Abstract
The metabolic syndrome (MetS) is a clustering of cardiovascular and cerebrovascular risk factors that are often comorbid with depressive symptoms. Individual components of the MetS also covary with the morphology of basal ganglia regions that are altered by depression. However, it remains unknown whether the covariation between the MetS and depressive symptomatology can be accounted for in part by morphological changes in the basal ganglia. Accordingly, we tested the hypothesis that increased depressive symptoms among individuals with the MetS might be statistically mediated by reduced grey matter volume in basal ganglia regions. The presence of the MetS was determined in 147 middle-aged adults using the criteria of the National Cholesterol Education Program, Adult Treatment Panel III. Basal ganglia volumes were determined on an a priori basis by automated segmentation of high-resolution magnetic resonance images. Depressive symptoms were assessed using the Patient Health Questionnaire. Even after controlling for demographic and other confounding factors, having the MetS and meeting more MetS criteria covaried with reduced globus pallidus volume. Meeting more MetS criteria and reduced pallidal volume were also related to depressive symptoms. Moreover, the MetS-depression association was statistically mediated by pallidal volume. In summary, reduced globus pallidus volume is a neural correlate of the MetS that may partly account for its association with depressive symptoms.
Keywords: basal ganglia, cardiovascular disease, depression, metabolic syndrome
1. Introduction
The metabolic syndrome (MetS) refers to a clustering of cardiovascular and cerebrovascular risk factors within the same individual. These risk factors include atherogenic dyslipidemia, elevated blood pressure, central adiposity, hypertriglyceridemia, and glucose dysregulation. Importantly, the MetS confers risk for cardiovascular and cerebrovascular disease morbidity and mortality more so than the risk conferred by any of its individual components [1–7]. The MetS also confers risk for type 2 diabetes mellitus, as well as associated impairments in cognitive function and adverse mental health outcomes, particularly dysphoric mood and major depressive disorder [8–10]. As reviewed below, several lines of evidence suggest that the MetS may be linked to risk for these diverse health outcomes via alterations in brain systems that are jointly involved in energy balance, metabolism, cardiovascular regulation, cognitive processes, and emotional functions that support hedonic or appetitive behaviors [11–14]. At present, however, the specific brain systems that may plausibly link the MetS to often comorbid health outcomes are incompletely understood [15–17]. Accordingly, a primary goal of the present study was to examine whether the basal ganglia may represent one such system, as there is cumulative evidence showing that aspects of basal ganglia morphology in particular relate to individual components and clinical sequelae of the MetS [18–20], as well as to comorbid health conditions that are specifically characterized by depressive symptomatology [21].
To elaborate, a growing number of structural neuroimaging studies show that individuals who exhibit features and correlates of the MetS also exhibit morphological changes in the basal ganglia, particularly reduced tissue volumes. For example, increased adiposity among otherwise healthy individuals has been linked to volumetric reductions in the putamen and globus pallidus of the basal ganglia [18,20]. In extension, individuals with type 2 diabetes mellitus – a condition that is often consequent to the MetS – also exhibit reduced volumes in the caudate, putamen, and globus pallidus [18]. Recent work has shown further that glucose dysregulation covaries with reduced volume in striatal and pallidal regions [19]. Together, these findings indicate that components of the MetS (e.g., adiposity, glucose dysregulation) and endpoints of the MetS (e.g., type 2 diabetes) associate with morphological, particularly volumetric, changes in basal ganglia regions. However, despite such prior associations, there are two questions that still remain open: (i) does the MetS itself, as opposed to its individual components, relate to reduced volumes within the basal ganglia, particularly among people without a clinical condition (e.g., type 2 diabetes)? And (ii), does meeting more MetS criteria – above and beyond its adiposity and glucose dysregulation components - relate to reduced volumes within the basal ganglia in a graded fashion? Findings bearing on these questions would help to address the issue of whether there is a neural (volumetric) correlate of the MetS that is not confounded by the presence of a clinical disorder, which would have further implications for understanding associated variation in and risk for mental health symptoms and health conditions that are often comorbid with this syndrome.
More precisely, and as noted above, the neural correlates of the MetS that may in turn relate to risk for comorbid conditions, particularly those associated with depressive symptoms, remain incompletely understood. This is despite prior speculations and indirect evidence that the MetS and depression may share common pathophysiological features within the brain [9], with structural or morphological changes in the basal ganglia being one specific and possibly shared feature [22]. In support of this possibility, reduced tissue volumes within the basal ganglia have been associated with major depression in postmortem samples [23,24]. Moreover, previous magnetic resonance imaging (MRI) studies on patients with major depressive disorder (MDD) have reported volumetric reductions in striatal and pallidal regions [21,25–28]. Secondly, case study evidence indicates that selective bilateral lesions to the globus pallidus result in depressive symptoms and concurrent weight gain [29]. In view of such findings and prior work relating individual MetS components and its clinical consequents to reduced basal ganglia volumes [18–20], it appears plausible that structural or morphological aspects of the basal ganglia may relate to the covariation between the MetS and depressive symptoms.
Accordingly, the present study examined the structural neural correlates of the MetS, with an a priori focus on anatomically defined regions of interest (ROIs) in the basal ganglia. Hence, based on prior associations between MetS components and basal ganglia volumes [18–20], we predicted that otherwise healthy individuals with the MetS, as well as those meeting more MetS criteria, would show reduced grey matter volume in basal ganglia ROIs, including the striatum (nucleus accumbens, putamen, caudate nucleus) and the globus pallidus, beyond the variance accounted for by demographic and confounding health-related factors. In addition, insofar as the MetS often exhibits comorbidity with depression [9], we next examined whether the presence of the MetS and meeting more MetS criteria would covary with depressive symptomatology. We predicted that greater depressive symptoms would be observed in individuals with the MetS relative to those without the syndrome, and among individuals meeting an increasing number of MetS criteria. Lastly, we explored the possibility that volumetric changes in basal ganglia regions might account for (statistically mediate) any observed association between the MetS and depressive symptoms.
2. Materials and methods
2.1. Participants
Participants were 155 otherwise healthy adults (78 men, 77 women; mean age, 40.7 ± 6.2 SD, range = 30–50 years) who were recruited by mass mailings to residents of Allegheny County, PA, USA. Inclusion criteria included no history of (1) cardiovascular disease (including treatment for or diagnoses of hypertension, stroke, myocardial infarction, congestive heart failure, and atrial or ventricular arrhythmias); (2) prior neurosurgery or neurological disorder; (3) any current treatment for or self-reported psychiatric disorder; (4) typical consumption of more than 15 alcoholic beverages per week; (5) daily use of corticosteroid inhaler; (6) current use of psychotropic, lipid lowering, or any cardiovascular medication, including any medication to control blood pressure; (7) metal implants or exposure; (8) colorblindness; and (9) claustrophobia. All participants demonstrated right-handedness, as assessed by the Edinburgh Handedness Inventory [30]. Women were excluded if pregnant (as verified by urine test).
The ethnicity of the sample was Caucasian/White (70.3%), African American/Black (21.9%), Asian (5.8%), and multiracial or other (1.9%). We have previously reported on the functional neural correlates of stressor-evoked baroreflex suppression [31] and insulin resistance [32] among a subset of participants from this study. All participants provided informed consent prior to completing study protocols, which were approved by the University of Pittsburgh Institutional Review Board.
All analyses were based on a final sample size of 147 participants (76 men, 71 women). This reduced sample size was due to missing variables needed to compute MetS criteria (n = 3), missing or poor quality structural neuroimaging data (n = 4), and a score on the Patient Health Questionnaire (PHQ-9) for one participant that was 3SD above the mean of the sample (see below). These excluded participants did not differ from the remaining sample in age, sex, metabolic factors studied here or in basal ganglia ROI volumes.
2.2. Assessment of the MetS and its components
Participants arrived for a neuroimaging protocol between 7:00AM and 11:00AM, after an 8-hour fast. This also included abstaining from exercising and consuming caffeine, alcohol, and tobacco products. Prior to neuroimaging, participants’ seated, resting BP was measured from the non-dominant arm with an oscillometric device (Critikon Dinamap 8100, Johnson & Johnson, Tampa, FL). Participants provided 3 BP measures taken 2 min apart after a ~20 min acclimation period, with the average of the last 2 of the 3 BP readings serving as the resting systolic (SBP) and diastolic (DBP) blood pressures. Participants’ waist circumference (in inches) was measured at the level of the umbilicus to the nearest ½ centimeter at end expiration. After each participant’s height, weight, and waist circumference were measured, a research nurse performed blood draws. Procedures to determine fasting serum lipids and glucose levels were performed in the Department of Primary Care Laboratory Services (Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center). Concentrations of total cholesterol and triglycerides were measured by a CHOL and triglyceride GPO reagent, respectively, using an enzymatic, timed-endpoint method on the SYNCHRON LX System (Beckman Coulter, Inc., Brea, California; [33–37]. The concentration of high-density lipoprotein (HDL) cholesterol was measured with a HDLD reagent on the SYNCHRON LX System, which uses an enzymatic, time-endpoint method to uniquely facilitate a detergent that solubilizes only the HDL lipoprotein particles (Beckman Coulter, Inc., Brea, California; [33,36]. Low-density lipoprotein (LDL) cholesterol concentrations were estimated by using the Friedewald calculation [38].
The MetS was defined by the criteria of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III [39,40]. According to NCEP criteria, the MetS is defined as the presence of three or more of the following: (1) serum triglycerides ≥ 150 mg/dL; (2) fasting serum glucose ≥ 110 mg/dL; (3) waist circumference ≥ 102 cm in men or ≥ 88 cm in women; (4) SBP ≥ 130 or DBP ≥ 85 mm Hg; (5) serum HDL cholesterol bold> 40 mg/dL in men or < 50 mg/dL in women. Given our screening criteria, we adopted a conservative NCEP guideline such that use of medication for hypertension or hyperglycemia was not factored into defining the presence of the MetS in this cohort. This conservative definition of the MetS ensured that presence of disease or medication use did not confound our results.
2.3. Magnetic resonance imaging
Imaging data were acquired on a 3Tesla Trio TIM whole-body scanner (Siemens, Erlangen, Germany), equipped with a 12-channel phased-array head coil. For each participant, we obtained T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) neuroanatomical images over 7 min 17 sec by these parameters: field of view = 256×208 mm, matrix size = 256×208 mm, time to repetition = 2100 ms, time-to-inversion = 1100 ms, time to echo = 3.29 ms, and flip angle = 8° (192 slices, 1mm thick, no gap). MPRAGE images were used to derive volumetric measures described below.
2.3.1. Volumetric processing
For the a priori segmentation and volumetric analysis of the left and right basal ganglia ROIs in line with study hypotheses (i.e., caudate nucleus, putamen, nucleus accumbens, and globus pallidus), we used the Oxford University Centre for Functional MRI of the Brain (FMRIB) Integrated Registration and Segmentation Tool (FIRST) in the FMRIB Software Library (FSL) version 4.0. FIRST is a semi-automated model-based subcortical segmentation tool that relies on a Bayesian framework, as well as shape and appearance models obtained from manually segmented images provided by the Center for Morphometric Analysis, Massachusetts General Hospital (Boston, MA). Volumetric labels are parameterized by a three-dimensional deformation of a surface model based on multivariate Gaussian assumptions. Specifically, FIRST searches through linear combinations of shape modes of variation for the most probable shape given the intensity distribution in the T1-weighted image (for a more detailed description of this method, see [41,42]).
This volumetric processing method runs a two-stage affine registration to a standard space template (Montreal Neurological Institute space) with 1 mm resolution using 12 degrees of freedom and a subcortical mask to exclude voxels outside of subcortical regions. Second, the caudate nucleus, putamen, nucleus accumbens, and globus pallidus were segmented with 30, 40, 50, and 40 modes of variation, respectively. Modes of variation were optimized based on a leave-one-out cross-validation using the training set [41]. Finally, boundary correction was implemented for each structure to classify boundary voxels as belonging to the structure or not using a statistical probability threshold (z score > 3.00; p < 0.001). The volume for each structure was then measured in mm3. Segmentations from each participant were visibly checked for any significant errors that could have occurred during the segmentation process (no errors were noted). The correlations between left and right hemisphere basal ganglia volumes across the entire sample were strong (rs ranged from 0.70 to 0.90, ps < 0.001). Hence, for volumetric analyses, we used the average of participants’ bilateral basal ganglia ROI volumes to reduce the number of statistical tests and because no laterality was hypothesized regarding the associations between the MetS, basal ganglia structures, and depression. Lastly, total brain volume was determined for each subject by calculating the sum of grey, white, and cerebrospinal fluid using the automated segmentation toolbox in FSL version 4.0. All volume measures (i.e., basal ganglia ROIs, total brain volume) were standardized (z-score transformed) to aid interpretation. Supplemental Figure 1 illustrates the basal ganglia ROIs. Supplemental Figure 1 illustrates all ROIs.
2.4. Assessment of depressive symptoms
Depressive symptoms were measured with the 9-item Patient Health Questionnaire (PHQ-9). The questionnaire was designed to aid in screening for affective disorders in primary care settings [43]. The PHQ-9 compares favorably with structured diagnostic interviews, particularly for major depressive disorder, (MDD) in both clinical populations as well as those with coronary heart disease [44–46]. This is in part due to the fact that each item on PHQ-9 assesses the frequency of depressive symptoms as per the 9 diagnostic criteria for MDD in Diagnostic and Statistic Manual of Mental Disorder Fourth Edition (DSM-IV). The PHQ-9 also demonstrates acceptable reliability and validity as a measure of depression severity [45]. In the present study, the PHQ-9 displayed acceptable internal consistency (α = 0.64).1
Participants reported the frequency of experiencing 9 depressive symptoms during the past two weeks, ranging from “not at all” (scored 0), “several days” (scored 1), “more than half the days” (scored 2), and “nearly every day” scored 3. Thus, possible scores on the PHQ-9 were 0–27. For our sample, PHQ-9 scores ranged from 0–6, which suggests mild depressive symptoms on average (see Table 1). This is consistent with our screening criteria, which excluded subjects with reported histories or current experiences of mood related disorders. Based on a recommended and established PHQ-9 cut-off score ≥ 10, we excluded one participant (PHQ-9 = 13) in the distribution from all analyses for possible major depression [45,46] as well as being more than 3 SDs above the group mean. There was another extreme PHQ-9 score, and this score was winsorized by transforming the data point to 3 SDs from the group mean. The estimate of the mean was computed without this one outlier.
Table 1.
Descriptive characteristics of the sample
| Full sample (n = 147) | Individuals meeting NCEP MetS criteria | Individuals not meeting NCEP MetS criteria (n = 123) | |
|---|---|---|---|
| Age, yrs | 40.5 ± 0.5 | 40.8 ± 1.3 | 40.5 ± 0.6 |
| Sex, m/f | 76/71 | 12/12 | 64/59 |
| Smoking status, never/former/current | 97/28/22 | 14/4/6 | 83/24/16 |
| Education, yrs | 17.2 ± 0.3 | 16.8 ± 0.6 | 17.3 ± 0.3 |
| Systolic BP, mmHg* | 120.1 ± 0.8 | 128.3 ± 1.8 | 119.5 ± 0.9 |
| Diastolic BP, mmHg* | 73.4 ± 0.8 | 77.9 ± 2.0 | 72.5 ± 0.8 |
| BMI, kg/m2* | 27.1 ± 0.4 | 32.1 ± 0.9 | 26.1 ± 0.4 |
| Waist, in* | 35.6 ± 0.4 | 41.2 ± 0.6 | 34.5 ± 0.4 |
| Hip, in* | 42.1 ± 0.5 | 45.2 ± 0.7 | 41.5 ± 0.5 |
| Triglycerides, mg/dL b* | 74.0 (63.0) | 147.0 (142.0) | 70.0 (41.0) |
| HDL cholesterol, mg/dL* | 49.6 ± 1.4 | 37.1 ± 1.1 | 52.0 ± 1.5 |
| Glucose, mg/dL b* | 87 (12.0) | 96.0 (23.0) | 86.0 (12.0) |
| Verbal IQ | 113.8 ± 0.7 | 112.2 ± 2.0 | 114.2 ± 0.8 |
| PHQ-9, total score | 1.0 ± 0.1 | 1.3 ± 0.4 | 0.9 ± 0.1 |
| Basal ganglia gray matter volumes, mm3 | |||
| Caudate nucleus | 6950.1 ± 69.7 | 6748.1 ± 160.6 | 6989.5 ± 76.9 |
| Putamen | 9360.3 ± 85.8 | 9162.3 ± 260.9 | 9398.9 ± 89.1 |
| Nucleus accumbens | 860.0 ± 16.0 | 807.6 ± 40.41 | 870.3 ± 17.3 |
| Globus pallidus* | 3264.5 ± 28.3 | 3130.7 ± 71.1 | 3290.6 ± 30.4 |
| Total brain tissue volume, mm3 (gray, white, cerebrospinal fluid) | 1429104.0 ± 11545.0 | 1424036.1 ± 32289.1 | 1430092.6 ± 12338.1 |
Note. NCEP = National Cholesterol Education Program; BP = blood pressure; mmHg = millimeters of mercury; BMI = body mass index; HDL = high-density lipoprotein; PHQ-9 = 9-item Patient Health Questionnaire; Verbal IQ = Estimated Verbal IQ score from the North American Adult Reading Test (Blair and Spreen, 1989).
Values are mean ± standard error of the mean, unless indicated otherwise.
Median (interquartile range).
Significant group difference between presence and absence of the MetS, p < 0.05.
2.4.1. Long-term test-retest stability of PHQ-9
Test-retest assessment of the PHQ-9 was performed on a subset of participants (n = 94) from the 155 participants who completed the PHQ-9 on two occasions, separated by a ~3 year delay. These reliability analyses were performed to determine the stability of PHQ-9. We found that PHQ-9 scores at Time 1 and Time 2 were significantly correlated (r = 0.50, p < 0.01), indicating a moderately stable dimension of individual difference assessed by this depression questionnaire. Additional analyses determined the stability of the PHQ-9 using SPSS 20 to compute the intra-class correlation coefficient (ICC) with a two-factor mixed-effects model and type consistency. The PHQ-9 showed moderate consistency over the 3 years (ICC = 0.59, p < 0.01), which further suggests the long-term stability of scores on this depressive questionnaire.
2.5. Assessment of covariates
Associations between the MetS, basal ganglia volumes, and depressive symptoms may be accounted for by (or confounded with) several factors, including age, sex, race (non-white versus white), years of education, and smoking status (i.e., categorically defined as 0 = non-smoker, 1 = smoker). These factors were treated as covariates. Of note, all covariates were chosen a priori based on existing research implicating them in association with the MetS, basal ganglia morphology, or psychological wellbeing. First, the prevalence of the MetS among U.S. adults is known to vary by age, sex, and race [47]. Second, education and smoking habits have been associated with the presence of the MetS [48,49]. Third, consistent with the age-, sex-, and smoking-related differences in the MetS, basal ganglia areas are susceptible to grey matter changes that vary by sex and age [50,51] in addition to smoking relapse [52]. Finally, depressive symptoms show sex, race, and age differences [53–55].
2.6. Statistical analyses
2.6.1. Demographic and cardio-metabolic variables
We used descriptive statistics to characterize participants with and without the MetS on age, years of education, smoking status, body mass index (BMI), and MetS components. Specifically, differences in demographic and cardio-metabolic risk factors as a function of presence of the MetS were analyzed using t-tests for continuous variables and chi-square test for categorical variables (see Table 1).
2.6.2. Volumetric variables
Several regression models were used to determine the effect of the MetS on basal ganglia volumes, adjusting for age, sex, race, education, smoking status, and total brain volume as covariates. The relationships between the MetS and volumes in basal ganglia ROIs were assessed first by regressing a variable coding the presence/absence of the MetS on standardized (mean = 0; standard deviation = 1) average bilateral volumes for the nucleus accumbens, caudate nucleus, putamen, and globus pallidus in a single logistic regression model. Accordingly, results reflect unique relationships between the MetS and basal ganglia volumes in a single model, as opposed to relationships revealed in separate models for each basal ganglia ROI, which would inflate type I error. We also conducted an additional logistic regression model whereby presence/absence of the MetS was based on individuals meeting criteria for MetS (i.e., 3 or more criteria) were compared against a subset of individuals meeting no criteria for any MetS component. Next, we examined associations between basal ganglia volumes and the number of MetS criteria met across the full sample of 147 participants in a similarly structured negative binomial model—regressing the number of MetS criteria met (i.e., criteria counts) on basal ganglia volumes. Hence, for these negative binomial models, the number of MetS criteria met (ranging from 0–5) was regressed on basal ganglia brain volumes together in one model with covariate adjustment. We also regressed a modified number of MetS criteria met (ranging from 0–3+) variable on basal ganglia volume to ensure that the few individuals that met criteria for 4 or 5 components did not influence the results.
Exploratory logistic regression analyses were also performed on the individual components of the MetS (i.e., low HDL cholesterol or elevated blood pressure, plasma glucose, triglycerides, or waist circumference) by regressing the presence/absence of each MetS component on basal ganglia volumes to determine which components were most closely related to basal ganglia volumes. Similar exploratory analyses with linear regression models were also performed on continuous measures of each syndrome component. Logarithmic transformations were applied to triglyceride values to correct distributional skew, and outliers (i.e., 3SD above or below the respective means) were removed from blood glucose (n = 4) and HDL cholesterol distributions (n = 1).
An alpha of 0.05 was used for all tests of statistical significance, while an alpha within the range of 0.06–1.0 was taken as a trend. To aid interpretation and clearly convey effect sizes, positive logistic regression coefficients are presented alongside odds ratios (OR; the exponentiated regression coefficient, eB), and negative logistic regression coefficients alongside inverse odds ratios (IOR; the inverse of the exponentiated regression coefficient, 1/eB). Negative binomial results are presented alongside the exponentiated regression coefficients, and these can be interpreted as the change in number of criteria met per unit change in predictor of interest. All analyses were conducted using SPSS version 20.
2.6.3 Depression variables
Similar regression models were used to test whether depressive symptoms were associated with the MetS (i.e., presence/absence, number of criteria met) and globus pallidus volumes with exception of the chosen covariates. Hence, logistic and negative binomial regression models were used to predict the presence/absence of the MetS and number of MetS criteria met (i.e., 0–5, 0–3+) by depressive symptoms, adjusting for age, sex, race, smoking, and education.
2.6.4. Exploratory mediation
To test whether basal ganglia volumes might meditate observed associations between the MetS and depressive symptoms, we used the product-of-coefficients strategy that tests the significance of the indirect effect (i.e., the path through a proposed mediator) by bootstrapping the product of the effect of the independent variable on the mediator (path a), and the effect of the mediator on the dependent variable accounting for the effect of the independent variable (path b). However, the sampling distribution of the indirect effect that is determined through the ab product of coefficients is subject to violations of normality when the possibility of mediation is present [56]. Hence, Preacher and Hayes [57] recommend using a bootstrapping approach whereby 5000 estimates of ab are used to calculate confidence intervals (CIs) and standard errors around the indirect effect. This bootstrapped mediation analysis approach was performed with the INDIRECT macro designed for SPSS [57].
In these mediation analyses, presence of the MetS or the number of MetS criteria met served as independent variables in separate models, depressive symptoms as measured by the PHQ-9 served as the dependent variable, basal ganglia volume(s) served as mediators, and age, sex, education, smoking status, race, and total brain volume were included as confounding covariates. We computed two mediation models with the INDIRECT macro for each operational definition of the MetS under study (i.e., presence/absence, number of criteria met). To model the indirect effect for number of criteria met, we first constructed k-1 dummy variables, with k being the number of possible number of criteria met. Next, each dummy variable was entered as an independent variable while the others were entered as covariates. This provided a test of the indirect effect of each category relative to reference category (i.e., meeting zero MetS criteria) in our dummy-coding scheme.
3. Results
3.1. Prevalence of MetS
There were 24 participants who met criteria for having the MetS and 123 subjects who did not. Thus, the prevalence of the MetS in this otherwise healthy and un-medicated sample was 16.3%, which is consistent with expected rates of the MetS demonstrated in national samples and similar community samples recruited from Allegheny County, Pennsylvania [49,58,59]. Table 1 shows descriptive statistics for the participants’ demographic and other characteristics.
Among the 24 with the MetS, 18 met three criteria, 4 met four criteria, and 2 met five criteria. Further, among these individuals meeting criteria for the MetS, the following frequencies were observed across syndrome components: low HDL cholesterol (n = 23), elevated waist circumference (n = 21), elevated systolic (n = 13) and diastolic (n = 6) blood pressure, elevated triglycerides (n = 12), and elevated fasting glucose (n = 10). Among the 123 without the MetS, 51 met zero criteria, 44 met one criterion, and 28 met two criteria. The following frequencies were observed across syndrome components among those who were without the MetS: low HDL cholesterol (n = 40), elevated waist circumference (n = 25), elevated systolic (n = 15) and diastolic (n = 12) blood pressure, elevated triglycerides (n = 7), and elevated fasting glucose (n = 7).
3.2. MetS and basal ganglia volume
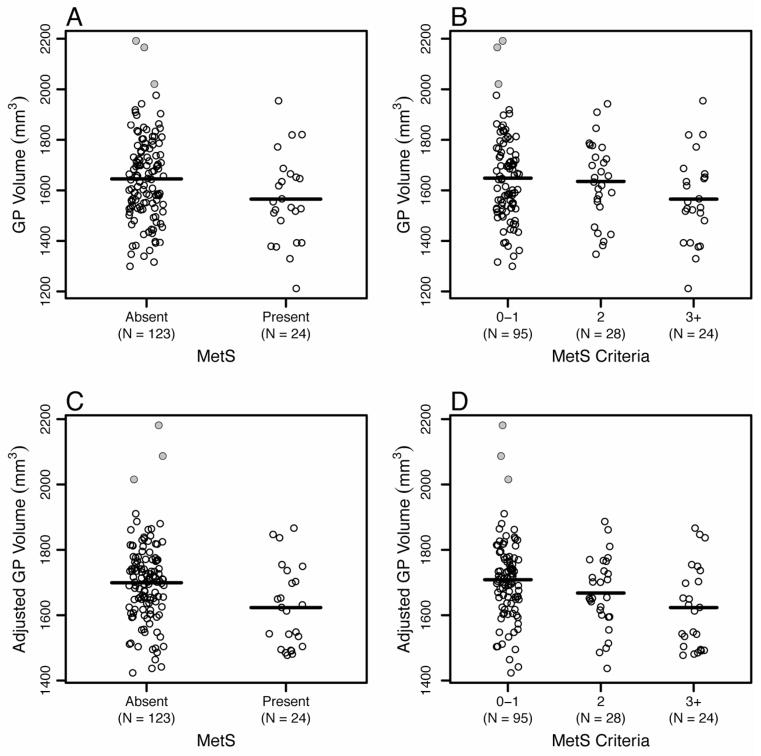
Presence of the MetS was associated with reduced grey matter volume within the basal ganglia. Hence, after accounting for demographic and health-related variables, reduced grey matter volume in the globus pallidus in particular was associated with an increased odds of having the MetS (B = −0.83, SE = 0.37, IOR = 2.30, p = 0.03). Specifically, a decrease of one standard deviation in globus pallidus volume was associated with 2.30 times increased odds of meeting criteria for the MetS. Because more than half of the individuals without the MetS met criteria for some MetS components (i.e., 72/123 met criteria for one or two components; see Section 3.1), we ran an additional logistic regression model comparing individuals with the MetS to a subset of individuals who did not meet criteria for any MetS component. The group differences were still present, such that reduced pallidal grey matter volume was specifically associated with a greater odds of having the MetS (B = −0.93, SE = 0.48, IOR = 2.54, p = 0.05). Similarly, negative binomial models revealed that only globus pallidus volume covaried inversely with the number of MetS criteria met while simultaneously accounting for all other basal ganglia ROI volumes (B = −0.27, SE = 0.11, eB = 0.76, p = 0.02), indicating that one standard deviation decrease in globus pallidus volume was associated with 76% increase in number of MetS criterion met.2 By contrast, neither presence of the MetS nor the number of MetS criteria met covaried positively or negatively with the volume of other basal ganglia ROIs; namely, the caudate, putamen, and nucleus accumbens (ps > 0.34). Figure 1 shows the associations of pallidal volume to the presence of the MetS and the number of MetS criteria met.
Figure 1.
Panels A and B plot unadjusted globus pallidus volume as a function of absence or presence of the metabolic syndrome and the number of metabolic syndrome criteria met. Panels C and D plot globus pallidus volumes that are adjusted for model covariates (age, gender, race, smoking status, and education, as well as grey matter, white matter and cerebral spinal fluid volumes). Significant group differences in globus pallidus volume were explained by individuals with the metabolic syndrome showing smaller pallidal volume. The shaded data points are the three outliers identified across both the pallidal and putamen volumes.
With respect to individual MetS components, reduced globus pallidus volume was associated with elevated waist circumference (B = −0.78, SE = 0.32, IOR = 2.18, p = 0.02) and low HDL cholesterol (B = −0.56, SE = 0.27, IOR = 1.75, p = 0.04) while there was a trend for elevated fasting glucose (B = −1.15, SE = 0.52, IOR = 3.15, p = 0.10). And although grey matter volume in the putamen did not covary with either presence of the MetS or number of MetS criteria met, reduced putamen volume was associated with elevated blood pressure (B = −0.74, SE = 0.35, IOR = 2.10, p = 0.04). Additional analyses also examined the association between continuous cardio-metabolic parameters defining the syndrome and basal ganglia volume. After adjusting for demographic and health-related factors, continuous measures of waist circumference (B = −0.12, t = −1.88, p = 0.06) and HDL cholesterol (B = 0.12, t = 1.82, p = 0.07) showed marginal associations with pallidal volume.
3.3. MetS and depressive symptoms
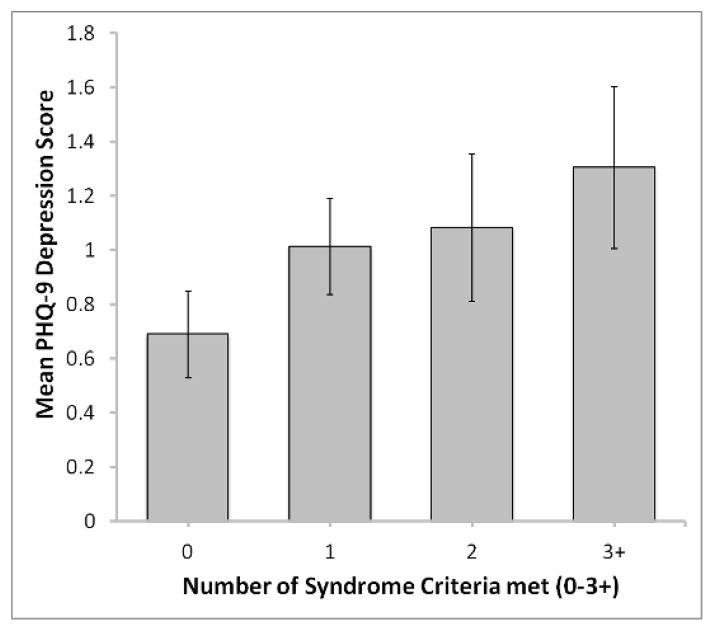
Depressive symptoms were positively associated with the number of MetS criteria met. Hence, after adjusting for age, sex, race, smoking, and education, greater severity in depressive symptoms was associated with meeting more MetS criteria (B = 0.10, SE = 0.05, eB = 1.11, p = 0.03). In an additional model wherein number of criteria met was defined as 0–3+, the dose-response relationship between number of criteria met and depressive symptoms trended toward significance (B = 0.09; SE = 0.05, eB = 1.09; p = 0.07). Contrary to prior research [60], however, we did not observe a significant association between presence/absence of the MetS and depressive symptoms, even in unadjusted analyses (ps ≥ 0.16).
3.4. Basal ganglia volume and depressive symptoms
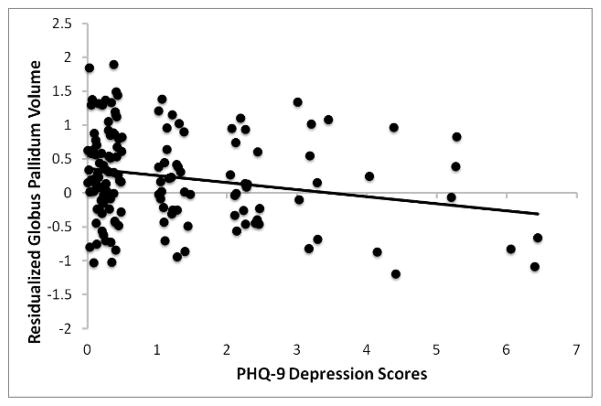
Reduced globus pallidus volume covaried with higher depressive symptoms (B = −0.16, t = −2.52, p = 0.01), after adjusting for total brain tissue volume (Figure 2). This association between depressive symptoms and pallidal volume persisted after additional adjustment for demographic and health-related covariates (p = 0.04).
Figure 2.
Scatterplot of the association between the volume of the globus pallidus (adjusted for total brain tissue volume) and PHQ-9 depression scores.
3.5. Mediation testing
The significant pattern of results between (i) number of MetS criteria met and globus pallidus volume and (ii) globus pallidus volume and depressive symptoms indicates that variation in pallidal grey matter volume may represent a candidate mediator linking number of MetS criteria met and depressive symptoms. Despite the absence of an total effect between presence of the MetS and depressive symptoms, we did test the hypothesis of a possible indirect effect through globus pallidus volume in accordance with prior recommendations regarding mediation testing [61]. We found a significant indirect effect with respect to presence/absence of the MetS and depressive symptoms through globus pallidus volume (ab indirect effect = 0.130; SE = 0.094; 95% CI = 0.0041, 0.398; p < 0.05). Similarly, we found that association of number of syndrome criteria met on depressive symptoms was mediated by globus pallidus volume (ab indirect effect = 0.147, SE = 0.108; CI = 0.0090, 0.4862 p < 0.05). This indirect effect was specific to meeting three syndrome criteria. Based on the direction of the paths, this mediation effect indicates that individuals with the MetS and/or meeting criteria for three MetS components may exhibit increased depressive symptoms as statistically mediated by decreases in pallidal regions of the basal ganglia.
3.6. Ancillary analyses
We identified 3 potential outlier cases (see Figure 1) exhibiting pallidal and putamen grey matter volumes that were 3SD above the mean of their respective brain volume distributions. Ancillary analyses were conducted to determine whether removal of these outliers would influence our observed results regarding the significant MetS-pallidal or pallidal-depressive symptom associations. Removal of these outliers did not alter any of the respective associations between the MetS, globus pallidus volume, and depressive symptoms; grey matter volume in the putamen was not associated with MetS or depressive symptoms after outlier adjustment. Hence, our reported findings persisted after accounting for the influence of these cases.
4. Discussion
The first novel finding of this study was that individuals with the MetS showed reduced grey matter volume in the globus pallidus, independent of demographic and health-related variables. Second, individuals meeting more MetS criteria showed a graded reduction in globus pallidus volume—suggesting a dose-response association, rather than a threshold effect of meeting binary MetS diagnostic criteria (i.e., presence vs. absence). However, neither the presence of the MetS nor the number of MetS criteria met associated significantly with grey matter volume in other basal ganglia regions. Third, as consistent with prior work [62], we found that individuals meeting more MetS criteria reported more depressive symptoms. Fourth, reduced globus pallidus volume mediated the association between meeting more MetS criteria and depressive symptoms. Finally, we found an indirect mediation effect of the MetS (i.e., presence, absence) on depressive symptoms through reduced globus pallidus volume, even though we observed no statistically significant total effect of the MetS on depressive symptoms. Taken together, there appears to be regional specificity within the basal ganglia—namely in the globus pallidus—with respect to volumetric reductions that are associated with the MetS and meeting more MetS criteria among otherwise healthy individuals. It is possible that these volumetric changes in the globus pallidus may correspond to a neural correlate that is common to the MetS and depressive symptomatology. However, the causal directions of association between the MetS, depressive symptomatology, and globus pallidus volume are unclear because of the correlational design of the present study.
Despite this design limitation, our novel finding of an inverse association between the MetS and pallidal volume appears consistent with prior animal and human evidence linking functional alterations within the basal ganglia to impairments in metabolism and energy balance [63]. This finding also appears consistent with those showing that individual components and correlates of the MetS, including adiposity [18], impaired fasting glucose [18], and insulin resistance [19], are associated with structural changes within the pallidal region of the basal ganglia. This finding also adds to an emerging literature indicating the MetS as a whole, as opposed to its individual components, relates to adverse brain changes and associated impairments in cognitive functions [17]. We note, however, that although we did not find adiposity or glucose associations with the volumes of other basal ganglia regions (e.g., putamen; [20]), other studies have reported similar null associations [64–66]. By contrast, our findings did replicate previous work [18], insofar as we found that both high fasting glucose and central adiposity (elevated waist circumference) covaried with reduced globus pallidus volume. Thus, in light of our findings and prior work, it may be that the link between the MetS and globus pallidus volume more closely reflects the associations of adiposity and glucose dysregulation with this region, as compared with other basal ganglia regions and other features and correlates of the MetS [67]. This interpretation is consistent with cumulative evidence that adiposity and glucose dysregulation are likely to be key etiological factors in the development of the MetS [39]. However, we cannot rule out the possibility that the MetS association with reduced globus pallidus volume is mediated by factors other than glucose dysregulation or central adiposity (e.g., systemic inflammatory mechanisms). Although, it is noteworthy that we found individuals meeting more MetS criteria exhibited a graded reduction in the grey matter volume of the globus pallidus, which suggests that (i) the other syndrome components are also linked to structural changes in this region, and (ii) the aggregate clustering of multiple MetS components exhibited a dose-response or graded association with the grey matter volume of this region, above-and-beyond just the presence of adiposity, glucose elevations, or other isolated MetS components per se. The latter observation appears consistent with work showing that the clustering of cardio-metabolic risk factors accounts for unique variation in MRI indicators of gross brain atrophy [68,69], and it adds to this work by showing an apparently specific neural correlate of the MetS (reduced globus pallidus volume) that is still observed after accounting for gross or total brain tissue volume.
The potential functional implications of reduced grey matter volume in the globus pallidus may relate to the role of this region in regulating physiologic processes relevant to the development of the MetS [13]. For instance, neurochemical and animal lesion studies suggest an influence of the globus pallidus on glucose metabolism [70], with pallidal lesions inducing hyperglycemia in rats [71]. More recent work has also shown that lesions to ventral regions of the pallidum lead to elevated glucose levels coupled with impaired glucose control following a regulatory challenge [72]. Critically, such pallidal lesions produce hyperglycemic levels that are enduring and comparable to pre-diabetic states in humans, states that are engendered by the MetS. Additional human case study evidence has also shown that a patient with lesions to the globus pallidus gained 20-lbs [29]. Although speculative, it may be that the grey matter changes in the globus pallidus linked to the MetS and, more specifically, impaired fasting glucose and adiposity might reflect alterations in energy balance functions served by the globus pallidus [73].
We also found that reduced pallidal volume was associated with more depressive symptomatology, which agrees with prior work [14,28]. Existing models of depression indicate that the globus pallidus and other basal ganglia regions are components of prefrontal and limbic circuits important for goal-directed behaviors and executive cognitive control processes that support emotion and mood regulation [74]. Interestingly, altered pallidal function has been associated with inhibited thalamo-cortical stimulation of prefrontal areas that mediate the regulation of negative affect [74,75]. Thus, the anatomical connections of the globus pallidus support the possibility that structural or volumetric changes in this region may relate to the pathophysiology of depression or depression risk by affecting emotion regulation functions supported by prefrontal and limbic circuits [75]. And here, our results appear to extend previous clinical findings by demonstrating that depressive-related variation in the grey matter volume of the globus pallidus might be best conceptualized along on a continuum that extends to otherwise healthy individuals. In view of such a conceptualization, it may be that volumetric and other features of basal ganglia morphology could reflect a so-called endophenotype associated with vulnerability to or risk for depression. In support of this possibility, two recent studies have proposed that depression vulnerability among otherwise healthy individuals might relate to volumetric reductions in the basal ganglia [76,77]. However, further research bearing on this issue is needed to determine whether the volumetric or other morphological changes in the pallidum emerge before or after the onset of depressive symptoms—especially in the context of comorbid metabolic dysregulation and MetS development [22].
In this context, it is noteworthy that in the present study individuals exhibiting more depressive symptoms also met more MetS criteria, which agrees with prior work on otherwise healthy adults [62,78,79]. Given that the MetS involves diverse physiological changes (e.g., systemic inflammatory states) that are associated with and implicated in the symptomatic expression of depression, it appears reasonable to expect that depressive symptoms would be more prevalent among individuals meeting more MetS criteria [80]. Our mediation findings extend prior findings linking peripheral physiological changes associated with the MetS and depressive symptomatology by identifying a potential mediating factor: reduced globus pallidus volume. As noted previously, this observation is consistent prior findings and speculations that the MetS and depressive symptoms may share common pathophysiological features [22], including alterations in central neurotransmitter systems that covary with metabolic dysregulation [58,59] and risk for depressive symptoms [81], as well as volume reductions in basal ganglia regions [74,82]. Again, however, the causal directions of associations and temporal ordering of depressive symptoms, volumetric changes, and depressive symptoms need to be tested in future longitudinal research.
In closing, there are several remaining study limitations and specific future directions that should be acknowledged. First, our present study does not permit inferences regarding the mechanisms that link the MetS to globus pallidus volume. Second, although our findings were specific to the globus pallidus, previous studies have implicated volumetric and white matter changes in medial temporal and other regions as a possible neural correlates of the MetS among adolescents [83]. Thus, future studies on adults should employ multimodal and voxel-wise methods that permit an examination of such changes in regions outside of the basal ganglia. This suggestion is reinforced by findings showing that individual components of the MetS covary with grey matter changes in the regions of the frontal and temporal lobes [18–20,64,84,85]. Finally, an unmeasured third-factor explanation, such as a shared genetic or heritable factor, may have accounted for the observed co-expression of the MetS, globus pallidus volume reductions, and depressive symptoms, which may not be functionally or causally related. Thus, longitudinal study designs that incorporate imaging assessments, as well as more comprehensive measures of mood-related symptoms, energy metabolism, and health behaviors (e.g., food intake and physical activity) are needed to extend the present findings to better understand the mechanisms and functional implications of the associations between the MetS, depressive symptoms, and grey matter volume changes in the basal ganglia.
Supplementary Material
Figure 3.
The association between number of metabolic syndrome (MetS) components and PHQ-9 depression score. Subjects with three, four, and five MetS components were combined.
Highlights.
Pallidal volume associated with the metabolic syndrome and depressive symptoms
The metabolic syndrome associated with depressive symptoms
Pallidal volume mediated this association
Acknowledgments
This work was funded by National Institutes of Health Grants HL089850 and HL101421 to Peter J. Gianaros. The authors thank Sara Snyder for her assistance in data collection and Lei K. Sheu for her assistance in data reduction.
Footnotes
The alpha coefficient of the PHQ-9 is important to consider when interpreting our findings among an otherwise healthy sample. Hence, the magnitude of this scale’s internal consistency can be expected to influence the magnitude of any observed covariation between depressive symptoms and the MetS or basal ganglia volumes. However, we note that significant associations were still observed here, despite the moderate value of this alpha coefficient of the PHQ-9.
In secondary analyses, similar models indicated that presence of MetS was independently related to left (B = −0.66, SE = 0.31, IOR = 1.94, p = 0.03) and right (B = −0.71, SE = 0.32, IOR = 2.04, p = 0.02) globus pallidus volume after adjusting for demographic and health-related factors, and total brain tissue volume. Further, number of MetS criteria met was also associated with left (B = −0.18, SE = 0.10, eB = 0.84, p = 0.06) and right (B = −0.23, SE = 0.10, eB = 0.79, p = 0.02) pallidal volume, although marginally with left regions. Again, the correlation between right and left globus pallidus volume was strong (r = 0.69, p italic> 0.01) which validated the use of average bilateral globus pallidus volume. Nonetheless, laterality effects did not appear to drive the observed volumetric associations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bataille V, Perret B, Dallongeville J, Arveiler D, Yarnell J, Ducimetiere P, et al. Metabolic syndrome and coronary heart disease risk in a population-based study of middle-aged men from France and Northern Ireland. A nested case-control study from the PRIME cohort. Diabetes Metab. 2006;32:475–9. doi: 10.1016/s1262-3636(07)70306-3. [DOI] [PubMed] [Google Scholar]
- 2.Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MV, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39:30–5. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Ma D, Liu M, Liu H, Feng S, Hao Z, et al. Association between metabolic syndrome and risk of stroke: a meta-analysis of cohort studies. Cerebrovasc Dis. 2008;25:539–47. doi: 10.1159/000131672. [DOI] [PubMed] [Google Scholar]
- 7.Sundström J, Risérus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ. 2006;332:878–82. doi: 10.1136/bmj.38766.624097.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao Z, Wu B, Wang D, Ming L. Association between metabolic syndrome and cognitive decline: a systematic review of prospective population-based studies. Acta Neuropsychiatrica. 2011;23:69–74. doi: 10.1111/j.1601-5215.2011.00527.x. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre RS, Rasgon NL, Kemp DE, Nguyen HT, Law CW, Taylor VH, et al. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep. 2009;9:51–9. doi: 10.1007/s11892-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 10.Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371:1927–35. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Tilve D, Stern JE, Tschop M. The brain and the metabolic syndrome: not a wireless connection. Endocrinology. 2006;147:1136–9. doi: 10.1210/en.2005-1586. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud H-R. Mind versus metabolism in the control of food intake and energy balance. Physiology & Behavior. 2004;81:781–93. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 14.Ring HA, Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatr. 2002;72:12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreier F, Kalsbeek A, Ruiter M, Yilmaz A, Romijn JA, Sauerwein HP, et al. Central nervous determination of food storage--a daily switch from conservation to expenditure: implications for the metabolic syndrome. Eur J Pharmacol. 2003;480:51–65. doi: 10.1016/j.ejphar.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 16.Van Dijk G, Buwalda B. Neurobiology of the metabolic syndrome: an allostatic perspective. Eur J Pharmacol. 2008;585:137–46. doi: 10.1016/j.ejphar.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 17.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–7. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–64. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care. 2013;36:443–9. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–25. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 21.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre RS, Kenna HA, Nguyen HT, Law CWY, Sultan F, Woldeyohannes HO, et al. Brain volume abnormalities and neurocognitive deficits in diabetes mellitus: points of pathophysiological commonality with mood disorders? Adv Ther. 2010;27:63–80. doi: 10.1007/s12325-010-0011-z. [DOI] [PubMed] [Google Scholar]
- 23.Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, et al. Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci. 1999;11:71–8. doi: 10.1176/jnp.11.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Bielau H, Trübner K, Krell D, Agelink MW, Bernstein H-G, Stauch R, et al. Volume deficits of subcortical nuclei in mood disorders A postmortem study. Eur Arch Psychiatry Clin Neurosci. 2005;255:401–12. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- 25.Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. Journal of Affective Disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Bonelli RM, Kapfhammer H-P, Pillay SS, Yurgelun-Todd DA. Basal ganglia volumetric studies in affective disorder: what did we learn in the last 15 years? J Neural Transm. 2006;113:255–68. doi: 10.1007/s00702-005-0372-7. [DOI] [PubMed] [Google Scholar]
- 29.Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, et al. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–8. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- 30.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 31.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan JP, Sheu LK, Critchley HD, Gianaros PJ. A Neural Circuitry Linking Insulin Resistance to Depressed Mood. Psychosomatic Medicine. 2012 doi: 10.1097/PSY.0b013e31824d0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 34.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 35.Pinter JK, Hayashi JA, Watson JA. Enzymic assay of glycerol, dihydroxyacetone, and glyceraldehyde. Arch Biochem Biophys. 1967;121:404–14. doi: 10.1016/0003-9861(67)90094-x. [DOI] [PubMed] [Google Scholar]
- 36.Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biochem. 1974;12:226. [PubMed] [Google Scholar]
- 37.Trinder P. Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Annals of Clinical Biochemistry. 1969;6:2. [Google Scholar]
- 38.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 39.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 40.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 41.Patenaude B, Smith SM, Kennedy D, Jenkinson M. FIRST: FMRIB’s integrated registration and segmentation tool. 2007 [Google Scholar]
- 42.Patenaude B, Smith SM, Kennedy D, Jenkinson M. Bayesian shape and appearance models. FMRIB Centre, University of Oxford; Oxford: 2007. [Google Scholar]
- 43.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 44.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study) Am J Cardiol. 2005;96:1076–81. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Löwe B, Spitzer RL, Gräfe K, Kroenke K, Quenter A, Zipfel S, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians’ diagnoses. J Affect Disord. 2004;78:131–40. doi: 10.1016/s0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- 47.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009:1–7. [PubMed] [Google Scholar]
- 48.Kim BJ, Kim BS, Sung KC, Kang JH, Lee MH, Park JR. Association of smoking status, weight change, and incident metabolic syndrome in men: a 3-year follow-up study. Diabetes Care. 2009;32:1314–6. doi: 10.2337/dc09-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manuck SB, Phillips JE, Gianaros PJ, Flory JD, Muldoon MF. Subjective socioeconomic status and presence of the metabolic syndrome in midlife community volunteers. Psychosom Med. 2010;72:35–45. doi: 10.1097/PSY.0b013e3181c484dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: a prospective MR imaging study. AJNR Am J Neuroradiol. 1998;19:1501–7. [PMC free article] [PubMed] [Google Scholar]
- 51.Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol. 2003;24:1849–56. [PMC free article] [PubMed] [Google Scholar]
- 52.Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology (Berl) 2010;210:577–83. doi: 10.1007/s00213-010-1862-3. [DOI] [PubMed] [Google Scholar]
- 53.Mirowsky J, Ross CE. Age and depression. J Health Soc Behav. 1992;33:187–205. discussion 206–212. [PubMed] [Google Scholar]
- 54.Nolen-Hoeksema S. Sex differences in depression. Stanford, Calif: Stanford University Press; 1990. [Google Scholar]
- 55.Riolo SA, Nguyen TA, Greden JF, King CA. Prevalence of depression by race/ethnicity: findings from the National Health and Nutrition Examination Survey III. Am J Public Health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–45. [PubMed] [Google Scholar]
- 57.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 58.Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. 2004;89:266–71. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- 59.Muldoon MF, Mackey RH, Korytkowski MT, Flory JD, Pollock BG, Manuck SB. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. J Clin Endocrinol Metab. 2006;91:718–21. doi: 10.1210/jc.2005-1654. [DOI] [PubMed] [Google Scholar]
- 60.Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Communication Monographs. 2009;76:408–20. [Google Scholar]
- 62.Muhtz C, Zyriax B-C, Klähn T, Windler E, Otte C. Depressive symptoms and metabolic risk: effects of cortisol and gender. Psychoneuroendocrinology. 2009;34:1004–11. doi: 10.1016/j.psyneuen.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Rubi B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let’s seek the balance. Endocrinology. 2010;151:5570–81. doi: 10.1210/en.2010-0745. [DOI] [PubMed] [Google Scholar]
- 64.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–64. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Widya RL, de Roos A, Trompet S, de Craen AJ, Westendorp RG, Smit JW, et al. Increased amygdalar and hippocampal volumes in elderly obese individuals with or at risk of cardiovascular disease. Am J Clin Nutr. 2011;93:1190–5. doi: 10.3945/ajcn.110.006304. [DOI] [PubMed] [Google Scholar]
- 66.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 2012;36:656–64. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz MW, Porte D. Diabetes, Obesity, and the Brain. Science. 2005;307:375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 68.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–11. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 69.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63:1591–9. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 71.Hahn Z, Karadi Z, Lenard L. Sex-Dependent Increase of Blood-Glucose Concentration after Bilateral Pallidal Lesion in the Rat. Acta Physiol Hung. 1988;72:99–102. [PubMed] [Google Scholar]
- 72.Egyed R, Karadi Z. Pathological glucose tolerance after kainate microlesions of the globus pallidus. Neurosci Res Commun. 2000;27:29–44. [Google Scholar]
- 73.Levin BE. Metabolic sensing neurons and the control of energy homeostasis. Physiology & Behavior. 2006;89:486–9. doi: 10.1016/j.physbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Bennett MR. The prefrontal–limbic network in depression: Modulation by hypothalamus, basal ganglia and midbrain. Progress in Neurobiology. 2011;93:468–87. doi: 10.1016/j.pneurobio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Lauterbach EC, Jackson JG, Wilson AN, Dever GE, Kirsh AD. Major depression after left posterior globus pallidus lesions. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:9–16. [PubMed] [Google Scholar]
- 76.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–37. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12:703, 767–75. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 78.Anstey KJ, Burns R, Butterworth P, Windsor TD, Christensen H, Sachdev P. Cardiovascular risk factors and life events as antecedents of depressive symptoms in middle and early-old age: PATH Through Life Study. Psychosom Med. 2009;71:937–43. doi: 10.1097/PSY.0b013e3181beab60. [DOI] [PubMed] [Google Scholar]
- 79.Panagiotakos DB, Kinlaw M, Papaerakleous N, Papoutsou S, Toutouzas P, Polychronopoulos E. Depressive symptomatology and the prevalence of cardiovascular risk factors among older men and women from Cyprus; the MEDIS (Mediterranean Islands Elderly) epidemiological study. J Clin Nurs. 2008;17:688–95. doi: 10.1111/j.1365-2702.2007.02056.x. [DOI] [PubMed] [Google Scholar]
- 80.McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CWY, Miranda A, et al. Should Depressive Syndromes Be Reclassified as “Metabolic Syndrome Type II”? Ann Clin Psychiatry. 2007;19:257–64. doi: 10.1080/10401230701653377. [DOI] [PubMed] [Google Scholar]
- 81.Meltzer HY. Role of Serotonin in Depressiona. Annals of the New York Academy of Sciences. 1990;600:486–99. doi: 10.1111/j.1749-6632.1990.tb16904.x. [DOI] [PubMed] [Google Scholar]
- 82.Vang FJ, Ryding E, Träskman-Bendz L, van Westen D, Lindström MB. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res. 2010;183:177–9. doi: 10.1016/j.pscychresns.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 83.Yau PL, Castro MG, Tagani A, Tsui WH, Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130:e856–864. doi: 10.1542/peds.2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jennings JR, Mendelson DN, Muldoon MF, Ryan CM, Gianaros PJ, Raz N, et al. Regional grey matter shrinks in hypertensive individuals despite successful lowering of blood pressure. J Hum Hypertens. 2011 doi: 10.1038/jhh.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ward MA, Bendlin BB, McLaren DG, Hess TM, Gallagher CL, Kastman EK, et al. Low HDL Cholesterol is Associated with Lower Gray Matter Volume in Cognitively Healthy Adults. Front Aging Neurosci. 2010:2. doi: 10.3389/fnagi.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.