Abstract
Androgen-deprivation is a mainstay of therapy for advanced prostate cancer but tumor regression is usually incomplete and temporary because of androgen-independent cells in the tumor. It has been speculated that these tumor cells resemble the stem/progenitor cells of the normal prostate. The purpose of this study was to examine the response of slow-cycling progenitor cells in the adult mouse prostate to castration. Proliferating cells in the E16 urogenital sinus were pulse labeled by BrdU administration or by doxycycline-controlled labeling of the histone-H2B GFP mouse. A small population of labeled epithelial cells localized at the junction of the prostatic ducts and urethra. Fluorescence-activated cell sorting (FACS) showed that GFP label-retaining cells were enriched for cells co-expressing stem cell markers Sca-1, CD133, CD44 and CD117 (4- marker cells; 60-fold enrichment). FACS showed, additionally, that 4-marker cells were androgen receptor positive. Castration induced proliferation and dispersal of E16 labeled cells into more distal ductal segments. When naïve adult mice were administered BrdU daily for 2 weeks after castration, 16% of 4-marker exhibited BrdU label in contrast to only 6% of all epithelial cells (P<0.01). In sham-castrated controls less than 4% of 4-marker cells were BrdU labeled (P<0.01). The unexpected and admittedly counter-intuitive finding that castration induced progenitor cell proliferation suggests that androgen deprivation therapy in men with advanced prostate cancer could not only exert pleiotrophic effects on tumor sub-populations but may induce inadvertent expansion of tumor stem cells.
Keywords: Prostate, progenitor cell, castration, proliferation, cancer stem cell
1. Introduction
The mouse prostate develops from the urogenital sinus (UGS). Before embryonic day 16 (E16), the UGS is comprised of an outer layer of mesenchyme surrounding an inner epithelial layer from which outgrowth occurs to form the prostate [1, 2]. At E16.5 –17.5 epithelial buds invade the surrounding mesenchyme and begin the process of ductal morphogenesis that generates the complex ductal structure of the adult prostate [3, 4, 5]. The adult mouse prostate has distinct anterior, dorsal-lateral and ventral lobes; each lobe is divided into proximal, intermediate and distal regions based on their relative location to the urethra [6, 7]. Prostate development is androgen dependent and involves intimate signaling between epithelial and mesenchymal cells. Maintenance of the adult prostate is also androgen-dependent, and the prostate undergoes rapid involution following castration. This involves epithelial apoptosis concentrated in the distal duct segments, loss of androgen-dependent differentiation in the remaining epithelium and remodeling of the periductal stroma [3]. This process is completely reversed by androgen supplement. The castration–regeneration cycle can repeat for many rounds without observable defects in regenerated prostate [3]. This observation suggested the presence of a progenitor cell population in the adult prostate capable of surviving androgen deprivation and sufficient to regenerate the ductal segments of the intact adult prostate.
Adult tissue progenitor cells possessing the ability for self-renewal and/or generation of lineage-committed cells are generally quiescent cells recruited into active proliferation during tissue regeneration and repair [8, 9]. The generally ‘slow cycling’ property of these cells has permitted localization by 3H thymidine, 5-bromo-2′-deoxyuridine (BrdU) and histone H2B- green fluorescent protein (GFP) labeling methods in a variety of tissues, such as mammary gland, hair follicles, small intestine, and cornea [10,11,12, 13,14,15]. The regenerative capacity of the prostate has been attributed to the existence of progenitor cells in the adult gland that survive castration-induced involution [16, 17, 18, 19, 20]. Several lines of evidence suggest that these progenitor cells reside within the proximal region of the prostate ducts. When adolescent mice were BrdU labeled and then subjected to multiple rounds of castration and testosterone supplementation to ‘wash out’ the BrdU label in dividing cells, the ‘slow cycling’ label-retaining cells were concentrated in the proximal duct segments [15]. In another set of experiments it was demonstrated that cells from the proximal duct have higher tissue regenerative ability when grafted under the renal capsule of recipient host animals [21, 22]. The studies reported here build upon those previous observations. We localize a slow-cycling cell population enriched for stem cell markers to the proximal duct segments and show the surprising and clinically important observation that castration induces these cells to proliferate and migrate to more distal sites in the ductal network.
2. Materials and Methods
2.1 Animal maintenance and tissue recovery
Wild-type and nude CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA). The transgenic mouse line expressing the histone H2B- green florescent protein (H2B-GFP) under the control of tetracycline- responsive element [23] was from Jackson Laboratories (Bar Harbor, ME); the mouse line expressing tetracycline responsive element under the control of CK5 promoter (tTA) was a generous gift from Adam B. Glick lab [48]. At the appropriate time prostates were harvested and analyzed for the presence of label retaining cells. The isolated prostate tissue was either enzymatic digested to single cells for FACS analysis or fixed in 10% neutral buffered formalin for embedding and sectioning.
All animal procedures were performed in accordance with institutional guidelines and approval by the Animal Care and Use Committee at the University of Wisconsin-Madison.
2.2 BrdU label retaining cells
To produce BrdU label-retaining cells labeled at E16, we injected (IP) pregnant dams at E16 with 0.5 ml of 50 mM BrdU in PBS. We found that a single injection of BrdU was sufficient to mark cells in the developing prostate, while at 10 weeks of age still retaining a small fraction of those cells.
2.3 GFP label retaining cells
To control for expression of GFP in the H2B-GFP pulse-chase experiments, the pregnant dam was fed a diet containing Doxycycline from before conception until E15.5 at which time the diet was changed to a standard feed without doxycycline for 24 hours after which the diet was maintained on the doxycycline containing feed until the animals were harvested. This 24-hour period of GFP expression was sufficient to label many cells and provide a population of label retaining cells in the adult animals at 10 weeks of age.
2.4 Castration
To determine the fate of the label retaining cells following androgen withdrawal we castrated animals, under an approved protocol, at 8 weeks of age and allowed them to recover for up to 14 days at which time the animals were sacrificed and prostates harvested.
2.5 Renal capsule graft
Rat urogenital sinus mesenchyme (rUGM) was isolated from 1% trypsin digested E18 rat urogenital sinus. A single cell suspension of UGM was collected after 0.5% collagenase digestion. FACS sorted 4-marker+ or 4marker− cells (1000 each) were mixed with 25000 UGM cells and resuspended in 15 μl of type I collagen (BD biosciences, Bedford, MA), and implanted under the renal capsule of athymic nude mice as described [15].
2.6 Histology
Dissected prostate tissues or renal grafts were fixed in 10% formaldehyde for 12 hours at 4 °C, appropriately orientated and embedded in 4% agarose. The samples were then sectioned at 200 μm in thickness with a vibratome. The vibratome sections were processed, paraffin embedded and sectioned. The 5 μm paraffin sections were dewaxed, and stained with Mayer’s hematoxylin and eosin (H&E) for histological evaluation.
2.7 Indirect immunofluorescent staining
Dewaxed sections were used for antigen retrieval which was performed in the citrate buffer and heated in the microwave oven for 30 to 50 minutes at 95% power. The tissue sections were blocked with 3% BSA in PBS plus 10% normal donkey serum. The primary antibodies used were: rabbit anti-androgen receptor (Santa Cruz, Dallas, Texas); rat anti-Ki67 (Dako North America, Carpinteria, CA); mouse anti-BrdU (Roche USA, Nutley, NJ). The secondary antibodies were from Molecular Probes (Eugene, Oregon). Following primary antibody incubation, the sections were washed with PBS, and the secondary antibody was applied at 1:200 at room temperature for 1 h. Then sections were washed with PBS and counter stained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured using an Olympus-BX51 fluorescent microscope (Olympus, Center Valley, PA) with a digital camera and SPOT advanced software or BD Pathway bioimaging systems (BD Biosciences, San Jose, CA).
2.8 FACS analysis of H2B-GFP label retaining cells for the stem cell markers (Sca-1, CD133, CD44 and CD117)
H2B-GFP mice were mated with K5-Tet (tTA, Tet off) mice and genotyped. Mice that were positive for both GFP and K5-Tet were used for experiments and double negative littermates were used as control. The prostate tissue was dissected from 10-week old mice in cold PBS under a dissecting microscope. The tissue was minced into 2–3 mm pieces and digested by collagenase and hyaluronidase (Stemcell Technologies, Inc., Vancouver, BC, Canada; Cat. No. 07912) at 37°C for 3 hours. This digestion was followed by a second digestion in 0.25% trypsin/EDTA in 2% BSA on ice for 30 minutes according to instructions (Stemcell Technologies, Inc., Vancouver, BC, Canada). We found digestion with 0.25% trypsin on ice for 30 minutes increased yield of single cells from prostate tissue without affecting FACS analysis of stem cell markers. The digested cells were passed through a 40 μm cell strainer to isolate single cells with an aliquot of cells used to determine cell concentration and checked for viability by trypan blue staining. Single cells were treated with mouse lineage depletion kit according to instructions (Miltenyi Biotec Inc., Auburn, CA). The lineage depleted cells (Lin−) then suspended in DMEM (Mediatech, Manassas, VA) containing 5% FBS followed by addition of primary antibodies directly coupled to specific fluorchromes (1:200, Sca-1, CD133, CD44 and CD117 as previously reported (Leong, et al., 2008) for 30 minutes on ice. DAPI was added to the cell suspension prior to FACS analysis to select viable cells. Single stem cell maker stained cells and isotype matched IgG were used to identify positive and negative signal. Fluorescence-minus-one (FMO) controls were used to determine gating in multicolor flow cytometry. FACS analysis was performed on a BD LSR-II flow cytometry (BD Biosciences, San Jose, CA). The cells were gated for single events and viability. The flow cytometry data was analyzed using FlowJo8.6 software. The relationship between GFP positive label retaining cells and stem cell marker expression was analyzed statistically.
2.9 FACS analysis of progenitor cell proliferation after castration by correlating of BrdU labeling and stem cell marker expression
Adult (8 week old) CD-1 mice were divided into 4 groups: Sham-BrdU, Sham-PBS, castration-BrdU and castration-PBS. 500 μl of 10 mM BrdU (Sigma, MO) was given by IP injection to castrated or sham mice daily for 14 days starting at the time of castration and 500 μl PBS was injected as control. On day 14 the prostate was processed and stained for the four stem cell markers as described above. After staining with antibodies to stem cell markers, the cells were fixed and stained with a BrdU Flow Kit (BD Bioscience, San Jose) as instructed. FACS analysis was performed as described above for the 4-markers and BrdU after gating for single events.
2.10 Quantitative and statistical analysis of the relationship between label retaining cells and other cell markers
The percentage of BrdU positive cells were determined by BrdU positive cells over total cells of interest. Each group has 3–5 animals, and the paraffin embedded prostate tissue from each animal was completely sectioned, and 5 sections were used for analysis. At least 10 of 200x high power field (HPF) images from each section was included in the analysis. Statistical analysis was conducted using one –way analysis of variance (ANOVA) and values were presented as mean ± SEM derived from at least three independent experiments, unless otherwise stated. The ANOVA was followed by Tukey’s post hoc test. P value less than 0.05 was regarded as statistically significant. Statistical analysis of IHC data was performed with assistance from the UW Madison Department of Surgery’s Statistical Core.
3. Results
3.1 Prenatal BrdU labeling yields label-retaining cells in the proximal duct segments
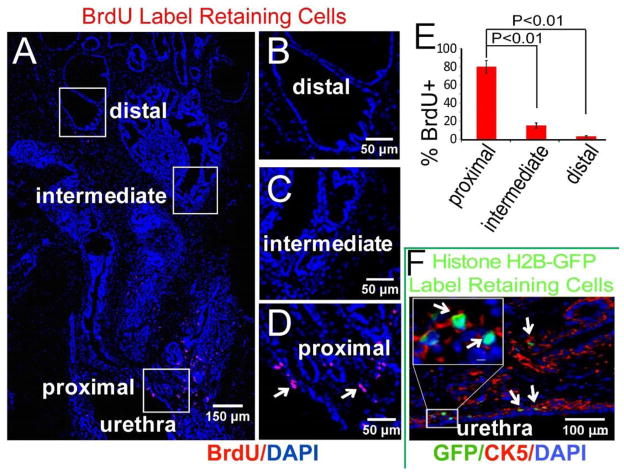
Previous studies have identified slow-cycling epithelial cells in the adult prostate by BrdU labeling during adolescence followed by multiple rounds of castration-induced involution and testosterone-induced re-growth. This approach yields a relatively abundant population of labeled cells preferentially localized to the proximal duct segments [15]. We speculated that initiation of prostate ductal budding (E16.5–17.5) would entail a robust expansion of prostate tissue-specific stem cells, primitive progenitor cells or both, and that pulse labeling with BrdU at E16 would yield a rarified population of label-retaining cells in the adult prostate that had remained mitotically quiescent since the earliest stages of prostate development. Pregnant dams were given a single dose of BrdU by intraperitoneal injection at E16 and the male offspring sacrificed at 10 weeks of age. The lower genitourinary tract was removed en bloc. The prostate and urethra were fixed, serially sectioned and immunostained for BrdU. We reproducibly observed a small number of labeled epithelial cells located at the junction of the prostatic main ducts and the urethra and in the proximal ducts (Figure 1). Epithelial labeling was confirmed by co-staining with epithelial markers. Labeled epithelial cells were rarely observed in intermediate and distal ducts. Note was made of scattered BrdU-labeled stromal cells that were most abundant in the region of the proximal ducts. Co-staining for BrdU and Ki67 or Caspase 3 revealed a very low proliferative index and rare instances of apoptosis among the labeled cells. We conclude that pulse BrdU labeling at E16 yields a population of slow-cycling labeled cells in the proximal duct segments of the adult prostate.
Figure 1. Location of label retaining cells in the adult mouse prostate.
(A) Wild-type CD-1 dams were injected with BrdU at E16 and the male offspring harvested at 10 weeks. Immunofluorescence staining localized BrdU label-retaining cells mostly to the proximal duct segments and junction with the urethra. (Montage image of sagittal sections of prostate). (B–D) Boxed regions in image A. (E) Distribution of LRCs in proximal, intermediate and distal regions (n=5). (F) H2B-GFP mice were mated and GFP expression from the cytokeratin 5 (CK5) promoter was activated in pregnant females for 24 hours at E16 by removing doxycycline from the water supply. The male offspring were harvested at 10 weeks, and fluorescence microscopy localized most GFP label-retaining cells to CK5 (red) positive cells.
3.2 Prenatal CK-5/GFP labeling yields labeled cells in the proximal duct segments
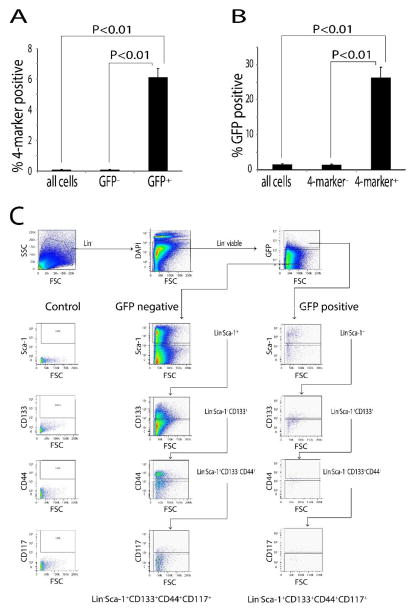
To corroborate this observation and facilitate FACS analysis of stem cell marker expression, cytokeratin 5 (CK-5) expressing cells were selectively pulse labeled at E16 using a doxycycline-controlled histone-H2B GFP mouse [23]. Histone labeling by this technique is effectively an equivalent way of introducing label into proliferating cells that is “washed out” by subsequent rounds of replication. However, it has the advantage that the histone-H2B GFP labelling is amenable to FACS analysis of living cells. The labeling technique differs from BrdU labeling in that only proliferating cells expressing CK5 are labeled with GFP. The male offspring were sacrificed at 10 weeks of age and the prostate and urethra were serially sectioned and imaged by immunofluorescence microscopy. We again observed (GFP) labeled epithelial cells localized to the proximal duct segments and their urethral junction (Figure 1F). Sca-1, CD133, CD44 and CD117 have been implicated as prostate stem cell markers since a single cell co-expressing these 4-markers can generate fully differentiated prostate tissue when combined with urogenital mesenchyme [24]. FACS analysis showed 6% of the GFP labeled cells in the 10 week adult prostate co-expressed Sca-1, CD133, CD44 and CD117. This contrasted with co-expression of these stem cell markers in only ~0.1% of the total epithelial cell population (Figure 2). Further analysis showed that 26% of cells co-expressing Sca-1, CD133, CD44 and CD117 were GFP positive even though GFP label was present in only 1.5% of the total epithelial cell population (Figure 2). These data show that pulse GFP labeling CK-5 expressing cells at E16 yields a population of label retaining cells located in the proximal duct segments that are significantly enriched for co-expression of prostate stem cell markers and, indeed, account for over a quarter of all co-expressing cells in the adult prostate.
Figure 2. GFP labeling at E16 yields a population of labeled cells enriched for co-expression of stem cell markers.
CK5/H2B-GFP mice were GFP-labeled at E16 and sacrificed at 10 weeks. Single cells were isolated by enzymatic digestion and lineage committed cells were depleted for Lin− cells. FACS analysis of Lin− was performed for co-expression of Sca-1, CD133, CD44 and CD117 (n=3). (A) Approximately 6 % of GFP positive cells co-expressed these 4 markers, while only 0.1% of the total cell populations co-expressed these 4 markers. (B) Approximately 26% of cells co-expressing these 4 markers were GFP positive, while only 1.5% of the total cell population was GFP positive. (C) Diagram of sequential analysis of flow cytometry data. Cells from the prostate were isolated by magnetic beads to obtain Lin− cells. These cells were gated by forward scatter (FSC) and side scatter (SSC). Then the cells were gated by DAPI exclusion (Lin− viable). Lin− viable cells were gated by GFP expression to separate GFP+ and GFP− cells. GFP+ and GFP− cells were analyzed by sequential gating for Sca-l, CD133, CD44 and CD117 and the percentage of 4-marker positive cells in GFP+ and GFP− groups were determined.
It is intuitively obvious and commonly accepted that prostate progenitor cells that participate in testosterone-induced regeneration of the castrated mouse prostate are not dependent on androgen for survival. However, it has not been established whether or not these cells express the androgen receptor. We performed staining for androgen receptor (AR) in BrdU label retaining cells. Mice were BrdU pulse labeled at E16 as described above and sacrificed at 10 weeks of age. Co-staining for BrdU and androgen receptor (AR) revealed approximately 75% of BrdU-labeled cells to be AR+ (Figure 3 A–B). This is less than the expression of AR in 95% of the total epithelial cell population, but the preponderance of AR expression in this population was unexpected and prompted us to examine the expression of AR in cells co-expressing Sca-1, CD133, CD44 and CD117. FACS analysis was performed on intact adult mice: we observed that 99.7% cells co-expressing Sca-1, CD133, CD44 and CD117 also express AR (Figure 3C).
Figure 3. The majority of prostate 4-marker cells stain positive for androgen receptor.
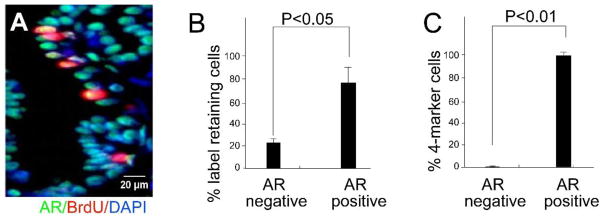
CD-1 mice were BrdU labeled at E16 and sacrificed at 10 weeks. Co-staining was performed for androgen receptor (AR) and BrdU. (A) A representative section of co-stained prostate epithelium. (B) Quantitative analysis of co-staining for AR and BrdU in the prostate epithelium (n=5). (C) Ten weeks old CD-1 mice were sacrificed and the epithelium subject to FACS analysis for AR, Sca-1, CD133, CD44 and CD117. Quantitative analysis shows that most 4-marker cells are androgen receptor positive (n=3).
3.3 Label retaining cells proliferate after castration
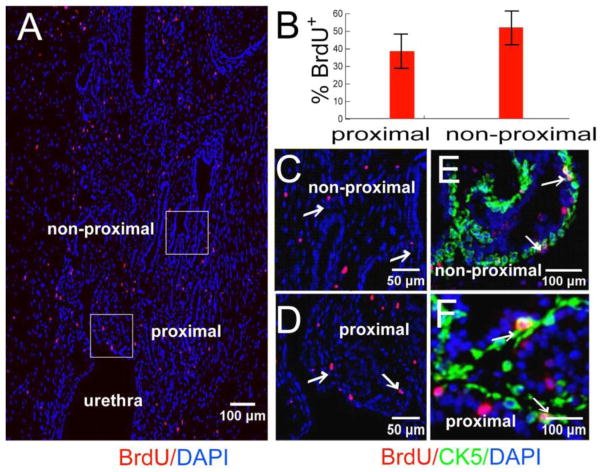
Castration of the adult mouse induces widespread epithelial apoptosis and glandular involution, however, several studies have shown that a low level of epithelial proliferation persists even while glandular involution is occurring [25, 26]. To examine the response of slow-cycling cells in the adult prostate to castration, E16-BrdU labeled mice were castrated or sham operated at 8 weeks of age and sacrificed 2 weeks later. In sham-operated mice, we observed label retaining cells located exclusively in the proximal duct segments and their junction with the urethra (Figure 1A–D). The labeled cells were usually solitary; they were occasionally paired. In castrated mice there was an increase in the total number of label retaining cells and dispersal of labeled cells into the non-proximal duct segments (Figure 4). Most labeled cells in the castrated mice were solitary; however, we frequently observed clusters of labeled cells. Clusters were a unique feature of the castrated mice (Figure 5A). From this finding, we inferred that label-retaining cells proliferate following castration. This inference was confirmed by Ki67 staining (Figure 5B and C).
Figure 4. BrdU labeled cells are dispersed post-castration.
CD-1 mice BrdU labeled at E16 were castrated at 8 weeks and sacrificed 2 weeks after castration. (A) Immunostaining for BrdU labeled cells (Montage image of sagittal sections of prostate). (B) Quantitative analysis for the location of BrdU labeled cells (n=5). (C and D) Boxed regions in A. (E and F) Colocalization of BrdU label-retaining cells (red) with CK5 (green) in non-proximal and proximal ducts.
Figure 5. Castration induces proliferation of BrdU labeled cells.
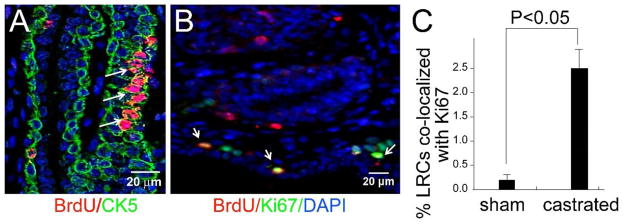
(A) Clusters of BrdU labeled cells (arrows) were observed in mice labeled and castrated as described in Figure 4. Similar clusters were never observed in the intact controls. Co-staining for BrdU and CK5 identified the cluster of labeled cells as basal cells. (B and C) Staining for Ki67 in mice labeled and castrated as described in Figure 4 revealed increased proliferation of BrdU labeled cells. (B) Immunofluorescence staining for BrdU (red), Ki67 (green) and DAPI (blue) shows co-localization of BrdU with Ki67. This is quite uncommon in intact controls. Quantitative analysis of co-staining for Ki67 and BrdU confirms co-staining is significantly increased castrated animals (C) (n=5).
3.4 Castration induces a selective proliferation of cells co-expressing stem cell markers
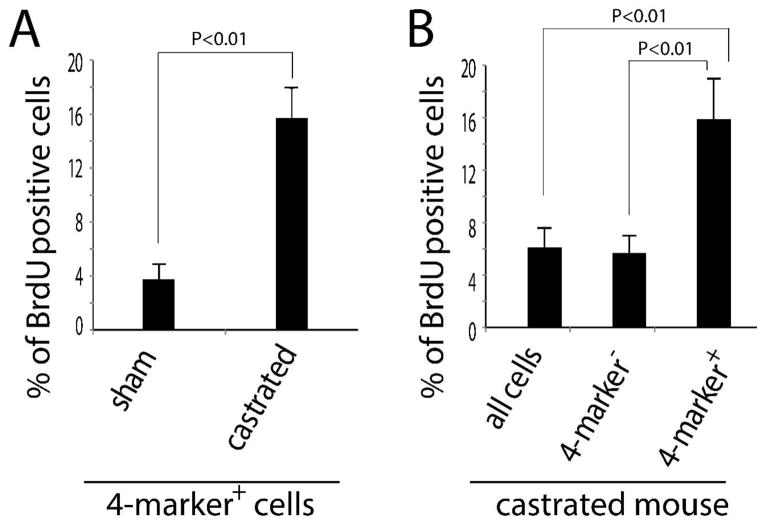
The observation that slow-cycling cells in the adult prostate proliferate in response to castration was surprising and prompted us to examine the behavior of cells co-expressing the stem cell markers Sca-1, CD133, CD44 and CD117. Adult males were castrated or sham operated and then administered BrdU daily by injection for 2 weeks. The prostates from sham-operated and castrated mice were harvested, enzymatically digested to single cells and subjected to FACS analysis to determine the proportion of BrdU labeling in cells co-expressing the stem cell markers Sca-1, CD133, CD44 and CD117. This analysis revealed a selective expansion of 4-marker cells following castration. We observed a significantly higher percentage of BrdU+ 4-marker cells in the castrated versus sham operated mice (16% versus 3.7%; Figure 6A). This finding indicates that castration increases proliferation of the 4-marker positive cell population. To distinguish a selective increase in progenitor cell proliferation from a generalized increase in epithelial proliferation, we compared labeling indices between 4-marker cells and the remainder. We found that 16% of cells co-expressing the four stem cell markers were BrdU+ whereas only 6% of the remaining epithelial cells were BrdU+ (Figure 6B) in castrated group.
Figure 6. Castration induces selective proliferation of 4-markers progenitor cells.
Eight week old mice were sham operated or castrated. BrdU was injected daily after surgery to label the proliferating cells, and mice were sacrificed 2 weeks after castration. (A) FACS analysis showed significantly higher labeling of 4-marker cells in the castrated versus sham operated mice (16% versus 3.7%; 3 independent experiments; Figure 6A). These data signify increased proliferation of 4-marker cells in the castrated mouse prostate. (B) Proliferation was greater in 4-marker progenitor cells than that in non- 4-marker positive cells in the castrated prostate group (n=3). Taken together, these data indicate that castration induces preferential proliferation of 4-marker cells.
3. Discussion
Epithelial proliferation is concentrated at the tips of the developing prostate ducts and early studies focused on the duct tip as the probable site of progenitor/stem cells [16]. Subsequent studies examining label retention and regenerative capacity implicated the proximal duct as the reservoir of stem/progenitor cells in the adult prostate [15, 16, 21, 22]. Further studies sorting for putative stem cell markers and testing for regenerative potential confirmed a relative abundance of stem cells in the proximal duct as compared to the intermediate and distal duct segments [26, 27, 21, 22]. Labeling with either BrdU or GFP at the onset of ductal budding yielded a small population of labeled cells in the adult prostate concentrated in the proximal ducts near their urethral origin. In our study, we also observed label-retaining stromal cells localized to stromal compartment surrounding the proximal ducts. This could be coincidence, but it does suggest the possibility that slow-cycling epithelial and stromal cells are co-localized within a specific niche in the adult gland and share regulatory signaling mechanisms [28, 29, 30]. The most striking findings were robust proliferation of these cells at E16, the preponderance of AR gene expression and the proliferative response to castration.
The ducts of the adult mouse prostate are line by a pseudostratified epithelium composed of basal cells, luminal cells and rare neuroendocrine cells. The identity and location of stem cells within this epithelial layer is still a matter of debate. At one time basal cells were widely believed to contain progenitor cells capable of differentiating into basal, luminal and neuroendocrine cells [19, 2]. This view has been challenged by recent observations suggesting that stem cells may also reside in luminal cell layer [31]. Single cells co-expressing of the markers used in our studies (Lin− Sca-1+CD133+CD44+CD117+) have been shown capable of regenerating fully differentiated prostate epithelium [24]. We found GFP label retaining cells were enriched for co-expression of Lin− Sca-1+CD133+CD44+CD117+. While only a fraction of label retaining cells co-expressed the four stem cell markers, they accounted for approximately a quarter of all the cells co-expressing these markers. This was unexpected and suggests that initiation of prostate ductal budding is associated with a uniquely robust proliferation of epithelial stem/progenitor cells. This is to our knowledge the first evidence that initiation of prostate organogenesis is accompanied by a burst of proliferation among cells that will become tissue specific stem or progenitor cells in the adult organ. We speculate that this proliferative burst potentially creates a window of vulnerability of these reserve cells to mutation or imprinting changes that could predispose to neoplasia in the adult. Whether a similar burst of proliferation among stem/progenitor cells in other developing organs remains to be determined.
AR is present in the nucleus of most epithelial and stromal cells of the intact adult prostate. Luminal cells are predominantly, if not exclusively, androgen positive whereas only half of all basal cells are [32]. AR in the stroma mediates paracrine stimulation of epithelial proliferation while AR in epithelial cells stimulates luminal cell differentiation and protein synthesis. It has been assumed that prostate stem cells lack AR [33], however, there is some evidence to challenge this view as it applies to the human prostate [34] and the mouse prostate [31]. We found that most BrdU label retaining epithelial cells in the adult prostate were AR positive. More striking was the observation that four-marker positive cells are nearly all AR positive. Insofar as previous studies showed 14 in 97 four-marker positive cells exhibit the regenerative capacity bona fide stem cells in a tissue recombination assay [24], our data suggests that at least some, if not all, stem cells are AR positive.
AR has been shown to exert a growth inhibitory effect in luminal cells. In transgenic mice lacking epithelial AR, epithelial cells are less differentiated and hyper-proliferative [35]. It is possible that selective proliferation of slow-cycling and 4-marker cells after castration reflects a release of androgen-mediated inhibition of proliferation of cells that do not require androgen for survival. There is precedent for this in the breast where estrogen suppresses stem cell proliferation [36]. An alternative explanation for proliferation and migration of LRCs and 4-marker cells following castration would be the response to injury. Progenitor cells play a primary role in the regenerative response to injury in a variety of adult tissues [37, 38, 39, 40, 41] and it has been postulated that factors released by injured tissues stimulate stem cell proliferation and attract stem cells to the sites of injury [43, 44]. The effects of castration on the adult prostate are a combination of ischemic injury due to vascular disruption, epithelial apoptosis and acute inflammation [45; 46; 47]. Whether a direct response to decreased testosterone levels or in response to castration-induced injury, our observation may be relevant to the behavior of tumor stem cells in prostate cancer. To the extent that tumor stem cells phenocopy the features of normal adult stem/progenitor cells, our findings suggest that treatment of human prostate cancer with androgen deprivation could inadvertently produce an expansion of tumor stem cells.
Supplementary Material
Supplemental Figure 1. GFP label retaining cells enriches for stem cell marker Sca-1, CD44 and CD117 positive cells but not CD133 positive cells. H2B-GFP mice were mated, and GFP expression from the CK5 promoter was activated at E16 by removing doxycycline from the water supply for 24 hours. The male offspring were harvested at 10 weeks. A) Diagram of analysis of flow cytometry data. Cells isolated from prostate were sorted by magnetic beads to obtain Lin− cells. These cells were gated by forward scatter (FSC) and side scatter (SSC). Then the cells were gated by DAPI exclusion (Lin− viable). Lin− viable cells were gated by GFP expression to separate GFP+ and GFP− cells. GFP+ and GFP− cells were analyzed by gating for Sca-l, CD133, CD44 and CD117 and percentage of individual stem cell marker positive cells in GFP+ and GFP− groups were determined. B) Quantitative analysis of flow cytometry data, GFP+ VS GFP− cells (n=3).
Supplemental Figure 2. Sca-1, CD133, CD44 and CD117 positive cells (4-marker+) have greater in vivo proliferative capacity than 4-marker− cells. FACS sorted 4-marker+ (Sca-1+CD133+, CD44+ and CD117+) or 4-marker− cells (1000 each) were mixed with rat urogenital sinus mesenchyme (rUGSM) cells, resuspended in 15 μl of type I collagen (BD biosciences, Bedford, MA), and implanted under the renal capsule of athymic nude mice as described (15). (A) 4-marker+ or 4-marker− cells (1000 cells each) were mixed with rUGSM and grafted under renal capsule. Images of grafted tissue were shown. (B) The weight of the grafted tissue from 4-marker+ or 4-marker− cells were measured after 8 weeks growth under the renal capsule. Four marker+cells formed more prostatic tissue than 4-marker− cells (n=4, *P<0.01). (C and D) H&E staining of representative tissue sections of grafted tissues from 4-marker+ cells (C) or 4-marker− cells (D). Four-marker+ cells form larger grafts, and more glandular structures were formed compared to 4-marker− cells. These data suggest the 4-marker+ cell population enriched for progenitor cells that have higher proliferative capacity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Price D. Comparative aspects of development and structure in the prostate. Natl Cancer Inst Monogr. 1963;12:1–27. [PubMed] [Google Scholar]
- 2.Wang Y, Hayward S, Cao MK. Thayer and G. Cunha, Cell differentiation lineage in the prostate. Differentiation. 2001;68 (4–5):270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- 3.Sugimura GR, Cunha A, Donjacour A. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34(5):973–983. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- 4.Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151(5):1427–32. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- 5.Hayward SW, et al. Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle. Acta Anat (Basel) 1996;155 (2):81–93. doi: 10.1159/000147793. [DOI] [PubMed] [Google Scholar]
- 6.Salm, et al. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 8.Morrison S, Spradling AC. Stem cells and niches. Mechanisms that promote tissue maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–97. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Cotsarelis G, Cheng SZ, Dong G, Sun T, Lavker R. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 11.Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A. 2000;26;97(20):10960–5. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;18;102(4):451–61. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 13.Berardi AC, Wang A, Levine JD, Lopez P, Scadden DT. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267(5194):104–8. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 14.Thorgeirsson SS. Hepatic stem cells in liver regeneration. FASEB J. 1996;10(11):1249–56. Review. [PubMed] [Google Scholar]
- 15.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinbara H, Cunha GR, Boutin E, Hayashi N, Kawamura J. Evidence of stem cells in the adult prostatic epithelium based upon responsiveness to mesenchymal inductors. Prostate. 1996;29(2):107–116. doi: 10.1002/(SICI)1097-0045(199608)29:2<107::AID-PROS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1996;37(3):149–160. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Hudson DL, O’Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest. 2000;80(8):1243–1250. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- 19.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 20.Foster CS, Dodson A, Karavana V, Smith PH, Ke Y. Prostatic stem cells. J Pathol. 2002;197(4):551–565. doi: 10.1002/path.1194. [DOI] [PubMed] [Google Scholar]
- 21.Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci U S A. 2005;102:7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the Epithelial Stem Cell Niche in Skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456(7223):804–8. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 25.Alison MR, Wright NA, Morley AR, Appleton DR. Cell proliferation in the prostate complex of the castrate mouse. J Microsc. 1976;106(2):221–37. doi: 10.1111/j.1365-2818.1976.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 26.Evans GS, Chandler JA. Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate. 1987;11(4):339–51. doi: 10.1002/pros.2990110406. [DOI] [PubMed] [Google Scholar]
- 26.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104(1):181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goto K, Salm SN, Coetzee S, Xiong S, Burger PE, Shapiro E, Lepor H, Moscatelli D, Wilson EL. Proximal prostatic stem cells are programmed to regenerate a proximal–distal ductal axis. Stem Cells. 2006;24(8):1859–1868. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 28.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110(4):1001–20. doi: 10.1242/dev.110.4.1001. Review. [DOI] [PubMed] [Google Scholar]
- 29.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97(25):13473–5. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slack JM. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461(7263):495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirosevich J, Bentel JM, Zeps N, Redmond SL, D’Antuono MF, Dawkins HJ. Androgen receptor expression of proliferating basal and luminal cells in adult murine ventral prostate. J Endocrinol. 1999;162(3):341–350. doi: 10.1677/joe.0.1620341. [DOI] [PubMed] [Google Scholar]
- 33.Oldridge EE, Pellacani D, Collins AT, Maitland NJ. Prostate cancer stem cells: Are they androgen-responsive? Molecular and Cellular Endocrinology. 2012;360(1–2):14–24. doi: 10.1016/j.mce.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Heer R. Hunterian Lecture. Characterisation of human prostate epithelial progenitor differentiation in response to androgens. Ann R Coll Surg Engl. 2011;93(6):424–8. doi: 10.1308/10.1308/147870811X589245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu CT, Altuwaijri Saleh, Ricke William A, Huang Shu-Pin, Yeh Shuyuan, Zhang Caixia, Niu Yuanjie, Tsai Meng-Ying, Chang Chawnshang. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104(31):12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simões BM, Piva M, Iriondo O, Comaills V, López-Ruiz JA, Zabalza I, Mieza JA, Acinas O, Vivanco MD. Estrogen reduces the proportion of stem cells in the normal human mammary gland and in breast cancer cells. Breast Cancer Research and Treatment. 2011;129 (1):23–35. doi: 10.1007/s10549-010-1169-4. [DOI] [PubMed] [Google Scholar]
- 37.Amcheslavsky A, Jing J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4(1):49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunt EM, Blomenkamp K, Ahmed M, Ali F, Marcus N, Teckman J. Hepatic progenitor cell proliferation and liver injury in alpha-1-antitrypsin deficiency. J Pediatr Gastroenterol Nutr. 2010;51(5):626–30. doi: 10.1097/MPG.0b013e3181e7ff55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang F, Clarke JD, Ferretti P. FGF-2 Up-regulation and proliferation of neural progenitors in the regenerating amphibian spinal cord in vivo. Dev Biol. 2000;225(2):381–91. doi: 10.1006/dbio.2000.9843. [DOI] [PubMed] [Google Scholar]
- 41.Deierborg T, Roybon L, Inacio AR, Pesic J, Brundin P. Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere-derived cells into neurons and oligodendrocytes. Neuroscience. 2010;171(4):1386–1396. doi: 10.1016/j.neuroscience.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 42.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nature Reviews Genetics. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 43.Inui S, Sakaguchi N. Establishment of a murine pre-B cell clone dependent on interleukin-7 and stem cell factor. Immunol Lett. 1992;34(3):279–88. doi: 10.1016/0165-2478(92)90225-d. [DOI] [PubMed] [Google Scholar]
- 44.Bodine D. Mobilization of peripheral blood ‘stem’ cells: where there is smoke, is there fire? Exp Hematol. 1995;23(4):293–5. [PubMed] [Google Scholar]
- 45.Hayek OR, Shabsigh A, Kaplan SA, Kiss AJ, Chen MW, Burchardt T, Burchardt M, Olsson CA, Buttyan R. Castration induces acute vasoconstriction of blood vessels in the rat prostate concomitant with a reduction of prostatic nitric oxide synthase activity. J Urol. 1999;162(4):1527–31. [PubMed] [Google Scholar]
- 46.Kerr JFR, Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch B Cell Pathol. 1973;13(2):87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- 47.Wu CT, Chen WC, Lin PY, Liao SK, Chen MF. Androgen deprivation modulates the inflammatory response induced by irradiation. BMC Cancer. 2009;25(9):9248. doi: 10.1186/1471-2407-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115(5):788–94. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee SO, Tian J, Huang CK, Ma Z, Lai KP, Hsiao H, Jiang M, Yeh S, Chang C. Suppressor role of androgen receptor in proliferation of prostate basal epithelial and progenitor cells. J Endocrinol. 2012;213(2):173–82. doi: 10.1530/JOE-11-0474. [DOI] [PubMed] [Google Scholar]
- 50.Norman JT, Cunha GR, Sugimura Y. The induction of new ductal growth in adult prostatic epithelium in response to an embryonic prostatic inductor. Prostate. 1986;8:209–220. doi: 10.1002/pros.2990080302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. GFP label retaining cells enriches for stem cell marker Sca-1, CD44 and CD117 positive cells but not CD133 positive cells. H2B-GFP mice were mated, and GFP expression from the CK5 promoter was activated at E16 by removing doxycycline from the water supply for 24 hours. The male offspring were harvested at 10 weeks. A) Diagram of analysis of flow cytometry data. Cells isolated from prostate were sorted by magnetic beads to obtain Lin− cells. These cells were gated by forward scatter (FSC) and side scatter (SSC). Then the cells were gated by DAPI exclusion (Lin− viable). Lin− viable cells were gated by GFP expression to separate GFP+ and GFP− cells. GFP+ and GFP− cells were analyzed by gating for Sca-l, CD133, CD44 and CD117 and percentage of individual stem cell marker positive cells in GFP+ and GFP− groups were determined. B) Quantitative analysis of flow cytometry data, GFP+ VS GFP− cells (n=3).
Supplemental Figure 2. Sca-1, CD133, CD44 and CD117 positive cells (4-marker+) have greater in vivo proliferative capacity than 4-marker− cells. FACS sorted 4-marker+ (Sca-1+CD133+, CD44+ and CD117+) or 4-marker− cells (1000 each) were mixed with rat urogenital sinus mesenchyme (rUGSM) cells, resuspended in 15 μl of type I collagen (BD biosciences, Bedford, MA), and implanted under the renal capsule of athymic nude mice as described (15). (A) 4-marker+ or 4-marker− cells (1000 cells each) were mixed with rUGSM and grafted under renal capsule. Images of grafted tissue were shown. (B) The weight of the grafted tissue from 4-marker+ or 4-marker− cells were measured after 8 weeks growth under the renal capsule. Four marker+cells formed more prostatic tissue than 4-marker− cells (n=4, *P<0.01). (C and D) H&E staining of representative tissue sections of grafted tissues from 4-marker+ cells (C) or 4-marker− cells (D). Four-marker+ cells form larger grafts, and more glandular structures were formed compared to 4-marker− cells. These data suggest the 4-marker+ cell population enriched for progenitor cells that have higher proliferative capacity.