Abstract
In this edition of Blood, Bertaina et al report 3-year survival exceeding 90% by using haploidentical αβ+CD3+/CD19+-depleted allogeneic transplantation for children with nonmalignant disorders.1
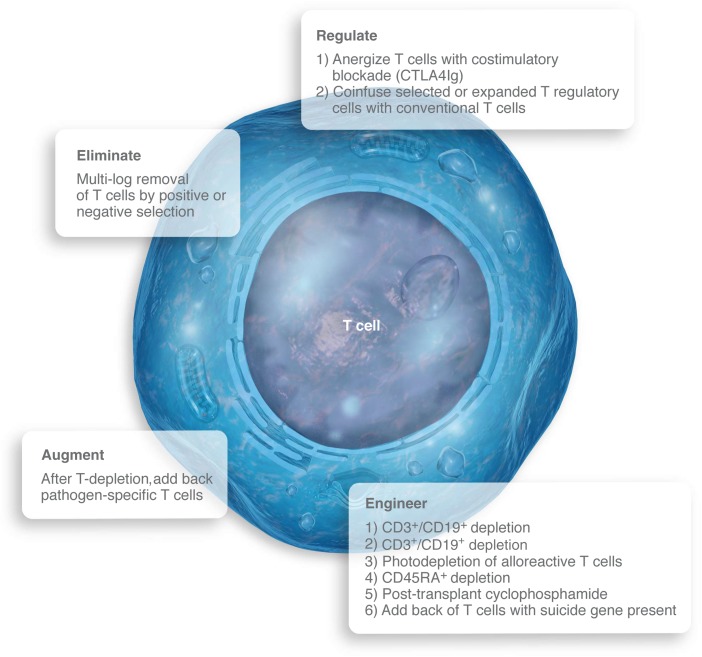
General categories and specific approaches that have been taken to facilitate and improve outcomes after haploidentical allogeneic hematopoietic cell transplantation. Professional illustration by Luk Cox, Somersault1824.
For those who lived through the early dark days of the development of haploidentical approaches, the notion that outcomes would become so good that one could consider using this approach relatively early in the course of these disorders (rather than as a last-ditch effort to save a child with end-stage disease) is nothing short of astonishing. In fact, there are currently so many promising haploidentical approaches that priority in the field over the next few years should be (1) testing the best ones compared with each other, (2) comparing haplo to other alternative donor sources such as cord blood, and (3) comparing haploidentical with standard approaches using matched unrelated and related donor sources.
Early progress in overcoming the major obstacle of haploidentical approaches, severe graft-versus-host disease (GVHD), came when centers developed efficient techniques for removing T cells in the graft (see figure). This elimination approach has been accomplished either by positive selection of CD34+ cells only for infusion or by negative selection, removing all CD3+ cells from the graft. Although elimination of T cells to less than 105 per kilogram of recipient weight controlled the GVHD problem, it spawned 2 more problems: increased graft rejection and profound and prolonged posttransplant immune deficiency. Giving megadose CD34+ infusions (>107 cells per kilogram) partially addressed the problem,2 but ongoing rejection issues have led to the use of profoundly immune suppressive preparative approaches, adding to never-ending posttransplant lymphopenia. Some groups have chosen to address the immune suppression challenge with augmentation of immune recovery by multipathogen-specific T cells.3 Although this is feasible and interesting, it requires expensive cell engineering and production available only at select centers.
An explosion of translational immunologic research over the past 2 decades has resulted in a variety of very promising techniques that either regulate GVHD-causing T cells or engineer grafts designed to partially or fully overcome the GVHD-rejection-infection triad. Some studies have attempted to anergize the graft to recipient tissues through T-cell costimulatory blockade.4 This approach theoretically allows grafts to retain immunity to nonanergized antigens such as infectious pathogens. Other groups are regulating GVHD by co-infusing selected or expanded T-regulatory cells along with specified doses of conventional T cells with the same end in mind.5
Bertaina et al used one of a series of variations of graft engineering that go beyond the nondiscriminating elimination of all of a given type of cell to instead selectively remove the bad cells and retain the good cells in the rich variety of cells in a graft. The first variation removed CD3+ and CD19+ cells, retaining natural killer cells, monocytes, and dendritic cells in the graft. Although immune recovery and mortality may be lower with this approach compared with earlier methods, a degree of rejection and slow immune recovery remains.6 The next generation in this technology (αβ+CD3+/CD19+ depletion used by Bertaina et al) recognizes that γδ+CD3+ T cells do not cause GVHD and may help with immune maintenance and recovery. This takes us one step closer to removing only alloreactive T cells, leaving other cells in the graft to do their work. A similar approach designed to remove only alloreactive cells has been built on the observation that alloreactive cells have been noted to be CD45RA+; thus, depletion of these cells leaves other cells in the mix that may be important for immune function.7 A similar attempt at selectively targeting alloreactive cells uses dibromorhodamine followed by photodepletion, a method that selectively depletes alloreactive T cells while sparing T regulatory cells.8
So which of these competing methods is best? The published data are limited, and comparative data between methods is absent, but as shown by Bertaina et al, although rejection was rare and was rescued by second grafts, an overall rejection rate of 17% means there is room for improvement. In addition, although Bertaina’s data show that a significant rise in the number of CD4RA+ and CD8RA+ cells occurred between 6 and 9 months after transplant, immune function was still very poor up to that point.1
Two other interesting forms of haplo-enabling graft engineering must be mentioned. Posttransplant cyclophosphamide has been noted to lead to high rates of engraftment and immune recovery with modest GVHD.9 That method takes advantage of the early proliferation of both donor and recipient alloreactive cells that occurs in the first few days after transplant. Cyclophosphamide is given on days 3 and 4 after transplant, causing an in vivo depletion of both donor and recipient alloreactive cells, which promotes engraftment and decreases GVHD. However, many other cells are affected by posttransplant cyclophosphamide; how this approach compares with other methods for GVHD and relapse prevention needs to be tested in randomized trials. A second approach that should be mentioned is that of transduction of donor T cells with suicide genes. The cells are then put in the graft and allowed to remain functional until GVHD occurs, at which point they are eliminated. Although this approach is technically cumbersome, it has resulted in major reduction of GVHD in patients who have undergone the procedure to date.10
So which of these approaches is best to tame haploidentical T cells and facilitate engraftment of this very convenient, nearly universally available stem cell source? With so many great candidates coming forward, the winning method or methods are likely to show that once-dreaded haploidentical approaches now rival fully HLA-matched transplantation and may soon become more widely embraced by transplant teams throughout the world.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–826. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 2.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 3.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–4406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340(22):1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 5.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 6.Federmann B, Bornhauser M, Meisner C, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica. 2012;97(10):1523–1531. doi: 10.3324/haematol.2011.059378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleakley M, Heimfeld S, Jones LA, et al. Engineering human peripheral blood stem cell grafts that are depleted of naïve T cells and retain functional pathogen-specific memory T cells. Biol Blood Marrow Transplant. 2014;20(5):705–716. doi: 10.1016/j.bbmt.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielke S, Nunes R, Rezvani K, et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood. 2008;111(8):4392–4402. doi: 10.1182/blood-2007-08-104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munchel A, Kesserwan C, Symons HJ, et al. Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep. 2011;3(Suppl 2):e15. doi: 10.4081/pr.2011.s2.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]