Abstract
Over 20 distinct cerebral cortical areas contain spatial map representations of the visual field. These retinotopic, or visuotopic, cortical areas occur not only in the occipital lobe but also in the parietal, temporal, and frontal lobes. The cognitive influences of visuospatial attention operate via these cortical maps and can support selection of multiple objects at the same time. In early visual cortical areas, spatial attention enhances responses of selected items and diminishes the responses to distracting items. In higher order cortex, the maps support a spatial indexing role, keeping track of the items to be attended. These maps also support visual short-term memory (VSTM) representations. In each hemisphere, all the known maps respond selectively to stimuli presented within the contralateral visual field. However, a hemispheric asymmetry emerges when the attentional or VSTM demands of a task become significant. In the parietal lobe, the right hemisphere visuotopic maps switch from coding only contralateral visual targets to coding memory and attention targets across the entire visual field. This emergent asymmetry has important implications for understanding hemispatial neglect syndrome, and supports a dynamic network form of the representational model of neglect.
Introduction
Visual perception often feels effortless; however, in many complex situations, there is far more information reaching our eyes than our cognitive systems can act on at one time. Although the retina performs massively parallel processing, visual cognition operates on no more than a few items at once. Given these limitations, what we do or do not perceive is largely determined by attentional mechanisms that select information for enhanced cognitive processing. Spatial representations are a fundamental aspect of information coding within the visual system and are available at the earliest stage of visual processing. This retinotopic structure is replicated, though with some representational biases, in cortical areas that process visual information in each of the four lobes of the cerebral cortex.1–17 The fact that spatial representations occur pervasively throughout the visual system, even within higher order cortical regions, points to the fundamental role of spatial coding within visual cognition. Spatial attention, a fundamental form of visual attentional selection, as well as other forms of selection (e.g., object-based and feature-based) are reflected within the spatial maps of visual cortex.1–19 Similarly, spatial maps of the parietal and frontal lobes are recruited during visual short-term memory (VSTM), which largely operates on object representations.20,21 This article summarizes functional Magnetic resonance imaging (MRI) based research explorations of the mechanisms of spatial attention in human cerebral cortex, focusing on findings from the occipital and parietal lobes.
James,22–24 commenting on experiments by von Helmholtz, proposed that visual attention selects a single object or region of visual space while largely neglecting the rest of the visual field. This idea is at the core of the notion of an ‘attentional spotlight’. This spotlight model was further refined by Eriksen and St James25–28 as a ‘zoom lens’ mechanism that selects one contiguous, convex spatial window. It has successfully addressed a wealth of psychophysical data; however, more recent work (e.g., Refs 1–3 and 29–33) indicates that visual attention may simultaneously select a small number of objects at once, while ignoring intervening regions. Visual cortical maps play a fundamental role in the coding of the attentional spotlight and have the capacity to support simultaneous processing of multiple spotlights. In higher order cortical areas, spatial maps are thought to play a central role in guiding attention and visual cognition. The visual system needs to keep track of where objects of interest are located1–5,8,10,12–14,17,29,34,35 and portions of the dorsal attention network appear to support this ‘spatial indexing’ role. VSTM briefly maintains a limited sampling from the visual world. Activity in the intraparietal sulcus (IPS) tightly correlates with the number of spatially distinct items stored in VSTM.36–40
Visual Cortical Spatial Maps in Occipital Lobe
To investigate the mechanisms of visual attention it is first important to review the organization of spatial maps in human visual cortex. The cortical representations of the visual field were first studied over 100 years ago by Inouye, an ophthalmologist working in the Russo-Japanese War.20,41,42 He observed a correlation between the location of shrapnel damage to the back of the skull and the extent and location of visual field loss experienced by the injured soldiers. From these observations, he correctly inferred that the occipital lobe contains a map-like representation of the visual field that is roughly rotated 180° from the orientation of the visual field (see Figure 1). These observations have been confirmed many times over the last century in human (e.g., Refs 22 and 24) and in nonhuman primates (e.g., Refs 25, 27, and 28). This cortical map is distorted in a way that reflects the density of photoreceptors in the retina; the fovea or center of the visual field has a large amount of cortex dedicated to it, while relatively smaller amounts of cortex are dedicated to the peripheral visual field.
Figure 1.
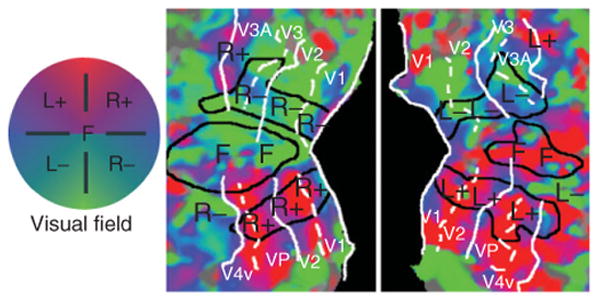
Retinotopic mapping of polar angle representations in early visual cortex. Left and right cortical hemispheric surfaces were reconstructed and inflated by computer; the occipital lobe representations were cut off and flattened to create these occipital patches. The color code reflects the preferred polar angle representations of each voxel obtained via functional mapping of retinotopy, using flashing checkerboard stimuli that slowly and periodically sweep through polar angles. (Reprinted with permission from Ref 31. Copyright 2004 Elsevier)
With the advent of functional MRI (fMRI), noninvasive methods using phase-encoded stimuli were developed for rapidly and accurately mapping visual field representations within human occipital cortex.1–3 The standard retinotopy method indexes the visual field using polar coordinates, mapping the polar angle and eccentricity dimensions using flickering checkerboard stimuli that slowly cycle across each spatial dimension in separate runs. The checkerboard stimuli induce traveling waves of activity across visual cortex. Phase-encoded analysis first identifies voxels that are significantly modulated at the temporal frequency of the stimulus and then uses the phase of the peak traveling wave signal to infer each voxel's preferred visual field location. The resulting phase information is typically displayed on inflated and flattened cortical surface representations for the occipital lobes (see Figure 1). In order for a region to qualify as a visuotopic map, it must contain voxels that are significantly modulated at the frequency of the periodic stimulus and the preferred phase of these voxels must change smoothly across the cortical area. When the gradient of preferred phases across the cortex reverses direction, this indicates a boundary between neighboring visuotopic areas. For instance, the preferred polar angle of voxels in the calcarine sulcus changes from the horizontal meridian for voxels in the fundus toward the vertical meridian as the bank of the sulcus curves into the lingual gyrus, and then the polar angle representation abruptly reverses direction. This direction reverse marks the boundary between areas V1 and V2. These fMRI methods have revealed no fewer than 20 distinct visual field maps tiling the occipital lobe and extending into portions of parietal and temporal cortices.1–5,8,10,12–14,17,34,35 In early visual cortex, areas V1, V2, and V3/VP, the representation of the fovea or center of the visual field converges at the occipital pole, while more peripheral representations lie anterior to the occipital pole. The dorsal portions of V1, V2, and V3 lie above the fundus of the calcarine sulcus and each represents the lower contralateral quadrant of the visual field, whereas the ventral regions lying below the calcarine fundus represent the upper contralateral quadrant. Anterior to V3, visual areas contain hemifield representations and their boundaries occur at vertical meridian representations. Area V3A lies anterior to dorsal V3, whereas hV4 lies anterior to ventral V3 (or VP).
These fMRI retinotopic mapping methods are an important initial step in the analysis of spatial attentional manipulations in early visual cortex. These maps can be obtained for each hemisphere of each individual subject, permitting researchers to identify the visual cortical representations of each of multiple visual stimuli placed in the visual field. These functionally defined representations can then be used as regions of interest (ROIs) to study the cortical effects of directing attention toward or away from a particular stimulus.
Attentional Modulation in V1 and Other Early Visual Cortical Areas
Our fMRI attention experiments began with the question of how visual cortical processing of a moving stimulus is influenced by attention.36 These experiments depended critically on the ability to distinguish the visual cortical representations of different parts of the visual field. The cortical representation of the center of the visual field (or fovea) occupies the center of the flattened occipital cortex patches and more peripheral eccentricities are represented as horizontal bands above and below the central region (see Figure 2(b) and (c)). The stimulus display was configured to exploit the eccentricity bias of the cortical map. A two-part stimulus (see Figure 2(a)) consisted of an annular region containing a rotating grating pattern and a central disk in which letters were displayed in a rapid serial visual presentation (RSVP) format. In this annulus-disk configuration, the RSVP letters would drive the central eccentricity band, whereas the motion annulus would drive the horizontal bands above and below the central region.
Figure 2.
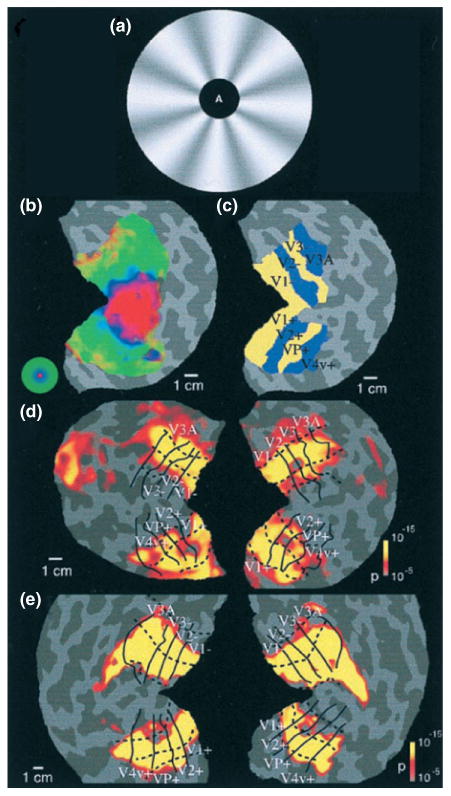
Attentional modulation of striate and extrastriate visual cortex. (a) Visual stimuli were composed of an annulus with rotating radial wedge patterns and a central target that was either a fixation point or single letters in a rapid serial visual presentation stream. (b) Functional mapping of visual eccentricity, with the foveal representation in the center of the flattened patch. (c) Functionally defined visual cortical areas. (d and e) Patterns of statistically significant increased activation for attend extrafoveal motion versus attend foveal letters for both hemispheres for two subjects, extending across all labeled retinotopic areas. (Reprinted with permission from Ref 36. Copyright 1999 National Academy of Sciences, USA)
Subjects held central fixation during this two-part display and directed their attention to one portion or the other. Eye position measurements performed in the scanner confirmed that subjects could hold central fixation during these experiments. Subjects performed alternating blocks of trials in which they either judged the rotation direction of the motion annulus or identified five consecutive letters appearing in the central RSVP stream. The central task was designed to be highly demanding in order to strongly draw attention away from the motion annulus. Comparison of fMRI activation between the two conditions revealed spatially specific attentional modulations across all early visual cortical areas, including V1, V2, V3, VP, V3A, and V4v. When attention was directed to the motion annulus, fMRI activation increased in the isoeccentricity bands corresponding to the cortical representation of the annulus (see Figure 2(d) and (e)). When attention was directed to the fovea, fMRI activation increased in the cortical representation of the fovea. To investigate whether the observed attentional modulations reflected an enhancement of activity at the attended locations or suppression of activity at the unattended locations or both, each attentional state (attend center and attend periphery) was paired with a condition in which the stimulus was passively viewed. Attentional modulations were observed in both spatial regions (in antiphase relationship), implying that spatial attention acts in a ‘push–pull’ manner, increasing responses at the cortical representations of the attended locations and diminishing responses at the nonattended cortical representations.
Our findings36 were supported by nearly simultaneous reports of V1 attention from other fMRI laboratories using widely different stimuli and task paradigms.41–45 This wave of studies resulted in the quick acceptance of the V1 attention finding.46 Significantly, Kastner and coworkers47 have shown robust spatial attention modulations in the lateral geniculate nucleus (LGN) of the thalamus, which provides the primary source of ascending visual input to V1 and is reciprocally connected with V1.
Multifocal Visual Attention
In real-world situations, such as driving, team sports, and videogame playing, we often have a need to attend to multiple objects while ignoring irrelevant distractors. There has been a long-standing debate as to whether spatial attention could be split to simultaneously attend to multiple distinct objects or regions of space. James23 famously defined attention as ‘the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneous objects or trains of thought’. This unifocal view of spatial attention dominated psychology until the turn of the millennium. However, numerous experiments, spanning behavioral, fMRI, EEG, and primate electrophysiology methods, demonstrate that multifocal attention exists and offers behavioral advantages at each location.29,31,32,48–52 Historically, three mechanisms have been proposed to account for attention to multiple objects: (1) The ‘multiple spotlight’ model suggests that spatial attention may be split to simultaneously attend to a small number of objects. (2) The ‘zoom lens’ model26,53,54 suggests that unifocal attention spreads out to select multiple objects. According to this model, attentional resources are diluted as the overall spatial extent of the zoom lens increases. Any distractors lying between targets of interest must be selected and thus additional processing would be required to ignore these selected distractors. (3) The ‘rapidly moving spotlight’ model55,56 suggests that a unifocal attentional spotlight rapidly moves between multiple locations. Variants of this model suggest either: (a) that the spotlight remains on while switching, thus briefly selecting regions and objects that lie between targets (much like the zoom lens model predicts) or (b) that the spotlight rapidly turns off when it moves and turns back on when it reaches a target of interest, requiring that the attentional spotlight switch between targets multiple times per second. The speed limit of the attentional switching is a major point of debate in this literature. When subjects intentionally move their attentional spotlight, it takes a minimum of 200–250 milliseconds to shift from one target to another,57–59 thus implying that a full cycle of attention to two distinct objects should take 400 milliseconds or longer.
We performed a series of experiments that demonstrate that spatial attention can be split into multiple distinct ‘spotlights’ of activation in human occipital cortical regions.31, 32 Because fMRI studies are limited by their temporal resolution and spatial attention is capable of moving as fast as every 200–500 milliseconds (e.g., Refs 57–59), fMRI methods are not ideal for ruling out rapid shifts of spatial attention. Thus, we employed a psychophysical task that excluded the possibility that spatial attention was rapidly switching between locations of interest.
Subjects were required to compare the identity of targets simultaneously displayed in two separated locations, a condition that we label as ‘Attend2′ (see Figure 3(a) and (b)). We used RSVP streams in which each digit was immediately masked by the following digit and there were no other overt cues to the appearance of a target. Subjects were required to report whether the target digits were the same or different. This task can only be performed at above chance levels if both targets are identified. These two RSVP streams were embedded in a display consisting of five RSVP streams, a central one surrounded by an equidistant peripheral stream, in each visual field quadrant (see Figure 3(a)). The central RSVP stream laid directly between the two attended RSVP streams in opposing visual field quadrants and contained only digits to maximize distraction. If information appearing in the central stream was selected along with the information in the two peripheral streams of interest, it would likely interfere with performance of the digit comparison task.
Figure 3.
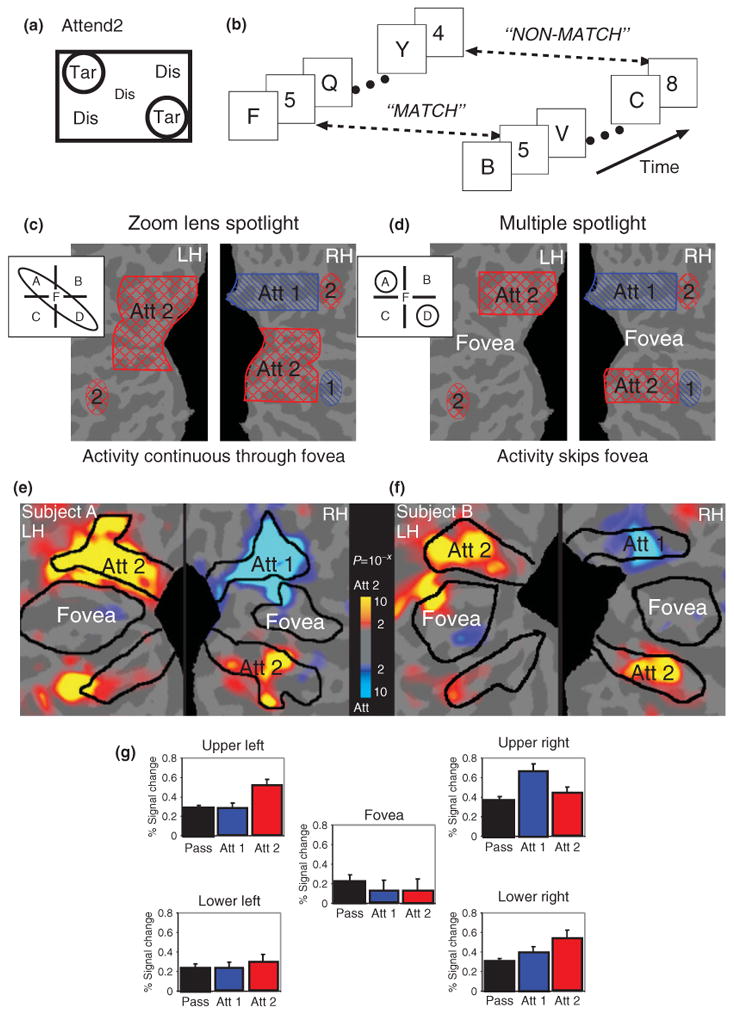
Multiple spotlights of spatial attention. (a and b) Multiple rapid serial visual presentation letter streams were presented. In the Attend2 condition, subjects were instructed to attend to two letter streams while ignore an intermediately positioned stream and two others. Digits would appear simultaneously in both streams and subjects had to report whether they were the same or different. (c and d) Predicted activation patterns for Attend2 versus Attend1 for the two competing hypotheses. (e and f) Activation patterns for Attend2 versus Attend1, for two subjects, supporting the multiple spotlight hypothesis. (g) Average activations in each of the five ROIs confirm that subjects split their attentional spotlight between two peripheral regions and spared the fovea in the Attend2 task. (Reprinted with permission from Ref 31. Copyright 2004 Elsevier)
Psychophysical performance in this task was investigated by parametrically varying the letter presentation duration of the RSVP streams. Threshold-level performance (d′ = 1) was observed at letter durations of 67 milliseconds. This rate is much faster than the minimum estimates for how quickly spatial attention can select a target, deploy to a new location, and select a second target (200–500 milliseconds). In addition to the Attend2 task, an ‘Attend1′ condition was included in which attention was covertly directed to a single peripheral RSVP stream (not one of the Attend2 streams), while viewing the same display. In the Attend1 task subjects had to identify digits appearing in the stream and report whether they matched a predefined target digit. Although the Attend1 task was somewhat easier than the Attend2 task, performance differences were far less than would be predicted if the Attend2 task was done by serial processing. Moreover, at threshold (d′ = 1) in the Attend1 task was 59 milliseconds, only 8 milliseconds shorter than for the Attend2 task. As no experiments have ever revealed evidence for 8 milliseconds attentional switching (or 125 times per second), we reject the hypothesis that subjects were rapidly moving a single attentional spotlight. Even recent work that argues for rapid switching (e.g., Refs 60 and 61) suggests substantially slower switch rates than could account for our findings. Thus, the psychophysical results demonstrate that spatial attention was simultaneously deployed to the two RSVP streams of interest.
Having thus ruled out the ‘rapid switching’ hypothesis for this task, two hypotheses then remained that could explain selection of the two peripheral streams. The zoom lens model predicts that attention stretches to select the two RSVP streams and necessarily also selects the distracting central RSVP stream (Figure 3(c)). The alternate hypothesis suggests that the spatial window of attention can be split into two distinct spotlights that select the two RSVP streams of interest while filtering out the central stream (Figure 3(d)). This question was addressed using fMRI of occipital lobe activation during task performance. Comparison of Attend2 versus Attend1 activation (see Figure 3(e) and (f)) revealed two hotspots of activation at the visual cortical representations of the two Attend2 streams. Critically, the cortical representation of the central RSVP stream was not activated (nor was it activated in comparisons with the passive viewing condition). This result demonstrates that the window of spatial attention may be split to select multiple distinct regions. This pattern was observed for all subjects. Group analysis revealed that this split attentional spotlight effect occurred in all ‘early’ visual cortical areas, including primary visual cortex. As expected, the Attend1 condition (vs Attend2) revealed a single hotspot of activation at the cortical representation of the attended RSVP stream (see Figure 3(e)–(g)). We replicated these findings using a configuration in which both targets and an intervening distractor were all placed in a single quadrant of the visual field, thus demonstrating that multiple attentional foci can be maintained within a single hemifield.31
In a follow-up experiment,32 we directly compared zoom lens and split spotlight attention deployed across one, two, or three stimuli. As expected, behavioral performance declined as the number of attended RSVP streams increased. There was no difference in performance between attending to two adjacent RSVP streams (Zoom2 condition) and attending to two RSVP streams separated by a distractor RSVP stream (Split2 condition). Moreover, performance in the split spotlight (Split2) condition was superior to performance when zoom lens attention selected all three RSVP streams (Zoom3) in which both conditions covered the same spatial extent. These behavioral patterns were mirrored in the fMRI BOLD signal attentional modulation observed in occipital cortex. Thus, multifocal attentional deployment to two targets exhibited no overhead costs, in terms of behavior or occipital lobe activation, relative to zoom lens selection of the same number of targets and multifocal attention exhibited a resource conservation benefit relative to zoom lens attentional selection over the same spatial extent.
Other paradigms have also revealed behavioral evidence for multifocal visual attention selection.29,52,62 Steady-state visually evoked potential (VEP) studies also provide strong evidence for the ability to simultaneously select two target locations while ignoring an intervening distractor region.18 Our findings have also been replicated in fMRI and electro-encephalography (EEG) experiments using different paradigms.63,64 Significantly, a recently published set of nonhuman primate electrophysiological experiments observed multifocal visual attention mechanisms at work within cortical area MT of the macaque.50 Furthermore, recent work by De Yoe and coworkers suggest that even when subjects are directed to attend to a single location in the visual field, individual subjects each have a highly reliable attentional signature that spreads to distant locations in the visual field.65,66
Spatial Maps in Parietal, Frontal, and Temporal Lobes
Topographic maps refer to areas defined by a preservation of the ordered progression of visual field locations from the retina and have been demonstrated throughout the cerebral cortex. Many perceptual and cognitive functions (from motion perception to object identification) rely upon integrating information from nearby locations in the visual field. Preserving a map structure may therefore decrease the length of many intracortical connections thereby increasing cortical efficiency.67 Furthermore, spatial location may be one of the organizing principles of white matter connections between cortical areas.68
Control of spatial attention in early visual cortex is likely directed by regions of the posterior parietal cortex (PPC) and lateral prefrontal cortex. Neuroimaging studies have revealed the existence of multiple visuotopic maps in parietal and frontal cortices.6–8,10,12,34,35,69,70 The PPC supports an extensive range of sensory and cognitive functions, including spatial representation, multimodal integration, attentional control, numerosity judgments, motor planning, and working memory.71–77 The ability to functionally define distinct cortical areas within this region therefore may help to elucidate the cortical organization of many cognitive functions (Figure 4).
Figure 4.
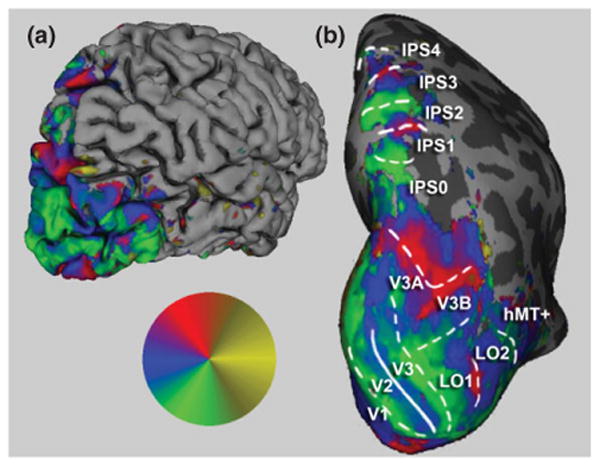
Visuotopically specific activation in posterior cerebral cortex. The angular position of the wedge resulting in the greatest significant (P < 0.05) response is indicated by the colored overlay (inset), with red representing the upper visual meridian, blue the contralateral horizontal meridian, and green the lower meridian. Potential ipsilateral responses are indicated in yellow. The right hemisphere of a single subject is shown. In (a), the reconstructed pial surface is shown from a posterior lateral view. Computational inflation of the folded cortical surface reveals visuotopically specific activation within the sulci (b). Reversals in the represented angular position correspond to areal boundaries in the early visual areas, and are shown as overlaid lines. Dashed lines indicate reversals at representations of the vertical meridian, while solid lines indicate reversals at horizontal meridian representations. On the basis of these scans, we find five hemifield maps along the medial bank of the intraparietal sulcus including areas IPS0 through IPS4.
In Ref 8, we employed direct stimulus-driven retinotopic mapping methods to demonstrate the existence of five topographically organized areas, IPS0, IPS1, IPS2, IPS3, and IPS4, within the medial bank of the IPS. Subjects were shown flickering checkerboard wedge stimuli that slowly rotated clockwise or counterclockwise, similar to stimuli used to demonstrate maps in the occipital cortex. Previously, Tootell et al.41 identified area V7 as a quarter visual field map lying anterior to occipital area V3A. We demonstrated that this was a full hemifield representation (see also Refs 6 and 7) that lies at the posterior end of the IPS and shares a foveal representation with IPS1. We therefore felt it was more appropriate to use nomenclature consistent with the other visuotopic regions in IPS and, as the regions lying anterior to this region within the IPS were previously named IPS1 and IPS2, we suggested the name IPS0. This nomenclature has been widely accepted in the literature (e.g., Refs 12 and 78–80). In addition, two previously unidentified maps (IPS3–4) were observed in our study and named consistent with this nomenclature.
In addition, multisensory topographic maps have been demonstrated lateral and anterior to those found in the IPS.17,69 There is currently no evidence that IPS0–4 are involved in sensory processing outside of the visual domain. Directly comparing topographic maps and activity from unimodal and multimodal sensory tasks, we have investigated tactile and auditory processing in IPS0–4. Swisher et al.8 observed that a tactile attention task activated voxels that abutted visuotopically mapped voxels in IPS0–4, but that there was minimal overlap between the two sets of activated voxels. In a recent study, we observed that auditory spatial attention activation also spared IPS0–4, but activated nonvisuotopically mapped regions of anterior and lateral IPS.81 Thus, our findings suggest that areas IPS0–4 function as predominantly unisensory maps.
In addition to maps in the occipital and dorsal parietal lobes, maps within dorsal regions of the frontal cortex have been demonstrated using delayed saccades, spatial and face working memory, pointing, and spatial attention. Two maps have been consistently demonstrated in the precentral sulcus using delayed saccades and spatial working memory, at the intersection with the superior and inferior frontal sulci.10,70 Within the prefrontal cortex, a face working memory task maps an additional area within the inferior frontal sulcus.10 Spatially directed attention can delineate maps across occipital, parietal, and frontal cortices in the absence of periodic changes in visual stimulation.6,35
In contrast to areas within the parietal and frontal cortices, areas in the temporal lobe have not been associated with processing spatial information, yet have recently demonstrated visual topography. These include areas extending from the occipital cortex into the temporal cortex, and each shows two hemifield representations that share a foveal confluence. On the lateral surface, temporal–occipital11,16 maps extend laterally from the two maps in lateral occipital82 complex. On the ventral surface, ventral occipital83 and parahippocampal13 maps extend from human V4 on the ventral occipital surface. Within these maps, temporal–occipital areas can be localized using global motion,11,16 whereas the other areas can be localized using object identification.13,83
VSTM Coding in Parietal Lobe Maps
VSTM refers to the encoding, maintenance, and retrieval of visually presented stimuli that are unlikely to be held in long-term memory.84 VSTM capacity, much like visual attentional capacity, is limited to approximately four objects.21 Human neuroimaging studies have demonstrated that activity in the IPS closely reflects the number of items held in VSTM.37,38,40 Areas involved in VSTM are proximal to topographically defined areas of the parietal cortex that have a role in visual attention, but the small size of topographic areas and individual variability makes it impossible to know, without performing visuotopic mapping in the same individuals, whether the areas underlying VSTM and the topographic maps are the same.
To determine whether the same visuotopically mapped IPS regions are also modulated by VSTM capacity, we conducted an experiment to directly investigate the role of topographic IPS in VSTM.8 Subjects performed retinotopic mapping in order to identify visuotopic areas IPS0–4, as well as VSTM fMRI scans, with varying degrees of memory load and visual field locations. In VSTM scans, subjects fixated a centrally located cross while-eight colored bars were presented in each of the left and right visual fields. Because visuotopic IPS areas are sensitive to visual drive,8 the number of visual stimuli was held constant across all conditions. Subjects were asked to remember the orientation (horizontal or vertical) of a subset of the bars that were distinguished from the distractors by color (red or blue). The number of the targets (1, 3, or 6) and their location (right visual field or left visual field) varied across blocks. In the remember-left conditions the targets were presented to the left while all of the stimuli on the right were distractors. In the remember-right conditions, the targets were presented to the right while all of the stimuli on the left were distractors.
We observed that activation in the human IPS reflected VSTM performance across varying memory loads within IPS0–2. However, activity during VSTM diverged from the more anterior topographically mapped regions (IPS3–4), activating a more lateral and inferior region that we termed anterior IPS (see Figure 5). Across IPS0-2 and anterior IPS, BOLD signal and K-score, a measure of the number of items effectively held in VSTM both increased and then reached a plateau as VSTM set size increased. This demonstrates that activity in IPS0-2 reflects the contents and capacity of VSTM. IPS3 appears to play a weaker role in VSTM; our evidence did not support a role for IPS4.
Figure 5.
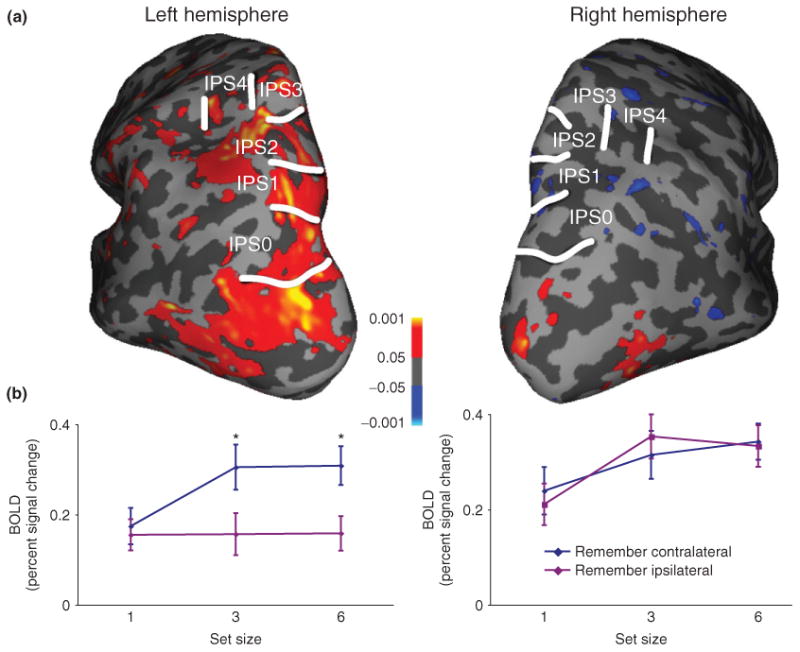
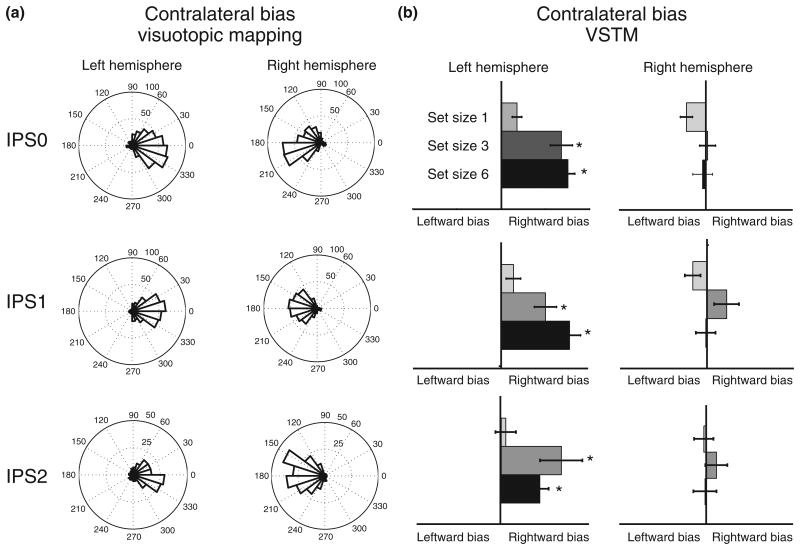
Group average contralateral bias (contralateral > ipsilateral). (a) Group average of areas showing significantly greater activity for contralateral than ipsilateral locations for set sizes 3 and 6 combined. (b) Activity for contralateral and ipsilateral conditions for each set size for IPS0–2 in each cortical hemisphere.
An Emerging Hemispheric Asymmetry
While each hemisphere of IPS0–4 contains a map of the contralateral visual field, the hemispheric symmetry of topographic maps in IPS sharply contrasts with findings from hemispatial neglect syndrome. Indeed, Saygin and Sereno35 showed that attention increased the power of the topographic signal across both hemispheres, with larger effects in the right hemisphere. Hemispatial neglect is characterized by spatial attention deficits in the contralesional visual field but rarely occurs after left hemisphere damage.85–90 While acute, structural damage in hemispatial neglect is normally found ventral to topographic regions of the PPC, neglect following damage to the IPS, although rare, results in similar behavioral deficits91 and diminished activity in dorsal PPC regions during the acute phase of neglect normalizes with the amelioration of neglect symptoms.92 These observations suggest that hemispatial neglect emerges from damage to a network that includes visuotopically mapped IPS.86 While several studies have investigated the contralateral bias during visual mapping and visual attention,6–8,35,93–95 there had not yet been a systematic study of spatial processing in IPS during VSTM, and there were few insights as to how to study the neglect network in normal healthy human subjects.
In our VSTM experiments,39 we asked whether contralateral bias, characteristic of visuotopic maps, is also found for VSTM representations in the parietal cortex. Within IPS0–2 the left hemisphere exhibited a strong contralateral bias for VSTM under high load (set size 3 and 6) as predicted by visuotopic mapping (see Figure 6). In contrast, no contralateral bias was observed for the right hemisphere. The left hemisphere exhibited increasing BOLD signal with increasing set size for contralateral stimuli only. In contrast, the right hemisphere exhibited increasing BOLD signal with increasing set size for both contralateral and ipsilateral memory targets. These results reveal a surprising, load-dependent, hemispheric asymmetry in VSTM processing in which the left hemisphere is strongly biased toward coding contralateral targets, but the right hemisphere robustly codes targets in both hemifields. Control studies ruled out eye position and response hand explanations of this hemispheric asymmetry.
Figure 6.
Comparison of contralateral bias during visuotopic mapping and visual short-term memory (VSTM). (a) Preferred visual angle for voxels significantly active during visuotopic mapping in regions IPS0–2 for the left and right hemispheres. (b) Contralateral bias [(contralateralsetsize−ipsilateralsetsize)/(contralateralsetsize+ ipsilateralsetsize)] during VSTM for the same regions of interest as in (a).
These results suggest an apparent contradiction. Visuotopic IPS shows a strong bias for stimuli in the contralateral visual field for both cortical hemispheres. This hemispheric symmetry contrasts with the asymmetry observed for VSTM activation. We repeated our analysis (see Figure 6) using only voxels with significant visuotopic mapping. While each ROI showed a consistent and hemispherically symmetric contralateral bias during visuotopic mapping, during VSTM the hemispheric asymmetry in the degree of contralateral bias persisted. In the left hemisphere, there was a significant contralateral bias for the higher set sizes (3 and 6). There was no significant effect of stimulus location for the right hemisphere for any ROI at any set size. These results confirm that the hemispheric asymmetry for VSTM emerges in IPS voxels that are strongly and symmetrically driven by contralateral visual representations.
Two different hypotheses could explain the difference in topographic representation within the right hemisphere. Right IPS might contain one neural population that performs mnemonic spatial indexing of the contralateral field and another population that performs nonspatial memory processes, while the left hemisphere might contain only contralateral spatial indexing neurons. This dual-population hypothesis implies that responses in right IPS should still be greater for contralateral than ipsilateral memory targets. Alternatively, right IPS could be driven by different sets of inputs that switch the effective spatial representation depending on which are more strongly activated by the task. This dual-input hypothesis suggests that bottom-up visual stimulation arrives only from the contralateral field, while mnemonic inputs arrive from the entire visual field. The dual-input hypothesis is consistent with our observations and with single-unit primate studies that have shown that the same neurons can alter their firing patterns depending upon the task demands both in terms of representation of items96 and the coordinate system in which objects are encoded.97 These mnemonic inputs might come from ventral parietal and superior temporal regions, the primary loci of lesions underlying hemispatial neglect, or from lateral anterior IPS, which exhibits the same hemispheric asymmetry for VSTM as IPS0–2, but does not exhibit contralaterally biased visuospatial maps (Boxes 1 and 2).
BOX 1. DYNAMIC REPRESENTATIONAL MODEL OF NEGLECT.
Attention maps in the brain have long been implicated in hemispatial neglect syndrome, a complex syndrome that reflects a disruption in spatial attention. Stimulus processing contralateral to the lesioned hemisphere is profoundly impacted. Curiously, neglect occurs almost exclusively after right hemisphere damage. To explain this hemispheric asymmetry, representational models of hemispatial neglect85,88,89 have suggested that the right hemisphere should contain bilateral maps, whereas the left hemisphere should contain only contralateral maps. Neuroimaging studies have observed multiple spatial maps in the dorsal parietal attentional network6–8,93; however, these reports have failed to observe significant hemispheric asymmetries in these maps. Dorsal parietal areas are not typically damaged by strokes that cause hemispatial neglect; however, these areas, including regions of IPS, show greatly reduced activity during the acute phase of neglect. As neglect symptoms recede, dorsal parietal activation increases. A pair of recent studies have reported asymmetries in visuotopic IPS regions39, 95 that emerge under visual attention or VSTM demands. These findings suggest a network model of hemispatial neglect in which temporoparietal damage causes a disruption of inputs to IPS.39,92,98 Our findings also provide fMRI evidence that the mechanisms underlying hemispatial neglect may be studied in normal human subjects.
BOX 2. TOPOGRAPHIC MAPPING ACROSS SUBJECT POPULATIONS.
Retinotopic mapping in visual cortex has been used to study functional activity within patients suffering from schizophrenia,99 hemispatial neglect,100 and within healthy aging populations.101 Developing robust topographic mapping techniques across cortical areas, therefore, may aid in understanding functional impairments within a number of populations. Direct visual stimulation has two main benefits for studying activity across subject populations. First, this technique can be used to delineate areas throughout occipital, parietal, and temporal cortices.8,35,78 In addition, because subjects perform a task at fixation, it is easier for retinotopy-naïve subjects to consistently maintain fixation, increasing the feasibility of mapping across subject populations. Recent studies suggest that directing attention toward versus away from the mapping stimulus improves mapping reliability.35,78 However, it is unknown whether the benefits on scan reliability of directing attention to the mapping stimulus negate the benefit of reduced eye movements in subjects inexperienced in performing visual experiments. In the above studies, in the condition in which the stimulus was unattended, subjects performed attentionally demanding tasks at fixation. Competitive interactions between attended and unattended stimuli are well documented.102,103 Attentionally demanding tasks may therefore siphon attentional resources away from the mapping stimulus. It is not clear to what extent task difficulty modulates this effect. It therefore remains to be seen how increasing attentional demands, both at fixation and within the stimulus, affects map reliability.
Conclusion
Visual field map representations are a pervasive feature of the cortical regions that process visual information. These map structures play a fundamental role in the guidance of attention. Visuospatial attention is directed by frontoparietal cortex, but the influences are observed in all visual cortical areas, including V1, and even the LGN. Attention boosts BOLD signal activation at the retinotopic cortical representations of attended targets, while suppressing activation for ignored distractors. In fMRI experiments, these spatial attention effects are additive/subtractive in nature. The visual system is limited in its attentional capacity, but this limit is not a single item, as implied by the spotlight model, but rather extends to a small number of objects. Multifocal spatial attention has been found to modulate early visual cortical representations of multiple targets, while treating intervening stimuli as distractors. Higher order cortical areas specifically in the parietal and frontal lobes control the direction of attention and short-term memory and play a role in limiting their capacity. The spatial representations in the parietal cortex can dynamically change with task demands, reflecting network influences from nonvisuotopic regions. These dynamic changes occur on the right hemisphere and may have an important role in understanding the neural basis of hemispatial neglect syndrome.
References
- 1.Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 2.Engel S. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- 3.DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen KA, Kay KN, Gallant JL. Topographic organization in and near human visual area V4. J Neurosci. 2007;27:11896–11911. doi: 10.1523/JNEUROSCI.2991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27:5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumoulin SO, Wandell BA. Population receptive field estimates in human visual cortex. Neuroimage. 2008;39:647–660. doi: 10.1016/j.neuroimage.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagler DJ, Riecke L, Sereno MI. Parietal and superior frontal visuospatial maps activated by pointing and saccades. Neuroimage. 2007;35:1562–1577. doi: 10.1016/j.neuroimage.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amano K, Wandell BA, Dumoulin SO. Visual field maps, population receptive field sizes, and visual field coverage in the human MT+ complex. J Neurophysiol. 2009;102:2704–2718. doi: 10.1152/jn.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcaro MJ, McMains SA, Singer BD, Kastner S. Retinotopic organization of human ventral visual cortex. J Neurosci. 2009;29:10638–10652. doi: 10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winawer J, Horiguchi H, Sayres RA, Amano K, Wandell BA. Mapping hV4 and ventral occipital cortex: the venous eclipse. J Vis. 2010;10:1. doi: 10.1167/10.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelissen K, Vanduffel W, Orban GA. Charting the lower superior temporal region, a new motionsensitive region in monkey superior temporal sulcus. J Neurosci. 2006;26:5929–5947. doi: 10.1523/JNEUROSCI.0824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. J Neurosci. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitada R, Kito T, Saito DN, Kochiyama T, Matsumura M, Sadato N, Lederman SJ. Multisensory activation of the intraparietal area when classifying grating orientation: a functional magnetic resonance imaging study. J Neurosci. 2006;26:7491–7501. doi: 10.1523/JNEUROSCI.0822-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller NG, Kleinschmidt A. Dynamic interaction of object- and space-based attention in retinotopic visual areas. J Neurosci. 2003;23:9812–9816. doi: 10.1523/JNEUROSCI.23-30-09812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci. 2002;5:631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- 20.Inouye T. Die Sehstroungen bei Schussverietzungen der korti-kalen Sehsphare. Leipzig, Germany: Engelmann; 1909. [Google Scholar]
- 21.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 22.Holmes G. Disturbances of vision by cerebral lesions. Br J Ophthalmol. 1918;2:353–384. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James W. The Principles of Psychology. New York: Dover; 1890. [Google Scholar]
- 24.Horton JC, Hoyt WF. The representation of the visual field in human striate cortex: a revision of the classic Holmes map. Arch Ophthalmol. 1991;109:816–824. doi: 10.1001/archopht.1991.01080060080030. [DOI] [PubMed] [Google Scholar]
- 25.Talbot S, Marshall W. Physiological studies on the neural mechanisms of visual location and discrimination. Am J Ophthalmol. 1941;24:1255–1263. [Google Scholar]
- 26.Eriksen CW, St James JD. Visual attention within and around the field of focal attention: a zoom lens model. Percept Psychophys. 1986;40:225–240. doi: 10.3758/bf03211502. [DOI] [PubMed] [Google Scholar]
- 27.Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Essen DC, Newsome WT, Maunsell JHR. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- 29.Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat Vis. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez GA, Franconeri SL. How many objects can you track? Evidence for a resource-limited attentive tracking mechanism. J Vis. 2007;7:1–10. doi: 10.1167/7.13.14. [DOI] [PubMed] [Google Scholar]
- 31.McMains SA, Somers DC. Multiple spotlights of attentional selection in human visual cortex. Neuron. 2004;42:677–686. doi: 10.1016/s0896-6273(04)00263-6. [DOI] [PubMed] [Google Scholar]
- 32.McMains SA, Somers DC. Processing efficiency of divided spatial attention mechanisms in human visual cortex. J Neurosci. 2005;25:9444–9448. doi: 10.1523/JNEUROSCI.2647-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettencourt KC, Somers DC. Effects of target enhancement and distractor suppression on multiple object tracking capacity. J Vis. 2009;9:1–11. doi: 10.1167/9.7.9. [DOI] [PubMed] [Google Scholar]
- 34.Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Saygin AP, Sereno MI. Retinotopy and attention in human occipital, temporal, parietal, and frontal cortex. Cereb Cortex. 2008;18:2158–2168. doi: 10.1093/cercor/bhm242. [DOI] [PubMed] [Google Scholar]
- 36.Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y. The role of the superior intraparietal sulcus in supporting visual short-term memory for multifeature objects. J Neurosci. 2007;27:11676–11686. doi: 10.1523/JNEUROSCI.3545-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 39.Sheremata SL, Bettencourt KC, Somers DC. Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load. J Neurosci. 2010;30:12581–12588. doi: 10.1523/JNEUROSCI.2689-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 41.Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Sasaki Y, Miyauchi S, Putz B, Fujimaki N, Nielsen M, Takino R, Miyakawa S. Attention-regulated activity in human primary visual cortex. J Neurophysiol. 1998;79:2218–2221. doi: 10.1152/jn.1998.79.4.2218. [DOI] [PubMed] [Google Scholar]
- 43.Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez A, Anllo-Vento L, Sereno M, Frank L, Buxton R, Dubowitz D, Wong E, Hinrichs H, Heinze H, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- 45.Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 46.Posner MI, Gilbert CD. Attention and primary visual cortex. Proc Natl Acad Sci. 1999;96:2585–2587. doi: 10.1073/pnas.96.6.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 48.Cave K, Bichot N. Visuospatial atention: beyond a spotlight model. Psychon Bull Rev. 1999;6:204–223. doi: 10.3758/bf03212327. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci. 2005;16:637–643. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 50.Niebergall R, Khayat PS, Treue S, Martinez-Trujillo JC. Multifocal attention filters targets from distracters within and beyond primate MT neurons’ receptive field boundaries. Neuron. 2011;72:1067–1079. doi: 10.1016/j.neuron.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Müller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003;424:309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- 52.Awh E, Pashler H. Evidence for split attentional foci. J Exp Psychol Hum Percept Perform. 2000;26:834–846. doi: 10.1037//0096-1523.26.2.834. [DOI] [PubMed] [Google Scholar]
- 53.Castiello U, Umiltà C. Size of the attentional focus and efficiency of processing. Acta Psychol. 1990;73:195–209. doi: 10.1016/0001-6918(90)90022-8. [DOI] [PubMed] [Google Scholar]
- 54.Müller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the “zoom lens” of visual attention. J Neurosci. 2003;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shulman G, Remington R, McLean J. Moving attention through visual space. J Exp Psychol Hum Percept Perform. 1979;5:522–526. doi: 10.1037//0096-1523.5.3.522. [DOI] [PubMed] [Google Scholar]
- 56.Tsal Y. Movements of attention across the visual field. J Exp Psychol Hum Percept Perform. 1983;9:523–530. doi: 10.1037//0096-1523.9.4.523. [DOI] [PubMed] [Google Scholar]
- 57.Weichselgartner E, Sperling G. Dynamics of automatic and controlled visual attention. Science. 1987;238:778–780. doi: 10.1126/science.3672124. [DOI] [PubMed] [Google Scholar]
- 58.Peterson MS, Juola JF. Evidence for distinct attentional bottlenecks in attention switching and attentional blink tasks. J Gen Psychol. 2000;127:6–26. doi: 10.1080/00221300009598568. [DOI] [PubMed] [Google Scholar]
- 59.Reeves A, Sperling G. Attention gating in short-term visual memory. Psychol Rev. 1986;93:180–206. [PubMed] [Google Scholar]
- 60.Hogendoorn H, Carlson TA, Verstraten FAJ. The time course of attentive tracking. J Vis. 2007;7:1–10. doi: 10.1167/7.14.2. [DOI] [PubMed] [Google Scholar]
- 61.Buschman TJ, Miller EK. Shifting the spotlight of attention: evidence for discrete computations in cognition. Front Hum Neurosci. 2010;4:194. doi: 10.3389/fnhum.2010.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention. Trends Cogn Sci. 2005;9:349–354. doi: 10.1016/j.tics.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Morawetz C, Holz P, Baudewig J, Treue S, Dechent P. Split of attentional resources in human visual cortex. Vis Neurosci. 2007;24:817–826. doi: 10.1017/S0952523807070745. [DOI] [PubMed] [Google Scholar]
- 64.Drew T, McCollough AW, Horowitz TS, Vogel EK. Attentional enhancement during multiple-object tracking. Psychon Bull Rev. 2009;16:411–417. doi: 10.3758/PBR.16.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Datta R, DeYoe EA. I know where you are secretly attending! The topography of human visual attention revealed with fMRI. Vision Res. 2009;49:1037–1044. doi: 10.1016/j.visres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brefczynski-Lewis JA, Datta R, Lewis JW, DeYoe EA. The topography of visuospatial attention as revealed by a novel visual field mapping technique. J Cogn Neurosci. 2009;21:1447–1460. doi: 10.1162/jocn.2009.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chklovskii D, Koulakov A. Maps in the brain: what can we learn from them? Annu Rev Neurosci. 2004;27:369–392. doi: 10.1146/annurev.neuro.27.070203.144226. [DOI] [PubMed] [Google Scholar]
- 68.Greenberg AS, Verstynen T, Chiu YC, Yantis S, Schneider W, Behrmann M. Visuotopic cortical connectivity underlying attention revealed with white-matter tractography. J Neurosci. 2012;32:2773–2782. doi: 10.1523/JNEUROSCI.5419-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- 70.Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- 71.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 72.Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 73.Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Curr Opin Neurobiol. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 74.Mesulam M, Small D, Vandenberghe R, Gitelman DR, Nobre AC. A Heteromodal Large-Scale Network for Spatial Attention. San Diego, CA: Academic Press; 2005. p. 6p. [Google Scholar]
- 75.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 78.Bressler DW, Silver MA. Spatial attention improves reliability of fMRI retinotopic mapping signals in occipital and parietal cortex. Neuroimage. 2010;53:526–533. doi: 10.1016/j.neuroimage.2010.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Res. 2011;51:718–737. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Helfrich RF, Becker HGT, Haarmeier T. Processing of coherent visual motion in topographically organized visual areas in human cerebral cortex. Brain Topogr. 2012 Apr 19; doi: 10.1007/s10548-012-0226-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Kong L, Michalka SW, Rosen ML, Sheremata SL, Swisher JD, Shinn-Cunningham BG, Somers DC. Auditory spatial attention representations in the human cerebral cortex. Cereb Cortex. 2012 Nov 23; doi: 10.1093/cercor/bhs359. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larsson J, Heeger DJ. Two retinotopic visual areas in human lateral occipital cortex. J Neurosci. 2006;26:13128–13142. doi: 10.1523/JNEUROSCI.1657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brewer AA, Liu J, Wade AR, Wandell BA. Visual field maps and stimulus selectivity in human ventral occipital cortex. Nat Neurosci. 2005;8:1102–1109. doi: 10.1038/nn1507. [DOI] [PubMed] [Google Scholar]
- 84.Phillips W. On the distinction between sensory storage and short-term visual memory. Percept Psychophys. 1974;16:283–290. [Google Scholar]
- 85.Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- 86.Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P. White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex. 2008;44:983–995. doi: 10.1016/j.cortex.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 87.Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129–133. doi: 10.1016/s0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- 88.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 89.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vallar G, Perani D. The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24:609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- 91.Gillebert CR, Mantini D, Thijs V, Sunaert S, Dupont P, Vandenberghe R. Lesion evidence for the critical role of the intraparietal sulcus in spatial attention. Brain. 2011;134:1694–1709. doi: 10.1093/brain/awr085. [DOI] [PubMed] [Google Scholar]
- 92.Corbetta M, Kincade M, Lewis C, Snyder A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 93.Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- 94.Shulman GL, Pope DLW, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30:3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci. 2010;30:148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toth LJ, Assad JA. Dynamic coding of behaviourally relevant stimuli in parietal cortex. Nature. 2002;415:165–168. doi: 10.1038/415165a. [DOI] [PubMed] [Google Scholar]
- 97.Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex. 2003;13:1009–1022. doi: 10.1093/cercor/13.10.1009. [DOI] [PubMed] [Google Scholar]
- 98.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martínez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vuilleumier P, Schwartz S, Verdon V, Maravita A, Hutton C, Husain M, Driver J. Abnormal attentional modulation of retinotopic cortex in parietal patients with spatial neglect. Curr Biol. 2008;18:1525–1529. doi: 10.1016/j.cub.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crossland MD, Morland AB, Feely MP, Hagen von dem E, Rubin GS. The effect of age and fixation instability on retinotopic mapping of primary visual cortex. Invest Ophthalmol Vis Sci. 2008;49:3734–3739. doi: 10.1167/iovs.07-1621. [DOI] [PubMed] [Google Scholar]
- 102.Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 103.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]