features including positive nodal disease and extra capsular spread (ECS). In the absence of these high risk features, our objective was to determine if perineural invasion (PNI) is an independent risk factor and if adjuvant radiation (XRT) improves disease control rates.
STUDY DESIGN
Historical cohort analysis.
SETTING
Tertiary university hospital.
METHODS
Eighty-eight OCSCC patients (46 males, 42 females; mean age, 56.7 years; median follow-up, 4.6 years) treated surgically with pN0 necks were studied. Overall 23% (20/88) were pN0/PNI+ and of those with PNI, 70% (14/20) underwent XRT. Survival analysis using Kaplan-Meier followed by multivariable Cox models was performed.
RESULTS
Multivariate analysis verified PNI to be associated with worse DFI (p=0.012) and LRC (p=0.005) and perivascular invasion (PVI) associated with worse DFI (p=0.05). Amongst pN0/PNI+ patients, those who received XRT demonstrated significantly improved DFI (mean 6.5yrs v. 1.7yrs; p=0.014) and LRC (mean 6.7yrs v. 1.9yrs; p=0.047). There was no improvement in OS (p=0.68) or DSS (p=0.8) in those receiving XRT.
CONCLUSIONS
PNI is an independent adverse risk factor in the absence of nodal metastasis and extracapsular spread. We observed a statistically significantly longer DFI and LRC when patients were treated with adjuvant radiation.
INTRODUCTION
Perineural invasion (PNI) has been classified as an intermediate risk factor for recurrence and decreased survival.1,2 When identified in the setting of nodal metastasis and extracapsular spread, the addition of adjuvant therapies is a well established method of treatment. Treatment decisions become more difficult in the pathologically negative neck with clear evidence of PNI when high risk factors such as extracapsular spread (ECS) and nodal metastasis are no longer a major factor in adding adjuvant therapy. Adjuvant therapies are not without risks and selecting the appropriate treatment regimen based on risk assessment, while maintaining optimal survival outcomes is vital to the overall management of patients with oral cavity squamous cell carcinoma (OCSCC).2
There is strong data supporting PNI as a risk factor for occult metastasis along with depth of invasion, size of primary tumor, differentiation and immunosuppression.3–5 The goal of identifying high risk groups in OCSCC and treating them appropriately has been shown in numerous trials to improve survival, although the effect of PNI biologically independent of other histologic risk factors has not been studied.1 We sought to evaluate the effect of PNI in OCSCC in patients who underwent a neck dissection and were found to have no pathologic evidence of regional metastasis (pN0), thus removing the confounding effect of N+ disease and ECS on outcomes. We hypothesized that in patients with pN0 necks, those with PNI (pN0/PNI+) would have a poorer prognosis compared to patients without PNI (pN0/PNI−). As a secondary outcome we assessed the role of adjuvant radiation in pN0 patients based on PNI status.
MATERIALS AND METHODS
Study Population and Eligibility Criteria
A historical cohort analysis of all patients treated primarily with surgery for OCSCC from 1998 – 2009 at a tertiary care center was performed. Two-hundred and ninety-nine patients with OCSCC were screened for the following inclusion criteria: previously untreated patients who underwent primary surgical extirpation with a selective neck dissection and no pathologic evidence of regional metastasis or positive margins. Eighty-eight patients were identified with no evidence of regional metastasis based on pathological analysis of their neck dissection specimen. Seventy – seven percent (68/88) of patient’s primary tumors had no evidence of perineural invasion (pN0/PNI−) while 23% (20/88) of patient’s primary tumors were found to have pathological evidence of perineural invasion (pN0/PNI+). Demographics of the pN0/PNI+ and pN0/PNI− cohorts are shown in Table 1. There were no differences between the pN0/PNI+ and pN0/PNI− groups by age, gender, smoking or alcohol status, T-classification, margin control, tumor grade, perivascular invasion, tumor subsite or median follow-up.
Table 1.
Demographics
| Perineural Invasion Positive | Perineural Invasion Negative | p-value | |
|---|---|---|---|
| Number (%) | 20 (23) | 68 (77) | |
| Mean Age (range) | 54.1 years | 57.4 years | 0.357 (NS) |
| Gender | 0.195 (NS) | ||
| Male (%) | 13 (65) | 33 (49) | |
| Female (%) | 7 (35) | 35 (51) | |
| T-classification (%) | 0.23 (NS) | ||
| T1 | 3 (15) | 21 (31) | |
| T2 | 4 (20) | 21 (31) | |
| T3 | 4 (20) | 9 (13) | |
| T4 | 9 (45) | 17 (25) | |
| AJCC Stage (%) | 0.23 (NS) | ||
| I | 3 (15) | 21 (31) | |
| II | 4 (20) | 21 (31) | |
| III | 4 (20) | 9 (13) | |
| IV | 9 (45) | 17 (25) | |
| Smoking Status | 0.339 (NS) | ||
| Yes | 13 (65) | 51 (75) | |
| No | 7 (35) | 17 (25) | |
| Mean Pack Years | 17.5 | 25.7 | 0.130 (NS) |
| Alcohol Status | 0.282 (NS) | ||
| Yes | 7 (35) | 32 (47) | |
| No | 13 (65) | 36 (53) | |
| Tumor Site (%) | |||
| Oral Tongue | 11 (55) | 26 (38) | 0.307 (NS) |
| Floor of Mouth | 4 (20) | 16 (24) | 1.0 (NS) |
| Alveolar Ridge | 2 (10) | 15 (22) | 0.342 (NS) |
| Buccal Space/RMT | 2 (10) | 11 (16) | 0.725 (NS) |
| Radiation Therapy | 0.002 | ||
| Yes | 14 (70) | 21 (31) | |
| No | 6 (30) | 47 (68) | |
| Median Follow-up | 57.0 months | 54.8 months | NS |
Treatment Plan
All patients were evaluated clinically and underwent direct laryngoscopy and esophagoscopy to confirm resectability and evaluate for second primaries. Primary extirpation with 1 cm margins was at the discretion of the attending surgeon. All patients in the study underwent neck dissection based on depth of invasion >2mm and clinical or radiographic evidence of regional metastasis and/or advanced stage (AJCC Stage III or IV). The extent of neck dissection was at the discretion of the operating surgeon based on tumor location. The minimum neck dissection was a selective level I–III unilateral neck dissection with the exception of one pN0/PNI− patient who underwent a selective level I–II neck dissection. Adjuvant radiation treatment was recommended for patients with positive margins and advanced T-classification, however there were 12 patients in this cohort with T3/T4 tumors who did not receive adjuvant radiation based on physician preference, patient refusal, and young age. In the pN0/PNI+ group, 6 patients did not receive adjuvant radiation based on young age, determination of the multidisciplinary tumor board or patient refusal. Patients with PNI (pN0/PNI+) were significantly more likely to undergo post-operative radiation therapy compared to patients without PNI (pN0/PNI−) (70% (14/20) v. 31% (21/68); p= 0.0034). In the pN0/PNI+ group, 1/20 (5%) refused radiation and received Xeloda alone.
Pathologic Analysis of Perineural Invasion
All patients identified in the database with PNI were analyzed by a head and neck pathologist (J.B.M.) for extent of perineural invasion, size of nerve invasion and intraneural invasion. Extent of PNI was categorized as focal (one focus of PNI), moderate (2–5 foci of PNI) or extensive (>5 foci of PNI). Size of nerve invasion was categorized as small (nerve size <1mm) or large (nerve size ≥1mm). Intraneural invasion was defined as cancer invasion through the nerve epineurium. Three patient blocks were unavailable for further analysis.
Statistical Analysis
The outcomes of interest were overall survival (OS), disease-specific survival (DSS), local-regional control (LRC) and disease-free interval (DFI). Overall Survival was defined as time from diagnosis of first primary to death by any cause; DSS was defined as the time from diagnosis of first primary to death from OCSCC, where the occurrence of a second primary was treated as a censored event. Local-regional control was defined as time from treatment to local and/or regional recurrence. Disease-free interval was defined as time from treatment to any type of recurrence. Patients who were alive and free of disease at last follow-up were censored at the last inquiry to the Social Security Death Index for survival and date of last follow-up for LRC and DFI.
The Kaplan-Meier method and the log-rank test were used to test for differences in the survival functions between strata defined by clinical variables. For descriptive purposes, we show the survival functions for patients with perineural invasion who did and did not undergo post-operative radiation therapy. Univariable and multivariable Cox models were used to explore the associations of clinical variables with time-to-event outcomes. Multivariate analysis was performed for OS, DSS, LRC and DFI using all potential covariates and implementing a backward selection algorithm with selection criteria set at a p-value of 0.15 to obtain parsimonious models. Each test may be limited by the sample size, especially in subgroup comparisons of the outcomes based on adjuvant radiation in the pN0/PNI+ group; therefore, a non-significant result does not eliminate the possibility that a relationship exists. All statistical analyses were done using SAS version 9.2 (SAS Institute, Carey, NC). A two-tailed p-value of .05 or less was considered statistically significant.
The data for this manuscript is approved by the IRB at the University of Michigan.
RESULTS
The overall failure rate was 25% (22/88). There was a significant difference in the local failure rate in the pN0/PNI+ group (35%, 7/20) compared to the pN0/PNI− group (12%, 8/68), (p=0.037). There was not a significant difference in the regional failure rate (10%, 2/20 vs. 10.2% 7/68), (p=1.0), or the distant failure rate (5%, 1/20 vs. 6% 4/68), (p=1.0).
Univariate analysis demonstrated pN0/PNI+ patients had significantly worse LRC (p=0.021), DFI (p=0.031), and DSS (p=0.031) than pN0/PNI− patients, while there was a trend towards a significant difference in OS (p=0.096) between the two groups (Figure 1A–C). The three and five year survival estimates between pN0/PNI+ and pN0/PNI− are shown in Table 2. Perivascular invasion conferred a worse DFI (p=0.028) and LRC (p=0.008) (Table 3). Given that all patients in this cohort were pN0 and there were only 22 disease related outcomes, there is inadequate sample to show expected differences with univariate testing of gender, tobacco use, tumor grade, T-classification, and AJCC Stage on OS, DSS, LRC, or DFI.
Figure 1.
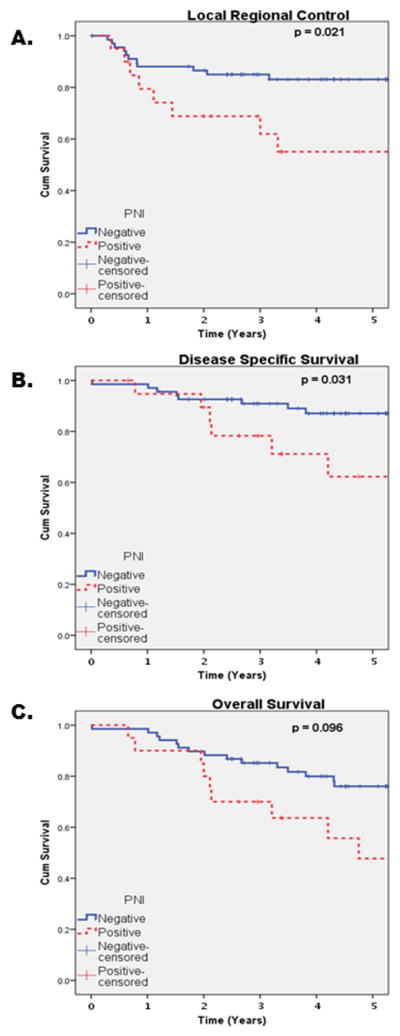
KM pN0/PNI+ v pN0/PNI− (A) Local-regional control, (B) Disease specific survival, (C) Overall Survival.
Table 2.
(A) Three and (B) Five Year Survival Estimates.
| (A) 3-year survival estimates | |||||
|---|---|---|---|---|---|
| pN0/PNI+ vs. pN0/PNI− (95% CI) | |||||
| n | Overall Survival | Disease Specific Survival | Disease Free Interval | Local Regional Control | |
| pN0/PNI+ | 20 | 70% (45–85) | 78% (52–91) | 64% (38–81) | 65% (40–82) |
| pN0/PNI− | 68 | 83% (72–90) | 90% (80–95) | 79% (66–87) | 84% (73–91) |
| pN0/PNI+ with Radiation vs. pN0/PNI+ without Radiation (95% CI) | |||||
| n | Overall Survival | Disease Specific Survival | Disease Free Interval | Local Regional Control | |
| PNI+ No Radiation | 6 | 83% (27–97) | 83% (27–97) | 33% (5–68) | 50% (11–80) |
| PNI+ Plus Radiation | 14 | 64% (34–83) | 76% (43–92) | 77% (45–92) | 71% (41–88) |
| (B) 5-year survival estimates | |||||
|---|---|---|---|---|---|
| pN0/PNI+ vs. pN0/PNI− (95% CI) | |||||
| n | Overall Survival | Disease Specific Survival | Disease Free Interval | Local Regional Control | |
| pN0/PNI+ | 20 | 48% (22–70) | 62% (33–82) | 50% (25–71) | 52% (27–72) |
| PN0/PNI− | 68 | 74% (61–83) | 86% (75–92) | 77% (64–86) | 82% (71–89) |
| pN0/PNI+ with Radiation vs. pN0/PNI+ without Radiation (95% CI) | |||||
| n | Overall Survival | Disease Specific Survival | Disease Free Interval | Local Regional Control | |
| PNI+ No Radiation | 6 | 42% (1–84) | 42% (1–84) | 33% (5–68) | 50% (11–80) |
| PNI+ Plus Radiation | 14 | 46% (18–70) | 65% (31–86) | 68% (34–87) | 63% (33–83) |
pN0 = pathologically N-0; PNI = Perineural invasion
Table 3.
Multivariate Analysis of pN0/PNI+ v pN0/PNI− for Overall Survival, Disesae-specific survival, local regional control and disease free interval.
| Variable | Standard Error | Wald chi-squared | p-Value | Hazard Ratio | beta-coefficient | 95% Hazard Ratio Confidence Interval | ||
|---|---|---|---|---|---|---|---|---|
| Overall Survival | Perineural Invasion | 0.40 | 2.84 | 0.092 | 1.97 | 0.34 | 0.84 | 4.35 |
| Disease Specific Survival | Perineural Invasion | 0.54 | 3.30 | 0.069 | 2.69 | 0.34 | 0.925 | 7.80 |
| Tumor Grade (Poor vs. Well) | 0.92000 | 4.620 | 0.032 | 7.225 | 1.23 | 1.191 | 43.848 | |
| Disease Free Interval | Perineural Invasion | 0.49 | 6.26 | 0.012 | 3.37 | 0.53 | 1.30 | 8.72 |
| Radiation | <0.001 | 2.56 | 0.11 | 1.00 | 0.0 | 1.00 | 1.002 | |
| Local-Regional Control | Perineural Invasion | 0.53 | 9.65 | 0.002 | 5.18 | 0.66 | 1.83 | 14.6 |
| Perivascular Invasion | 0.64 | 4.36 | 0.037 | 3.83 | 0.79 | 1.09 | 13.5 | |
| Radiation | 0.001 | 4.14 | 0.042 | 1.001 | 0.0 | 1.00 | 1.002 | |
Multivariable Cox Regression analysis controlling for age, gender, tobacco and alcohol use, positive margins, tumor grade, perivascular invasion, T-classification, AJCC stage, chemotherapy, and radiation therapy was performed. The variable pN0/PNI+ compared to pN0/PNI− showed significantly worse DFI (p=0.012) and LRC (p=0.005), while there was a trend toward significance in OS (p=0.09) and DSS (p=0.07). In this multivariate model, perivascular invasion was significantly associated with worse DFI (p=0.012). (Table 3)
To assess the effect of adjuvant radiation therapy on pN0/PNI+ patients, subset analysis of 14 pN0/PNI+ patients who received adjuvant radiation were compared to six pN0/PNI+ patients who did not receive adjuvant radiation. Local regional control rates were significantly improved in pN0/PNI+ patients treated with adjuvant radiation compared to those who did not receive adjuvant radiation (6.7 yrs v. 1.9 yrs, respectively; p=0.047) (Figure 2A). The mean disease free interval was significantly improved in pN0/PNI+ patients treated with radiation versus those who did not undergo radiation treatment (6.5 yrs. v. 1.7 yrs respectively; p=0.012). While adjuvant radiation for pN0/PNI+ patients resulted in improved LRC and DFI, it did not result in significant improvement for DSS (p=0.8) or OS (p=0.68). (Figure 2B and C)
Figure 2.
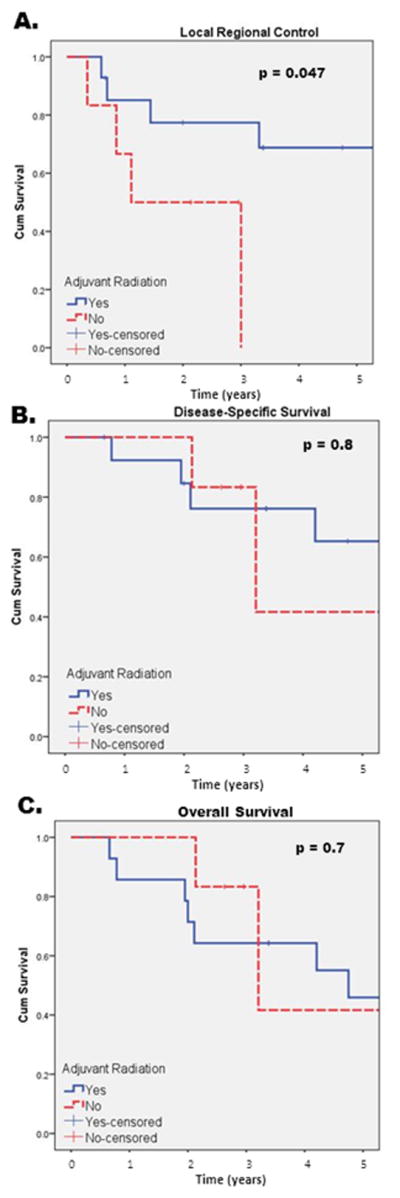
KM pN0/PNI+/XRT+ v. pN0/PNI+/XRT- (A) Local-regional control, (B) Disease specific survival, (C) Overall Survival.
To assess for potential bias between the pN0/PNI+ group that underwent and did not undergo adjuvant radiation, comparison of differences in T-classification and tumor grade was performed. In the pN0/PNI+ group who underwent adjuvant radiation, a greater proportion had advanced stage tumors (T3/4) compared to patients who did not undergo adjuvant radiation (71% v. 50% respectively). In this cohort, there was one patient with poorly differentiated squamous cell carcinoma. In the pN0/PNI+ group that received adjuvant radiation 82.3% had well-moderate differentiated tumors compared to 100% in pN0/PNI+ group who did not undergo adjuvant radiation. Despite more advanced T-classification in the pN0/PNI+ group receiving adjuvant radiation, LRC (p=0.047) and DFI (p=0.012) are significantly improved compared to the pN0/PNI+ group who did not undergo adjuvant radiation therapy. (Table 2)
To further assess treatment failures in the pN0/PNI+ group who received adjuvant radiation versus those that did not, descriptive analysis of all patients with local, regional or distant failures in the pN0/PNI+ group was performed. Comparison of pN0/PNI+ patients who received adjuvant radiation versus those that did not demonstrated 21% (3/14) vs. 67% (4/6) failed locally, 0% (0/14) vs. 17% (1/6) failed regionally and 7% (1/14) vs. 17% (1/6) had distant failures respectively.
The histologic descriptors of perineural invasion [extent of PNI (focal, moderate and extensive), type of nerve involved (small v. large) and intraneural v. extraneural invasion] were also analyzed. Fifty-three percent of pN0/PNI+ patients had extensive PNI (9/17) compared to 35% (6/17) having focal PNI and only 6% (1/17) with moderate PNI. Small nerves were predominantly involved (92%) compared to large nerves (8%) and the vast majority (79%) had extraneural invasion. There was no correlation of these histologic descriptors of PNI to type of failure, however this subset analysis was underpowered.
DISCUSSION
While PNI is often regarded as an intermediate risk factor, this study demonstrates that in the absence of pathologic evidence of nodal metastasis, PNI portends a worse DSS, LRC and DFI in patients treated surgically for OCSCC. PNI in the absence of nodal disease or positive margins increases the risk of local regional failure by 5-times and shortened disease-free interval by 3-times. Adjuvant radiation therapy appears to improve LRC and DFI in patients with PNI.
Several studies have sought to analyze the effect of PNI in OCSCC patients with N0 neck disease. Liao et al. analyzed 460 patients that with clinical T1-3 and N0 neck disease, however 15% of their patients did not undergo neck dissections.6 Similar to our study they found a significantly increased regional recurrence rate in the PNI+ group but found no benefit for their PNI+ patients undergoing adjuvant radiation treatment.
By using pathologically N0 patients, we are better equipped to analyze the true effect of PNI independent of known high-risk features. We sought to evaluate OCSCC patients with no evidence of regional metastasis based on pathologic analysis after neck dissection to more accurately define a group of patients that were biologically independent of the known risk factors (ECS, N+ and positive margins) for poor outcomes.
The next logical question after identifying PNI as an independent risk factor for poor outcome is to examine treatment options to modify the negative effects of PNI. In this study, adjuvant radiation appears to improve LRC and DFI despite more advanced stage tumors in the group receiving adjuvant radiation. The 2012 National Comprehensive Cancer Network (NCCN) recommends at least adjuvant radiation for T1-4N0 OCSCC with adverse features.7 While adjuvant radiation has risks, the risk must be weighed against outcomes.8–11 In the study cohort local treatment failures were significantly higher in the pN0/PNI+ group compared to the pN0/PNI− group while there was no difference comparing regional or distant failures. In patients who are pN0/PNI+, our findings support the NCCN guidelines for adjuvant radiation in pN0/PNI+ patients. In our analysis 83% of pN0/PNI+ patients who did not receive adjuvant radiation treatment experience local-regional failure whereas 21% who did receive adjuvant radiation treatment had local-regional failures. These findings are limited by sample size but the addition of adjuvant radiation seems to improve local regional control and disease free interval. Although we acknowledge the small sample size, this is one of the largest reviews of PNI as an isolated risk factor. Given the controversy in the literature regarding PNI as an absolute indication for adjuvant radiation, a larger randomized prospective trial would better answer the question of the role of adjuvant radiation for the pN0/PNI+ patient.6,8,9
Overall, PNI was shown to be an independent risk factor for reduced DSS, LRC and DFI. Previous work has shown histologic subtypes of PNI correlate with OS and LRC.12,13 However in the present study histologic subtypes of PNI did not correlate with risk of recurrence or poor outcomes. The number of patients in our subset analysis is small and would require a larger analysis to determine if the pattern of PNI is predictive of outcome. Interestingly, perivascular invasion was significantly associated with worse DFI and LRC. However, there were only 5 patients in the entire cohort with PVI. Given the small sample size, the effect of PVI is underpowered to make any valid conclusions.
Future studies evaluating a larger cohort of patients with different histologic subtypes of PNI may allow for a risk-stratification for prognosis and treatment. In addition, better understanding the molecular mechanisms of PNI is imperative to identifying high risk groups and potentially mediating the mechanism of perineural spread in HNSCC.
CONCLUSION
Perineural invasion portends a worse DSS, DFI and LRC in this cohort of patients with pN0 OCSCC. We observed statistically significant improvement in DFI and LRC treated with adjuvant radiation; it should be considered for patients with pN0/PNI+ OCSCC and must be further studied to define adjuvant treatment modalities in this subset of patients with OCSCC
Acknowledgments
Grant support: T32 training grant (T32 DC005356) (S.B.C); University of Michigan SPORE (P50 CA97248) (E.L.B.).
We would like to acknowledge Scott A. Mclean MD PhD, Vasu Divi, MD, Jeffrey S. Moyer MD, Mark E. Prince MD and Carol R. Bradford MD for their substantial contributions to the conception and design of the paper, analysis and interpretation of data, critical manuscript revisions and final approval of the version to be published.
Footnotes
This work was presented at the American Academy of Otolaryngology – Head and Neck Surgery Annual Meeting, Washington D.C., September 2012.
References
- 1.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Cooper JS. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist. 2005 Mar;10(3):215–24. doi: 10.1634/theoncologist.10-3-215. [DOI] [PubMed] [Google Scholar]
- 3.Liao CT, Lin CY, Fan KH, et al. Identification of a high-risk group among patients with oral cavity squamous cell carcinoma and pT1-2N0 disease. Int J Radiat Oncol Biol Phys. 2012 Jan 1;82(1):284–90. doi: 10.1016/j.ijrobp.2010.09.036. Epub 2010 Nov 13. [DOI] [PubMed] [Google Scholar]
- 4.Sparano A, Weinstein G, Chalian A, et al. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004 Oct;131(4):472–6. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Huang SH, Hwang D, Lockwood G, et al. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009 Apr 1;115(7):1489–97. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 6.Liao CT, Chang JT, Wang HM, et al. Does adjuvant radiation therapy improve outcomes in pT1-3N0 oral cavity cancer with tumor-free margins and perineural invasion? Int J Radiat Oncol Biol Phys. 2008;71:371–376. doi: 10.1016/j.ijrobp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) Oral Cavity Treatment Guidelines. 2012 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 8.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 10.Hinerman RW, Mendenhall WM, Morris CG, et al. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head Neck. 2004 Nov;26(11):984–94. doi: 10.1002/hed.20091. [DOI] [PubMed] [Google Scholar]
- 11.Fan KH, Wang HM, Kang CJ, et al. Treatment results of postoperative radiotherapy on squamous cell carcinoma of the oral cavity: coexistence of multiple minor risk factors results in higher recurrence rates. Int J Radiat Oncol Biol Phys. 2010 Jul 15;77(4):1024–9. doi: 10.1016/j.ijrobp.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 12.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005 Feb;29(2):167–78. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 13.Fagan JJ, Collins B, Barnes L, et al. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998 Jun;124(6):637–40. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]


