Abstract
Background
To describe the relationship of p16 and EGFR expression with survival in surgically treated patients who had oropharyngeal and oral cavity squamous cell carcinoma (OPSCC and OCSCC).
Methods
Tissue from 36 OPSCC and 49 OCSCC patients treated between 1997 and 2001 was imbedded and immunostained using a tissue microarray.
Results
p16 was positive in 57% and 13% of OPSCC and OCSCC patients, respectively. EGFR was positive in 60% and 63% of OPSCC and OCSCC patients, respectively. In OPSCC patients, p16 expression was associated with improved disease specific survival (DSS), overall survival (OS), and time to recurrence (TTR) (p <0.01, <0.01, <0.01). EGFR expression was associated with poorer DSS, OS, and TTR (p <0.01, =0.01, <0.01). For OPSCC, when examining both p16 and EGFR expression as combined biomarkers, high p16 expression coupled with low EGFR expression was associated with improved disease-specific survival (pp16 = 0.01; pEGFR= 0.01). OCSCC patients showed no association between biomarker and outcome.
Conclusions
For patients with OPSCC, high p16 and low EGFR were associated with improved outcome, suggesting a predictive role in surgically treated patients.
Keywords: oropharyngeal neoplasm, oral cavity neoplasm, p16(INK4A), EGFR protein, human papilloma virus
Introduction
Optimal treatment of oropharyngeal squamous cell carcinoma (OPSCC) and oral cavity squamous cell carcinoma (OCSCC) should be guided by both patient characteristics and tumor biology. Conventional treatment of locally advanced OPSCC/OCSCC involves multimodality therapy, which includes a combination of surgery, radiotherapy and cisplatin-based chemotherapy. Primarily, clinical factors guide decisions on the type of treatment prescribed to the patient. More recently, however, prognostic biological markers, in addition to clinical parameters, have improved the ability to predict outcome, particularly in patients with OPSCC. Among them, expression of p16 and epidermal growth factor receptor (EGFR), assessed through immunohistochemistry, have been shown to help further characterize the behavior of tumors, and ultimately may aid in the design of clinical trials that will select for patients requiring treatment escalation or de-escalation.
p16 is an excellent surrogate marker for high-risk human papillomavirus (HPV) in patients with OPSCC, reflecting the inactivation of the retinoblastoma tumor suppressor protein by the oncoprotein E7.(1) Many studies have demonstrated the correlation between p16 expression and improved survival, particularly in patients with OPSCC and particularly in those treated with nonsurgical therapies.(1–3) EGFR plays an important role in the regulation of cellular proliferation and survival in epithelial tumors. Alterations in EGFR signaling pathways lead to decreased apoptosis of tumor cells, enhanced invasiveness, cell migration, angiogenesis, and metastasis.(4) Many studies have demonstrated an association between elevated expression of EGFR and poorer survival, though the majority of these studies focus on non-surgical treatment of patients with OPSCC.(2, 3, 5, 6)
At the University of Michigan, standard treatment for patients with locally advanced OPSCC includes a combination of chemotherapy and radiotherapy. A recent trial (UMCC-9921) demonstrated the efficacy of both p16 and EGFR in predicting survival in patients with OPSCC treated with non-surgical modalities.(2) Patients with increased expression of p16 had improved survival, while those with elevated expression of EGFR had poorer survival. Less is known about the ability of these biomarkers to predict outcome in patients with OPSCC who are treated surgically. Further, the ability of these biomarkers to predict survival in patients with OCSCC has yet to be investigated. The objective of this study was to evaluate and contrast biomarker expression in surgically treated patients with OPSCC and OCSCC.
Materials and Methods
Study Design
This was a prospective cohort study.
Study Population
Eligibility criteria for the study included patients with previously untreated squamous cell carcinoma of the oropharynx (OPSCC) or oral cavity (OCSCC) who were treated with primary surgical extirpation. Patients were excluded if they presented with a synchronous primary neoplasm of the head and neck, a history of previous head and neck cancer, a history of previous surgery or radiation therapy to the upper aerodigestive tract, or distant metastatic disease. 85 patients were enrolled from 1997–2001 and followed prospectively for outcome (disease status and survival). These 85 patients were separated into two cohorts: 36 patients with OPSCC and 49 with OCSCC. These cohorts were each analyzed separately. The cohort sizes were small because of the stringent inclusion criteria. The inclusion criteria of no prior treatment limited the cohort size the most.. Table 1 demonstrates baseline demographics, staging, and treatment modality of the OPSCC and OCSCC patient cohorts. Patients continue to undergo regular follow-up, and have currently been followed for 9 years. With respect to the 36 patients who comprised the OPSCC cohort, the five-year disease-specific and overall survival were 59% and 38%, respectively. At 5 years of follow-up, 42% (15/36) of patients were alive and free of disease, 3% (1/36) were alive with disease, 36% (13/36) were dead from disease, 6% (2/36) were dead of second primary neoplasm, and 14% (5/36) were dead of intercurrent illness.
Table 1.
Patient and Tumor Characteristics for OCSCC and OPSCC. SD, standard deviation.
| Oral Cavity n = 49 | Oropharynx n = 36 | |
|---|---|---|
| Patient Characteristic | ||
|
| ||
| Age: mean (SD) | 57.2 (17.0) | 52.3 (11.0) |
|
| ||
| Gender | ||
| Male | 17 (35%) | 53 (14%) |
| Female | 32 (65%) | 31 (86%) |
|
| ||
| Smoking History | ||
| Never | 3 (6%) | 2 (6%) |
| Past | 36 (73%) | 29 (81%) |
| Current | 6 (12%) | 4 (11%) |
| Unknown | 4 (8%) | 1 (3%) |
|
| ||
| Adjuvant Treatment | ||
| None | 15 (30%) | - |
| Radiotherapy | 33 (66%) | 31 (86%) |
| Chemoradiotherapy | 2 (4%) | 5 (14%) |
|
| ||
| Tumor Characteristic | ||
|
| ||
| T stage (clinical) | ||
| T1 | 5 (10%) | 1 (3%) |
| T2 | 15 (31%) | 9 (25%) |
| T3 | 10 (20%) | 12 (33%) |
| T4 | 19 (39%) | 14 (39%) |
|
| ||
| N stage (pathologic) | ||
| N0 | 22 (45%) | 5 (14%) |
| N1 | 8 (16%) | 4 (11%) |
| N2 | 19 (39%) | 24 (69%) |
| N3 | - | 2 (6%) |
|
| ||
| AJCC Stage | ||
| I | 4 (8%) | - |
| II | 8 (16%) | - |
| III | 9 (18%) | 6 (17%) |
| IV | 26 (57%) | 30 (83%) |
|
| ||
| Histologic Grade | ||
| Well | 9 (20%) | 2 (6%) |
| Moderate | 30 (65%) | 19 (54%) |
| Poor | 7 (15%) | 14 (40%) |
|
| ||
| Perineural Invasion | 10 (20%) | 4 (11%) |
|
| ||
| Perivascular Invasion | 7 (14%) | 8 (22%) |
|
| ||
| Nodal ECS | 12 (24%) | 21 (58%) |
The five-year disease-specific and overall survival estimates for the 49 patients with OCSCC were 60% and 54%, respectively. At 5 years of follow-up, 59% (29/49) of patients were alive and free of disease, 29% (14/49) were dead of disease, 2% (1/49) were dead of a second primary tumor, 8% (4/49) were dead of intercurrent illness, and 2% (1/49) were lost to follow-up.
Variables Under Study
Patients were evaluated at baseline for clinical covariates (age, gender, tobacco exposure, alcohol history, tumor site, and stage). Tobacco history and alcohol status were classified as ‘never’, ‘past’ (quit 6 months ago), or ‘current’. Staging was based on the 2002 American Joint Committee on Cancer staging system. Pre-treatment biopsies of the primary tumor were used to construct a tissue microarray (TMA) to evaluate the expression of biomarkers (p16 and EGFR). After surgical extirpation, pathology specimens were evaluated for perineural/perivascular invasion, histologic grade, and nodal characteristics.
Immunostaining and Scoring
Tumor specimens used to construct the TMA were deparaffinized, rehydrated, and peroxidase-quenched (Dako Cytomation, Glostrup, Denmark). For antigen retrieval, slides were incubated with citrate buffer (p16; 30 minutes at 92°C) or with pepsin (EGFR; 10 minutes at 37°C) and were blocked with horse serum (30 minutes at 25°C). Primary antibody, p16/16P04, (Lab-Vision, Fremont CA) and EGFR/31G7 (Zymed Laboratories, South San Francisco, CA), were added for 1 hour and were probed with avidin/biotin peroxidase (ABC Kit; Vector Laboratories, Burlingame, CA).(2)
Antibody binding was scored by a pathologist (K.G.C) who was blinded to the clinical outcome. Based on previous experience from our institution(2, 7), p16 expression was scored based on the proportion of tumor staining positive using an ordinal scale from 1 to 4: 1 was less than 5%; 2, (5% to 20%); 3 (21% to 50%); and 4 (51% to 100%). Patients with a score greater than 1 were considered positive for p16. EGFR expression was scored based on level of intensity using a scale from 1 to 4; 1 equal to no staining; 2, low intensity; 3, moderate; and 4, high intensity. Patients with a score greater than 2 were considered positive for EGFR. Scores for three cores of tumor from each patient were averaged. Of the 36 patients with OPSCC eligible for the study, 35 had sufficient specimens for immunostaining. Of the 49 patients with OCSCC eligible for the study, 46 had sufficient specimens for immunostaining.
Response Variables
Patients were evaluated for the following outcomes: disease-specific survival (DSS), as defined by the time from primary surgery to death from OCSCC or OPSCC, overall survival(OS), as defined by the time from primary surgery to all-cause mortality and time to recurrence, as defined by the time from primary surgery to the first recurrence.
Statistical Analysis
Baseline patient and tumor characteristics were tabulated. Patient-level averages for proportion of p16 expression and intensity of EGFR expression were used in the analysis. For the assessment of bivariate associations between markers and covariates, rank-based statistical methods were employed. The Kaplan-Meier method and the log-rank test were used to compare the homogeneity of survival rates within and between categories of discrete variables. Only patients who had sufficient specimen for immunostaining were considered for the association between markers and survival. Cox proportional hazards models were used to assess the markers’ effects beyond the effects of the clinical covariates. Likelihood ratio statistics were used to determine statistically significant differences between the models. All statistical analyses were done with SAS version 9.0 (SAS Institute, Carey, NC). A two-tailed P value of .05 or less was considered statistically significant.
The Institutional Review Board at the University of Michigan granted approval for this study.
Results
Prevalence of p16 and EGFR
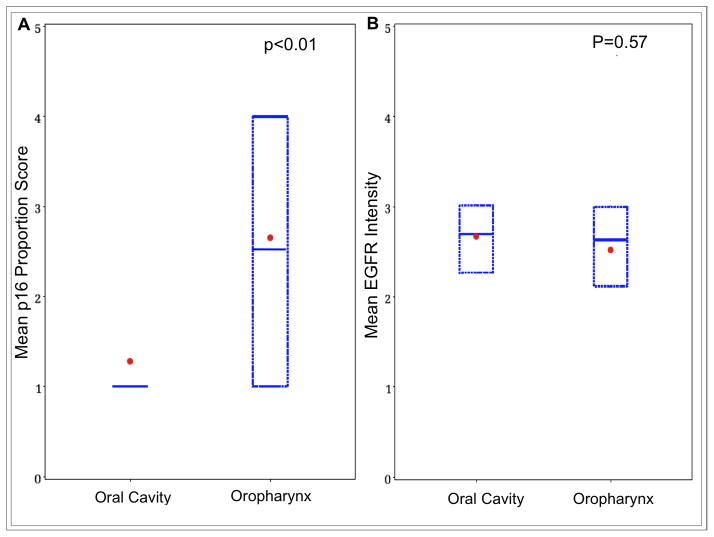
The prevalence of p16-positivity in patients with OPSCC and OCSCC were 57% (20/35) and 13% (6/46), respectively. The mean proportion of cells staining positive for p16 was higher in the oropharynx (Figure 1a, p<0.01). Of the 20 patients who were positive for p16 in the oropharynx, 95% (19/20) had a p16 score of 4. In contrast, of the 6 patients who were positive for p16 in the oral cavity, only 50% (3/6) had a score of 4.
Figure 1.
Comparison of mean score of immunostain across subsites. (a) Box plot shows a significantly lower mean score of p16 proportion in OCSCC versus OPSCC. (b) A similar mean score of EGFR intensity is seen between OCSCC and OPSCC. Dot, mean; box, 25th to 75th percentile; horizontal bar, median.
The prevalence of EGFR-positivity in the oropharynx and oral cavity were 60% (21/35) and 63% (29/46), respectively. In addition, there was no statistically significant difference in mean intensity of EGFR staining between oropharyngeal and oral cavity tumors (Figure 1b, p=0.57).
Biomarker and Survival Analysis
p16 Survival Analysis
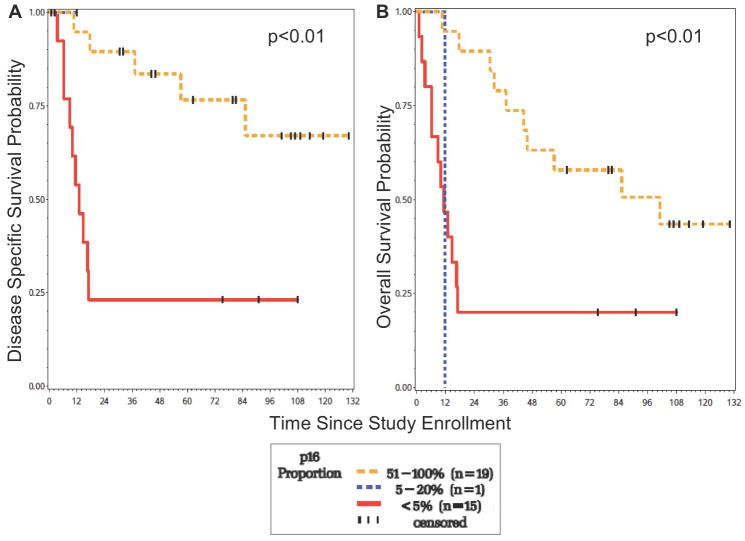
For OPSCC, patients with a higher p16 expression experienced significantly improved DSS (p<0.01) and OS (p<0.01) compared to those with a low p16 expression at nine years of follow-up (Figure 2). In addition, patients with a higher p16 expression had a significantly longer time to recurrence (p<0.01). At 5 years, those with the highest p16 staining proportion (51–100%, score = 4) had a DSS of 75% versus those with a staining proportion of less than 5% (score = 1), who had a DSS of 23%. Similarly, those with the highest p16 staining proportion (51–100%, score = 4) had an OS of 57% versus those with a staining proportion of less than 5% (score = 1), who had an OS of 20%.
Figure 2.
Survival of OPSCC patients according to p16 proportion. (a) Those with a high p16 expression have significantly improved DSS (b) Those with a high p16 expression have improved OS.
43% (15/35) of the patients with OPSCC were negative for p16 expression and this finding was associated with a poorer DSS and OS. Of these 15 patients, 9 were dead of disease at 5 years of follow-up. The pattern of recurrence included 1/9 local, 1/9 regional, 4/9 distant, 2/9 local/regional, and 1/9 local/regional/distant. In summary, distant disease was seen in 5/9 patients with OPSCC who were negative for p16 expression.
57% (20/35) of the OPSCC patients were positive for p16 expression and this was associated with improved survival. Of these 20 patients, 4 were dead of disease at 5 years of follow-up. Interestingly, in this group of p16-positive patients, all 4 died of distant disease.
In OCSCC, due to the low prevalence of p16 (13%), the ability to stratify patients by this biomarker was not feasible when performing survival analysis.
EGFR Survival Analysis
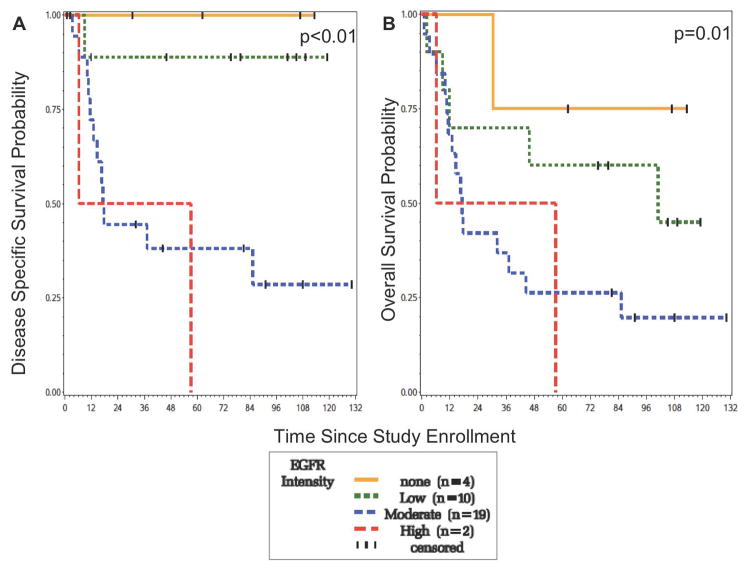
For OPSCC, patients with none or low EGFR expression (score = 1,2) experienced significantly improved disease-specific survival (p<0.01) and overall survival (p=0.01) compared to those with a moderate or high level of expression (score = 3, 4) at nine years of follow-up (Figure 3). In addition, patients with a lower EGFR expression had a significantly increased time to recurrence (p<0.01). At 5 years, those with none or low expression had a disease-specific survival of 95% versus those with a moderate or high expression, who had a disease-specific survival of 38%. Similarly, those with none or low expression had an overall survival of 68% versus those with a moderate or high expression, who had an overall survival of 25%.
Figure 3.
Survival of OPSCC patients according to EGFR intensity. (a) Those with none or low EGFR expression have significantly improved DSS (b) Those with none or low EGFR expression have significantly improved OS.
60% (21/35) of the OPSCC patients were positive for EGFR expression and this was associated with poorer DSS and OS. Of these 21 patients, 12 were dead of disease at five years of follow-up. Most patients died of distant disease. The pattern of recurrence was 9/12 distant, 2/12 local/regional, and 1/12 local recurrence.
40% (14/35) of the OPSCC patients were negative for EGFR expression and this was associated with improved DSS and OS. Of these 14 patients, only 1 died of disease at five years of follow-up, and this was from a regional recurrence.
Combined Marker Survival Analysis
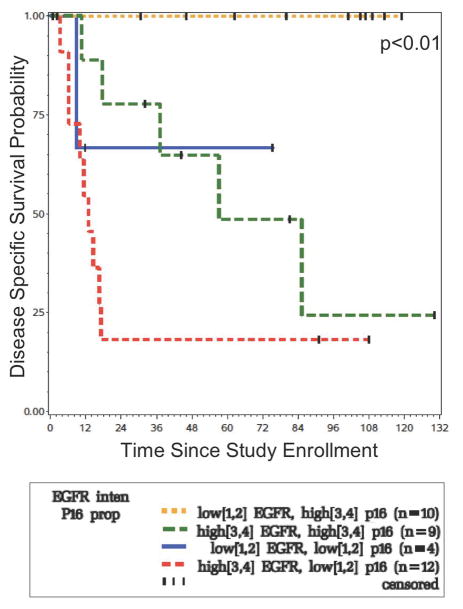
For OPSCC, when examining both p16 and EGFR expression as combined markers in a multivariable model, high p16 expression coupled with low EGFR expression were associated with improved disease-specific survival (pp16 = 0.01; pEGFR = 0.01) (Figure 5). At five years, the disease-specific survival for the 10 patients with high p16 proportion and low EGFR intensity was 100%. In contrast, the disease-specific survival for the 12 patients with low p16 proportion and high EGFR intensity was 17%.
Figure 5.
Disease Specific Survival of OPSCC patients according to combined EGFR intensity and p16 proportion. Those with low EGFR/high p16 have the best DSS, while those with high EGFR/low p16 have the worst DSS.
Clinical Covariates and Outcome
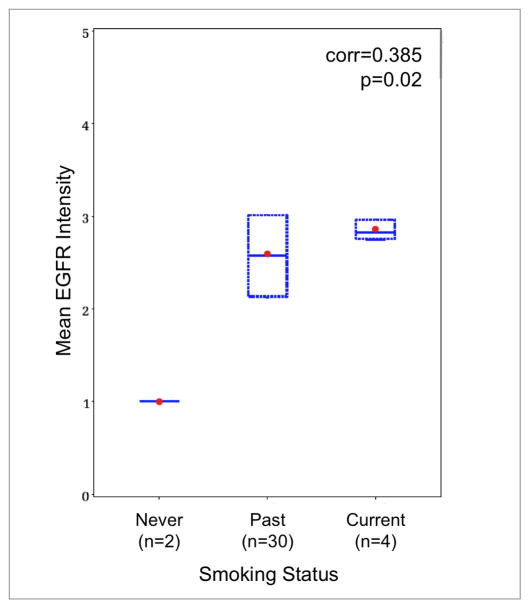
In patients with OPSCC, 58% (21/36) had evidence of extracapsular spread in the lymph nodes and 22% (8/36) had evidence of perivascular invasion in the primary tumor. In univariate analysis, extracapsular spread and perivascular invasion were each associated with poorer DSS (p = 0.026 and 0.014, respectively). However, multivariable regression changed these findings. When a multivariable regression was performed on ECS, perivascular invasion, and EGFR, only EGFR remained a significant predictor of disease specific survival (p = 0.002). For patients with OPSCC, higher levels of EGFR expression were associated with a positive smoking history, either past or current (r = 0.385, p = 0.025) (Figure 4). This finding must be tempered by the fact that of the 35 patients, the majority (29/35) were past smokers, while only 2 were never smokers and 4 were current smokers. In patients with OCSCC, there was a similar lack of never smokers (3/45). Most patients had a past smoking history (36/45), but were not current smokers (6/45).
Figure 4.
Correlation of mean EGFR intensity with smoking status in patients with OPSCC. Higher levels of EGFR expression were associated with either past or current smoking status (r=0.385, p=0.02) Dot, mean; box, 25th to 75th percentile; horizontal bar, median; corr, Spearman’s Correlation Coefficient.
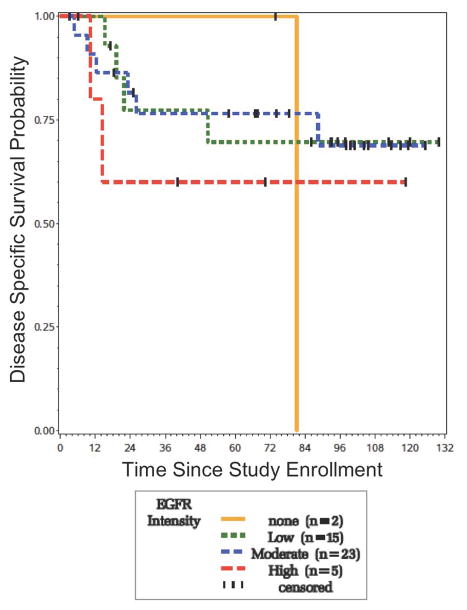
For patients with OCSCC, several clinical covariates were found to be associated with survival. On bivariate analysis, the presence of perineural invasion in the primary tumor (10/49 patients), nodal disease (27/49 patients), and extracapsular spread (found in 12/27 patients with nodal disease) were all associated with poorer disease-specific survival (p = 0.001, 0.047, and 0.004, respectively) and overall survival (p = 0.001, 0.005, and 0.005, respectively). No statistically significant difference was seen between those who were positive versus those who were negative for EGFR expression. However, patients with a score of 4 had a trend toward both earlier disease-related events and poorer 5-year DSS (Figure 6). After multivariable regression with perineural invasion, nodal disease, extracapsular spread, and the biomarkers, the clinical covariates remained statistically significant predictors of survival.
Figure 6.
Survival of OCSCC patients according to EGFR intensity. Those with the highest expression of EGFR have a trend toward poorer survival compared to those with none, low or moderate staining.
Discussion
The objective of this study was to characterize the expression of two biomarkers, p16 and EGFR, in two separate cohorts of surgically treated patients: the first, patients with OPSCC and, the second, patients with OCSCC. For patients with OPSCC, the results of this study demonstrate a distinct survival advantage for those with high levels of p16 expression and a survival disadvantage for those with high levels of EFGR expression. As combined markers, p16 and EGFR identified, with more accuracy, those patients with the best and worst survival. Several studies in the literature have demonstrated the importance of both p16 and EGFR in predicting survival of patients with OPSCC, however most are based on treatment with primary radiotherapy and cisplatin-based chemotherapy.(2, 3, 6–13) This study is unique in that it evaluates the predictive power of these two biomarkers in a two surgically treated cohorts of patients with OPSCC, and shows that p16 and EGFR are predictive in surgically treated patients. Further, the OPSCC patients included in this study were treated at a time when surgery was the standard treatment for OPSCC at the University of Michigan.
p16 expression assessed through immunohistochemistry has now been accepted as a reliable biomarker for the HPV E7 oncoprotein(12), thus acting as a surrogate marker for high risk HPV infection in OPSCC.(1, 2, 11, 14, 15) A previous study at the University of Michigan has demonstrated a very strong association between p16 and HPV copy number (p <0.001).(2) Further, the importance of p16 expression may extend beyond that of being a surrogate marker of HPV infection. Lassen et al argue that p16 positivity may identify HPV infections in OPSCC that are transcriptionally active and thus may predict patient outcome more accurately than HPV detection.(11)
A few studies have explored the role of HPV in surgically treated patients with OPSCC.(16) Licitra et al performed a retrospective review of 90 consecutive patients with OPSCC, all treated with surgery +/− radiotherapy. Patients were evaluated for HPV DNA type 16 and 18, in association with a second biomarker, TP53. Multivariable regression analysis demonstrated that those tumors that were both HPV-positive and contained wild type TP53 had significantly improved survival.(17) Ukpo et al performed a retrospective review of 102 patients with OPSCC, treated with surgery +/− radiotherapy. This study did not demonstrate any association between HPV status and survival.(18) Conversely, Rich et al performed a retrospective analysis of patients treated with transoral laser microsurgery +/− adjuvant therapy. Increased expression of p16, assessed in tumors from 73 patients, was associated with improved survival. Interestingly however, patients with positive HPV DNA (determined by in-situ hybridization techniques) did not have significantly improved survival.(15) Similar to our study, the majority of patients in this study (93%) received adjuvant radiotherapy. In our study, those patients with OPSCC who had high levels of p16 expression had over 50% improved disease-specific survival in comparison to those with low levels of p16 expression at 5 years of follow-up. While the biologic basis for the survival advantage conferred by p16 up-regulation is unknown, a plausible explanation that prevails in the literature is that tumors expressing p16 are less hypoxic and therefore respond with less accelerated repopulation when irradiated, thus making them more susceptible to radiation.(11) The prevalence of p16 positivity in large series evaluating patients with OPSCC treated with conventional chemoradiation ranges from 19–95%.(3, 7, 9–11, 14–16, 18, 19) Although this range is quite large, studies that evaluate the prevalence more recently tend to have a higher rate, suggesting an increase in the prevalence over time. The prevalence in our study (57%) is consistent with the studies that have assembled patient cohorts from a similar time period.
The prevalence of HPV in OCSCC is not well established, but a few studies quote a range from 3% – 12%.(9, 20) Our study demonstrated a p16 prevalence of 13%, in keeping with studies in the literature. p16 is much less likely to be a surrogate marker for HPV status in the oral cavity and, due to the low prevalence of p16 in our OCSCC cohort, a survival analysis stratifying patients by p16 status was not feasible.
The role of EGFR expression in predicting outcome for surgically treated patients with OPSCC has yet to be elucidated. Preuss et al conducted a retrospective case series of patients with OPSCC treated with surgery +/− radiotherapy and found no relation between EGFR expression and survival.(4) Many studies, however, have demonstrated the relationship between EGFR expression and poorer survival in patients treated with primary chemoradiotherapy.(3, 5, 6, 8, 13, 21) Further, the majority of patients in these studies demonstrate locoregional treatment failure, suggesting that tumors with high EGFR intensity may be radioresistant. In this surgical cohort, patients with high levels of EGFR expression also experienced poorer survival but the pattern of recurrence was different. The majority of patients treated surgically failed distantly, suggesting that surgery may result in better local/regional control but still requires an additional modality to address distant disease.
Most studies evaluating the role of EGFR expression in OCSCC combine OCSCC with other subsites of the head and neck.(6, 8, 13, 21) One study that looked solely at OCSCC found no association between EGFR expression and survival.(22) Our study suggests that patients with the highest score of EGFR intensity were associated with worsened survival and shorter time to recurrence when compared to those with lower EGFR intensity scores. This is consistent with our findings in the OPSCC cohort. A study with a larger sample size may be able to demonstrate a statistically significant difference in survival in the OCSCC cohort. Further, using a higher cut-point for the score that establishes EGFR positivity may also strengthen the ability to detect a difference with respect to survival.
Consistent with a small number of studies in the literature, EGFR expression was associated with smoking status in patients with OPSCC.(2, 23, 24) However, smoking status was not an independent risk factor for worsened survival in this study. The analysis was hampered by a low frequency of patients with no smoking history. This study also suggested that both extracapsular spread and perivascular invasion, when considered alone, were negative prognosticators for those with OPSCC. One of the mechanisms in which EGFR expression translates into advanced local/regional disease is through angiolymphatic invasion.(25) In this study, the addition of EGFR status to the survival model showed that EGFR expression was more predictive of survival than extracapsular extension and perivascular invasion, perhaps suggesting that EGFR may represent a range of invasive behaviors that encompass nodal metastasis, ECS and perivascular invasion.
This study found that high p16/low EGFR expression was associated with improved survival in an OPSCC surgical cohort whereas these markers were not useful for prediction in patients with OCSCC. The use of a combination of p16 and EGFR expression to predict survival in OPSCC has only been reported in patients treated with primary chemoradiotherapy.(2, 3) Based on the results of this study, we recommend that the patient with OPSCC should have the primary tumor stained for both EGFR and p16 regardless of treatment modality.
Conclusion
This study suggests that in a surgically treated cohort of patients with OPSCC, increased p16 expression predicts improved survival whereas increased EGFR expression predicts poorer survival. Used in combination, the two biomarkers may identify those who are likely to do well with treatment versus those who will do poorly. In addition, the prevalence of p16 differs significantly between the oral cavity and oropharynx subsites, whereas the prevalence of EGFR is similar between the two sites. For OCSCC, clinical nodal status, ECS, and perineural invasion remain more important markers of prognosis than p16 or EGFR.
Acknowledgments
The authors thank Tamara Miller, RN, BSN for assistance with prospectively following the patients in this cohort and Christopher Postma, BBA for assistance with manuscript preparation.
References
- 1.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 2.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120(8):1731–8. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 4.Preuss SF, Weinell A, Molitor M, et al. Survivin and epidermal growth factor receptor expression in surgically treated oropharyngeal squamous cell carcinoma. Head Neck. 2008;30(10):1318–24. doi: 10.1002/hed.20876. [DOI] [PubMed] [Google Scholar]
- 5.Numico G, Russi EG, Colantonio I, et al. EGFR status and prognosis of patients with locally advanced head and neck cancer treated with chemoradiotherapy. Anticancer Res. 30(2):671–6. [PubMed] [Google Scholar]
- 6.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6. [PubMed] [Google Scholar]
- 7.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26(19):3138–46. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74(2):553–61. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 10.Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, Kelsey KT. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8(10):3187–92. [PubMed] [Google Scholar]
- 11.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24(25):4170–6. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 14.Nichols AC, Faquin WC, Westra WH, et al. HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140(2):228–34. doi: 10.1016/j.otohns.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Rich JT, Milov S, Lewis JS, Jr, Thorstad WL, Adkins DR, Haughey BH. Transoral laser microsurgery (TLM) +/− adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119(9):1709–19. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: A united states multicenter study. Head Neck. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 17.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630–6. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 18.Ukpo OC, Pritchett CV, Lewis JE, Weaver AL, Smith DI, Moore EJ. Human papillomavirus-associated oropharyngeal squamous cell carcinomas: primary tumor burden and survival in surgical patients. Ann Otol Rhinol Laryngol. 2009;118(5):368–73. doi: 10.1177/000348940911800509. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104(3):336–44. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 20.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95(23):1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 21.Dassonville O, Formento JL, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol. 1993;11(10):1873–8. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 22.Smid EJ, Stoter TR, Bloemena E, et al. The importance of immunohistochemical expression of EGFr in squamous cell carcinoma of the oral cavity treated with surgery and postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65(5):1323–9. doi: 10.1016/j.ijrobp.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 16(4):1226–35. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentzen SM, Atasoy BM, Daley FM, et al. Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol. 2005;23(24):5560–7. doi: 10.1200/JCO.2005.06.411. [DOI] [PubMed] [Google Scholar]