Abstract
Prevalence of asthma and allergy has increased over the past 2–3 decades in Westernized countries. Despite increased understanding of the pathogenesis of asthma and allergic diseases, control of severe asthma is still difficult. Asthma is also associated with high prevalence of anxiety in particular adolescents. There is no effective treatment for food allergy. Food allergy is often associated with severe and recalcitrant eczema. Novel approaches for treatment of asthma and food allergy and comorbid conditions are urgently needed. Traditional Chinese medicine (TCM), used in Asia for centuries, is beginning to play a role in Western health care. There is increasing scientific evidence supporting the use of TCM for asthma treatment.
This review article discusses promising TCM interventions for asthma, food allergy and comorbid conditions and explores their possible mechanisms of action. Since 2005, several controlled clinical studies of “anti-asthma” herbal remedies have been published. Among the herbal medicines, anti-asthma herbal medicine intervention (ASHMI) is the only anti-asthma TCM product that is a US FDA investigational new drug (IND) that has entered clinical trials. Research into ASHMI’s effects and mechanisms of actions in animal models is actively being pursued. Research on TCM herbal medicines for treating food allergy is rare. The herbal intervention, Food Allergy Herbal Formula-2 (FAHF-2) is the only US FDA botanical IND under investigation as a multiple food allergy therapy. Published articles and abstracts, as well as new data generated in preclinical and clinical studies of ASHMI and FAHF-2 are the bases for this review. The effect of TCM therapy on food allergy associated recalcitrant eczema, based on case review, is also included.
Laboratory and clinical studies demonstrate a beneficial effect of ASHMI treatment on asthma. The possible mechanisms underlying the efficacy are multiple. Preclinical studies demonstrated the efficacy and safety of FAHF-2 for treating food allergy in a murine model. A clinical study demonstrated that FAHF-2 is safe, well tolerated, and exhibited beneficial immunomodulatory effects. A clinical report showed that TCM treatment reduced eczema scores and improved quality of life. Herbal interventions, ASHMI and FAHF-2 may be further developed as botanical drugs for treating asthma and food allergy. TCM may also be of benefit for comorbid conditions such as anxiety and recalcitrant eczema. More controlled studies are warranted.
In conclusion, novel approaches for treatment of asthma and food allergy and comorbid conditions such as anxiety and eczema are urgently needed. This article discusses promising interventions for such conditions from traditional Chinese medicine (TCM) and explores their possible mechanisms of action.
Keywords: Herbal interventions, traditional Chinese medicine, ASHMI, FAHF-2, asthma, food allergy
BACKGROUND
Asthma
Asthma affects approximately 25 million Americans,(1) and is the leading childhood chronic disease, affecting 9.6% of American children.(2) The prevalence of asthma in children increased 3 fold in the past 3 decades.(3;4) Airway hyperreactivity (AHR) to a variety of stimuli is a characteristic feature of asthma. In atopic subjects, re-exposure to the relevant antigen triggers an immediate early phase response (EPR), often followed by a late-phase response (LPR) 6–12 hours later.(5–7) The EPR is triggered by mast cell degranulation and release of mediators, such as histamine. (8;9) LPR is associated with infiltration of eosinophils.(5) However other cells including neutrophils, T cells and macrophages are also recruited during LPR. Chronic inflammation may result in airway remodeling, prominent features of which are subepithelial fibrosis, mucous cell hyperplasia/metaplasia and increased smooth muscle mass.(10–14) These structural changes may be responsible for the accelerated progressive decrease in lung function of some patients (15;16) and be significant determinants of AHR.(17) It has been demonstrated that memory T helper 2 (Th2) cells play a central role in the pathology of allergic asthma via releasing Th2 cytokines.(18) GATA-3 is a master Th2 transcription factor regulating Th2 cytokine expression.(19–22) It is also widely accepted that abnormal airway smooth muscle (ASM) function characterized by increased contraction and/or reduced smooth muscle relaxation, with or without airway remodeling, contributes to the excessive airway narrowing and AHR, characteristic of asthmatic attacks.(23) The complexity of this chronic disease is a great challenge to developing interventions that exhibits a broad spectrum of pharmacological actions on pathogenic changes of asthma. Current standard asthma management is primarily directed towards suppressing airway inflammation with inhaled corticosteroids (ICSs) and relieving bronchoconstriction with bronchodilators. This standard therapy controls symptoms in most, but not all, asthmatics. (24;25) Severe asthma is difficult to control. (24–26) Most asthmatic children still suffer attacks.(4) Relapse after therapy withdrawal is common.(27) ICSs are generally safe and efficacious with few side effects. However, prolonged use of ICSs, especially at higher doses has been accompanied by concern about both systemic and local side effects, (28;29) including osteoporosis, (28–31) hypothalamic-pituitary-adrenal axis suppression, (32) immune suppression resulting in increased susceptibility to infections such as esophageal candidiasis (33) and frequent upper respiratory tract infection in children, (34) development of cataracts in elderly patients,(35;36) mood changes,(37) and pharyngitis.(38) There is an urgent need to develop novel approaches for treatment of asthma.
[Callout] Inhaled corticosteroids (ICSs) are generally safe and efficacious with few side effects. However, prolonged use of ICSs, especially at higher doses has been accompanied by concern about both systemic and local side effects.
Food Allergy
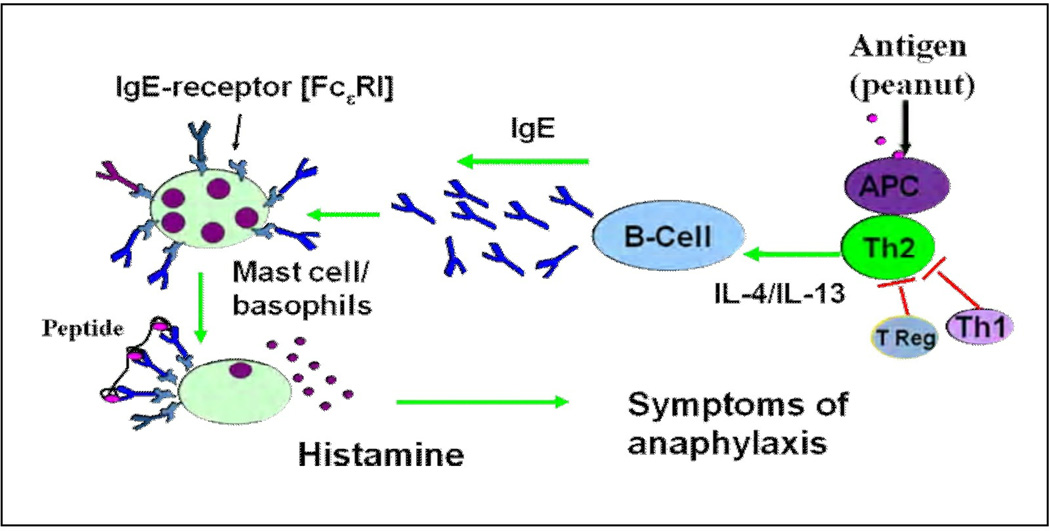
Food allergy (FA) is a significant health problem in Westernized countries, affecting 6% of children, and 3–4 % of adults in the US.(39) Peanut ( PN) and tree nut allergies account for the majority of fatal and near-fatal anaphylactic reactions in the U.S.(40;41) The prevalence of childhood peanut allergy (PNA) has tripled since 1997. The prevalence of PN and tree nut allergy in children younger than 18 years is 2.1%.(42) The reasons for the food allergy epidemic are unknown, but there are several hypotheses, including the hygiene hypothesis,(43) the overall allergy march(44) and epigenetics. (43;45–47) Although the precise mechanisms involved in the development of PNA have not fully elucidated, several mechanisms involved in PNA have been established in humans and in animal models. In genetically predisposed persons, ingestion of PN allergen induces predominant Th2 responses partially due to inadequate Th1 and /or Treg cross-control of Th2 responses. Th2 cytokines such as IL-4 and IL-13 promote B cell activation - switching to IgE production. These IgE antibodies then bind to the high affinity Fc epsilon receptor (Fc epsilonRI) on basophils and mast cells. In patients with food hypersensitivity, re-exposure to the relevant foods triggers degranulation of mast cells (basophiles) resulting in the release of histamine and other mediators (such as PAF), which provoke symptoms of anaphylaxis. Thereafter, if there is no further allergen exposure, some of the T cells and B cells will undergo apoptosis, but others will become long lived memory cells that might be the important driving force for the persistent food allergy (Fig 1). The signs and symptoms of an immediate reaction to food occur within minutes to 2 hours after ingestion. The symptoms and severity vary depending upon the routes and extent of mast cells degranulation. Mast cells are abundant in the skin, lungs, and gastrointestinal tract. In general, symptoms may be limited to the GI tract i.e. GI anaphylaxis manifested by vomiting, diarrhea, and abdominal pain. In other cases, the spread of allergen through the blood-stream leads to degranulation of mast cells in the skin which is manifested by hives, swelling, and pruritus; in the airway, manifested by choking, coughing, wheezing, and difficulty breathing. In the most severe cases, the cardiovascular system is also involved, with subsequent anaphylactic shock response.
Figure 1.
Immunopathogenesis of food allergy. This figure outlines some of the important aspects of the allergic cascade in PNA. Green arrows indicate positive regulation and red arrows indicate negative regulation.
At present, the primary treatments for PNA remain PN avoidance and appropriate use of rescue medication. In 1997, Nelson et al(48) investigated conventional allergen-specific immunotherapy and concluded that this therapy is not satisfactory because of the unacceptable number of adverse reactions and poor maintenance of tolerance afterwards.(48;49) Since 2009, several studies (50–52) reported results of a newer immunotherapy strategy- oral immunotherapy (OIT) for PNA. PN OIT appeared to increase the patients’ threshold to PN protein while on daily immunotherapy (desensitization),(50;52) but there is, as yet, no evidence that the protection persists post-therapy (tolerance induction). PN OIT also causes allergic reactions(50;52;53) although less severe than subcutaneous PN immunotherapy .(54;55) Anti-IgE therapy, that neutralizes free IgE, has shown only modest and transient benefits in food allergy.(56) Thus, development of improved and effective therapies for PNA is an ongoing challenge.
COMPLEMENTARY AND ALTERNATIVE MEDICINE IN UNITED STATES
An NIH/National Center for Complementary and Alternative Medicine (NCCAM) survey reported that more than 50% of Americans have used some form of CAM treatment. The chronic nature of allergic diseases and the lack of satisfactory treatment lead many families of children with allergy and asthma to seek CAM treatments.(57) Although the results of surveys on CAM use by children varies between the studies, the reported rates by children with asthma ranges from 33% to 89%.(57) A recent survey reported that a majority of pediatricians believe that their patients have a positive attitude toward, and are using CAM. Because patients consider CAM therapies to be safe and effective, pediatricians would also consider referring to a CAM practitioner and receiving more education on CAM(58) so that they might better discuss the implications of using these therapies and potentially improve adherence to the prescribed medication regimen, and allergy and asthma management.(59) Increased use of CAM by allergy patients is also a driving force for allergists to gain more insight into CAM. The American Academy of Allergy, Asthma and Immunology (AAAAI), Complementary & Alternative Practice in Allergy Committee conducted a survey of allergists in 2007. Of 450 responders, 80% were interested in learning more about CAM. The participants clearly reflected their interest in learning more about various CAM modalities, with 88% extremely interested in Herbal and Botanical Medicine. This highlights the needs to develop safe and effective CAM therapy. The AAAAI Natural Medicines Comprehensive Database provides assistance for allergists in their practice (http://www.aaaai.org/members.stm). The AAAAI CAM committee promotes CAM research in allergy and asthma and provides insight into integrative medicine practice.
TRADITIONAL CHINESE HERBAL MEDICINES FOR ASTHMA AND FOOD ALLERGY
Traditional Chinese medicine (TCM) has a long history of human use in China and other Asian countries, such as Korea and Japan, for treating and preventing disease, and is part of main stream medicine in these countries. Main components of TCM include herbal therapy, acupuncture, acupressure/massage, mind-body therapy and dietary therapy. Since TCM is part of Asian mainstream medicine, the cost is covered by insurance in these countries. TCM is also beginning to play a role in the health care system in the US. Acupuncture needles have been approved by the FDA as medical device; Herbal medicines are viewed as dietary supplements and their cost is not covered by insurance. In recent years the US FDA has provided guidance for investigating botanical drug products, including complex formulas containing several herbs, focusing on efficacy, safety and consistency.(60) The National Institutes of Health (NIH)/ NCCAM defines TCM as Whole Medical Systems.(61) The NCCAM/NIH provides grants to support clinical and basic research on CAM. Several publications including ours indicate that TCM has potential for treating asthma, (62) managing allergic rhinitis (63) and improving quality of life of atopic dermatitis patients.(64) Therefore, in the near future, some TCM remedies may become botanical drugs i.e. prescription drug via clinical investigation.
Since 2005, several controlled clinical studies of “anti-asthma” herbal remedies including anti-asthma herbal medicine intervention (ASHMI),(65) DCT,(17) and AST-1 (66) have been published. Of these ASHMI is the only anti-TCM herbal product that received US FDA IND approval and entered clinical trial in the US. Research into ASHMI’s active compounds is actively being pursued. Research on TCM herbal therapy for food allergy is limited. The food allergy herbal formula-2 (FAHF-2) also received US FDA IND approval and is undergoing clinical trials for multiple food allergies. Thus, this review is not a systematic review or meta analysis, but rather a focus on up-to-date translational studies of safety, efficacy and mechanisms of action of the herbal interventions, ASHMI and FAHF-2 for asthma and food allergy respectively in murine models and clinical trials. In addition, potential effects of TCM on food allergy-associated recalcitrant eczema, based on case review, have also been included.
DEVELOPMENT OF ANTI-ASTHMA HERBAL MEDICINE INTERVENTION AS BOTANICAL DRUG FOR TREATMENT OF ASTHMA
ASHMI is an extract of 3 Chinese herbal medicines: Ling-Zhi (Ganoderma Lucidum, G lucidum), Ku-Shen (Sophora Flavescentis, S flavescentis ), and Gan-Cao (Glycyrrhiza uralensis, G urilensis). It was derived from a 14 herb formula, MSSM-002, that has been used in China-Japan Friendship Hospital for treating childhood asthma. We tested ASHMI pharmacological and immunological actions and mechanisms in vivo and in vitro models and began to investigate its safety and efficacy in asthma patients (Table1). Animal research was conducted in accordance with the National Institutes of Health (US) guidelines for the care and use of laboratory animals and approved by Institutional Animal Care and Use Committee. The human studies have been approved by the Mount Sinai School of Medicine Institutional Review Board. Each subject provided informed consent. The followings are the major findings in preclinical and clinical studies related to ASHMI.
Table 1.
Major Known Actions and Mechanisms of ASHMI and FAHF-2
| ASHMI (IND) | FAHF-2 (IND) |
|---|---|
| Animal model (BALB/C mice) | Animal model (C3H/HeJ mice) |
| 1). Reduces bronchoconstriction, airway inflammation & remodeling; BALF histamine & LTC4, IL-4, IL-5, IL-13 levels and serum IgE levels(67;116) 2). Increases BALF IFN-γ, IL-10 and TGF-β levels (116) 3). Long-lasting post treatment tolerance to antigen exposure (117) 4. High safety profile(116) |
1). Completely prevents peanut anaphylaxis and histamine release (104) 2). Reverses established peanut allergy(105) 3). Pharmacological and synergistic effect of constituents in FAHF-2(103) 4). Prolonged post-treatment protection against peanut anaphylaxis via CD8+ T cells(106) 6). High safety profile(104) |
| Mechanistic studies | Mechanistic studies |
|
Mouse cell lines and tracheal rings 1). G. Uralensis flavonoids inhibits Th2 cytokine production by memory Th2 cells (D10 G4.1, Th2 clone) (84) 2) G. lucidum triterpenoids inhibit TNF-α production by mouse macrophage (RAW267.4)(118) 3). G. Uralensis flavonoids inhibit TNF-α production by brain microglial cells (BV2)(119) S. flavescenes flavonoids inhibit Ach induced ASM contraction(93) |
Mouse and rat cell lines 1). FAHF-2 exhibits long lasting beneficial effects on T cells and B cells in vivo(106) 2). FAHF-2 suppresses IgE-mediated proliferation and FcεR1 expression, and histamine release by MC/9 mast cells(107) 3. Phellodendri alkaloids inhibit IgE-mediated degranulation of mast cell/basophils cells (RBL-2H3) via blocking the Syk signaling pathway(107) |
|
Human cell lines and primary cells: 1). G. uralensis flavonoids inhibit eotaxin production by human fetal lung fibroblasts (HFL-1)(120) 2). S. flavescenes flavonoids inhibit IgE production by human myeloma cells (U266)(121) 3). ASHMI increases PGI2 production by human airway smooth muscle cells (HASM)117) 4). ASHMI inhibits IL-5, and increases IL-10 production by PBMCs from asthma patients (99) |
Human cell lines and primary cells: 1). Phellodendri alkaloids inhibit IgE production by human B cells(107) 2) FAHF-2 alkaloid rich fraction suppress human primary mast cell deganulation in response to IgE analog and complement stimulation(107) |
| Clinical trials | Clinical trials |
| 1). RCT, dose escalation safety study. Adult 18 individuals (ASHMI, n=12; placebo, n=6); one week treatment duration(97) Major findings: ASHMI is safe and well tolerated 2). RCT, 91 individuals (n=45 on ASHMI and n=46 on prednisone, 4 week treatment duration(65) Major findings: a. Like prednisone, significantly improved lung function (FEV1 and PEF) (but less effective than prednisone); Reduced symptom scores and β2 agonist use, suppressed IgE and Th2 responses; no adverse effects on the major organs; b. Unlike prednisone, ASHMI had no significant effect on body weight, no negative effects on adrenal function; a beneficial immunoregulatory effect on Th1/Th2 balance 3). RCT, 51 children, ages 5–11yrs (ICS+ASHMI, n=25; ICS+Placebo, n=26 [ICS alone]; 3 month treatment duration(98) Major findings: ICS+ASHMI was safe and well tolerated, more effective than ICS alone in improving clinical symptom |
1). RCT, acute phase I. 18 individuals (FAHF-2, n=12; placebo, n= 6), ages 12–45 yrs with peanut and/or tree nut, fish and shellfish allergy; 1 week treatment duration(122) Major findings: a. Safe and well tolerated b. Ex vivo immunological study showed FAHF-2 treatment reduced IL-5, no changes on IFN-γ by PBMCs compared to placebo c. In vitro immunological study showed reduction of IL-5 and induction of IL-10 by PBMCs after 3 day culture with FAHF-2 2. Open label extended phase I, FAHF-2, n=14, 6 months treatment duration(123) Major findings: a. Safe and well tolerated b. Inhibited ex vivo basophil activation after 4 and 6 months of treatment c. Trend of reduction of basophil and eosinophil numbers Phase II study (RCT) is ongoing |
IND, investigational new drug; ICS, inhaled corticosteroid (budesonide); β2AR, β2 adrenergic receptor; PGI2, prostaglandin I2; ASM, airway smooth muscle; PBMCs, peripheral blood mononuclear cells; PEF, Peak expiratory flow; FEV1, forced reparatory volume of the first second, FEV1% VC; RCT, randomized controlled trial. Ach, acetylcholine; S. flavescenes, Sophora flavescenes; G. uralensis, Glycyrrhiza uralensis; G. Lucidum, Ganoderma Lucidum.
Preclinical Studies: Actions and Mechanisms of Actions of Anti-asthma Herbal Medicine Intervention in Animal Model
Broad Actions of Anti-asthma Herbal Medicine Intervention in Animal Model
Zhang et al [21] investigated the pharmacological and immunological actions of ASHMI in a murine asthma model. In this study BALB/c mice were sensitized weekly intraperitoneally (i.p.) twice with ovalbumin (OVA) and alum followed by 3 weekly intratracheal(i.t.) challenges with OVA, then 2 consecutive daily challenges (4th and 5th challenges) 4 weeks later. To determine whether ASHMI affected the progression of chronic asthma, ASHMI was administered intragastrically (i.g.) twice daily for 6 weeks. ASHMI abolished early-phase airway responses, which was associated with significantly reduced plasma and bronchoalveolar lavage fluid (BALF) histamine and leukotriene C4 levels, and decreased plasma OVA-specific IgE levels. It was also shown that late-phase airway responses were prevented, which was evidenced by significantly reduced numbers of BALF eosinophils, decreased collagen content of lungs, fewer goblet cells in the airways, as well as reduced levels of the Th2 cytokines, IL-4, IL-5, IL-13 but increased IFN-γ, IL-10 and TGF- ß levels in BALF. A potential direct effect of ASHMI on airway constriction was demonstrated by inhibition of acetylcholine (Ach) induced constriction of murine tracheal rings ex vivo. This effect was not mediated by ß2-adrenergic receptor stimulation. ASHMI also increased production of PGI2, indicating that the potent smooth muscle relaxer PGI2 played a role in ASHMI-mediated airway relaxation.
Antiasthma Herbal Medicine Intervention Is Effective In Later Onset Asthma
Busse et al (67) subsequently investigated the effects of ASHMI on characteristics of allergic asthma in an aged mouse model of late onset asthma. Although asthma is typically characterized as a childhood onset disease, it also develops later in life. Airway disease may be more severe in later-onset asthma compared with earlier-onset disease;(68) hospitalization and mortality rates are highest, and there is also a higher systemic steroid requirement in patients older than 65 years.(69;70) Thus, later-onset asthmatic patients may be at increased risk for oral corticosteroid induced potential adverse effects. Osteoporosis, increased fracture risk, muscle weakness, glucose intolerance, and easy bruising, are of particular concerns in older patients. Therefore, developing non-steroid interventions for late onset asthmatic patients is important. In this study ASHMI treatment of young and aged OVA mice resulted in significantly decreased lung inflammation, airway mucous cell metaplasia, and messenger RNA copy numbers of the mucin gene MUC5AC, as well as reduced collagen production.
Antiasthma Herbal Medicine Intervention Provides More Persistent Benefits than Dexamethasone
Srivastava et al [23–24] evaluated the persistence of ASHMI beneficial effects following discontinuation of therapy as compared to that of OVA challenge induced airway hyper-reactivity (AHR) and pulmonary eosinophilic inflammation that did not re-occur following OVA re-challenge up to 8 weeks post-therapy. Decreased allergen-specific IgE and Th2 cytokine levels, and increased IFN-γ levels also persisted at least 8 weeks post-therapy. In contrast, Dexamethasone (Dex)-treated mice exhibited similarly reduced airway inflammation, Th2 cytokines and OVA-specific IgE levels immediately after 6 weeks therapy but were not protected at 8 weeks post-therapy. IFN-γ levels in BALF, and serum corticosterone levels were reduced by Dex treatment. To determine the contribution of IFN-γ to ASHMI effects, mice were given IFN- γ neutralizing antibody concomitantly with ASHMI. ASHMI effects were eliminated by neutralization of anti-IFN-γ antibody, but not anti-TGF-ß antibody during therapy.
[Callout] In studies involving animal models of airway hyperreactivity, Srivastava et al showed that anti-asthma herbal medicine intervention (ASHMI) provides more persistent benefits than dexamethasone.
Mechanistic Studies: Chemical and Biological Characterization of Active Compounds in Antiasthma Herbal Medicine Intervention
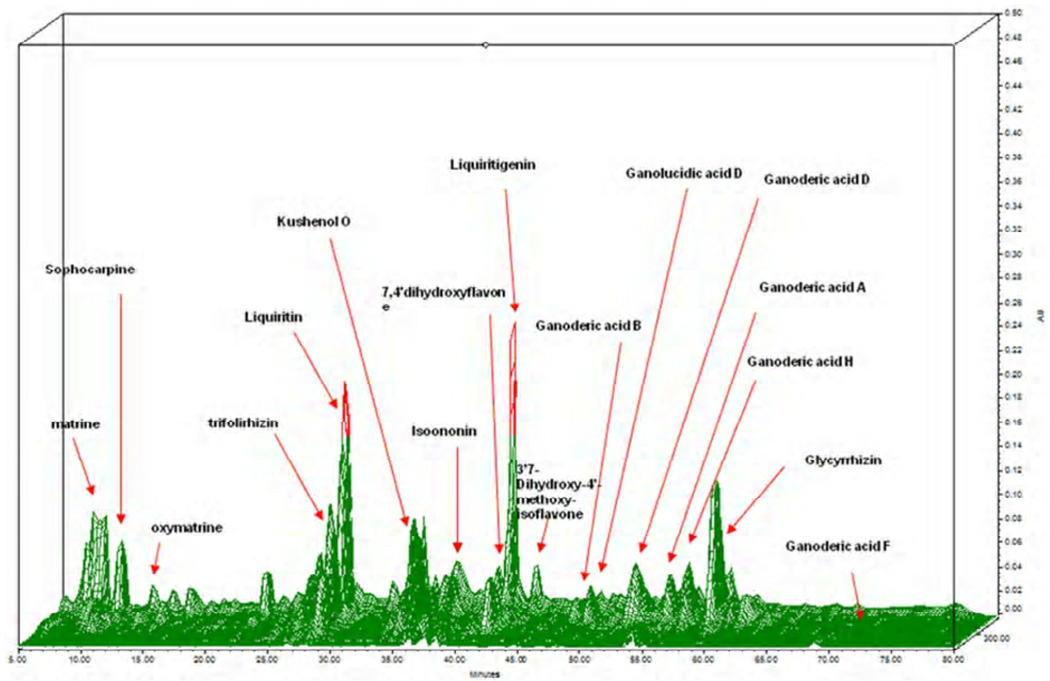
Research on herbal medicine development presents unique challenges because TCM formulas frequently contain many unknown components. The US FDA has issued guidelines for industrial botanical products focusing on safety and efficacy.(71) Standardization of raw herbs, the manufacturing process, and the final product, as well as the safety data on limit testing of pesticides, heavy metals, and microbials, are key components in obtaining approval. Use of high-performance liquid chromatography (HPLC) is recommended by the FDA as a means of monitoring the consistency of raw herbs and the final product. We generated HPLC fingerprint of ASHMI and characterization of corresponding peaks and chemical markers (Fig 3). However, the active compounds responsible for ASTHMI therapeutic effect are largely unknown.
Figure 3.
3D- HPLC-DAD fingerprint of the ASHMI extract. Peak analysis and assignment were performed using standard samples and/or LC-MS method. Matrine, sophocarpine, oxymatrine, kushenol O, trifolirhizin correspond to the compounds from Radix Sophorae Flavescentuis. Ganoderic acid B, D, A, H, and F and ganolucidic acid D correspond to Ganoderma lucidum; Liquiritin, liquiritigenin, 7, 4'dihydroxyflavone, Isoononin, 3’7-Dihydroxy-4’-methoxy-isoflavone, and glycyrrhizic acid correspond to compounds from Radix glycyrrhiza. D- HPLC-DAD, 3 dimensional high high-performance liquid chromatography with diode array detection; LC-MS, Liquid chromatography -Mass Spectroscop(116)
Isolation and identification of active constituents will improve herbal product quality control and lead to better understanding of the mechanisms of action. Modern chromatographic techniques, such as HPLC, together with mass spectrometry and nuclear magnetic resonance (NMR) techniques make isolation and purification and identification of chemical constituents from complex mixtures feasible. (72–74) A phytochemical database of Chinese herbal constituents and bioactive plant components has recently been established .(75) Of these phytochemical classes, alkaloids, polyphenols, and terpenes are the most frequently identified biologically active compounds in TCMs.(76) However, because most previous phytochemical studies of herbal medicines focused mainly on isolation and identification of new compounds, and because limited amounts of material were isolated, few isolated compounds are commercially available. Therefore, studies on pharmacological activities and mechanisms of action relevant to asthma are limited. The critical step to identifying active compounds in a mixture is isolation of compounds. Given our understanding of pathological mechanisms of asthma, we have focused on isolation and identification of active compounds that contribute to ASHMI anti-inflammatory effects and inhibit airway smooth muscle (ASM) contractility. The major findings are summarized below:
Glycyrrhiza Uralensis Flavonoids Inhibit Eotaxin and Th2 Cytokine Production
Gan Cao, Glycyrrhiza uralensis (G uralensis) commonly called “licorice” is one of the most commonly used herbs in TCM. Gan Cao means sweet herb. Licorice products are most often consumed as flavoring and sweetening agents in food products. Based on phytochemistry, G uralensis contains mainly terpenoids and flavonoids. The licorice triterpenoid glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 and stat 6 by a human lung fibroblast cell line(77;78). However, their effects on asthma have not been reported and other potential anti-inflammatory constituents such as flavonoids in G. uralensis have not been fully investigated, partially due to lack of commercially available flavonoid compounds. Eosinophilic airway inflammation is a major feature of allergic asthma and eotaxin is involved in recruitment of eosinophils to sites of antigen-induced inflammation in asthmatic airways. (79) Because in humans, lung fibroblasts are the major source of eotaxin,(80) inhibition of eosinophil recruitment by suppression of fibroblast eotaxin production is a potentially valuable approach to pharmacological intervention in asthma. Our group has conducted a systematic bioassay-guided purification of G. uralensis which yielded 5 flavonoids: liquiritin, liquiritigenin, isoliquiritigenin, 7, 4′-dihydroxyflavone and isoononin. The structures of the compounds were established by 1H, 13C NMR and LC-MS studies.(81) The potential ability of these isolated pure compounds, and of glycyrrhizin, to inhibit eotaxin secretion by human fetal lung fibroblasts (HFL-1) was tested. We found that G uralensis flavonoids inhibited eotaxin secretion were more potent than triterpenoid glycyrrhizin (commercially available) and that liquiritigenin, isoliquiritigenin, and 7, 4′-dihydroxyflavone being more effective than liquiritin. A dose response study showed the IC50 concentrations of liquiritigenin, isoliquiritigenin, and 7, 4′-dihydroxyflavone were 4.2, 0.92 and 0.21 µg/mL, respectively. (82)
Consequently, we investigated effects of these 3 flavonoids on a Th2 cell line ( D10 G4.1) that produces robust Th2 cytokines to antigen ( conalbumin) stimulation, and on the in vivo effect of 7, 4′-dihydroxyflavone. We found that 7, 4′-dihydroxyflavone was also the most potent licorice flavonoid in suppression of Th2 cell cytokine production by D10 cells, which was associated with suppression of GATA3 expression. 7, 4′-dihydroxyflavone also showed inhibition of eosinophils and Th2 cytokines without suppression of IFN-γ in an animal model of asthma. Abstracts describing these results have been published (83;84).
Glycyrrhiza Uralensis Flavonoids Inhibit Microglial Cell TNF-α Secretion and Increases Brain Derived Neurotropic Factor Secretion
Anxiety and depression are often associated with poor asthma control and poor quality of life.(85–90) The prevalence of serious psychological distress among adults with asthma (7.5%) is more than double that of the overall US population (3.0%) and adults without asthma (2.6%). (91) Twenty to 40% of adolescents with asthma experience significant symptoms of anxiety.(92) Inflammatory cytokines such as TNF-α in the peripheral and central nervous system are associated with anxiety. Some conventional medications used to treat asthma can enhance anxiety/depression. (37) ASHMI has been shown to decrease inflammation in asthma patients and a murine asthma model, and suppression of TNF-α production by macrophages in vitro. Brain derived neurotropic factor (BDNF) has been suggested to be beneficial for anxiety/depression. We determined the effects of the three herb formula ASHMI and purified compounds on production of TNF-α and BDNF by mouse microglial cells. In this study, BV-2 mouse microglial cells (1x 105) were cultured in DMEM-10% FBS media for 48 hours until confluent. The cells were then pre-treated with various doses (15.6–500µg/mL) of ASHMI or extracts of 3 individual herbs in ASHMI for 8 hours and stimulated with LPS (500 ng/mL) for 24 hours. In addition individual compounds isolated from Gan-Cao (GC1-5) were also tested (0.78–25µg/mL). TNF-α levels in the supernatants were measured by ELISA and viability was determined by MTT assay. ASHMI produced dose dependent inhibition of TNF-α secretion (IC50:116.8µg/mL). Of the three herbs Gan-Cao produced significant inhibition of TNF-α at all doses tested ( IC50:16.9µg/mL). Ling-Zhi at 500 µg/ml and Ku-Shen at 250 µg/ml and 500 µg/ml showed significant inhibition of TNF-α levels. Of the 5 flavonoids isolated from Gan-Cao, GC-2, GC-3, and GC-4 showed dose dependent inhibition of TNF-α, and IC50 values were 1.18µg/mL, 2.21µg/mL and 4.86µg/mL, respectively, GC3 being more effective. GC3 also inhibited NF-κB and induction of BDNF. ASHMI and its licorice flavonoids have an anti-inflammatory effect on mouse microglia cells and enhance BDNF production. ASHMI may provide additional benefits for patients with asthma and comorbid anxiety/depression. Clinical studies are warranted.
Effects of Active Compounds from Antiasthma Herbal Medicine Intervention on Airway Smooth Contraction
Since we previously showed that ASHMI has a direct effect on airway smooth muscle contractility, to determine the active continuants responsible for this action, we tested effects of 3 individual constituents in ASHMI on constriction of murine tracheal rings isolated from ovalbumin sensitized and challenged mice (asthmatic mice). Only Ku Shen (Sophora flavescens Ait), exhibited potent inhibition of airway smooth muscle (ASM) contraction to acetylcholine provocation. A dichloromethane extracted fraction of this herb, containing mainly flavonoids, retained the inhibitory effect of Ku Shen on ASM contraction. We therefore focused on this fraction and generated 19 sub-fractions using PreP- HPLC, three of which contains major peaks named 10S, 11S and 12S. 11S significantly suppressed tracheal ring contraction induced by 10−6M to 10−4M Ach compared with that in physiological salt solution (PSS) (sham) treated tracheal rings (p<0.01–0.05). 12S only showed a minor effect and 10S showed no inhibitory effect on tracheal ring contraction at any tested dose of Ach. This study indicated that 11S might be the major player in ASHMI responsible for inhibition of Ach induced ASM hypercontractility and might be a novel ASM relaxation agent for potential asthma treatment.(93) Further characterization of this compound by NMR is underway.
Clinical Studies
Wen et al (94) reported the first double-blind, randomized, placebo-controlled trial investigating the efficacy and tolerability of an anti asthma herbal medicine intervention ASHMI in 91 patients 18–60 years of age with moderate-to-severe symptomatic asthma as compared to oral prednisone therapy. Subjects in the ASHMI group (45 patients) received oral ASHMI capsules (4 capsules, tid, 0.3 g/capsule) and placebo tablets similar in appearance to prednisone. Subjects in the prednisone group (46 patients) received oral prednisone tablets (20mg qd in the morning) and “ASHMI placebo capsules” for 4 weeks. Treatment was administered daily over 4 weeks. This study found that post treatment lung function (FEV1 and peak expiratory flow values) was significantly improved in both ASHMI (64.9± 6 3.6 to 84.2± 6 5.0; P < .001) and prednisone (65.2± 6 3.7 to 88.4 ± 6 8.0; P < .001) groups. The improvement was slightly but significantly greater in the prednisone group (P < .05). There was a significant and a similar degree of reduction in clinical symptom scores in both treated groups (median [range], ASHMI, 5.0 [4–8] to 2.0 [0–4]; P < .001; prednisone, 5.0 [4–7] to 2.0 [0–4]; P < .001), use of β2-bronchodilators (median [range], ASHMI, 4.7 [3.5–5.7] to 0.9 [0.14–2.3]; P <.001; prednisone, 4.7 [3.5–5.6] to 0.6 [0.3–1.0]; P <.001). ASHMI had no significant effect on body weight (increases in body weight post-therapy 2.8 kg in the prednisone group vs 0.8 kg in ASHMI). Further serum IgE levels and Th2 cytokine levels were significantly reduced in both groups. However, in contrast to prednisone, ASHMI significantly increased serum IFN-γ levels.
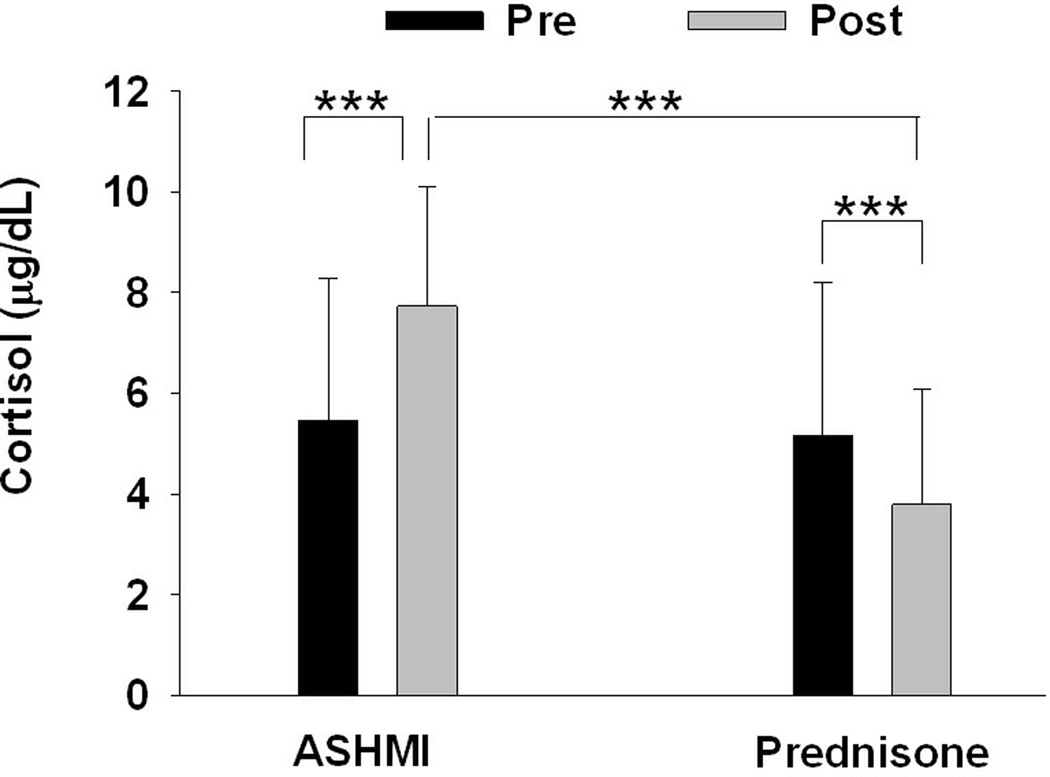
Additional interesting finding in the study by Wen et al was a beneficial effect of ASHMI treatment on adrenal function. Pretreatment cortisol levels were slightly below normal (6–23 micro gram/dl) in both groups. After treatment, subjects in the prednisone treatment group showed a significant reduction in serum cortisol levels after treatment (5.1 ±3.0 to 3.7 ±2.3 micro gram/dl; P < .001). In contrast, patients in the ASHMI treatment group showed increased levels of serum cortisol (5.4 ± 2.8 to 7.7 ± 2.3 micro gram/dl; P < .001; Fig 2), which were within the normal range. The difference between groups was statistically significant (P < .001). Thus, ASHMI restored adrenal function.
Figure 2.
Effect of treatment with ASHMI or Prednisone on serum cortisol levels (7:30–8:30 am) determined by radioimmunoassay. To ensure consistency of the results, all blood samples were drawn between 7:30–8:30 A.M. (before treatment and 48 hours after treatment). Data are Means±SD. *** indicates p<0.001. Normal level are8–23 µg/dL
[Callout] In a study comparing ASHMI with prednisone in the treatment of moderate-to-severe symptomatic asthma, in which the subjects showed pretreatment serum cortisol levels slightly below normal, prednisone significantly reduced cortisol levels further while ASHMA increased them to the normal range. ASHMI thus restored adrenal function.
This result might be attributed to glycyrrhizin (a component of Gan-Cao), which affects the conversion of cortisol to cortisone, by inhibition of the 11-β-hydroxysteroid dehydrogenase enzyme activity. (95) Gan-Cao has been used for adrenal insufficiency.(96) However, it is also possible that the increase in cortisol levels may be due to the lack of suppression of adrenal functions. Further research is required to understand the precise mechanisms. No significant adverse effects were observed. All hematological, electrocardiogram and liver and kidney function test results were normal in both groups. Thus ASHMI appeared to be effective and well tolerated, and may offer benefits comparable to standard corticosteroid therapy for some patients.
Kelly-Piper(97) et al. conducted a double blind, placebo controlled, dose escalation safety trial (phase I) in 18 adult asthma individuals and showed that ASHMI is safe and well tolerated. All subject’s laboratory test (complete blood counts and chemistry) results were within the normal range.
Busse et al(98) reported a study to determine the safety, tolerability and immunological benefits of complementary ASHMI in children 5–14 years of age with persistent asthma with or without allergic rhinitis. Subjects were randomly assigned to receive standard inhaled corticosteroid treatment (Budesonide -Pulmicort Turbohaler) plus ASHMI as complementary therapy (Standard+ ASHMI group, n=28) or inhaled cortical steroid treatment plus placebo (standard + placebo, n=28). 51 asthma patients completed the trial including 26 patients in the complementary ASHMI group and 25 from the standard group. We found that patients in the complementary ASHMI group showed significantly greater reduction of serum total IgE (p<0.05) and higher serum levels of IFN-γ (P<0.001) and cortisol levels (p<0.05) than group after 3 months of treatment as compared to the Standard+ placebo group.
Patil at al (99) showed ASHMI in vitro treatment of peripheral blood mononuclear cells (PBMCs) from patients with moderate to severe asthma (n=31) for 72 hours significantly suppressed IL-5 production without affecting IFN-γ and cell viability, and increased IL-10 production to antigen stimulation compared to untreated PBMCs. This in vitro study provided direct evidence that ASHMI has immunomodualtory effects. A phase II study involving 60 patients is underway.
Taken together these studies showed that ASHMI was safe and well tolerated in human trials and showed beneficial clinical and immunological effects. However, the study size is generally small. ASHMI also showed multiple beneficial effects on asthma related pathological mechanism in animal model of asthma. Identification of active compounds in ASHMI will enhance our understanding of the pharmacological mechanisms of ASHMI and accelerate the process for developing ASHMI as botanical drug in the US. Asthma is a serious disease, the lack of control of which can be very dangerous; until very high-level DBRCTs are conducted on ASHMI, clinicians should stick with current pharmaceutical guidelines, possibly with the incorporation of herbal therapeutics.
DEVELOPMENT OF FOOD ALLERGY HERBAL FORMULA-2 AS BOTANICAL DRUG FOR TREATMENT OF FOOD ALERGY
The term “food allergy” is not described in the classical TCM literature, but the food allergy-like phenomenon has long been recognized. Wu Mei Wan is a classical Chinese formula for treating intestinal parasite infection -first documented by Dr. Zhang Zhong Jing in a classical Chinese medical text compiled ~2000 years ago. This formula along with other classical TCM formulas has been translated into English by Bensky et al “Chinese Herbal Medicine: Formulas and Strategies”.(100) The indications of Wu Mei Wan include intermittent attacks of abdominal pain, a stifling sensation, irritability, and warmth in the chest (anxiety), accompanied by vomiting, diarrhea after eating, and cold hands and feet. Based on our understanding on food allergy clinical expression and immunopathological mechanisms, and TCM formula principle and updated research in TCM and allergy, we developed food allergy herbal formula-1 (FAHF-1) derived from Wu Mei Wan, containing 11 herbs and then refined that formula to make FAHF-2 that contains 9 herbs. FAHF-2 pharmacological and immunological actions and mechanisms were tested in in vivo and in vitro models and phase I studies were conducted in patients with PN and/or tree nut, fish, shell fish and sesame allergies (Table1). Animal research was conducted in accordance with the National Institutes of Health (US) guidelines for the care and use of laboratory animals and approved by Institutional Animal Care and Use Committee. The human studies have been approved by the Mount Sinai School of Medicine Institutional Review Board. Each subject provided informed consent. The followings are the major findings in preclinical and clinical studies related to FAHF-2.
Preclinical Studies
Effect of Food Allergy Herbal Formula-2 on Peanut Allergy in Animal Model
PNA is potentially life threatening. We previously found that an herbal formula, food allergy herbal formula (FAHF)-1, blocked PN-induced anaphylaxis in a murine model when challenged immediately posttherapy.(101) We then tested whether FAHF-2, an improved herbal formula, from which 2 herbs, Zhi Fu Zi (Radix Lateralis Aconiti Carmichaeli Praeparata) and Xi Xin (Herba Asari), were eliminated, is equally effective toFAHF-1, and if so, whether protection persists after therapy is discontinued. The raw herb quality was ascertained according to the standards required by the Pharmacopoeia of the People’s Republic of China.(102) Based on organoleptic and microscopic examination, the raw herbs used in FAHF-2 were identified as Prunus mume, Zanthoxylum schinifolium, Angelica sinensis, Zingiber officinalis, Cinnamomum cassiae, Phellodendron chinense, Coptis chinensis, Panax ginseng, Ganoderma lucidum. The quality and ratio of herbs used has been described previously. (103) Product quality was monitored by HPLC fingerprinting according to the FDA’s Guidance for Industry Botanical Drug Products. (71) In this study, mice allergic to PN treated with FAHF-2 for 7 weeks were challenged 1, 3, or 5 weeks posttherapy. The results showed that after challenges, all sham-treated mice developed severe anaphylactic signs, significant decrease in rectal temperatures, significantly increased plasma histamine levels, and marked vascular leakage. In contrast, no sign of anaphylactic reactions, decreased core body temperatures, or elevation of plasma histamine levels was observed in FAHF-2–treated mice in 5 separate experiments. IgE levels were significantly reduced by FAHF-2 treatment, and remained significantly lower as long as 5 weeks post therapy. Splenocytes from FAHF-2–treated mice showed significantly reduced IL-4, IL-5, and IL-13, and enhanced IFN-g production to recall PN stimulation in vitro. FAHF-2 also showed high safety margin.(104) We also found FAHF-2 induced PN tolerance in mice after establishment of peanut allergy. This effect was associated with up-regulation of interferon-gamma by CD8+T cells.(105)
[Callout] Food allergy herbal formula-2 (FAHF-2) prevents and reverses peanut allergy in an animal model of peanut anaphylaxes.
Pharmacologic and Immunologic Effects of Individual Herbs in Food Allergy Herbal Formula-2 on Peanut Allergy
We then investigated the pharmacological actions of individual herbs comprising FAHF-2 on PN-induced anaphylactic reactions in a murine model of PNA, and determined if all nine herbs were necessary to prevent anaphylactic reactions, or if a simplified formula containing fewer herbs would be equally effective. Our results showed that some individual herbs reduced PN-induced anaphylactic symptoms but no single herb offered full protection from anaphylactic symptoms equivalent to FAHF-2 (Table 2). The herbs had highly variable effects on histamine release, as well as PN-specific serum IgE and IgG2a levels. The herbs also had variable effects on IL-4, IL-5 and IFN-γ levels. A simplified formula comprised of only the most efficacious individual herbs showed only partial efficacy and was not able to reproduce the effects of FAHF-2. This finding suggested that component herbs of FAHF-2 may be working synergistically to produce the curative therapeutic effects produced by the whole formula, which appears to be the best option for future clinical trials.(103)
Table 2.
Effect of FAHF-2 and its Individual Constituents on PN Induced Anaphylaxis
| Herbal Treatment |
Anaphylactic reactions n/total |
% of mice with a reaction |
Anaphylactic score Median (range), p values vs Sham |
|---|---|---|---|
| Sham | 9/11 | 81 | 2 (0–4), |
| FAHF-2 | 0/8 | 0 | 0 (0), p<0.001 |
| HB | 1/4 | 25 | 0 (0–2), p= 0.008 |
| GJ | 3/5 | 60 | 2 (0–2), p= 0.058 |
| HL | 3/5 | 60 | 2 (0–4), p= 0.058 |
| CJ | 5/8 | 63 | 2(0–4), p= 0.140 |
| LZ | 3/4 | 75 | 2.5 (0–3), p= 0.590 |
| WM | 6/7 | 86 | 2 (0–4), p= 0.387 |
| GZ | 6/7 | 86 | 2 (0–3), p= 0.330 |
| DG | 5/5 | 100 | 3 (2–3), p= 0.640 |
| RS | 4/4 | 100 | 3 (2–3), p= 0.386 |
| Naive | 0/15 | 0 | 0 (0–0), p<0.001 |
Anaphylactic reaction rates and median scores obtained 30 minutes after oral PN challenge using the scoring system: 0 - no signs; 1 - scratching and rubbing around the snout and head (mild reaction); 2 - pufFiness around the eyes and snout, diarrhea, pilar erecti, reduced activity, and/or decreased activity with increased respiratory rate (moderate reaction); 3 - wheezing, labored respiration, cyanosis around the mouth and the tail (severe reaction); 4 - no activity after prodding, or tremor and convulsion (near fatal reaction); 5 - death.Sham, PN allergic mice fed water; FAHF-2, food allergy herbal formula -2; HB, Huang Bai; GJ; Gan Jang; HL, Huang Liang; CJ, Chuan Jiao; LZ, Ling Zhi; WM, Wu Mei; GZ, Gui Zhi; DG, Dang Gui; RS, Ren She.
Food Allergy Herbal Formula-2 Silences Peanut-induced Anaphylaxis for Prolonged Post-treatment Period via IFN-γ-producing CD8+ Cell
Because, in mice, FAHF-2 treatment reestablished tolerance to PN after PN hypersensitivity was fully established, and because this effect persisted for 4 weeks post therapy,(105) we sought to determine if FAHF-2 mediated protection could be extended long-term and explored the mechanisms underlying its persistent immunomodulatory effects. In this study, PN-allergic mice received FAHF-2 daily orally for seven weeks, and then received seven oral PN challenges at 4 to 10 week intervals over 36 weeks. For mechanistic studies, some mice received CD4+ or CD8+ T cell-depleting antibodies or interferon-γ neutralizing antibodies. Anaphylactic symptoms, body temperatures and plasma histamine levels were recorded following each challenge, and PN-specific immunoglobulin levels and cytokine profiles of splenocytes, mesenteric lymph node cells and purified CD4+ and CD8+ T cells were determined.
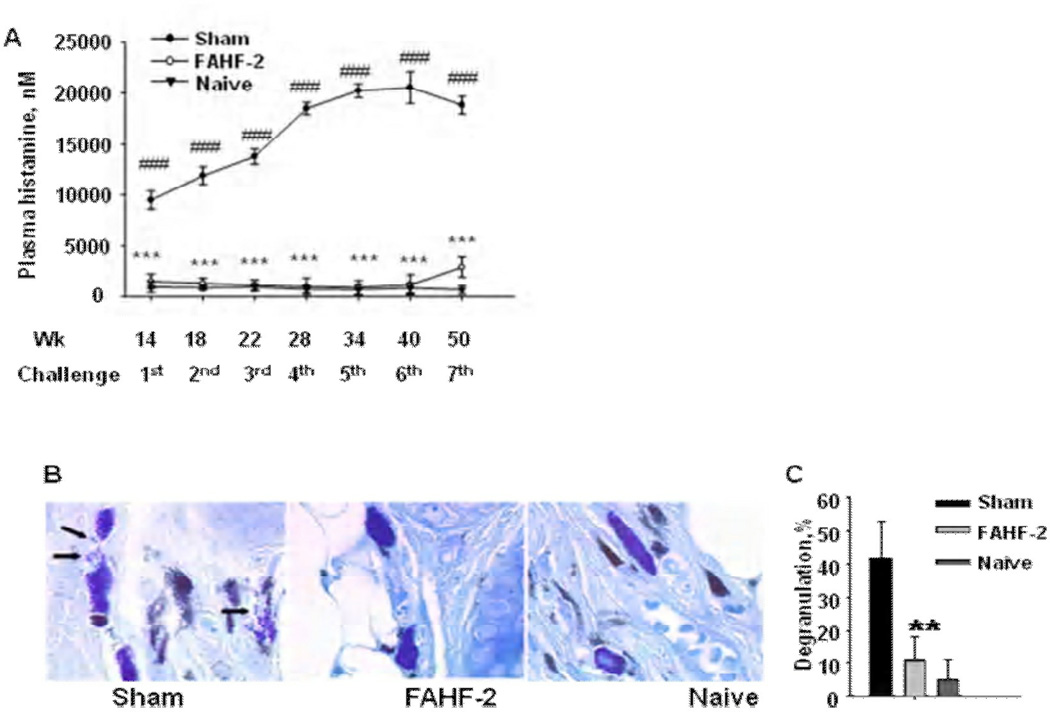
The results showed that FAHF-2 treatment protected mice from anaphylaxis for over 36 weeks after discontinuing treatment (Table II). PN-specific IgE levels were reduced up to 50%, whereas IgG2a levels were increased up to 60%, and these effects persisted overtime. Clinical protection was also accompanied by markedly suppression of plasma histamine levels which was associated with significant suppression of mast cell degranulation (Fig 4). At week 50, the experiment was terminated and mice were sacrificed. Splenocytes and mesenteric lymph node cells from these mice showed significant suppression of Th2 cytokines (IL-4, IL-5 and IL-13) and enhancement of IFN-γ production (Fig 5). Furthermore, Th2 cytokine production by CD4+ T cells from FAHF-2-treated mice was reduced up to 75%, whereas CD8+ T cell IFN-γ production was increased by up to 85% at the final challenge. Neutralization of INF-γ and depletion of CD8+ T cells markedly attenuated FAHF-2 efficacy. (106) This study showed that FAHF-2 provides long-term protection from anaphylaxis by inducing a beneficial shift in allergen-specific immune responses mediated largely by elevated CD8+ T cell interferon-γ production.
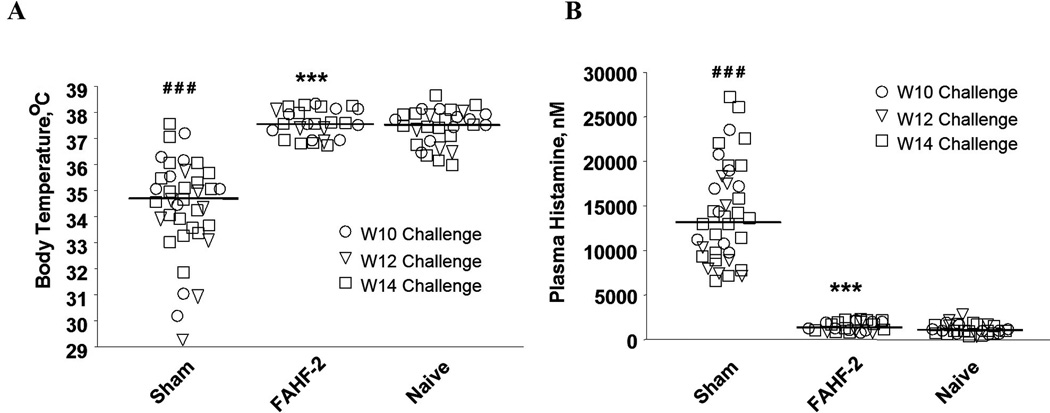
Figure 4.
(A) FAHF-2 prevented a drop in core body temperatures. Rectal temperatures were measured 25 min following i.g. PN challenge in mice challenged at wks 10 (○), 12(∇), and 14 (□). Bars indicate the medians of temperature from totals of 5 sets of experiments (sham, n=38; FAHF-2, n=26; naïve, n=29). ###, p<0.001 vs naïve; and ***, p< 0.001 vs sham. (B) FAHF-2 blocked histamine release. Data are plasma histamine levels 30 min post challenge the same mice as in A. ###, p<0.001 vs naïve; and ***, p< 0.001 vs sham.
Figure 5.
FAHF-2 treatment prevented histamine release and mast cell degranulation. (A) Plasma histamine levels: Blood was collected immediately after scores and temperatures were recorded following each post-therapy challenge, and plasma was obtained. Histamine levels were measured using enzyme immunoassay. Data points indicate group means ± SEM, n = same as indicated above. The scale of the x axis is not linear. (B). Illustration of mast cell degranulation: Ear tissue samples taken 40 min after 7th challenge and stained with toluidine blue. Mast cells were considered degranulated if at least five granules appeared outside cell body. Arrows indicate deregulated mast cells. The image was captured using 100 X objective. (C) Percent of degranulated mast cells: Mast cells were counted using light microscopy at a 100x. A total of 500 cells were counted from 3 sections of each ear sample for calculation of the percentage of degranulated cells. Data points indicate group means ± SEM, n=4–5 mice /group from one set of experiment. **, p<0.01; ***, p<0.001 vs Sham. # # #, p<0.001 vs naive.
[Callout] FAHF-2 silences peanut (PN)-induced anaphylaxis for a prolonged post-treatment period via IFN-γ-producing CD8+T cell.:
Food Allergy Herbal Formula -2 Protection against Peanut Anaphylactic Reaction Is via Inhibition of Mast Cells and Basophils
Mast cells and basophils are key effector cells of IgE-mediated anaphylactic reactions. Using a well-established murine model of PN anaphylaxis, we found that the food allergy herbal formula-2 (FAHF-2) abrogated anaphylactic reactions in mice and this protection persisted for at least 36 weeks after therapy was discontinued.(106) The increased IFN-γ production by CD8+ T cells, as shown previously, may be an important mechanism underlying FAHF-2 modulated potent and long-term protection.(106)( However, neutralization of IFN-γ or depletion of CD8+ T cells blocked the suppression of IgE and Th2 cytokine production induced by FAHF-2, while protection from anaphylaxis still existed up to 4 weeks post therapy. We therefore hypothesized that FAHF-2 may also directly inhibit mast cells/basophils. To confirm this possibility, we investigated whether FAHF-2 inhibits mast cell/basophil numbers and IgE mediated activation. In this study, PN allergic mice (PNA mice) were treated with FAHF-2 intragastrically (i.g.) for 7 weeks and challenged (i.g.) with PN 1 day and 4 weeks post treatment. Peripheral blood basophil numbers and peritoneal mast cell numbers and FcεRI expression were determined. Direct effects of FAHF-2 on murine mast cell line MC/9, and effects of four fractions and three compounds isolated from FAHF-2 on rat basophilic leukemia cells (RBL-2H3) and human skin mast cells degranulation, and on the IgE mediated Syk signaling pathway were determined. The results showed that while all sham-treated PNA mice developed anaphylaxis, FAHF-2-treated PNA mice were protected against anaphylaxis following PN challenge at 1 day and 4 weeks post therapy. Reduction of peripheral blood basophil numbers began after 1 week of treatment and continued for at least 4 weeks post therapy. The number of FcεRI expression by peritoneal mast cells was also significantly decreased 4 weeks post therapy. FAHF-2 treated MC/9 cells showed significantly reduced IgE-induced FcεRI expression, FcεRI γ mRNA subunit expression, proliferation, and histamine release upon challenge. Fraction 2 (F2) from FAHF-2 inhibited RBL-2H3 cell and human mast cell degranulation. Three compounds from F2- berberine, palmatine and jatrorrhizine inhibited RBL-2H3 cell degranulation via suppressing Syk phosphorylation.(107) This study demonstrates that FAHF-2 reduction of basophils and mast cell numbers as well as suppression of IgE-mediated mast cell activation may contribute to FAHF-2 persistent protection against PN anaphylaxis.
[Callout] FAHF-2 protection against PN anaphylactic reaction is via inhibition of mast cells and basophils.
Clinical Studies
Safety and Immunological Effects in Acute Phase I Study
Because FAHF-2 has been shown to have potent therapeutic effects in animal model of PN anaphylaxis and high safety profile in preclinical study, and because all individual herbs in FAHF-2 have long history of human use and are lawfully used in the US, FAHF-2 received approval of investigational new drug (IND) by US FDA to study its safety and efficacy in patients ages 12 – 45 years of old with PN and/or tree nut, fish and shellfish allergies. This clinical study included both phase I (safety study) and phase II (efficacy study). Phase I study included acute phase I study and extended phase I study. We have completed both phase I studies. The acute phase I is a randomized, double-blinded, placebo-controlled, dose escalation trial to evaluate the safety and tolerability of FAHF-2 in subjects with food allergy. In this study, subjects received one of three doses of FAHF-2 or placebo: 2.2 grams (4 tablets), 3.3 grams (6 tablets) or 6.6 grams (12 tablets) three times a day for 7 days. Four active and two placebo subjects were treated at each dose level. Vital signs, physical examination, laboratory data, pulmonary function tests and electrocardiogram data were monitored. Immunomodulatory studies were also performed. Nineteen food allergic participants were included in the study. Two subjects (one FAHF-2 and one placebo) reported mild gastrointestinal symptoms. One patient withdrew from the study due to an allergic reaction that was unlikely related to the study medication. No significant differences were found in vital signs, physical examination, laboratory data, pulmonary function tests and electrocardiogram data obtained at pre- and post- treatment visits. Significantly decreased IL-5 levels were found in the active treatment group after 7 days. In vitro studies of PBMCs cultured with FAHF-2 also demonstrated a significant decrease in IL-5 and an increase in IFN-γ and IL-10 levels. This data showed that FAHF-2 is safe and well tolerated by subjects with food allergy and also showed beneficial immnunoregulatory effects, which is favorable for induction of food allergy tolerance.
Clinical Safety of Food Allergy Herbal Formula-2 2 and Its Inhibitory Effect on Basophils from Patients with Food Allergy: Extended Phase I Study
Basophils comprise less than 1% of leukocytes, but are critical to allergic reactions, as are tissue mast cells. CD63 expression, a marker of basophil activation, (108) correlates with release of histamine from intracytoplasmic granules and is triggered by IgE and allergen cross-linking of FcεRI receptors. The basophil activation test (BAT) is a flow cytometry-based assay that detects basophil CD63 expression, requires the use of very small quantities of blood, and does not require isolation of cells. The real time flow cytometry read-out makes this assay a convenient tool for longitudinal clinical studies. (52;109–111) A recent study by Jones et al. found that basophil activation was significantly reduced by 4–6 months of oral immunotherapy (OIT) (52) and that inhibition of basophils, but not IgE, correlated with clinical protection. Since peanut, tree nut, fish, and shellfish allergies are typically lifelong, chronic treatment is essential. To further ensure the safety and tolerability of this formulation prior to phase II study, we conducted an open label, single dose, 6 month extension of the phase I study. Although the primary outcome of this study was to evaluate the long term safety and tolerability of FAHF-2, based on substantial animal study results, and the fact that this is a 6 month study, we determined the effects of FAHF-2 on basophils in individuals before, during and after FAHF-2 treatment. Of the eighteen patients enrolled, 14 completed the study. No significant drug-associated differences in laboratory parameters, pulmonary function studies, or electrocardiographic findings before and after treatment were found. There was a significant reduction (p< 0.05) in basophil CD63 expression in response to ex vivo stimulation after 6 months of FAHF-2 treatment. There was also a trend towards a reduction of circulation basophil and eosinophil numbers after treatment. It was concluded FAHF-2 was safe, well-tolerated, and had an inhibitory effects on basophils in an extended phase I clinical study. A controlled phase II study is warranted.
In summary, in animal of PNA FAHF-2 treatment completely protected PN allergic mice from anaphylaxis despite multiple oral PN challenges for over six months-1/4 of the mouse lifespan. This effect was associated with sustained suppression of Th2 responses (IgE and Th2 cytokines) and enhancement of Th1 responses (IgG 2a and IFN-γ). Elevation of IFN- γ via CD8+T cells and persistent inhibition of basophils may contribute to FAHF-2 long term protection. Clinical study showed that FAHF-2 is safe and well tolerated by patients with food allergy.
[Callout] FAHF-2 treatment completely protected PN allergic mice from anaphylaxis despite multiple oral PN challenges for over six months, which is 1/4 of the mouse lifespan. This effect was associated with sustained suppression of Th2 responses (IgE and Th2 cytokines) and enhancement of Th1 responses (IgG 2a and IFN-γ). Elevation of IFN- γ via CD8+T cells and persistent inhibition of basophils may contribute to FAHF-2 long term protection. Clinical study also showed that FAHF-2 is safe and well tolerated by patients with food allergy.
There were significant suppression of Th2 responses, basophil activation, and increased IFN- γ and IL-10 suggesting beneficial immunological responses, which are favorable for food allergy treatment. Therefore, FAHF-2 appeared to have multiple targets on food allergy related immunopathological mechanisms as illustrated in Fig 1.
TRADITIONAL CHINESE MEDICINE FOR FOOD ALLERGY ASSOCIATED RECALCITRANT ECZEMA
Atopic dermatitis (AD) is a chronic, inflammatory and pruritic skin disease that affects up to 20% of children. (112;113). Regardless of dietary modifications, daily hydration therapy, and topical corticosteroids, some of these children continue to suffer from severe skin lesions-recalcitrant eczema. Clinically, 40% of refractory, moderate-to-severe atopic dermatitis is associated with food hypersensitivity.(114) TCM has been used for centuries to treat eczema. However, an effect on recalcitrant eczema has not been previously reported. Wisniewski et al (115) report a case series of 14 patients [median age 5.4 yrs (IQR 0.5 – 52)] with persistent recalcitrant AD who were treated with TCM at Ming Qi Natural Health Center in Manhattan between August 2006 and May 2008. A retrospective analysis of patients with AD who received TCM, consisting of Shizheng Herbal Tea, Herbal Bath Additive, Herbal Cream, and acupuncture. The SCORAD index for AD severity was calculated, with scores >40 – 103 considered severe, 15– 40 moderate, and <15 mild. The Dermatology Life Quality Index (DLQI) was calculated on a scale of 0–30, with a score of 30 representing highest impairment to life quality. Baseline median (IQR) SCORAD and DLQI scores were 89 (42–103) and 17 (10–30), compared to 11 (0–62) and 1 (0–14) after a median of 8 months (3–24) treatment (t-test, P<.0001 and P<0.0001). 60–90% reduction in SCORAD was reached in 13/14 patients after 3.3mo (1.6–8.6). Greater than 50% improvement in DLQI scores was achieved in 13/14 patients in 2.4mo (0.7–5.9) (Fig 5). Peripheral eosinophilia decreased from 1000±700 mc/L to 500±200 (P=0.03) with no change in total blood counts. No abnormality of liver and kidney function was observed. Patients reported a reduction in use of steroids, antibiotics, and antihistamines within 3 months of TCM treatment. These results showed that TCM is both a safe and effective treatment for patients with persistent AD, especially those with severe disease and significant life quality impairment.(115) Food allergy associated eczema is often accompanied by elevation of total and food allergy specific-IgE levels. Interestingly, there was a reduction of food specific IgE levels following eczema clinical improvement (Li et al. manuscript in preparation). It is possible that improvement of food allergy-associated eczema might be beneficial for food allergy treatment. At present there is no other report about TCM effect on food allergy associated eczema beyond this retrospective clinical analysis. Although the clinical effects are promising, controlled study is required to further investigate the efficacy and safety of these herbal treatments for food allergy-associated recalcitrant eczema.
CONCLUSION
TCM is beginning to play a role in the US health care system. There is increasing scientific evidence demonstrating that TCM has potential for treating asthma and food allergy, and associated conditions. Our goals are to accelerate clinic trials and to identify the active compounds that target on specific asthma and food allergy aspects, and on comorbid conditions such as asthma associated anxiety and food allergy associated severe and recalcitrant eczema.
Figure 6.
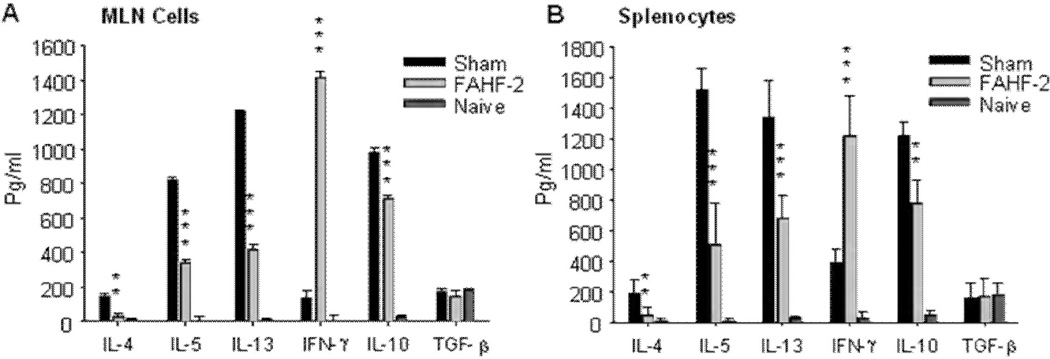
FAHF-2 treatment reduced Th2 cytokine levels and increased IFN-γ secretion by specific modulation of CD4+ and CD8+ T cells. MLN cells and SPC were collected from each group of mice immediately following evaluation of clinical effect and blood drawing following the 7th challenge (wk 50). Single MLN cells and SPCs were prepared and stimulated with crude CPE for 72 hours. Cytokines in MLN culture supernatants (A) and SPC culture supernatants (B) were measured by ELISA.Data are shownas Means ± SEM of pooled cultures from representative of one of two experiments measured in triplicate (n= 5 mice per group). **, p<0.01; ***, p<0.001 vs sham. MLN, mesenteric lymph node cells; SPC splenocytes; CPE, crude peanut extract.
Table 3.
Persistent Protection of FAHF-2 Against PN Induced Anaphylaxis
| Challenge (Time– Point) |
PNA/Sham | PNA/FAHF-2 | Naïve | |||
|---|---|---|---|---|---|---|
| Individual Score (N=12–13) |
Median Score |
Individual Score (N=8) |
Median Score |
Individual Score (N=5–10) |
Scores Median |
|
| 1st (W14) | 13/13 (2–4) | 3 | 0/8 (0) | 0* | 0/5 (0) | 0 |
| 2nd (W18) | 13/13 (3–4) | 4 | 0/8 (0) | 0* | 0/5 (0) | 0 |
| 3rd (W22) | 13/13 (3–4) | 4 | 0/8 (0) | 0* | NC | ND |
| 4th (W28) | 13/13 (2–4) | 4 | 0/8 (0) | 0* | NC | ND |
| 5th (W34) | 13/13 (3–4) | 4 | 0/8 (0) | 0* | NC | ND |
| 6th (W40) | 13-13 (3–5) | 4 | 0/8 (0) | 0* | NC | ND |
| 7th (W50) | 12/12 (3–4) | 4 | 3/8 (0–2) | 0* | 0/10(0) | 0 |
Anaphylactic signs were evaluated 30 minutes following oral challenge utilizing the scoring system previously described in table I. Sham, PN allergic mice fed water; FAHF-2, food allergy herbal formula -2. P<0.05 vs Sham.
ACKNOWLEDGEMENT
Grant Support
These studies are supported by grants 1 P01 AT002625-01 and R01 1R01AT001495-01A1 and 2 R01 AT001495-05A1 from the National Institutes of Health/the National Center for Complementary and Alternative Medicine/ a grant from the Food Allergy Initiative and Winston Wolkoff Children’s Holistic Medicine for Allergy Foundation to Dr. Xiu-Min Li
I would like to thank my colleagues and collaborators including Hugh Sampson, MD, Kamal Srivastava, PhD, Jullie Wang, MD, Sangita Patil, PhD, Ying Song, MD, Nan Yang, PhD, Joseph Albans, MS, Wei Zhao MD, PhD, Mia Oh, MD, Julia Wisniewski, MD, Anna Nowak-Wegrzyn, MD, Joseph Goldfarb, PhD, Jacob Kattan, MD, Changda Lui, PhD, Bangho Liang, BS, Sylvan Wallenstein Ph, Sally Noone RN, Brian Schofield, JD, Scott Sicherer, MD and summer student interns including William Slotkin, Peter Slotkin, Thomas Slotkin, and Rebeca Chan for their initial contributions
AUTHOR’S DISCLOSURE
Xiu-Min Li receives grant support from the Food Allergy Initiative and the NIH and is a consultant for the Food Allergy Initiative. Xiu-Min Li and Hugh Sampson have shares of US Patent PCT/US 05/08600 on FAHF-2, PCT/US05/08600 on ASHMI, and Herbal Springs LLC.
ABBREVIATIONS
- AHR
airway hyperresponsiveness
- ASM
airway smooth muscle
- ASHMI
Anti-asthma herbal medicine intervention
- BDNF
Brian derived neurotropic factor
- CAM
complementary and alternative medicine
- DCT
Ding Chuan Tang, classical formula
- FAHF-1
Food allergy herbal formula-1
- FAHF-2
Food allergy herbal formula-2
- IND
Investigational new drug
- FDA
Food and drug administration
- NCCAM
National Center for Complementary and Alternative Medicine
- PN
peanut
- PNA
peanut allergy
- TCM
traditional Chinese medicine
REFERENCES
- 1.CDC. [Accessed September 18, 2010];National Center for Health Statistics: Asthma. 2010 http://www.cdc.gov/nchs/fastats/asthma.htm.
- 2.American Academy of Allergy AaI. Asthma statistics. 2010. Ref Type: Statute. [Google Scholar]
- 3.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 5.Metzger WJ, Hunninghake GW, Richerson HB. Late asthmatic responses: inquiry into mechanisms and significance. Clin Rev Allergy. 1985;3:145–165. doi: 10.1007/BF02992980. [DOI] [PubMed] [Google Scholar]
- 6.Busse WW, Vrtis RF, Dick EC. The role of viral infections in intrinsic asthma: activation of neutrophil inflammation. Agents Actions Suppl. 1989;28:41–56. [PubMed] [Google Scholar]
- 7.Lemanske RF, Kaliner MA. Late-phase allergic reactions. In: Middleton Jr, Reed CE, Ellis EF, Adkinson NF, Yunginger JW, editors. Allergy: Principles and Practice. St. Louis: C. V. Mosby, Co.; 1993. p. 320. [Google Scholar]
- 8.Pauwels R. The relationship between airway inflammation and bronchial hyperresponsiveness. Clin Exp Allergy. 1989;19:395–398. doi: 10.1111/j.1365-2222.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 9.Drazen JM, Arm JP, Austen KF. Sorting out the cytokines of asthma. J Exp Med. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 11.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104(8):1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar RK. Understanding airway wall remodeling in asthma: a basis for improvements in therapy? Pharmacol Ther. 2001;91(2):93–104. doi: 10.1016/s0163-7258(01)00149-8. [DOI] [PubMed] [Google Scholar]
- 13.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1518–1523. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 14.Howarth PH. The influence of pharmacological therapy on airway remodeling in asthma. In: Howarth PH, Wilson JW, Bousquet J, Rak S, Pauwels RA, editors. Airway Remodeling. New York: Marcel Dekker, Inc.; 2001. pp. 167–188. [Google Scholar]
- 15.Jeffery PK, Godfrey RW, Adelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am Rev Respir Dis. 1992;145(4 Pt 1):890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JW. What causes airway remodelling in asthma? Clin Exp Allergy. 1998;28(5):534–536. doi: 10.1046/j.1365-2222.1998.00298.x. [DOI] [PubMed] [Google Scholar]
- 17.Boulet LP, Laviolette M, Turcotte H, Cartier A, Dugas M, Malo JL, et al. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest. 1997;112(1):45–52. doi: 10.1378/chest.112.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Christodoulopoulos P, Cameron L, Nakamura Y, Lemiere C, Muro S, Dugas M, et al. TH2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression. J Allergy Clin Immunol. 2001;107(4):586–591. doi: 10.1067/mai.2001.114883. [DOI] [PubMed] [Google Scholar]
- 19.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272(34):21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 21.Hendriks RW, Nawijn MC, Engel JD, van DH, Grosveld F, Karis A. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur J Immunol. 1999;29(6):1912–1918. doi: 10.1002/(SICI)1521-4141(199906)29:06<1912::AID-IMMU1912>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16(1):3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 23.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4(1):2. [PMC free article] [PubMed] [Google Scholar]
- 24.Lemanske RFJ. A review of the current guidelines for allergic rhinitis and asthma. J Allergy Clin Immunol. 1998;101:S392–S396. doi: 10.1016/s0091-6749(98)70228-3. [DOI] [PubMed] [Google Scholar]
- 25.Hall IP. The beta-agonist controversy revisited. Lancet. 2004;363(9404):183–184. doi: 10.1016/S0140-6736(03)15369-X. [DOI] [PubMed] [Google Scholar]
- 26.The National Institute of Allergy and Infectious Diseases (NIAID) [accessed September 19, 2010];Asthma and Allergic Diseases Cooperative Research Centers (U19) 2010 http://grants.nih.gov/grants/guide/rfa-files/RFA-AI-10-013.html.
- 27.Sheth A, Reddymasu S, Jackson R. Worsening of Asthma with Systemic Corticosteroids. J Gen Intern Med. 2005 doi: 10.1111/j.1525-1497.2005.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999;159(9):941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 29.Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126(1):213–219. doi: 10.1378/chest.126.1.213. [DOI] [PubMed] [Google Scholar]
- 30.Toogood JH, Crilly RG, Jones G, Nadeau J, Wells GA. Effect of high-dose inhaled budesonide on calcium and phosphate metabolism and the risk of osteoporosis. Am Rev Respir Dis. 1988;138(1):57–61. doi: 10.1164/ajrccm/138.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Packe GE, Robb O, Robins SP, Reid DM, Douglas JG. Bone density in asthmatic patients taking inhaled corticosteroids: comparison of budesonide and beclomethasone dipropionate. J R Coll Physicians Lond. 1996;30(2):128–132. [PMC free article] [PubMed] [Google Scholar]
- 32.Zollner EW. Hypothalamic-pituitary-adrenal axis suppression in asthmatic children on inhaled corticosteroids (Part 2)--the risk as determined by gold standard adrenal function tests: a systematic review. Pediatr Allergy Immunol. 2007;18(6):469–474. doi: 10.1111/j.1399-3038.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima C, Matsuse H, Tomari S, Obase Y, Miyazaki Y, Shimoda T, et al. Oral candidiasis associated with inhaled corticosteroid use: comparison of fluticasone and beclomethasone. Ann Allergy Asthma Immunol. 2003;90(6):646–651. doi: 10.1016/S1081-1206(10)61870-4. [DOI] [PubMed] [Google Scholar]
- 34.Noonan M, Leflein J, Corren J, Staudinger H. Long-term safety of mometasone furoate administered via a dry powder inhaler in children: Results of an open-label study comparing mometasone furoate with beclomethasone dipropionate in children with persistent asthma. BMC Pediatr. 2009;9:43. doi: 10.1186/1471-2431-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116(4):652–657. doi: 10.1016/j.ophtha.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116(4):652–657. doi: 10.1016/j.ophtha.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Cordina M, Fenech AG, Vassallo J, Cacciottolo JM. Anxiety and the management of asthma in an adult outpatient population. Ther Adv Respir Dis. 2009;3(5):227–233. doi: 10.1177/1753465809347038. [DOI] [PubMed] [Google Scholar]
- 38.Bhalla RK, Taylor W, Jones AS, Roland NJ. The inflammation produced by corticosteroid inhalers in the pharynx in asthmatics. Clin Otolaryngol. 2008;33(6):581–586. doi: 10.1111/j.1749-4486.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Sampson HA. Food allergy. J Clin Invest. 2011;121(3):827–835. doi: 10.1172/JCI45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 41.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 42.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Pali-Scholl I, Renz H, Jensen-Jarolim E. Update on allergies in pregnancy, lactation, and early childhood. J Allergy Clin Immunol. 2009;123(5):1012–1021. doi: 10.1016/j.jaci.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worth A, Sheikh A. Food allergy and atopic eczema. Curr Opin Allergy Clin Immunol. 2010 doi: 10.1097/ACI.0b013e3283387fae. [DOI] [PubMed] [Google Scholar]
- 45.Bjorksten B. Genetic and environmental risk factors for the development of food allergy. Curr Opin Allergy Clin Immunol. 2005;5(3):249–253. doi: 10.1097/01.all.0000168790.82206.17. [DOI] [PubMed] [Google Scholar]
- 46.Prescott SL, Clifton V. Asthma and pregnancy: emerging evidence of epigenetic interactions in utero. Curr Opin Allergy Clin Immunol. 2009;9(5):417–426. doi: 10.1097/ACI.0b013e328330634f. [DOI] [PubMed] [Google Scholar]
- 47.van PN, Le GG, McConnell MJ. Epigenetic regulation of Th2 cytokine expression in atopic diseases. Tissue Antigens. 2008;72(2):91–97. doi: 10.1111/j.1399-0039.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 48.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–751. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 49.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90:256–262. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 50.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126(1):83–91. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 51.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 52.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. 300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varshney P, Steele PH, Vickery BP, Bird JA, Thyagarajan A, Scurlock AM, et al. Adverse reactions during peanut oral immunotherapy home dosing. J Allergy Clin Immunol. 2009;124(6):1351–1352. doi: 10.1016/j.jaci.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–277. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]
- 55.Plaut M, Sawyer RT, Fenton MJ. Summary of the 2008 National Institute of Allergy and Infectious Diseases-US Food and Drug Administration Workshop on Food Allergy Clinical Trial Design. J Allergy Clin Immunol. 2009;124(4):671–678. doi: 10.1016/j.jaci.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 56.Leung DY, Sampson HA, Yunginger JW, Burks AWJ, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348(11):986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 57.Slader CA, Reddel HK, Jenkins CR, Armour CL, Bosnic-Anticevich SZ. Complementary and alternative medicine use in asthma: who is using what? Respirology. 2006;11(4):373–387. doi: 10.1111/j.1440-1843.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 58.Sawni A, Thomas R. Pediatricians' attitudes, experience and referral patterns regarding Complementary/Alternative Medicine: a national survey. BMC Complement Altern Med. 2007;7:18. doi: 10.1186/1472-6882-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mark JD. Integrative medicine and asthma. Pediatr Clin North Am. 2007;54(6):1007–1023. doi: 10.1016/j.pcl.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 60.US Food and Drug Administration (FDA) Center for Drug Evaluation and Research. Guidance for Industry Botanical Drug Products. 2004. Revised ed. [Google Scholar]
- 61.nccam. 2008 Jan 28; ( http://nccam.nih.gov/health/backgrounds/wholemed.htm.
- 62.Li XM, Teper A, Zou ZM, Srivastava K, Schofield B, Sampson H. The Chinese herbal formula, MSSM-002, can reverse the established Th2 phenotype, which is accompanied with down regulation of the Th2 transcription factor GATA-3. J Allergy Clin Immunol (Abstract) 2002;109:S155. [Google Scholar]
- 63.Jindal V, Ge A, Mansky PJ. Safety and efficacy of acupuncture in children: a review of the evidence. J Pediatr Hematol Oncol. 2008;30(6):431–442. doi: 10.1097/MPH.0b013e318165b2cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hon KL, Leung TF, Ng PC, Lam MC, Kam WY, Wong KY, et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2007 doi: 10.1111/j.1365-2133.2007.07941.x. [DOI] [PubMed] [Google Scholar]
- 65.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma 23. J Allergy Clin Immunol. 2005;116(3):517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 66.Chang TT, Huang CC, Hsu CH. Clinical evaluation of the Chinese herbal medicine formula STA-1 in the treatment of allergic asthma. Phytother Res. 2006;20(5):342–347. doi: 10.1002/ptr.1843. [DOI] [PubMed] [Google Scholar]
- 67.Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, et al. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann Allergy Asthma Immunol. 2010;104(3):236–246. doi: 10.1016/j.anai.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 69.Ariano R, Panzani RC, Augeri G. Late onset asthma clinical and immunological data: importance of allergy. J Investig Allergol Clin Immunol. 1998;8(1):35–41. [PubMed] [Google Scholar]
- 70.Quadrelli SA, Roncoroni AJ. Is asthma in the elderly really different? Respiration. 1998;65(5):347–353. doi: 10.1159/000029294. [DOI] [PubMed] [Google Scholar]
- 71.The US Food and Drug Administration (FDA) Center for Drug Evaluation and Research. Guidance for Industry Botanical Drug Products. 2004. Revised ed. [Google Scholar]
- 72.Huang X, Kong L, Li X, Chen X, Guo M, Zou H. Strategy for analysis and screening of bioactive compounds in traditional Chinese medicines. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1–2):71–84. doi: 10.1016/j.jchromb.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 73.Efferth T, Kahl S, Paulus K, Adams M, Rauh R, Boechzelt H, et al. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese materia medica with activity against tumor cells. Mol Cancer Ther. 2008;7(1):152–161. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- 74.Zhang HY, Hu CX, Liu CP, Li HF, Wang JS, Yuan KL, et al. Screening and analysis of bioactive compounds in traditional Chinese medicines using cell extract and gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2007;43(1):151–157. doi: 10.1016/j.jpba.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 75.Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical databases of Chinese herbal constituents and bioactive plant compounds with known target specificities. J Chem Inf Model. 2007;47(2):254–263. doi: 10.1021/ci600288m. [DOI] [PubMed] [Google Scholar]
- 76.Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical informatics of traditional Chinese medicine and therapeutic relevance. J Chem Inf Model. 2007;47(6):2316–2334. doi: 10.1021/ci700155t. [DOI] [PubMed] [Google Scholar]
- 77.Matsui S, Sonoda Y, Sekiya T, izu-Yokota E, Kasahara T. Glycyrrhizin derivative inhibits eotaxin 1 production via STAT6 in human lung fibroblasts. Int Immunopharmacol. 2006;6(3):369–375. doi: 10.1016/j.intimp.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 78.Matsui S, Matsumoto H, Sonoda Y, Ando K, izu-Yokota E, Sato T, et al. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int Immunopharmacol. 2004;4(13):1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2(3):150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato E, Haniuda M, Numanami H, Ushiyama T, Tsukadaira A, Takashi S, et al. Histamine and serotonin stimulate eotaxin production by a human lung fibroblast cell line. Int Arch Allergy Immunol. 2002;128(Suppl 1):12–17. doi: 10.1159/000059413. [DOI] [PubMed] [Google Scholar]
- 81.Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patil SP, Bolleddulla P, Bezdecny S, Doddaga S, Rusinova E, Li X-M. Anti-inflammatory effects of a purified herbal flavanoid 7, 4' dihydroxy flavone in vivo and in a murine model. J Allergy Clin Immunol (Abstract) 2009;123(2):S122. [Google Scholar]
- 84.Yang N, Zhu-ge J, Zhang W, Jayaprakasam, Goldfarb J, Li X-M. Flavonoids from Gan-Cao (Radix Glycerrhizae) Inhibit Th2 Memory Cell Cytokine Production. J Allergy Clin Immunol (abstract) 2010;2(125):S 198. [Google Scholar]
- 85.Deshmukh VM, Toelle BG, Usherwood T, O'Grady B, Jenkins CR. The association of comorbid anxiety and depression with asthma-related quality of life and symptom perception in adults. Respirology. 2008;13(5):695–702. doi: 10.1111/j.1440-1843.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 86.Di MF, Verga M, Santus P, Giovannelli F, Busatto P, Neri M, et al. Close correlation between anxiety, depression, and asthma control 2. Respir Med. 2010;104(1):22–28. doi: 10.1016/j.rmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Eisner MD, Katz PP, Lactao G, Iribarren C. Impact of depressive symptoms on adult asthma outcomes 84. Ann Allergy Asthma Immunol. 2005;94(5):566–574. doi: 10.1016/S1081-1206(10)61135-0. [DOI] [PubMed] [Google Scholar]
- 88.Kovacs M, Stauder A, Szedmak S. Severity of allergic complaints: the importance of depressed mood 5. J Psychosom Res. 2003;54(6):549–557. doi: 10.1016/s0022-3999(02)00477-4. [DOI] [PubMed] [Google Scholar]
- 89.Lavoie KL, Bacon SL, Barone S, Cartier A, Ditto B, Labrecque M. What is worse for asthma control and quality of life: depressive disorders, anxiety disorders, or both? 26. Chest. 2006;130(4):1039–1047. doi: 10.1378/chest.130.4.1039. [DOI] [PubMed] [Google Scholar]
- 90.Thomas M, Price D. Impact of comorbidities on asthma 11. Expert Rev Clin Immunol. 2008;4(6):731–742. doi: 10.1586/1744666X.4.6.731. [DOI] [PubMed] [Google Scholar]
- 91.Oraka E, King ME, Callahan DB. Asthma and serious psychological distress: prevalence and risk factors among US adults, 2001–2007. Chest. 2010;137(3):609–616. doi: 10.1378/chest.09-1777. [DOI] [PubMed] [Google Scholar]
- 92.McGrady ME, Cotton S, Rosenthal SL, Roberts YH, Britto M, Yi MS. Anxiety and asthma symptoms in urban adolescents with asthma: the mediating role of illness perceptions. J Clin Psychol Med Settings. 2010;17(4):349–356. doi: 10.1007/s10880-010-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang B, Slotkin T, Yang N, Li X-M. Inhibitory Effect of Sub-fractions containing mainly flavonoids in KuShen (Radix Sophorae Flavescentis) on Airway Smooth Muscle Contraction. J Allergy Clin Immunol (Abstract) 2011 In press. [Google Scholar]
- 94.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116(3):517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 95.WHO monographs on selected medicinal plants. Volume 1: Radix Glycyrrhizae. 1999. pp. 183–194. [Google Scholar]
- 96.Peirce A. The American Pharmaceutical Association Practical Guide to Natural Medicines. New York: William Morrow and Company, Inc.; 1999. [Google Scholar]
- 97.Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Wisnivesky J, et al. Safety and tolerability of an antiasthma herbal formula (ASHMI™) in adult asthmatics: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. Journal of Alternative and complementary Medicine. 2009;15(7):735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Busse P, Busse P, Srivastava K, Wei C-H, Li G-H, Yu N, et al. Safety, tolerability and beneficial immunological responses of complementary ASHMI therapy to inhaled corticosteroids therapy in children with persistent allergic asthma with or without allergic rhinitis. Allergy Clin Immunol. 2009 (Abstract in press) [Google Scholar]
- 99.Patil P, W-S Ji, Singha A, Hendricks DE, Busse P, Sampson H, et al. In vitro immunomodulatory effect of ASHMI (Anti-asthma Herbal Medicine Intervention) on PBMCs from asthmatics. J Allergy Clin Immunol. 2009 (Abstract in press) [Google Scholar]
- 100.Bensky D, Barolet R. Chinese Herbal Medicine: Formulas & Strategies. Seattle: Eastland Press; 1990. [Google Scholar]
- 101.Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108(4):639–646. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- 102.The State Pharmacopoeia Commission of The People's Republic of China. Pharmacopoeia of the People's Republic of China. People's Medical Publishing House; 2005. Version 6 ed. [Google Scholar]
- 103.Kattan JD, Srivastava KD, Zou ZM, Goldfarb J, Sampson HA, Li XM. Pharmacological and immunological effects of individual herbs in the Food Allergy Herbal Formula-2 (FAHF-2) on peanut allergy. Phytother Res. 2008;22(5):651–659. doi: 10.1002/ptr.2357. [DOI] [PubMed] [Google Scholar]
- 104.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115(1):171–178. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–855. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 106.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123(2):443–451. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 107.Song Y, Qu C, Srivastava K, Yang N, Busse P, Zhao W, et al. Food allergy herbal formula 2 protection against peanut anaphylactic reaction is via inhibition of mast cells and basophils. J Allergy Clin Immunol. 2010;126(6):1208–1217. doi: 10.1016/j.jaci.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. 2007;72(3):196–203. doi: 10.1002/cyto.b.20142. [DOI] [PubMed] [Google Scholar]
- 109.Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, et al. Characterization of a hypoallergenic recombinant Bet v 1 variant as a candidate for allergen-specific immunotherapy. Int Arch Allergy Immunol. 2008;145(3):193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- 110.Nagao M, Hiraguchi Y, Hosoki K, Tokuda R, Usui T, Masuda S, et al. Allergen-induced basophil CD203c expression as a biomarker for rush immunotherapy in patients with Japanese cedar pollinosis. Int Arch Allergy Immunol. 2008;146(Suppl 1):47–53. doi: 10.1159/000126061. [DOI] [PubMed] [Google Scholar]
- 111.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009;123(4):789–794. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larsen F, Hanifin JM. Epidemiology of Atopic Dermatitis. Immunology and Allergy Clinics of North America. 2002;22(1):1–24. Ref Type: Journal (Full) [Google Scholar]
- 113.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43(4):649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 114.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101(3):E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 115.Wisniewski J, Oh M, Nowak-Wegrzyn A, Steenburgh-Thanik E, Sampson H, Li XM. Efficacy and Safety of Traditional Chinese Medicine for Treatment of Atopic Dermatitis (AD) J Allergy Clin Immunol (abstract) 2010;123(2):S37. [Google Scholar]
- 116.Zhang T, Srivastava K, Wen MC, Yang N, Cao J, Busse P, et al. Pharmacology and immunological actions of a herbal medicine ASHMI on allergic asthma. Phytother Res. 2010;24(7):1047–1055. doi: 10.1002/ptr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Srivastava K, Zhang T, Yang N, Sampson H, Li XM. Anti-Asthma Simplified Herbal Medicine Intervention-induced long-lasting tolerance to allergen exposure in an asthma model is interferon-gamma, but not transforming growth factor-beta dependent. Clin Exp Allergy. 2010;40(11):1678–1688. doi: 10.1111/j.1365-2222.2010.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chuang Y, Liu C, Yang N, Liang B, Song Y, Li X-M. Inhibitory Effect of Constituents Isolated from Ganoderma lucidum on LPS-stimulated Macrophage cells TNF-á Production. J Allergy Clin Immunol. 2011 (Abstract) In press. [Google Scholar]
- 119.Alban J, Patil SP, Li X. Flavonoids In ASHMI (Anti-asthma Herbal Medicine Intervention) Inhibit Microglial Cell TNF-a Secretion. Journal of Allergy and clinic Immunol. 2010;Volume 125(Issue 2):AB206. [Google Scholar]
- 120.Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Slotkin P, Ling B, Liu C, Li XM, Yang N. Inhibition of IgE Production By Chemical Constituents Isolated From Qumai (Dianthus Superbus) Journal of Allergy and Clini Immunol. 2011;Volume 127(Issue 2):AB241. [Google Scholar]
- 122.Wang J, Patil S, Yang N, Ko J, Lee J, Noone S, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula (FAHF-2) in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. Ann Allergy Asthma Immunol. 2010;105(1):75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Patil P, Song Y, Noone S, Yang N, Wallenstein S, Sampson HA, et al. Clinical safety of FAHF-2, and inhibitory effect on basophils from patients with food allergy - extended phase I study. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.06.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]