Abstract
Background
Behavioral inhibition, a temperament identified in early childhood, is often associated with dysregulated attention and affective processing, particularly in response to threat. Longitudinal studies find that the manifestation of perturbed attention and affective processing often dissipates with age. Yet, childhood behavioral inhibition continues to predict perturbed brain function into adulthood. This suggests that adults with childhood behavioral inhibition may engage compensatory processes to effectively regulate emotion-related attention. However, it is unknown whether perturbations in brain function reflect compensation for attention bias to emotional stimuli generally, or to threatening contexts more specifically. The present study tests these possibilities.
Methods
Adults with and without a history of stable childhood behavioral inhibition completed an attention-control task in the context of threatening and nonthreatening stimuli while undergoing functional magnetic resonance imaging. Participants were asked to identify the gender of fearful (threatening) and happy (nonthreatening) faces, while ignoring both the face emotion and overlaid congruent (low attention control, LAC) or incongruent (high attention control, HAC) gender words.
Results
When fearful faces were present, adults with stable childhood behavioral inhibition exhibited more activity in striatum, cingulate, and dorsolateral prefrontal cortex for HAC trials compared with LAC trials, relative to those without behavioral inhibition. When happy faces were present, the opposite activation pattern emerged. No group differences in behavior were observed.
Conclusions
Among adults, stable childhood behavioral inhibition predicts neural, but not behavioral, responding when attention control is engaged in discrete emotional contexts. This suggests a mechanism by which adults may compensate for the behavioral manifestation of threat-based attention biases.
Keywords: personality, magnetic resonance imaging, emotions, attention, risk factor, anxiety
INTRODUCTION
Behavioral inhibition, a temperament identified in the first years of life, is characterized by fear of novelty, heightened vigilance to threat, reticent behavior in social contexts, and an increased risk for developing social anxiety disorder.[1–5] Several functional magnetic resonance imaging (fMRI) studies suggest that although behavioral signs of behavioral inhibition may not persist into adulthood, its latent neural correlates remain.[6–12] Although the absence of behavioral effects may relate to the relatively small sample size used in these studies, lack of effects may also be due to the emergence of compensatory regulatory mechanisms, reflected by differences in brain function. As such, threatening stimuli, which elicit behaviorally observable heightened fear and vigilance among children with behavioral inhibition,[13, 14] may still exert unique influence on neural processing into adulthood. The current fMRI study tests the hypothesis that threatening and nonthreatening emotional stimuli differentially modulate the neural mechanisms engaged by attention in adults with or without a history of stable childhood behavioral inhibition.
Mounting evidence suggests that attention plays an important role in shaping how individuals perceive and respond to social contexts. For example, enhanced attention to social threat (i.e., threat bias) occurs in anxious children[15–17] and adults.[18] Like children with anxiety disorders, behaviorally inhibited children also exhibit a threat bias. Specifically, behaviorally inhibited adolescents and children, relative to noninhibited children, are more likely to attend to angry faces than neutral faces.[13, 14] This pattern of response is, in turn, associated with concurrent social withdrawal, a behavioral sequela of behavioral inhibition.[13, 14] Conversely, some evidence suggests that adolescents not characterized as behaviorally inhibited in childhood may exhibit a behavioral attention bias toward happy stimuli.[13]
A small but growing literature links stable childhood behavioral inhibition to perturbed brain function, but not behavior, later in life. Stable childhood behavioral inhibition predicts heightened amygdala and striatal activity, but not behavioral response, to affective stimuli in adolescence[6–8, 10] and adulthood.[9, 19] Likewise, stable childhood behavioral inhibition predicts heightened medial prefrontal cortex (mPFC) activity, but not behavioral response, when attention control is engaged in adolescence[20] and adulthood.[9] Thus, stable childhood behavioral inhibition has long-lasting effects on the neural response to, but not necessarily on the behavior elicited by, both affective stimuli and attention control.
Less is known about whether the emotional valence of affective stimuli modulates brain activity required to engage attention control in adults characterized in childhood with stable behavioral inhibition. One recent fMRI study found that, unlike anxious patients,[21, 22] adults with stable childhood behavioral inhibition exhibit heightened striatal and mPFC activity when high levels of attention control are engaged in the presence of emotional stimuli.[9] This suggests that some adults with stable childhood behavioral inhibition may engage compensatory processes to regulate emotion-related attention effectively. Given that electrophysiological response in mPFC during attention control differentially relates to the expression of anxiety and psychopathology in shy[23] and stable childhood behavioral inhibition in adolescents,[20] it is plausible that such compensatory processes could reduce the risk for psychopathology in adulthood. It is unknown if potential compensatory mechanisms engaged by emotion-related attention control differentially respond in the presence of threatening (e.g., angry) and nonthreatening (e.g., happy) stimuli. This is an important distinction, given that hypervigilance to threat is a key behavioral correlate of early behavioral inhibition. Thus, it is unclear if heightened striatal and mPFC activity in adults with stable childhood behavioral inhibition reflects compensation for attention bias to emotional stimuli generally, or to threatening stimuli more specifically.
The present fMRI study addresses this issue. Here, adults with and without a history of stable childhood behavioral inhibition performed a gender-based Stroop task, which requires high attention control (HAC) or low attention control (LAC), in the context of threatening and nonthreatening emotional faces.[24] We hypothesized that in adults, a history of stable childhood behavioral inhibition would predict heightened activity in fronto-striatal regions for trials that require HAC, relative to LAC trials. However, we hypothesized that this effect would be modulated by emotional context, and thus occur in the presence of fearful, but not happy faces.
METHODS
PARTICIPANTS
Participants were selected from a larger longitudinal study of temperament (for details see[25, 26]). Reactions to novel stimuli were assessed at 4 months of age (N = 433); infants with high and low reactivity were enrolled in the longitudinal study (N = 153). Inhibited responding to novel visual and auditory stimuli was assessed at 14 and 24 months. Social reticence during standardized social interactions was assessed at 4 and 7 years. Parent-reported temperamental shyness was assessed at each time point. Data at each time point were standardized by Z-score, and used to create a single composite score of BI. Following prior methods,[6–9, 19, 27] children were categorized as behaviorally inhibited (BI) or nonbehaviorally inhibited (non-BI), based on whether they were in the upper or lower half of the Z-score composite distribution. Participants were recontacted at approximately 20 years of age. Of those who agreed to participate (~70%), a subset was excluded for contraindicated fMRI, use of medication with central nervous system effects, or psychopathology requiring immediate clinical attention. A lifetime anxiety diagnosis was present in the majority of BI participants excluded for medication or acute psychopathology (N = 13/15; 87.67%), but fewer than half of the excluded non-BI participants (N = 8/17; 38.89%).
The current study included 35 young adults: 21 BI, and 14 non-BI. Composite scores for each group (Table 1) were comparable to prior fMRI studies.[6–9, 19, 27] Relative to included participants, composite scores for those excluded for medication or acute psychopathology did not differ for BI (M ± SD = .51 ± .50), but were higher for non-BI participants (−.42 ± .24; P < .05). BI and non-BI participants did not differ on demographics, anxiety (Liebowitz Social Anxiety Scale, LSAS[28]), depression (Beck Depression Inventory, BDI[29]), or presence of current Structured Clinical Interview for DSM-IV (SCID[30]), or lifetime psychiatric disorders, assessed at 10, 14, and 15 years of age (Kiddie-Sads-Present and Lifetime Version KSADS-PL).[31] Most participants (N = 31) completed an additional experiment while scanning (order counterbalanced), which has been reported elsewhere.[9]
TABLE 1.
Participant characteristics
| Non-BI
|
BI
|
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Demographics | ||||
| N (female/male) | 6/8 | 11/10 | ||
| Age (years) | 20.01 | 1.41 | 19.45 | 1.46 |
| IQ | 112.58 | 12.52 | 116.32 | 8.78 |
| Psychological characteristics | ||||
| BI composite score (14 months–7 years) | − 0.61 | 0.19 | 0.69 | 0.63 |
| Current depression (BDI) | 2.42 | 2.50 | 3.06 | 3.40 |
| Current social anxiety (LSAS) | 25.09 | 16.34 | 22.05 | 15.47 |
| Lifetime diagnostic frequency (N) | 6 | 11 | ||
| Anxiety | 4 | 6 | ||
| Depression | 2 | 5 | ||
| Substance use | 1 | 3 | ||
| Current diagnostic frequency (N) | 2 | 3 | ||
| Anxiety | 2 | 3 | ||
BI, behaviorally inhibited temperament; BDI, Beck Depression Inventory; LSAS, Liebowitz Social Anxiety Scale.
IMPLICIT EMOTION-PROCESSING TASK
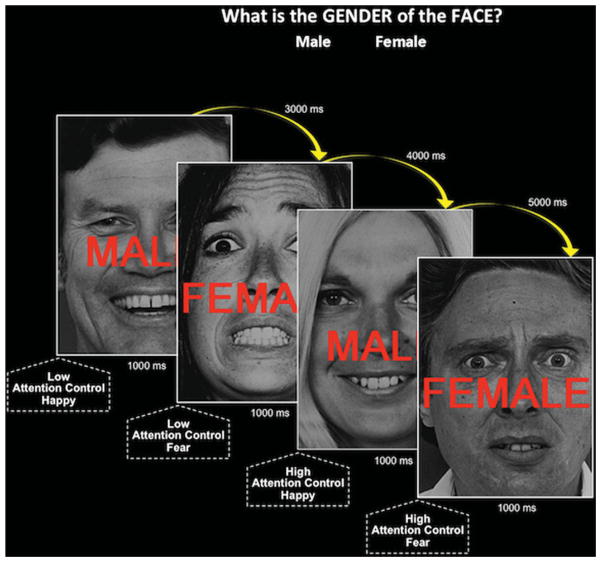
While undergoing an fMRI scan, participants were presented with photographs of happy or fearful, male or female faces overlaid with the words “MALE” or “FEMALE” (Fig. 1[24]). They were instructed to ignore the overlaid words, and indicate the gender of individual in the photograph by button press. Because of a prepotent tendency to read words, greater attention control is required to ignore incongruent, relative to congruent overlaid gender words, reflected by a slowing in response time (RT) to identify the gender of the face.[32] Half of the trials required LAC (e.g., female face overlaid with the word FEMALE), whereas the other half were required high attention control (HAC; e.g., female face overlaid with the word MALE).
Figure 1.
Task design as depicted with a sample of the experimental paradigm’s stimulus presentation sequence. LAC trials: gender of the photograph and overlaid word was congruent. HAC trials: gender of the photograph and overlaid word was incongruent.
Stimuli were presented (via projection) in a pseudorandom order, and counterbalanced for gender and facial expression of the photograph and overlaid word (EPrime; Sharpsburg, PA). Stimuli were presented for 1,000 ms, with a varying interstimulus interval of 3,000–5,000 ms (M = 4,000 ms). Data were acquired during a single 13-min functional run with 148 trials. To minimize fatigue, the run included four blocks of 37 trials, with 8 s of rest separating each block. Participants were required to achieve 90% accuracy on 10 practice trials prior to scanning. Trials with a RT of >±2 SDs from the mean for each type of trial, within each block, were excluded from analyses (3.85 ± 1.55 trials).
fMRI DATA ACQUISITION
Neuroimaging data were acquired with a GE 3T-scanner (Waukesha, WI). For each subject, 340 functional image volumes with 35 contiguous axial 3-mm slices (in-plane resolution = 2.5 × 2.5 mm) were obtained with a T2*-weighted echo-planar sequence (repetition time/echo time (TR/TE) = 2,300/25 ms, flip = 90°; field of view (FOV) = 240 mm, matrix = 96 × 96). To facilitate anatomical localization and coregistration of functional data, a high resolution structural scan was acquired (axial plane) with a T1-weighted magnetization-prepared spoiled gradient-recalled echo sequence (echo time/inversion time (TE/TI) = min full/725 ms, flip = 6°; FOV = 220 mm, matrix = 256 × 256, in-plane resolution, 0.86 × 0.86 mm).
DATA ANALYSIS
fMRI data were preprocessed and analyzed with AFNI,[33] correcting for slice timing and coregistering to the high resolution structural scan. Data were smoothed (6 mm full width at half maximum), spatially normalized to standard Talairach space, and resampled, resulting in 2.5 mm3 voxels. Temporally adjacent TRs with a Euclidean Norm motion derivative >0.3 mm were censored (6.29 ± 8.91% TRs per participant) and omitted from analyses.
For first-level fMRI analyses, separate regressors were created for each stimulus event. Events were classified by two criteria: (1) whether the gender of the face (male/female) was congruent or incongruent with the overlaid gender label (MALE/FEMALE), and thus engaged LAC or HAC; (2) whether the emotional valence of the facial expression was fearful or happy. Thus, four task-specific regressors were modeled for LAC and HAC events that occurred in the context of fearful or happy faces. Three additional low-frequency events (error trials, posterror trials, and the first trial of each block) were modeled but excluded from analysis.[24] Task-specific regressors were convolved with a γ-variate basis function approximating the blood oxygen level dependent (BOLD) response.[34] Additional regressors modeled motion residuals and baseline drift. This analysis produced a β-coefficient and associated t-statistic for each voxel and regressor. Percent signal-change maps were generated by dividing signal intensity at each voxel by the mean voxel intensity, and multiplying by 100.
Group-level analyses were conducted with a repeated measure ANOVA with three factors: group (BI, non-BI), attention control (LAC, HAC), and emotional valence (fear, happy). This assessed whether stable childhood behavioral inhibition differentially predicted neural activity depending on the level of attention control required to identify the gender of the face in the presence of happy and fearful expressions. Behavioral RT data were assessed in SPSS with a corresponding repeated measure ANOVA.
The current study did not have a sufficiently large sample to assess potential two-way interactions between stable childhood BI and lifetime psychiatric diagnosis. However, given the link between BI and risk for psychopathology, exploratory analyses were conducted to compare participants with and without lifetime psychopathology. A first analysis classified participants based on the presence (N = 17) or absence (N = 18) of any lifetime psychiatric diagnosis. A second analysis classified participants based on the presence (N = 10) or absence (N = 18) of a lifetime anxiety-disorder diagnosis. These classifications were made regardless of stable childhood behavioral inhibition. In a third analysis, disorder-related differences were examined specifically in BI participants. Thus, adults with stable childhood BI were classified based on the presence (N = 10) or absence (N = 11) of any lifetime psychiatric diagnosis. Small sample size prohibited an equivalent analysis for lifetime anxiety diagnosis.
A priori regions of interest (ROIs) were defined anatomically[35] based on prior studies that find childhood behavioral inhibition predicts dysregulated function in amygdala,[10–12] striatum (nucleus accumbens, putamen, and caudate),[6–9] and medial cortex (frontal pole, medial orbitofrontal gyrus, superior frontal gyrus, rostral, and caudal anterior cingulate, posterior cingulate).[6, 8, 9] Analyses considering each ROI were thresholded by an overall significance level (false detection probability) based on 10,000 Monte Carlo simulations (mean estimated spatial correlation of 8.79 × 8.80 × 8.12 mm FWHM; Al-phaSim). After correcting for the small volume of each ROI, simulations determined the minimum number of contiguous voxels needed to identify significant activity at P < .005, with an overall family-wise error rate of α < .05, in amygdala (ke = 2; 31 mm3), striatum (ke = 13; 203 mm3), and medial cortex (ke = 27; 419 mm3). For exploratory whole brain analyses, simulations determined that a cluster size of 70 contiguous voxels (1,094 mm3) was needed to achieve a threshold of P < .005, with an overall family-wise error rate of α < .05. For activation clusters exhibiting three-way interactions, subject-level percent signal-change values were extracted and plotted to facilitate interpretation. Finally, correlation analyses assessed the relation between RT and percent signal change in activation clusters exhibiting three-way interactions. After using a Bonferroni procedure to correct for the relatively large number of tests conducted in this analysis, significance level was thresholded at P < .005.
RESULTS
BEHAVIOR
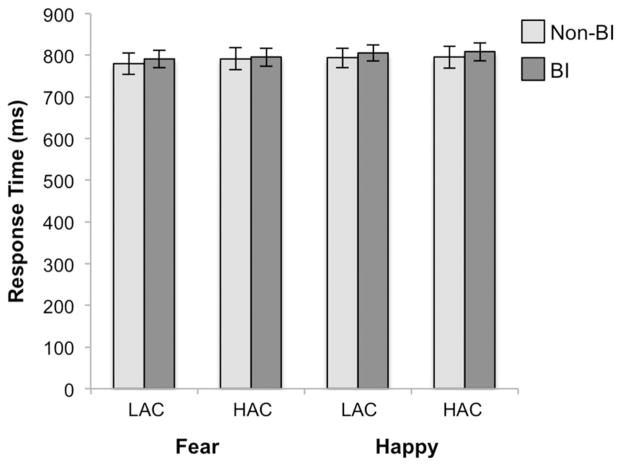
As Expected, RTs were slower for HAC compared with LAC trials F (1,34) = 25.06, P < .001 (Fig. 2). There was no main effect of group, emotional valence, or any interactions between group, emotional valance, and attention control. See Supporting Information for accuracy data.
Figure 2.
Group by attention control by emotional valence for RT. Bar graph depicts average RT plotted with standard error bars. BI, behaviorally inhibited; LAC, low attention control; HAC, high attention control.
BRAIN FUNCTION
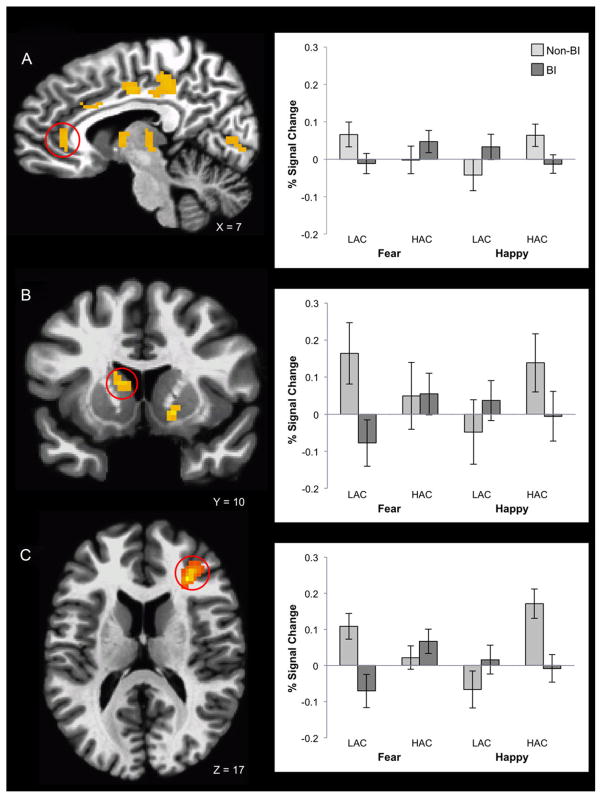
In line with our prediction, behavioral inhibition status in childhood predicted brain response as a function of attention control and emotional context (Table 2A). ROI analyses revealed three-way group-by-attention control-by-emotion valence interactions in medial cortex and striatal regions (Fig. 3A and B). Whole brain analyses revealed additional three-way interactions in dorsolateral PFC (dlPFC; Fig. 3C) extending to orbitofrontal cortex, as well as thalamus, cuneus, and globus pallidus. For all regions, unique group-by-attention control interactions occurred for each emotional valence (Table 2B). When fearful faces were present, BI adults exhibited more activity than non-BI adults for HAC compared with LAC trials. The opposite pattern emerged when happy faces were present, such that BI adults exhibited less activation than non-BI adults for HAC compared with LAC trials (Table 2B). RT did not relate to brain function in the 11 clusters with significant three-way interactions. See Supporting Information for analyses that consider group-by-attention control interactions separately for each emotional valence, and main effects of attention control.
TABLE 2.
(A) Activation clusters identified in three-way interaction: group (BI, non-BI) × attention control (high, low) × emotional valence (fear, happy); (B) decomposition of group × attention control for each emotional valence; (C) decomposition of attention control for each emotional valence, within each group (BI, non-BI)
| Region | (A) Group × emotion × attention control
|
(B) Group × attention control
|
(C) BI: high versus low attention control
|
Non-BI: high versus low attention control
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak MNI coordinates | Happy | Fear | Happy | Fear | Happy | Fear | ||||||
| x | y | z | Cluster size | F | Partial η2 | F | F | t | t | t | t | |
| ROI analyses | ||||||||||||
| Amygdala | – | – | – | – | – | – | – | – | – | – | – | |
| Striatum | ||||||||||||
| Caudate | − 14 | 11 | 16 | 25 | 13.29 | .31 | 7.68** | 10.43*** | − .73 | 2.24* | 3.59*** | − 3.97*** |
| Ventral striatum | 19 | 4 | − 1 | 22 | 15.41 | .31 | 8.39** | 6.34* | − 1.72 | 2.86** | 2.37* | − 0.94 |
| Putamen | 29 | − 7 | 9 | 20 | 13.47 | .30 | 20.64*** | 1.77 | − 4.59*** | .83 | 2.23* | − 1.01 |
| Medial cortex | ||||||||||||
| Perigenual anteior cingulate | 6 | 35 | 8 | 48 | 15.21 | .30 | 6.11* | 9.83*** | − 1.68 | 1.08 | 1.75 | − 4.28*** |
| Anterior mid cingulate | 11 | 20 | 38 | 38 | 15.67 | .35 | 8.64** | 9.13*** | − 1.57 | 2.16* | 2.31* | − 2.19* |
| Posterior mid cingulate | 6 | − 13 | 42 | 35 | 18.21 | .32 | 10.23*** | 10.43*** | − 1.55 | 2.71* | 2.74* | − 1.99 |
| Dorsal posterior cingulate | 11 | − 37 | 40 | 127 | 21.35 | .37 | 10.46*** | 7.07** | − 0.75 | 2.20* | 3.63*** | − 1.92 |
| Whole brain analysis | ||||||||||||
| Dorsolateral prefrontal cortex | 29 | 31 | 17 | 281 | 46.22 | .51 | 14.66*** | 18.92*** | − .72 | 3.80*** | 2.70* | − 3.56*** |
| Thalamus | − 4 | − 22 | 0 | 165 | 26.71 | .42 | 12.91*** | 7.27** | − 1.46 | 2.02 | 4.19*** | − 1.98 |
| Cuneus | − 19 | − 76 | 0 | 162 | 16.57 | .35 | 7.29** | 7.41** | − 0.63 | 1.94 | 2.72* | − 2.24* |
| Globus pallidus | 19 | 1 | − 1 | 112 | 16.23 | .42 | 13.35*** | 10.06*** | − 1.98 | 2.56* | 2.82* | − 2.01 |
P < .05;
P < .01;
P < .005;
MNI = Montreal Neurological Institute.
Figure 3.
Group by attention control by emotional valence interaction in the mPFC (A), striatum (B), and dorsolateral dlPFC (C). Bar graphs depict average percent signal-change values extracted from the activation cluster, plotted with standard error bars. BI, behaviorally inhibited; LAC, low attention control; HAC, high attention control.
SECONDARY ANALYSES: LIFETIME PSYCHIATRIC DIAGNOSIS
There were no main effects or interactions between presence and absence of lifetime psychiatric disorders, or anxiety disorders more specifically, for behavioral (Ps > .30) or brain response to attention control or emotional valence.
DISCUSSION
The current study examined associations among stable childhood behavioral inhibition and adult behavior and brain response during attention control in the context of threatening and nonthreatening emotional faces. As expected, stable childhood behavioral inhibition differentially predicted neural response based on level of attention control and the emotional valence of stimuli. Specifically, in the presence of fearful faces, adults with stable childhood behavioral inhibition, relative to adults with no such history, exhibited greater activity in cingulate cortex, dlPFC, and striatum for HAC compared with LAC trials. The opposite pattern emerged in the presence of happy faces. The expected behavioral effect was elicited across the studied sample, which manifested as changes in attention control. These changes were indexed by slower RT to incongruent relative to congruent trials. However, stable childhood behavioral inhibition did not predict degree of RT slowing. Thus, temperament predicted neural but not behavioral response patterns over a span of 15 years, on a task where the expected behavioral response was present in the sample as a whole.
These findings suggest that stable childhood behavioral inhibition does not predict patterns of behavior on an emotion-based attention-control task. However, stable childhood behavioral inhibition may predict patterns of neural activity engaged when greater attention control is required. For example, threatening task events requiring attention control produced heightened activity in cingulate cortex, striatum, and dlPFC in adults with a history of behavioral inhibition relative to those with no such history. This is consistent with prior work linking perigenual anterior cingulate cortex (ACC)[21, 22, 36] and dlPFC activity to attention engagement and reduced interference from distracting stimuli.[24, 37] Thus, threatening stimuli may capture attention more strongly in adults with versus without stable childhood behavioral inhibition. However, engagement of additional, compensatory mechanisms in adults with behavioral inhibition may eliminate behavioral expressions of these underlying differences in attention capture. Complementary findings emerged for another task implemented in an overlapping sample.[9]
Of note, adolescents without stable childhood behavioral inhibition exhibit a behavioral attention bias toward happy stimuli.[13] Thus, heightened brain activity in fronto-striatal regions may facilitate attention control in the presence of happy faces in adults without childhood behavioral inhibition. Such a pattern may reflect the signature of childhood exuberance, a temperament associated with high levels of approach and risk-taking behaviors.[38–40] This pattern also suggests that non-BI adults resemble the normative population more closely than BI adults. Like non-BI adults, healthy adults exhibit enhanced activity in striatum and dlPFC when attention control is engaged in the presence of happy stimuli,[41, 42] but diminished activity in such regions and mPFC in the presence of threatening stimuli.[41, 43, 44] However, given the lack of a priori hypothesis about brain regions engaged by nonthreatening stimuli, and the heterogeneity of individuals categorized based on the absence, rather than presence, of a specific temperament, these results must be interpreted with caution.
Although children with stable behavioral inhibition are at an increased risk of developing anxiety disorders,[45, 46] clinical levels of anxiety occur in only a subset of this vulnerable population.[1]Our data suggest that compensatory control of attention to threat may help account for the fact that most do not develop anxiety. Specifically, we found that adults with stable childhood behavioral inhibition, most of whom were currently psychiatrically healthy (>75%), showed heightened activity in frontal and striatal areas relative to adults without stable childhood behavioral inhibition. Adolescents with stable childhood behavioral inhibition exhibit enhanced striatal response to the threat of negative peer evaluation[27] or monetary reward omission, and to the ultimate failure to receive monetary rewards.[6, 8] Thus, there appears to be continuity in dysregulated striatal response to threat across development. However, adults with stable childhood behavioral inhibition may be better able to regulate their response to threat-related contexts by virtue of enhanced engagement of top-down attention control mechanisms in PFC. Such compensatory mechanisms may have contributed to the resilience of participants included in the study, relative to those excluded due to use of psychotropic medication and severe acute psychopathology.
Indeed, mounting evidence suggests that attention control may moderate the association between behavioral inhibition and anxiety symptoms during childhood and adolescence. For example, BI children and adolescents, relative to non-BI peers, exhibit enhanced electrophysiological response in mPFC during attention tasks, which in turn predict symptoms of anxiety and poor social functioning over time.[20, 23, 47] Moreover, children and adolescents with a history of behavioral inhibition, who are also hypervigilant toward threat, are more likely than those without this attention bias to exhibit concurrent socially withdrawn and anxious behavior.[13, 14] Thus, the inability to engage compensatory processes, thereby overcoming early attention biases, may influence development of anxiety in BI children. This suggests that attention-based training, potentially using methods that have shown promise in anxious patients,[48, 49] may benefit children with stable behavioral inhibition, who are at risk for developing anxiety.
It is important to note that the brain mechanisms engaged by attention control in anxious patients and those with a history of stable childhood behavioral inhibition include distinct and overlapping features. Unlike those with a history of stable behavioral inhibition, anxious adults exhibit diminished activity in perigenual ACC and dlPFC when high levels of attention control are engaged in emotion-based contexts.[21, 22, 43, 50] Moreover, this diminished activity is typically coupled with heightened amygdala activity,[21, 22, 51] a finding not observed in the present study. Yet, like adults with stable childhood behavioral inhibition, anxious adults exhibit greater activity in dorsal anterior cingulate when HAC is required in the context of incidental emotional stimuli.[51] Given the well-established link between dorsomedial PFC and threat detection,[51] our data suggest that, like anxious adults, individuals with a history of behavioral inhibition demonstrate an enhanced sensitivity to detect threat; however, unlike anxious adults, individuals with a history of behavioral inhibition appear to have developed mechanisms to dampen their otherwise exaggerated behavioral response to threat.
A true test of this hypothesized compensatory mechanism would require a comparison of adults with and without stable childhood behavioral inhibition who have or have not gone on to develop anxiety disorders. Unfortunately, the composition and size of the sample in the current study was ill suited to test this hypothesis. The modest sample size partially results from our exclusion of participants using psychotropic medications or in need of acute psychiatric care. Additionally, these exclusion criteria may have obscured the association between stable childhood behavioral inhibition and heightened expression of anxiety, which is observed in the longitudinal cohort from which this sample was drawn.[1] Future studies should consider whether the lasting neural signature of stable childhood behavioral inhibition detected in the current study manifests specifically among resilient at-risk adults, as opposed to those who develop psychopathology.
Other limitations of the current study also relate to its composition and small sample size. Although several prior fMRI studies[6–9, 19, 27] combine measures of reactivity during infancy and social reticence during early childhood to classify BI and non-BI groups, others[11, 12] classify groups using data from infancy alone. Given this, it is possible the present sample of adults with stable childhood behavioral inhibition were more socially reticent during early childhood than adults studied by Schwartz et al.[11, 12] Additionally, although the size of effects at the brain level was quite large (partial η2 range = .30–.51), the small sample size suggests the need for a cautious interpretation of the data. Finally, the experimental paradigm implemented here did not have sufficient power to test for “conflict adaptation” effects[9, 21, 36] by contrasting threatening and non-threatening incongruent trials preceded by incongruent or congruent trials. Thus, we are unable to determine the extent to which participants with and without childhood behavioral inhibition implement this specific form of regulation in discrete emotional contexts.
In summary, the current findings indicate that stable childhood behavioral inhibition predicts neural, but not behavioral, responding when attention control is engaged in discrete emotional contexts. This adds to our understanding of the long-lasting effects of stable childhood behavioral inhibition and suggests a mechanism by which adults may compensate for the behavioral manifestation of threat-based attention biases. Large-scale studies that classify participants based both on history of stable childhood behavioral inhibition history and adult anxiety-disorder status are needed to substantiate this hypothesized relation.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Health, National Institute of Mental Health, and grants from the National Institute of Mental Health Grant (NAF: R01 MH 074454) and the National Institute of Child Health and Development Grant (NAF: 5R37 HD 017899-20). We would like to thank Jamie A. Mash, B.A., for her assistance in preparing this manuscript.
Contract grant sponsor: National Institute of Mental Health Grant; Contract grant number: NAF: R01 MH 074454; Contract grant sponsor: National Institute of Child Health and Development Grant; Contract grant number: NAF: 5R37 HD 017899-20.
References
- 1.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158(10):1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 3.Hirshfeld DR, Rosenbaum JF, Biederman J, et al. Stable behavioral inhibition and its association with anxiety disorder. J Appl Anal Comput. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Hirshfeld-Becker DR, Biederman J, Henin A, et al. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28(3):225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 5.Fox NA, Henderson HA, Marshall PJ, et al. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Haim Y, Fox NA, Benson B, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helfinstein SM, Benson B, Perez-Edgar K, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and noninhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarcho JM, Fox NA, Pine DS, et al. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol Psychol. 2013;92(2):306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz CE, Kunwar PS, Greve DN, et al. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol Psychiatry. 2012;17(10):1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz CE, Wright CI, Shin LM, et al. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Edgar K, Bar-Haim Y, McDermott JM, et al. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Edgar K, Reeb-Sutherland BC, McDermott JM, et al. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J Abnormal Child Psychol. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagan J, Reznick JS, Clarke C, et al. Behavioral-inhibition to the unfamiliar. Child Dev. 1984;55(6):2212–2225. [Google Scholar]
- 16.Roy AK, Vasa RA, Bruck M, et al. Attention bias toward threat in pediatric anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2008;47(10):1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin KH, Lynch D, Coplan R, et al. Birds of a feather … behavioral concordances and preferential personal attraction in children. Child Dev. 1994;65(6):1778–1785. doi: 10.1111/j.1467-8624.1994.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 18.Bar-Haim Y, Lamy D, Pergamin L, et al. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Hardee JE, Benson BE, Bar-Haim Y, et al. Patterns of neural connectivity during an attention bias task moderates associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott JM, Perez-Edgar K, Henderson HA, et al. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etkin A, Prater KE, Hoeft F, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168(9):968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 23.Henderson HA. Electrophysiological correlates of cognitive control and the regulation of shyness in children. Dev Neuropsychol. 2010;35(2):177–193. doi: 10.1080/87565640903526538. [DOI] [PubMed] [Google Scholar]
- 24.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18(6):1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 25.Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 1996;67(2):523–540. [PubMed] [Google Scholar]
- 26.Fox NA, Henderson HA, Rubin KH, et al. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- 27.Guyer AE, Benson B, Choate VR, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. doi: 10.1017/S0954579413000941. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fresco DM, Coles ME, Heimberg RG, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31(6):1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JB. SCID-I/NP (for DSM-IV) Non-Patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 31.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 32.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 33.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 34.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 35.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral-based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Degnan KA, Hane AA, Henderson HA, et al. Longitudinal stability of temperamental exuberance and social-emotional outcomes in early childhood. Dev Psychol. 2011;47(3):765–780. doi: 10.1037/a0021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dennis T. Emotional self-regulation in preschoolers: the interplay of child approach reactivity, parenting, and control capacities. Dev Psychol. 2006;42(1):84–97. doi: 10.1037/0012-1649.42.1.84. [DOI] [PubMed] [Google Scholar]
- 40.Stifter CA, Putnam S, Jahromi L. Exuberant and inhibited toddlers: stability of temperament and risk for problem behavior. Dev Psychopathol. 2008;20(2):401–421. doi: 10.1017/S0954579408000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerestes R, Ladouceur CD, Meda S, et al. Abnormal pre-frontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012;42(1):29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- 42.Erk S, Kleczar A, Walter H. Valence-specific regulation effects in a working memory task with emotional context. Neuroimage. 2007;37(2):623–632. doi: 10.1016/j.neuroimage.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Krug MK, Carter CS. Adding fear to conflict: a general purpose cognitive control network is modulated by trait anxiety. Cogn Affect Behav Neurosci. 2010;10(3):357–371. doi: 10.3758/CABN.10.3.357. [DOI] [PubMed] [Google Scholar]
- 44.Mullin BC, Perlman SB, Versace A, et al. An fMRI study of attentional control in the context of emotional distracters in euthymic adults with bipolar disorder. Psychiatry Res. 2012;201(3):196–205. doi: 10.1016/j.pscychresns.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Essex MJ, Klein MH, Slattery MJ, et al. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Dev. 2004;75(1):251–263. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- 48.Hakamata Y, Lissek S, Bar-Haim Y, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bar-Haim Y. Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. J Child Psychol Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 50.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosc. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 51.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]