Abstract
Asthma is a serious health problem worldwide, particularly in industrialized countries. Despite a better understanding of the pathophysiology of asthma, there are still considerable gaps in knowledge as well as a need for new classes of drugs. ASHMI™ (Anti-asthma Herbal Medicine Intervention) is an aqueous extract of Ganoderma lucidum (Fr.) P. Karst (Ling Zhi), Sophora flavescens Aiton (Ku Shen) and Glycyrrhiza uralensis Fisch. ex DC (Gan Cao). It prevents allergic asthma airway hyper-reactivity in mice and inhibits acetylcholine (ACh) induced airway smooth muscle (ASM) contraction in tracheal rings from allergic asthmatic mice. The purpose of this research was to identify individual herb(s) and their active compound(s) that inhibit ASM contraction. It was found that Sophora flavescens (S. flavescens), but not Ganoderma lucidum (G. lucidum) or Glycyrrhiza uralensis (G. uralensis) aqueous extracts, inhibited ASM contraction in tracheal rings from asthmatic mice. Bioassay-guided isolation and identification of flavonoid fractions/compound(s) via methylene chloride extraction, preparative HPLC fractionation, and LC-MS and NMR spectroscopic analyses showed that trifolirhizin is an active constituent that inhibits acetylcholine mediated ASM contraction or directly relaxes pre-contracted ASM independent of β2-adrenoceptors.
Keywords: Sophora flavescens, Leguminosae, Asthma, Traditional Chinese herbal medicine, ASHMI, Ku Shen, trifolirhizin, airway smooth muscle contraction
1. Introduction
Allergic asthma, a growing health concern worldwide, particularly in industrialized countries (Akinbami et al., 2009; Braman, 2006; CDC, 2011), is a complex disease associated with an elevation of IgE production, abnormally high levels of Th2 cytokines, and airway inflammation. The immediate allergic asthmatic response is the release of histamine and leukotrienes from IgE-activated mast cells that leads to bronchial constriction and airway hyper-reactivity (AHR) (Barrios et al., 2006). Increased airway smooth muscle (ASM) contraction and reduced ASM relaxation in chronic asthma contributes to the excessive airway narrowing and AHR characteristic of asthmatic attacks (Billington et al., 2003; Holgate et al., 2003)
Despite a better understanding of the pathophysiology of asthma, there are still considerable gaps in knowledge as well as a need for new classes of drugs. Current standard asthma management is primarily directed towards suppressing airway inflammation with inhaled corticosteroids (ICSs) and relieving bronchoconstriction with bronchodilators. This standard therapy controls symptoms in most, but not all asthmatic patients while on treatment (Hall, 2004; Lemanske, 1998). Severe asthma is difficult to control (Hall, 2004; Lemanske, 1998; The National Institute of Allergy and Infectious Diseases (NIAID), 2010). ICSs are generally safe, however, their prolonged use, especially at high doses has been accompanied by concern about both systemic and local side-effects (Lipworth, 1999; Roland et al., 2004). These include osteoporosis (Lipworth, 1999; Packe et al., 1996; Roland et al., 2004; Toogood et al., 1988), hypothalamic-pituitary-adrenal axis suppression (Zollner, 2007), immune suppression resulting in increased susceptibility to infections such as esophageal candidiasis (Fukushima et al., 2003) and frequent upper respiratory tract infections in children (Noonan et al., 2009), development of cataracts in elderly patients (Wang et al., 2009), mood changes (Cordina et al., 2009), and pharyngitis (Bhalla et al., 2008). Long-term treatment with β2-adrenoceptor (β2-AR) agonists that directly relax ASM contraction, can result in bronchodilator resistance (Salpeter et al., 2004), cardiovascular diseases and arrhythmias (Plusa, 2010). Therefore, development of new alternative or complementary therapies for this disease is becoming increasingly important.
In recent years, interest and use of complementary and alternative medicine (CAM) has been increasing (Bielory et al., 2004; Li, 2009). Traditional Chinese medicine (TCM) has a long history of human use in China and other Asian countries for treating and preventing diseases including asthma, and is part of main stream medicine in these countries. Furthermore, TCM is beginning to play a role in the US health care system. There is also increasing scientific understanding of the mechanisms of actions of certain TCMs for allergy and asthma (Li et al., 2009). Kao et al. (2001) showed that the TCM formula xiao-qing-long-tang (XQLT) inhibited bronchoconstriction and airway eosinophil infiltration in ovalbumin-sensitized guinea pigs, and suppressed airway smooth muscle contraction (Kao et al., 2001). It was previously demonstrated that ASHMI™, an aqueous (aq.) extract of G. lucidum, S. flavescens and G. uralensis, eliminated both the immediate airway response and late-phase AHR, and significantly reduced pulmonary inflammation and airway remodeling in a murine allergic asthma model (Busse et al., 2010; Li et al., 2000; Zhang et al., 2010). In a clinical trial, ASHMI™ treatment reduced the use of β2-AR agonists, while significantly improving objective lung function and reducing symptom scores of asthmatics to a similar extent as conventional treatment, and showed a high safety profile (Kelly-Pieper K et al., 2009; Wen et al., 2005). It was further found that, in addition to its anti-inflammatory effect, ASHMI™ inhibited ex vivo acetylcholine-induced ASM contractility in tracheal rings of allergic asthmatic mice without activating β2-ARs (Zhang et al., 2010). However, the compounds responsible for inhibiting ASM contraction have not been identified.
The goal of this study was to identify active components in ASHMI™ that inhibit ASM contraction. It was first found that S. flavescens, but not G. lucidum or G. uralensis aq. extracts,inhibited acetylcholine-induced ex vivo ASM contraction in tracheal rings from asthmatic mice. The efficacy of this single herb extract was equivalent to that of ASHMI™. S. flavescens contains a variety of flavonoids and alkaloids (Abdel-Kader, 2010; Bensky D et al., 1993; Liu et al., 2011; Zhang et al., 2007). Given the ubiquitous distribution of flavonoids in plants, their relatively low toxicity compared to other active plant compounds, and the findings that some flavonoids have anti-allergy and anti-inflammatory properties (Kawai et al., 2007), and based on our preliminary study(Liang B et al., 2010), a bioassay-guided fractionation was conducted to identify flavonoid compounds in S. flavescens that inhibit ASM contraction. For the first time, it is demonstrated that a flavonoid-rich fraction (MC-F1) of S. flavescens inhibits ASM contraction to ACh stimulation, and that the compound, trifolirhizin (1) isolated from this fraction is an active compound that inhibits ASM contraction.
2. RESULTS AND DISCUSSION
2.1. S. flavescens inhibited acetylcholine-induced ASM contraction in tracheal rings from asthmatic mice
To determine which herbal constituents in ASHMI™ have a direct inhibitory effect on ASM contraction, an asthmatic mouse model was induced by ovalbumin (OVA) sensitization and challenge as described previously (Zhang et al., 2010). Tracheal rings from asthmatic mice were treated with an aq. extract of ASHMI™ (100 μg/ml), or individual herb aq. extracts, G. lucidum (35μg/ml), S. flavescens (45μg/ml) and G. uralenisis (20μg /ml), for 30 min prior to ex vivo acetylcholine provocation. The ASHMI™ concentration was based on our previous publication (Zhang et al., 2010). Concentrations of individual herb extracts were equivalent to their individual concentrations in ASHMI™. ASHMI™ treatment significantly reduced acetylcholine -induced ASM contraction in tracheal rings over a range of 10−6 M −10−4 M acetylcholine (Fig. 1A, p<0.001 vs control phosphate buffered saline (PBS)-treated tracheal rings). These results are consistent with our previous publication (Zhang et al., 2010). S. flavescens extracts at a concentration equivalent to that in ASHMI™ inhibited acetylcholine - induced ASM contraction to the same degree as ASHMI™ (p<0.001, Fig. 1A, and B, myography tracing). G. lucidum and G. uralensis at their ASHMI™ equivalent concentrations did not inhibit contraction (Fig. 1A).
Fig 1. S. flavescens inhibits acetylcholine-induced ASM contraction.
(A). Effect of ASHMI™ and three individual herb extracts (in concentrations equivalent to that in ASHMI™ ) on acetylcholine (ACh)-induced contraction of ASM in tracheal rings from sensitized/challenged BALB/c mice. Acetylcholine contractions are expressed as a percent of 60 mM KCl contractions and the graph shows mean ± SEM. (n=3 tracheal ring per treatment) (***, p<0.001 significantly different from the control group ) The concentrations of ASHMI, S. flavescens, G. lucidum, and G. uralensis were 100, 45, 35, and 20 μg/ml respectively. (B). Representative myography tracings of control (PBS) (upper tracing) and S. flavescens-treated (lower tracing) tracheal rings from sensitized/challenged BALB/c mice.
These findings demonstrated that S. flavescens is the herb in ASHMI™ responsible for inhibition of ASM contraction. S. flavescens is one of the most widely used traditional Chinese medicinal herbs. It has been commonly used for the treatment of viral hepatitis, cancer, and eczema (Bensky D et al., 1993; The State Pharmacopoeia Commission of The People's Republic of China, 2005), as well as bronchial asthma (Hoang et al., 2007; Yu et al., 1999). To our knowledge, this is the first study demonstrating that S. flavescens has a direct effect on ASM contraction.
2.2. Identification of the S. flavescens flavonoid fraction and subfraction that inhibit acetylcholine-induced ASM contraction
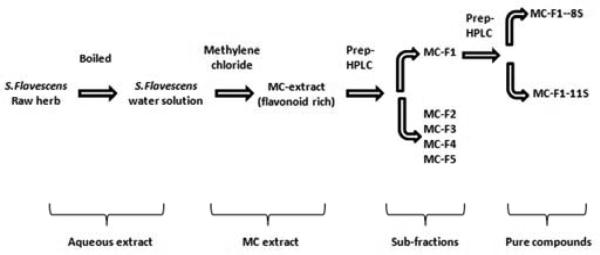
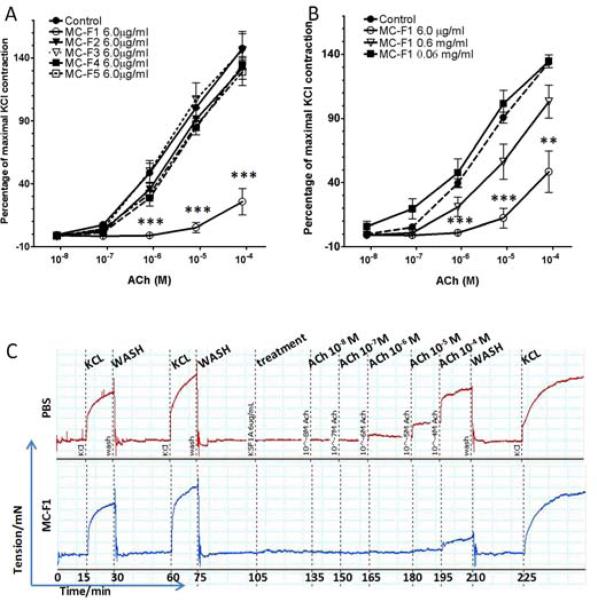
A methylene chloride (MC) extract from an aq. extract of S. flavescens was first obtained (designated MC extract, Fig. 2). Preliminary work, reported in an abstract (Liang B et al., 2010), showed that 6.0 μg/ml MC extract (equivalent to 84.5μg/ml of aq S. flavescens extract) inhibited ASM contraction to 10−4 M acetylcholine. In the current study, the effect of the MC extract (6.0 μg/ml) on ASM contraction in tracheal rings from asthmatic mice to incremental concentrations of acetylcholine was determined, ranging from 10−8 to 10−4 M. The MC extract significantly inhibited acetylcholine -induced contraction (Fig. 3, p<0.001 vs. control). Next, 5 sub-fractions (MC-F1, 2, 3, 4 and 5) were obtained using preparative HPLC and each was tested at 6.0 μg/ml. Only MC-F1 significantly suppressed ASM contraction to acetylcholine (Fig. 4A, p<0.001 vs. control). Other fractions did not have a significant effect (Fig. 4A vs. control), even at 2-5 times higher concentrations (data not shown). The inhibition of ASM contraction by MC-F1 was concentration-dependent (Fig. 4B) and non-toxic as contractile responses to KCl were present after buffer washings (Fig. 4C). These findings show that sub-fraction MC-F1 contains the active flavonoid(s), and thus it was subjected to further fractionation and identification of single compounds.
Fig 2. Fractionation method of aq. S. flavescens extract.
MC: Methylene Chloride. MC-F1~5: sub-fractions of methylene chloride extract of aq. S.flavescens. Two individual compounds were isolated and named MC-F1-8S and MC-F1-11S.
Fig 3. MC-extract inhibition of acetylcholine-induced ASM contraction.
30 min pretreatment of sensitized/challenged mouse tracheal rings with MC-extract (6.0 μg/ml) was followed by challenges with acetylcholine (ACh) (10−8 M to 10−4 M).. Acetylcholine contractions are expressed as a percent of 60 mM KCl contractions and the graph shows mean ± SEM. (n=3 tracheal rings per group) (***, p≤0.001, significantly different from control group).
Fig 4. MC-F1 sub-fraction inhibition of acetylcholine -induced ASM contraction.
(A). Acetylcholine (ACh)-stimulated mouse tracheal rings were pre-treated with either MC-F1, 2, 3, 4, or 5 at a concentration of 6.0 μg/ml for 30min and then stimulated with acetylcholine. Acetylcholine contractions are expressed as a percent of 60 mM KCl contractions. (B). Acetylcholine -stimulated mouse tracheal rings pre-treated with MC-F1 at 0.06, 0.6, and 6.0 μg/ml. Graph shows mean ± SEM. (n=3 tracheal rings per group), (**, p≤0.01; ***, p≤0.001 significantly different from control group). (C). Representative myography tracing of control (PBS) (upper tracing) or MC-F1 (6.0 μg/ml) treated (lower tracing) tracheal rings demonstrating the marked attenuation of acetylcholine-induced contractions in rings pre-treated with MC-F1.
2.3. Isolation and identification of 3',7-dihydroxy-4'-methoxy-isoflavone and trifolirhizin from MC-F1
The MC-F1 fraction was further fractionated by preparative HPLC and 19 subfractions were collected. Based on analytical HPLC chromatograms subfractions 8 and 11 each contained a single peak, designated MC-F1-8S and MC-F1-11S (purity > 90%). The remaining fractions containing either mixtures (not pure) or flow-through solvent were combined for further investigation. The structures of these compounds (Fig. 5) were identified as trifolirhizin (1) (11S) and 3',7-dihydroxy-4'-methoxy-isoflavone (2) (8S) using liquid chromatography mass spectrometry (LC-MS), 1H and 13C NMR spectroscopy. LC-MS was first used to analyze these two subfractions. The UV spectrum data and total ion chromatography (TIC) are shown in supplemental data, Fig. 1A. Mass spectral data of MC-F1-11S indicated that m/z 285, 447, and 464 corresponded to [M-Glc]+, [M+H]+, and [M+NH4]+, respectively. Mass spectral data for MC-F1-8S showed a strong [M+H]+ ion at m/z 285 (Supplemental data Fig. 1B). The molecular weights (MW) of MC-F1-11S and MC-F1-8S were then determined to be 446 and 284 respectively. Both the 1H NMR and 13C NMR spectroscopic data of trifolirhizin (1) was collected (Supplemental data Table 1) and agree with previously reported data (Abdel-Kader, 2010; Hyun et al., 2008; Zhang et al., 2007; Zhou et al., 2009). Based on the HPLC, LC-MS and 1H NMR spectroscopic data of MC-F1-8S (Supplemental data Table 2) and a previous publication (Hyun et al., 2008), its structure was identified as 3',7-dihydroxy-4'-methoxy-isoflavone (2).
Fig 5.

Chemical structures of trifolirhizin (1) and 3',7-dihydroxy-4'-methoxy-isoflavone (2).
2.4. Trifolirhizin inhibited ASM contraction
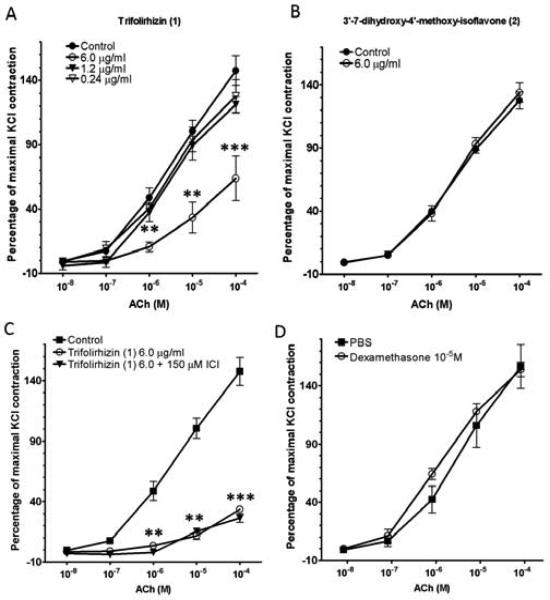
Trifolirhizin (1) significantly inhibited acetylcholine -induced contraction at 6.0 μg/ml, but not at lower concentrations (Fig. 6A, p<0.01-0.001). 3',7-dihydroxy-4'-methoxy-isoflavone (2) (6.0 μg/ml) had no effect on ASM contraction to acetylcholine (Fig. 6B). β2-AR agonists are commonly used therapeutics to prevent ASM contraction or facilitate ASM relaxation. To determine whether trifolirhizin's (1) effect on ASM contraction involved β2-ARs, sensitized murine tracheal rings were preincubated with or without trifolirhizin (1) (6.0 μg/ml) and with or without the β2-AR antagonist ICI 118,551 (150 μM) for 30 min before acetylcholine contractions. The β2-AR antagonist had no effect on ability of trifolirhizin (1) to impair an acetylcholine contraction (Fig. 6C). Dexamethasone is commonly used as a treatment for acute asthma. Therefore, the ability of an acute dexamethasone pretreatment to inhibit acetylcholine-induced contractions in sensitized murine tracheal rings was also tested. Dexamethasone (10μM) did not inhibit the acetylcholine -induced ASM contractions (Fig. 6D).
Fig 6. Effects of compounds isolated from MC-F1 on ASM contraction.
(A). The effect of trifolirhizin (1) on acetylcholine -induced ASM contraction. (B). The effect of 3',7-dihydroxy-4'-methoxy-isoflavone (2) on acetylcholine (ACh)-stimulated ASM contraction. (C). β2-AR antagonist ICI 118,551 (150 μM) did not block the trifolirhizin (1) inhibiton of acetylcholine-induced contractions. (D) Pretreatment with dexamethasone (10 μM) did not affect acetylcholine-induced contractions. Acetylcholine contractions are expressed as a percent of 60 mM KCl contractions and graph shows mean ± SEM. (n=3 tracheal rings per group), significantly different from control group (**, p≤0.01; ***, p≤0.001 compared to control).
Studies of pharmacological actions of trifolirhizin (1) are limited. It has been shown to inhibit inflammatory responses (Zhou et al., 2009), and melanin synthesis by melanoma cells (Yu et al., 1999). However, it has not previously been evaluated for a potential effect on ASM. The current study is thus the first demonstration that trifolirhizin (1) inhibits ASM contraction. A previous open label study reported that S. flavescens aq. extract produced remarkable clinical improvement of asthma symptoms, reduction of 2-AR agonist use, dose of inhaled corticosteroid, and improvement of PEF in refractory asthmatics (Hoang et al., 2007). Hoang et. al. suggested that the two major matrine-type alkaloids, matrine and oxymatrine, may be the primary active components in the herbal mixture responsible for the anti-asthma effect (Hoang et al., 2007). However, S. flavescens contains not only matrine-type alkaloids, it also contains active flavonoids which may contribute other anti-asthmatic effects (Jin et al., 2010). In another open label study, S. flavescens aq. extract was given by inhalation. Wheezing and chest pain diminished, and in some cases disappeared in 10 minutes, and this effect lasted for up to 36 hours (Zhang et al., 2010). The current study is, however the first to identify the S. flavescens flavonoid trifolirhizin (1) as an active compound that contributes to S. flavescens inhibition of ASM contraction. Mechanisms of ASM modulation are complex, but mainly involve G protein-coupled receptors (GPCRs) controlling intracellular calcium and the responsiveness of the actin myosin complex. Gs-coupled receptors, including β2-ARs and prostaglandin E2 receptors, primarily mediate relaxation as a consequence of cAMP signaling. Gq and Gi-coupled receptors include muscarinic M3, histamine H1 and leukotriene CysLT1 mediate contraction (Zhang et al., 2007). Activation of Gq-coupled receptors results in activation of phospholipase C, increased intracellular Ca+2 and ASM contraction. Activation of receptors coupled to Gi (such as adenosine A1/A3, and muscarinic M2) leads to inhibition of adenylyl cyclase, and activation of phospholipase C. Aberrant GPCR signaling in ASM may contribute to elevated ASM tone in asthma. Our previous study showed that ASHMI inhibition of ASM contraction is β2-AR independent (Zhang et al., 2010), and that was confirmed for trifolirhizin (1) in the present study where pretreatment with the β2-AR antagonist, ICI 118,551 did not block its effect of pretreatment on impairment of acetylcholine in sensitized murine tracheal rings (Fig. 6C).
2.5. Trifolirhizin relaxed the pre-contracted tracheal rings
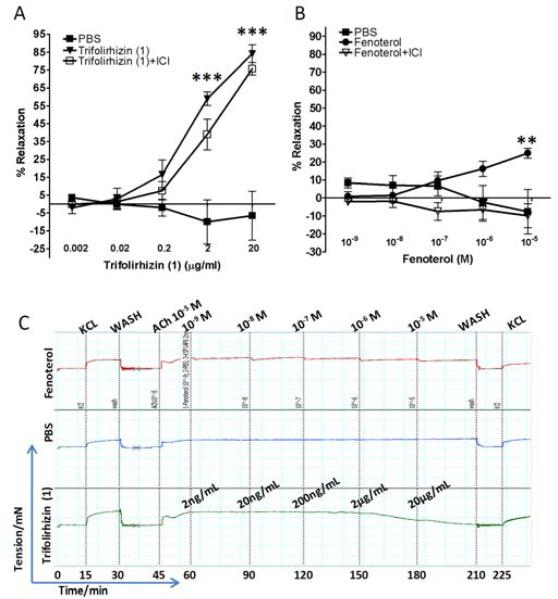
The primary therapy for acute constriction of airway smooth muscle is β2-AR agonists which are inhaled for the acute relaxation of hyper-responsive airway smooth muscle. Studies with trifolirhizin (1) were performed to determine whether it could relax pre-contracted airway smooth muscle and how the magnitude of relaxation compared to a classic β2-AR agonist fenoterol. In order to investigate the relaxation effect of trifolirhizin (1) on ASM, it was tested on acetylcholine pre-contracted sensitized murine tracheal rings, and it relaxed the pre-contracted tracheal rings stimulated by 10−5 M acetylcholine in a dose-dependent manner. The maximal relaxation was 85% of the initial contractile tone at a trifolirhzin (1) concentration of 20μg/ml (Fig. 7A). In parallel studies, the β2-AR agonist fenoterol only achieved 25% relaxation even at the highest concentration tested (10−5 M). It was also observed that fenoterol relaxed the contraction of tracheal rings immediately after the addition, but slowly lost its effect with increasing time of incubation which is consistent with desensitization of the β2-AR, a mechanism thought in part responsible for β 2-agonists’ limitations in the treatment of acute allergic asthma. Co-incubation of the β2-AR antagonist, ICI 118,551 with trifolirhizin (1) was without effect on its inhibition capability (Fig.7A), while ICI 118,551 completely blocked the effect of fenoterol (Fig. 7B).
Fig 7. Relaxation effects of trifolirhizin (1) or the β2-AR agonist fenoterol on acetylcholine pre-contracted ASM.
(A). The relaxation effect of trifolirhizin (1) on acetylcholine -stimulated tracheal rings with or without ICI 118,551 (ICI). (B). The relaxation effect of the β2-AR agonist fenoterol on acetylcholine -contracted tracheal rings with or without ICI 118,551; Relaxation is expressed as the percent of residual contractile tone remaining at 15 min after trifolirhizin (1) or fenoterol addition. (C). Representative myography tracings of relaxation experiment using fenoterol (upper tracing), PBS control (middle tracing) or trifolirhizin (1)- (lower tracing) treated sensitized mouse tracheal rings (***, p≤0.001 compared to PBS control). Note the magnitude of total relaxation achieved with trifolirhizin (1) compared to the β2-AR agonist fenoterol.
2.6. Quantification
The amount of bioactive flavonoid trifolirhizin (1) present in the S. flavescens was quantified by HPLC, using a calibration at five different concentrations 5.0 to 15.0 μg/ml. The calibration curves were plotted using the peak areas of each condition and the equation of the peak area and the concentration was generated. Its concentration in the aqueous extract of S. flavescens was approximately 6.7 μg/100 mg. Previous in vitro use of trifolirhizin (1) involved concentrations ranging from 10-20 μM to 100-200 μM in the cultures of mouse J774A.1 macrophages (McGraw et al., 2003) , or 50μM in the cultures of melanoma B16 cells . The trifolirhizin (1) concentration used in our ex vivo study was 6.0 μg/ml (13.4 μM), which is in the lower range of concentrations shown to be active in other systems. Although effective concentrations for ASM were comparable to those previously reported, they reflect higher concentrations than would occur in ASHMI™. Whether this reflects the presence of other ASM-relaxant compounds in ASHMI™, or potentiation of trifolirhizin (1) by other ASHMI™ components, is yet unknown.
3. CONCLUSIONS
It is demonstrated here for the first time, that S. flavescens prevents tracheal ring acetylcholine-induced ASM contraction. Using bioassay-guided chemical and HPLC fractionation methods, and LC-MS and NMR spectroscopic analyses, the flavonoid trifolirhizin (1) was isolated, and showed that, at micromolar concentrations, it could not only inhibit ASM contraction to acetylcholine, but also relaxed pre-contracted ASM. Its effect is not mediated through the β2-AR and the detailed cell signaling mechanism(s) require further investigation. These findings may lead to the development of a new class of ASM modulating drugs for asthma.
4. Experimental procedures
4.1. General procedures, Chemicals and reagents
The medicinal herbs used in this study were of Chinese origin. All were inspected for identity and quality by pharmacists trained and licensed in identification and processing of herbal medicines and only herbs meeting quality and safety standards (The State Pharmacopoeia Commission of The People's Republic of China, 2005) were used. Aq. extracts of the individual herbal medicines, G. lucidum, S. flavescens and G. uralensis were provided by Sino-lion (Zhong Shi) Pharmaceutical Company (Weifang, China). G. lucidum (60kg), S. flavescens (27kg), and G. uralensis (9 kg) were soaked in 10 volumes of water for 1 hour, boiled for 2 hours, and the decoction was collected. The decoction was then concentrated under reduced pressure (P = 0.08 Mpa), and sprayed to fine liquid drops, and thermally dried by filtered hot air. Based on the ratio of individual raw herbs G. Lucidum, S. flavescens and G. uralensis in the ASHMI™ formula (62.5%, 28.1% and 9% respectively), and the yield of extracts of G. lucidum, S. flavescens, and G.uralenisis (6 %, 20 % and 17 % respectively), ASHMI™ used in this study was a combination of the 3 herbal extracts in a ratio of 35%, 45%, 20% of G. lucidum, S. flavescens and G. uralenisis respectively. HPLC grade CH2Cl2, EtOAc, CH3CN with 0.1% HCO2H, KCl, and Al (OH)3 (Alum, Imject®) were purchased from Fisher Scientific (Pittsburgh, PA). Albumin (OVA, Grade VI), Acetylcholine chloride (ACh, 99%), ICI 118,551 hydrochloride (>98% TLC), dexamethasone, and fenoterol hydrobromide were purchased from Sigma Aldrich (St Louis, MO).
4.2. Experimental Animals
Six-week old, BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facility at Mount Sinai School of Medicine. Standard guidelines for the care and use of animals were followed (Institute of Laboratory Animal Resources Commission of Life Sciences, 1996).
4.3. Preparation of flavonoid rich fraction from S. flavescens by methylene chloride extraction
Methylene chloride (MC) extraction was to isolate the less-polar compound soluble extract (Fig. 2), from a dried S. flavescens aqueous extract (101.27 g) dissolved in distilled H2O (4.0 L), which was then extracted with CH2 Cl2 (3.0 L) for 24hr in a liquid-liquid extractor (Sigma-Aldrich) at 100°C using a 2 L heating mantle (EM2000/CX1, Barnstead International, Dubuque, IA). The CH2Cl2 extract of S. flavescens (MC-extract) was dried with a rotary evaporator (Rotavapor R-210, BÜCHI, Switzerland) and lyophilized (LYPH·LOCK 6 freeze dry system, LABCONCO, Kansas City, MO). Total 3.06 g of MC-extract was collected.
4.4. Preparation of MC sub-fractions and individual compounds by preparative HPLC
A 100 mg/ml MC-extract solution was prepared by dissolving the MC-extract powder into a mixture of H2O and CH3 CN (1:1, v/v) containing 0.1% HCO2H and loaded into a preparative HPLC system. Separation was performed on a C18 reversed phase preparative column (XBridge™ Prep C18 5μm OBDTM, 19×150 mm, Waters, Milford, MA) with distilled H2O as mobile phase A and CH3CN containing 0.1% HCO2H as mobile phase B at a constant flow rate of 20 ml/min and detected under photodiode array (PDA) detector at wavelength 254nm. The initial gradient was 30% B for 20 min; increased to 50% in 4 min; increased to 100% B in 2 min; maintained at 100% for another 2 min. Five sub-fractions were collected according to a time based collection method (MC-F1~5).
Further separation of MC-F1 sub-fractions was performed using preparative HPLC. 19 subfractions were collected, and each fraction was analyzed using analytical HPLC. Those fractions containing individual peaks were dried in vacuo, and their structures determined using NMR and LC-MS. Fractions containing multiple-peaks were saved for further separation.
4.5. LC-MS and NMR method
Mass spectra data were collected on a micromass LCT premier TOF mass spectrometer (Waters Corporation, Milford, MA) coupled with a Waters Alliance 2695 HPLC system in a positive electrospray mode (ESI+). 1H and 13C NMR spectra were obtained on a JOEL instrument (at 300 MHz for 1H NMR and 75 MHz for 13C NMR) using DMSO-d6 as the solvent.
4.6. HPLC quantification
Quantification of the biologically active compounds present in S. flavescens was performed by HPLC. The analyses were carried out with a Waters Alliance 2695 separation module (Waters) equipped with PDA detector. The separation was performed on a Zorbax-C18 (150 × 4.6 mm) column with mobile phase A (0.1% HCO2H) and B (CH3CN) at a flow rate of 1.0 ml/min. The linear gradient from 2% to 46% B over 75 min was used for separation. Samples were dissolved in mobile phase mixture (1:1, v/v). UV wavelength was monitored from 210 nm to 400 nm. Five different concentrations of standard compound (5, 8, 10, 12, and 15 μg/ml) were prepared and analyzed with HPLC and the Empower software (Waters). The calibration curve was generated by using peak area obtained at 254 nm of standard at different concentrations. The R2 was 0.9995.
4.7. Animal sensitization and challenge
Mice were sensitized by intraperitoneal (i.p.) injection of OVA (200μg) plus 2 mg of Al(OH)3 (alum) in PBS (400 μl) on days 0 and 7. Mice were challenged intratracheally (i.t.) with OVA (100 μg) in PBS (50μl) weekly for 2 weeks (days 14 and 21). Mice were challenged i.t. once daily with OVA (100μg, 50 μl) on 2 consecutive days (days 28 and 29) 48 hr before sacrifice and tissue harvesting.
4.8. Measurement of ASM contractility ex vivo
Forty-eight hrs after the last i.t. OVA challenge, mice were sacrificed and tracheas were excised and cut into 3 tracheal rings as previously described. Each tracheal ring was attached to a pair of mounting pins in the organ chamber of a Myograph System Model 610M system (AD Instruments, Denver, CO) and bathed in a chamber containing physiological salt solution (PSS (In g/L- NaCl: 6.95, KCl: 0.35, MgSO4·7H2O:0.28, KH2PO4: 0.1561, CaCl2·2H2O: 0.36, NaHCO3: 2.1, EDTA: 0.01, Glucose: 1.09, pH=7.4). All chambers were maintained at 37°C and were continuously bubbled with 100 % O2. The change of tracheal ring tension was recorded by Power Lab Chart recording software (AD Instruments). After incubation at 37°C for 15 min at baseline tension, passive tension applied to the tracheal ring was adjusted to 1.00 mN. Following stabilization of passive tension, tracheal rings were contracted using 60mM KCl twice, to assess ASM contractility. Tracheal rings were washed with PSS buffer 3 times and allowed to rest at 1.00mN for 30 min after each stimulation. ASHMI™, individual herb extracts, and fractions were dissolved in PBS. The individual compounds and dexamethasone were dissolved in DMSO and then re-suspended in PBS (DMSO < 0.1%). Each drug was added to the chamber at the concentrations indicated and the pre-incubation time was 30 min. Tracheal rings were then contracted with incremental doses of acetylcholine (from 10−8 M to 10−4 M). In some experiments, the β2-AR antagonist ICI 118,551 was added 15 minutes prior to addition of trifolirhizin (1) to the organ chamber. PBS (vehicle) treatment was used as control.
In order to investigate the relaxation effect of trifolirhizin (1), tracheal rings were first contracted with acetylcholine at10−5 M for 15 min and then incubated with different concentrations of trifolirhizin (from 2 ng/ml~20 μg/ml) or fenoterol (10−9 M~10−5 M) in the presence or absence of ICI 118,551.
After completing the experiments, all tracheal rings were washed and stimulated with KCl to verify viability. Responses to acetylcholine were expressed as percent of contraction to 60 mM KCl.
4.9. Statistical analyses
Data were analyzed using SigmaStat 3.5 software (SPSS Inc. Chicago, IL). For data that passed normality testing, differences between groups were analyzed by One Way Analysis of Variance (One way ANOVA) followed by pair wise testing using Bonferroni’s adjustment. For data that appeared skewed (non-normal), differences between groups were analyzed by One Way ANOVA on Ranks followed by all pair wise comparisons. P values ≤0.05 were considered significant.
Supplementary Material
S. flavescens inhibited acetylcholine-induced murine airway smooth muscle (ASM) contraction.
3',7-dihydroxy-4'-methoxy-isoflavone and trifolirhizin were isolated from the methylene chloride extract of S. flavescens.
Trifolirhizin inhibited the acetylcholine-induced ASM contraction through a non- 2-adrenoceptor mediated pathway.
Acknowledgement
This study was supported by NIH/NCCAM grant # 1P01 AT002644725-01 “Center for Chinese Herbal Therapy (CHT) for Asthma” to X.-M. Li. and partially supported by a Utah State University faculty startup fund to J. Zhan, and partially supported by NIH/NCCAM Ruth L. Kirchstein Pre-doctoral fellowship award # F31AT003769 to KD Srivastava.
US Patent PCT/US05/08600 on ASHMI™ has been issued to Drs. Xiu-Min Li, Hugh Sampson Ming Chun Wen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Kader MS. Preliminary pharmacological study of the pterocarpans macckian and trifolirhizin isolated from the roots of Ononis vaginalis. Pak. J Pharm. Sci. 2010;23:182–187. [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Barrios RJ, Kheradmand F, Batts L, Corry DB. Asthma: pathology and pathophysiology. Arch. Pathol. Lab Med. 2006;130:447–451. doi: 10.5858/2006-130-447-APAP. [DOI] [PubMed] [Google Scholar]
- Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. Revised Edition Seattle: 1993. pp. 82–83. [Google Scholar]
- Bhalla RK, Taylor W, Jones AS, Roland NJ. The inflammation produced by corticosteroid inhalers in the pharynx in asthmatics. Clin Otolaryngol. 2008;33:581–586. doi: 10.1111/j.1749-4486.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- Bielory L, Russin J, Zuckerman GB. Clinical efficacy, mechanisms of action, and adverse effects of complementary and alternative medicine therapies for asthma. Allergy Asthma Proc. 2004;25:283–291. [PubMed] [Google Scholar]
- Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003;4:2–25. [PMC free article] [PubMed] [Google Scholar]
- Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, Srivastava K, Li XM. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann. Allergy Asthma Immunol. 2010;104:236–246. doi: 10.1016/j.anai.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC [January 18, 2012];National Center for Health Statistics: Asthma. 2011 http://www.cdc.gov/nchs/fastats/asthma.htm.
- Cordina M, Fenech AG, Vassallo J, Cacciottolo JM. Anxiety and the management of asthma in an adult outpatient population. Ther. Adv. Respir. Dis. 2009;3:227–233. doi: 10.1177/1753465809347038. [DOI] [PubMed] [Google Scholar]
- Fukushima C, Matsuse H, Tomari S, Obase Y, Miyazaki Y, Shimoda T, Kohno S. Oral candidiasis associated with inhaled corticosteroid use: comparison of fluticasone and beclomethasone. Ann. Allergy Asthma Immunol. 2003;90:646–651. doi: 10.1016/S1081-1206(10)61870-4. [DOI] [PubMed] [Google Scholar]
- Hall IP. The beta-agonist controversy revisited. Lancet. 2004;363:183–184. doi: 10.1016/S0140-6736(03)15369-X. [DOI] [PubMed] [Google Scholar]
- Hoang BX, Shaw DG, Levine S, Hoang C, Pham P. New approach in asthma treatment using excitatory modulator. Phytother. Res. 2007;21:554–557. doi: 10.1002/ptr.2107. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR., Jr. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J. Allergy Clin. Immunol. 2003;111:S18–S34. doi: 10.1067/mai.2003.25. [DOI] [PubMed] [Google Scholar]
- Hyun SK, Lee WH, Jeong d.M., Kim Y, Choi JS. Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin from Sophora flavescens on tyrosinase and melanin synthesis. Biol. Pharm. Bull. 2008;31:154–158. doi: 10.1248/bpb.31.154. [DOI] [PubMed] [Google Scholar]
- Jin JH, Kim JS, Kang SS, Son KH, Chang HW, Kim HP. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J Ethnopharmacol. 2010;127:589–595. doi: 10.1016/j.jep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Kao ST, Lin CS, Hsieh CC, Hsieh WT, Lin JG. Effects of xiao-qing-long-tang (XQLT) on bronchoconstriction and airway eosinophil infiltration in ovalbumin-sensitized guinea pigs: in vivo and in vitro studies. Allergy. 2001;56:1164–1171. doi: 10.1034/j.1398-9995.2001.00982.x. [DOI] [PubMed] [Google Scholar]
- Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y, Ohkawara T, Hagihara K, Yamadori T, Shima Y, Ogata A, Kawase I, Tanaka T. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007;56:113–123. doi: 10.2332/allergolint.R-06-135. [DOI] [PubMed] [Google Scholar]
- Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Wisnivesky J, Li X-M, Kattan M. Safety and tolerability of an antiasthma herbal formula (ASHMI™) in adult asthmatics: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. Journal of Alternative and complementary Medicine. 2009;15:735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemanske RFJ. A review of the current guidelines for allergic rhinitis and asthma. J. Allergy Clin. Immunol. 1998;101:S392–S396. doi: 10.1016/s0091-6749(98)70228-3. [DOI] [PubMed] [Google Scholar]
- Li XM. Complementary and alternative medicine in pediatric allergic disorders. Curr. Opin. Allergy Clin Immunol. 2009;9:161–167. doi: 10.1097/ACI.0b013e328329226f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Brown L. Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J Allergy Clin Immunol. 2009;123:297–306. doi: 10.1016/j.jaci.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XM, Huang CK, Zhang TF, Teper AA, Srivastava K, Schofield BH, Sampson HA. The chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol. 2000;106:660–668. doi: 10.1067/mai.2000.110102. [DOI] [PubMed] [Google Scholar]
- Liang B, Brown LL, Srivastava K, Birmingham N, Li X. Inhibitory effect of Ku-Shen sub-fractions on murine airway smooth muscle contraction. J Allergy Clin Immunol. 2010;125:S 200–200. (abstract) [Google Scholar]
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch. Intern. Med. 1999;159:941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- Liu G, Dong J, Wang H, Hashi Y, Chen S. Characterization of alkaloids in Sophora flavescens Ait. by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. J Pharm. Biomed. Anal. 2011;54:1065–1072. doi: 10.1016/j.jpba.2010.12.024. [DOI] [PubMed] [Google Scholar]
- McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by beta-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway beta-agonist paradox. J. Clin. Invest. 2003;112:619–626. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan M, Leflein J, Corren J, Staudinger H. Long-term safety of mometasone furoate administered via a dry powder inhaler in children: Results of an open-label study comparing mometasone furoate with beclomethasone dipropionate in children with persistent asthma. BMC. Pediatr. 2009;9:43–50. doi: 10.1186/1471-2431-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packe GE, Robb O, Robins SP, Reid DM, Douglas JG. Bone density in asthmatic patients taking inhaled corticosteroids: comparison of budesonide and beclomethasone dipropionate. J R. Coll. Physicians Lond. 1996;30:128–132. [PMC free article] [PubMed] [Google Scholar]
- Plusa T. Agonists of beta2 adrenergic receptor in the therapy of obstructive diseases. Pol. Merkur Lekarski. 2010;28:8–12. [PubMed] [Google Scholar]
- Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126:213–219. doi: 10.1378/chest.126.1.213. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann. Intern. Med. 2004;140:802–813. doi: 10.7326/0003-4819-140-10-200405180-00010. [DOI] [PubMed] [Google Scholar]
- The National Institute of Allergy and Infectious Diseases (NIAID) [September 19, 2010];Asthma and Allergic Diseases Cooperative Research Centers (U19) 2010 http://grants nih gov/grants/guide/rfa-files/RFA-AI-10-013html.
- The State Pharmacopoeia Commission of The People's Republic of China Pharmacopoeia of the People's Republic of China. Version. 2005;6:1–791. [Google Scholar]
- Toogood JH, Crilly RG, Jones G, Nadeau J, Wells GA. Effect of high-dose inhaled budesonide on calcium and phosphate metabolism and the risk of osteoporosis. Am. Rev. Respir. Dis. 1988;138:57–61. doi: 10.1164/ajrccm/138.1.57. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116:652–657. doi: 10.1016/j.ophtha.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, Mu DZ, Du JB, Li GH, Wallenstein S, Sampson H, Kattan M, Li XM. Efficacy and tolerability of anti-asthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J. Allergy Clin. Immunol. 2005;116:517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Yu Q, Wang P, Liu L, et al. Ku-Shen. Chinese edition Beijing: 1999. [Google Scholar]
- Zhang L, Xu L, Xiao SS, Liao QF, Li Q, Liang J, Chen XH, Bi KS. Characterization of flavonoids in the extract of Sophora flavescens Ait. by high-performance liquid chromatography coupled with diode-array detector and electrospray ionization mass spectrometry. J Pharm. Biomed. Anal. 2007;44:1019–1028. doi: 10.1016/j.jpba.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Zhang T, Srivastava K, Wen MC, Yang N, Cao J, Busse P, Birmingham N, Goldfarb J, Li XM. Pharmacology and immunological actions of a herbal medicine ASHMI on allergic asthma. Phytother. Res. 2010;24:1047–1055. doi: 10.1002/ptr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lutterodt H, Cheng Z, Yu LL. Anti-Inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J Agric. Food Chem. 2009;57:4580–4585. doi: 10.1021/jf900340b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner EW. Hypothalamic-pituitary-adrenal axis suppression in asthmatic children on inhaled corticosteroids (Part 2)--the risk as determined by gold standard adrenal function tests: a systematic review. Pediatr. Allergy Immunol. 2007;18:469–474. doi: 10.1111/j.1399-3038.2007.00539.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.