Abstract
Although the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are mediated through binding and activation of the aryl hydrocarbon receptor (AhR), the subsequent biochemical and molecular changes that confer immune suppression are not well understood. Mice exposed to TCDD during an acute B6-into-B6D2F1 graft-vs-host response do not develop disease, and recently this has been shown to correlate with the generation of CD4+ T cells that express CD25 and demonstrate in vitro suppressive function. The purpose of this study was to further characterize these CD4+ cells (TCDD-CD4+ cells) by comparing and contrasting them with both natural regulatory CD4+ T cells (T-regs) and vehicle-treated cells. Cellular anergy, suppressive functions, and cytokine production were examined. We found that TCDD-CD4+ cells actively proliferate in response to various stimuli but suppress IL-2 production and the proliferation of effector T cells. Like natural T-regs, TCDD-CD4+ cells do not produce IL-2 and their suppressive function is contact dependent but abrogated by costimulation through glucocorticoid-induced TNFR (GITR). TCDD-CD4+ cells also secrete significant amounts of IL-10 in response to both polyclonal and alloantigen stimuli. Several genes were significantly up-regulated in TCDD-CD4+ cells including TGF-β3, Blimp-1, and granzyme B, as well as genes associated with the IL12-Rb2 signaling pathway. TCDD-CD4+ cells demonstrated an increased responsiveness to IL-12 as indicated by the phosphorylation levels of STAT4. Only 2% of TCDD-CD4+ cells express Foxp3, suggesting that the AhR does not rely on Foxp3 for suppressive activity. The generation of CD4+ cells with regulatory function mediated through activation of the AhR by TCDD may represent a novel pathway for the induction of T-regs.
Introduction
The 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)3 is a widespread environmental contaminant that induces profound immune suppression in mice. Although the immunosuppressive effects of TCDD are mediated through binding and activation of the aryl hydrocarbon receptor (AhR) (1, 2), the subsequent biochemical and molecular changes that confer suppression are still not well elucidated. After binding TCDD, the AhR translocates to the nucleus where it dimerizes with the AhR nuclear translocator (ARNT). This basic helix-loop-helix PER-ARNT-SIM ligand-activated transcription factor can then bind core consensus sequences of DNA (5′-GCGTG-3′) known as dioxin-responsive elements to cause specific changes in gene expression (3, 4). Dioxin-responsive elements have been identified in the promoter regions of several genes important for cell activation, proliferation, and differentiation (5, 6). In 2002, Kerkvliet et al. demonstrated that T cells are direct AhR-dependent targets of TCDD (7), and yet the implications of AhR activation during the process of effector T cell differentiation are not clear, with anergy, deletion, and/or induction of regulatory T cells suggested in recent reports (8, 9, 10, 11, 12, 13).
Regulatory T cells (T-regs) are a subset of T cells with immunosuppressive capabilities. Although the concept of suppressor T cells was described as far back as the early 1970s (14), the naturally occurring CD4+CD25+ T-regs were not described until the mid-1990s (15). Subsequently, it was identified that natural T-regs express Foxp3, a transcription factor required for their development and regulatory function (16, 17). Additional populations of adaptive regulatory T cells with distinct markers and activity have also since been described, including inducible CD4+CD25+ T-regs (18, 19), CD4+CD25−Foxp3− T-regs (20, 21), TGF-β-induced CD4+ T-regs (22, 23), IL-10-induced CD4+ T-regs (Tr1) (24, 25), and CD8+CD28−Foxp3+ cells (26). The ability of immunosuppressive agents to induce T-regs has also been previously demonstrated. For example, the combination of vitamin D3 and dexamethasone has been shown to induce IL-10-secreting T-regs in culture (27, 28). Rapamycin has been shown to cause de novo induction and maintenance of T-regs in culture (29) and to generate IL-10-secreting donor T cells in a lymphohematopoietic graft-vs-host (GVH) model (30).
Our previous studies have shown that TCDD suppresses the CD4+ T cell-dependent CD8+ CTL response in a C57BL/6 (B6)-into-B6D2F1 (F1) acute GVH response mouse model. In this model, the presence of AhR in both grafted CD4+ and CD8+ T cells is necessary for the full suppression of CTL in TCDD-treated mice (7). The alloreactive donor-derived CD4+ T cell population in TCDD-treated mice (TCDD-CD4+ cells) consists primarily of proliferating CD25+ cells that co-express CTLA-4 and glucocorticoid-induced TNFR (GITR) at 48 h after adoptive transfer (9). Furthermore, TCDD-CD4+ cells share some functional characteristics with regulatory T cells, including the ability to potently suppress the proliferation of anti-CD3 Ab-stimulated naive CD4+ T cells in culture, and a lack of proliferation in response to anti-CD3 Ab stimulation (9).
The studies presented in this article provide an ex vivo characterization of TCDD-CD4+ cells, including direct comparisons with natural T-regs. The purpose was to identify effector mechanisms as well as changes in gene and protein expression that help to explain the function and/or generation of TCDD-CD4+ cells. TCDD-CD4+ cells share several characteristics with natural T-regs but exhibit unique properties, including the ability to retain suppressive function in culture during proliferation. TCDD-CD4+ cells produce significant amounts of IL-10 in response to polyclonal and alloantigen stimuli and express elevated levels of several gene transcripts, including TGF-β3, Blimp-1, granzyme B, and IL-12Rb2. Little is known about the effects of TCDD on regulatory T cells and whether the induction of T-regs is one of the mechanisms by which TCDD suppresses the immune system. Activated AhR may act as an alternative to Foxp3 during activation-induced differentiation of naive T cells to produce T-regs in TCDD-treated mice.
Materials and Methods
Mice
B6 and F1 mice were purchased from The Jackson Laboratory; B6.PL-Thy1a/CyJ (Thy1.1+; originally purchased from The Jackson Laboratory) were maintained as a breeding colony on-site. All animals were kept in a pathogen-free animal facility at Oregon State University (Corvallis, OR) and treated according to animal use protocols approved by the Institutional Animal Care and Use Committee at Oregon State University.
TCDD preparation and treatment
TCDD (99% purity; Cambridge Isotope Laboratories) was dissolved in anisole (J. T. Baker) and diluted in peanut oil. The anisole/peanut oil solution alone served as vehicle control. Host F1 mice were dosed with 15 μg/kg TCDD or with vehicle control by oral gavage within 24 h before the adoptive transfer of splenic Thy1.1+ donor T cells.
Preparation and injection of Thy1.1+ donor T cells
Splenocyte suspensions were prepared by dissociation of spleens between frosted microscope slides in HBSS containing 2.5% FBS, 50 μg/ml gentamicin, and 20 mM HEPES followed by a 10-s water lysis of RBC. T cells (CD4+ and CD8+) were isolated from pooled splenocytes using a Pan T cell isolation kit and an autoMACS separator (Miltenyi Biotec) to >90% purity. In some experiments the cells were labeled with 2 μM CFSE (Molecular Probes) before adoptive transfer. Sex-matched F1 host mice were injected i.v. via the tail vein with 2 × 107 B6 donor T cells. Host spleens were then harvested 48 h after the transfer.
Purification of Thy1.1+ donor CD4+ T cells during GVH response
Donor Thy1.1+ CD4+ cells were isolated from F1 host splenocytes using a combination of panning and magnetic sorting methods. In this procedure, pooled splenocyte suspensions were isolated on the second day of the GvH response from vehicle or TCDD-treated host mice and resuspended in HBSS containing 10% FBS, 50 μg/ml gentamicin, and 20 mM HEPES. The cells were added to non tissue culture treated petri plates coated with anti-mouse IgG (Jackson ImmunoResearch Laboratories) and then incubated (4°C) to allow B cells to adhere. The remaining cell suspensions were transferred to new petri plates coated with anti-mouse IgG and anti-CD8a and incubated (4°C) to allow CD8+ cells and additional B cells to adhere. Next, the remaining suspended cells were stained with PE-labeled anti-mouse Thy1.1 (clone OX-7; BD Pharmingen) and sorted with anti-PE microbeads on an autoMACS separator (Miltenyi Biotec). The purified cells (>80% CD4+Thy1.1+) are designated as VEH-CD4+ cells or TCDD-CD4+ cells from vehicle- and TCDD-treated hosts, respectively. In some experiments, the dividing VEH-CD4+ cells and TCDD-CD4+ cells were identified by CFSE dilution and were sorted from pooled host spleens using a MoFlo cell sorter to >95% purity.
Splenocyte cultures
Splenocytes were cultured in RPMI 1640 medium containing 10% FBS, 50 μg/ml gentamicin, and 50 μM 2-ME (cRPMI). For suppression assay cultures, naive B6 splenocytes were fractionated using a mouse CD4+CD25+ regulatory T cell isolation kit and an autoMACS separator (Miltenyi Biotec). Isolated fractions included CD4+CD25+ natural T-regs, CD4+ CD25− responders that were labeled with CFSE (2 μM; Molecular Probes), and T cell-depleted accessory cells that were either gamma irradiated (3 kilorad) or mitomycin C treated (50 μg/ml) before culture. Naive CD4+CD25+ natural T-regs or donor CD4+ cells isolated from TCDD- or vehicle-treated F1 mice 48 h after adoptive transfer of donor T cells were titrated into culture (1:1 to 1:16 suppressor to responder ratio) with 2 × 105responder CD4+ T cells, 1 × 105 accessory cells (ACs), and 0.25 μg/ml soluble anti-CD3 Ab (BD Biosciences) in cRPMI. Cells and supernatants were harvested 72 h later and dilution of CFSE in responder cells was measured by flow cytometry.
To some cultures containing purified donor Thy1.1+ CD4+ cells, exogenous rIL-2 (IL-2, eBioscience), IL-12 (eBioscience), or plate-bound anti-CD3 Ab was added as indicated. IL-2, IL-17A (eBioscience), TGF-β1 (Anogen), and TGF-β3 (R&D Systems) were measured in supernatants by ELISA as per the manufacturer’s instructions. IL-10, IL-4, and IFN-γ were measured with FlowCytomix Simplex kits (Bender MedSystems) according to the manufacturer’s instructions on a Beckman Coulter FC-500 flow cytometer. Abs added to neutralize or ligate molecules in the assay included anti-mouse IL-10 (clone JES5-2A5; BD Pharmingen), anti-mouse GITR (clone DTA-1; eBioscience), and purified rat IgG2a (eBioscience) as isotype control. Recombinant soluble mouse TGF-βRII/mouse Fc (R&D Systems) was added to bind and sequester TGF-β1 and TGF-β3.
To determine requirements for cell contact, a Corning HTS Transwell-96 tissue culture system with a 0.4-μm polycarbonate membrane was used to separate cultures containing TCDD-CD4+ cells (or natural T-regs) (2.5 × 104) with ACs (5 × 104) (top insert) from CFSE-labeled CD4+ responders (5 × 104) and ACs (5 × 104) (bottom insert) in cRPMI containing 0.5 μg/ml soluble anti-CD3 Ab.
Dendritic cell isolation and cultures
F1 dendritic cells (DCs) were derived from bone marrow cells flushed from tibias with a 25-gauge needle containing HBSS medium with 2.5% FBS and 50 μg/ml gentamicin. Cells were dissociated through a 100-μm nylon mesh cell strainer and cultured in non-tissue culture-treated 100 × 15-mm petri dishes in cRPMI containing 15 ng/ml GM-CSF (eBioscience). After 3 days of culture, floating and loosely adherent cells were collected and recultured in fresh cRPMI supplemented with GM-CSF for an additional 7 days of culture. The DCs were then used within an additional 14 days. Removal of adherent DCs was achieved by a 15-min incubation (4°C) with 5 mM EDTA followed by gentle-trituration. Maturation of DCs was achieved by incubation with 500 ng/ml LPS (Escherichia coli0111:B4, Sigma) for 24 h.
Flow cytometry
Splenocytes were washed and stained on ice in Dulbecco’s PBS containing 1% BSA and 0.1% sodium azide. Cells were first incubated with rat IgG (Jackson ImmunoResearch Laboratories) for Fc receptor blocking and then stained with optimal concentrations of different combinations of anti-mouse mAbs including biotinylated-Thy1.1 (clone OX-7), allophycocyanin-Cy7-CD4 (clone GK1.5), PE-CD25 (clone PC61), or allophycocyanin-CD25 (clone PC61) from BD Biosciences. Foxp3 (clone FJK-16s) was measured using a PE anti-mouse/rat Foxp3 staining kit (eBioscience). STAT4 was measured using BD Phosflow reagents including mouse PE-STAT4 (clone 38/p-Stat4) as per the manufacturer’s instructions. Samples lacking one of the individual stains (called fluorescence minus one) or Ab isotypes were used as staining controls. Viability of unfixed cells was measured with 7-aminoactinomycin D (Calbiochem) or ethidium monoazide (Sigma-Aldrich) for fixed cells. A minimum of 5,000 donor CD4+ events or >10,000 nondonor events were collected per sample on a Beckman Coulter FC-500 flow cytometer. Data analysis and software compensation were performed using WinList (Verity Software).
Real-time RT-PCR
IL-2 mRNA levels were measured in cultured cells using the RNAqueous-4 PCR kit (Ambion) followed by cDNA synthesis. PCRs were conducted using the SuperScript III Platinum two-step quantitative RT-PCR kit (Invitrogen). The following are the sequences of the IL-2-specific and 18S ribosomal subunit-specific (31) primers and probes used; IL-2: 5′-CCTGAGCAGGATGGAGAATTACA-3′ (forward), 5′-TCCAGAACATGCCGCAGAG-3′ (reverse) (BioSource International), and 5′-FAM-CCCAAGCAGGCCACAGAATTGAAAG-BHQ1-3′ (probe) (Integrated DNA Technologies); 18S ribosomal subunit: 5′-CTTTGGTCGCTCGCTCCTC-3′ (forward), 5′-CTGACCGGGTTGGTTTTGAT-3′ (reverse), and 5′-FAM-TGCCGACGGGCGCTGACC-BHQ1–3′ (probe) (BioSource International). The real-time PCR protocol was one cycle at 50°C for 2 min, 1 cycle at 95°C for 2 min, and then 40 cycles at 95°C for 20 s and 61°C for 1 min. RT-PCRs were performed using a Bio-Rad iCycler instrument.
mRNA levels of other genes were measured by semiquantitative RT-PCR (qPCR) in VEH-CD4+ cells or TCDD-CD4+ cells (n = 3; two pooled mice per n; >90% Thy1.1+ purity) using the RNeasy mini kit and SuperArray ReactionReady first strand cDNA synthesis kit (Qiagen). PCRs were performed with RT2 real-time SYBR Green/ROX master mix (SuperArray Bioscience) including gene-specific primers, and normalized for β-actin expression. Reactions were performed on an Applied Biosystems 7500 sequence detection instrument using the following protocol: one cycle at 95° for 10 min and then 40 cycles at 95° for 15 s and 60° for 1 min followed by a denaturation step. Results were analyzed using ABI 7500 system software. The same VEH-CD4+ and TCDD-CD4+ cDNA samples were also used to measure the expression of 84 genes associated with T cell differentiation using a RT2 Profile mouse Th1-Th2-Th3 PCR array (SuperArray BioScience). The assay and data analysis were conducted as per the manufacturer’s instructions.
Statistical analyses
Results are presented as the mean ± SEM for individual mouse and/or culture well replicates as indicated. Unpaired t tests were performed using GraphPad Prism and GraphPad software where p < 0.05 (*), p < 0.005 (**), and p < 0.0005 (***) indicate statistical significance.
Results
TCDD does not induce Foxp3+ cells during an acute GVH response
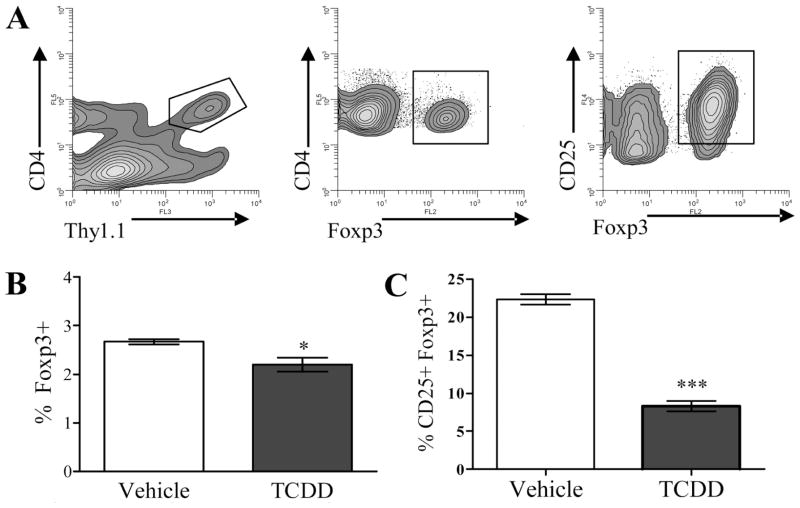
Foxp3, a Forkhead/winged helix transcription factor, is expressed by natural CD4+CD25+ T-regs and is necessary for their development and suppressive function (32). Because donor CD4+ cells express a CD25high phenotype during the second day of the B6-into-F1 acute GVH response in TCDD-treated F1 host mice and demonstrate suppressive function in vitro (9), it was important to determine whether these cells also express Foxp3. B6 donor T cells were identified in spleens from F1 hosts treated with TCDD (TCDD-CD4+) or vehicle control (VEH-CD4+) by their expressions of the congenic marker Thy1.1, from which Foxp3, and CD25 were measured (Fig. 1A). As shown in Fig. 1B, Foxp3 was expressed at a low frequency in both TCDD-CD4+ cells and VEH-CD4+ cells (2–3% of cells), and significantly fewer TCDD-CD4+ cells expressed Foxp3 (2.2% ± 0.15) than VEH-CD4+ cells (2.7% ± 0.1; p < 0.05). When the coexpression of CD25 and Foxp3 was examined, only 9% of the CD25+ TCDD-CD4+ cells expressed Foxp3 compared with 21% of the CD25+ VEH-CD4+ cells (Fig. 1C). Furthermore, the Foxp3+cells present in the TCDD-CD4+ population were not among those actively proliferating in response to alloantigen according to CFSE dilution (data not shown), suggesting that they represent residual natural T-regs present in the donor inoculum at the time of adoptive transfer. These data suggest that TCDD does not induce de novo generation of Foxp3+ CD4+ cells or expand the existing population. This is further supported by the finding that depleting CD25+ cells from the donor cell inoculum before transfer into hosts does not influence the effects of TCDD on TCDD-CD4+ cells (9). Foxp3 protein expression was also measured in F1 host CD4+ T cells and the percentages that were Foxp3+ were not different between TCDD-treated (13.1% ± 0.4) and vehicle-treated mice (14.0% ± 0.7).
FIGURE 1.
The frequency of Foxp3+ donor CD4+ cells is decreased in mice exposed to TCDD during acute GVH response. A, Using flow cytometry, B6 donor CD4+ cells were identified in vehicle- or TCDD-treated F1 host mice at 48 h after adoptive transfer by their expression of the congenic marker Thy 1.1, from which Foxp3, and/or CD25 were measured. B and C, The percentage of Thy1.1+ CD4+ cells (B) and percentage of Thy1.1+CD4+ CD25+ cells (C) expressing Foxp3 is shown. These data are representative of two separate experiments (n = 3 mice per treatment group). Asterisks indicate statistically significant difference in the percent of Foxp3+ cells compared with vehicle control (t test; *, p < 0.05; ***, p < 0.0005).
TCDD-CD4+ cells do not produce IL-2 and suppress IL-2 production by responder T cells
Another important feature of natural T-regs is their lack of IL-2 production despite high CD25 expression. To determine whether this feature was shared by TCDD-CD4+ cells, soluble IL-2 protein was measured in the supernatants of anti-CD3 Ab-stimulated naive CD4+ T cells, TCDD-CD4+ cells, or natural T-regs at 24, 48, and 72 h. The highest levels of IL-2 were found at 48 h, at which time the supernatants from anti-CD3 Ab-stimulated naive CD4+ T cells contained 766 ± 34.9 pg/ml IL-2, compared with 19.6 ± 7.5 pg/ml and 8.9 ± 0.8 pg/ml IL-2 in TCDD-CD4+ and natural T-reg supernatants, respectively (Fig. 2A). We suspected that the low levels of IL-2 in TCDD-CD4+ and natural T-reg supernatants came from small numbers of contaminating effector CD4+ T cells still present in the magnetically sort-purified populations. Hence, IL-2 was further examined at the mRNA level early in culture (14 h) by qRT-PCR for the same purified populations of T cells. IL-2 was detected in RNA isolated from anti-CD3 Ab-stimulated CD4+ T cells but was below detectable levels in RNA isolated from stimulated TCDD-CD4+ cells and natural T-regs, suggesting that, like natural T-regs, TCDD-CD4+ cells do not produce significant amounts of IL-2.
FIGURE 2.
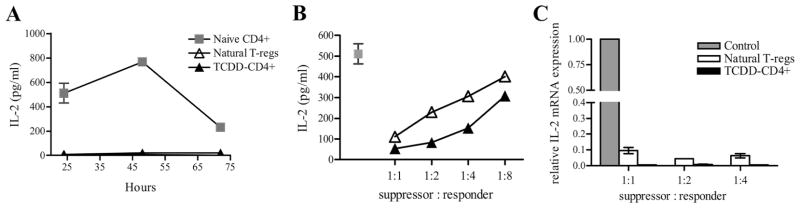
TCDD-CD4+ cells suppress IL-2 production by responder CD4+ T cells. A, IL-2 protein levels were measured by ELISA in supernatants from cultures containing anti-CD3 Ab and irradiated ACs cocultured with either naive CD4+ cells, natural T-regs, or magnetically sorted TCDD-CD4+ cells at 24, 48, and 72 h (triplicates). B, IL-2 was measured in suppression assay supernatants harvested at 24 h containing naive CD4+ responder cells alone or cocultured with either natural T-regs or TCDD-CD4+ cells titrated in at 1:1 to 1:8 suppressor to responder ratios. C, The relative expression of IL-2 was determined by qRT-PCR (normalized for 18S ribosomal subunit expression) in cells harvested from a suppression assay at 14 h including naive CD4+ responders cultured alone or cocultured with TCDD-CD4+ cells or natural T-regs at 1:1 to 1:4 suppressor to responder ratios.
In studies performed to measure suppressive function, TCDD-CD4+ cells were titrated into a suppression assay culture consisting of naive CD4+CD25− responder cells labeled with CFSE, soluble anti-CD3 Ab, and mitomycin C-treated or gamma-irradiated accessory cells. The dynamics of IL-2 expression in this assay were examined to determine whether the suppressed proliferation of responder T cells in the presence of TCDD-CD4+ cells was due to a lack of IL-2 in culture. As shown in Fig. 2B, an inverse relationship existed between increasing levels of IL-2 in the supernatants and decreasing numbers of TCDD-CD4+ cells (and natural T-regs) in the culture. To determine whether TCDD-CD4+ cells were sequestering or depleting IL-2 due to the high levels of CD25 being expressed, exogenous IL-2 was added to cultures containing only TCDD-CD4+ cells, natural CD25+CD4+ T-regs, or no cells (to control for degradation of IL-2 in culture over time), and then IL-2 levels were measured in the supernatants by ELISA 72 h later. We found that cultures containing TCDD-CD4+ cells had at least as much remaining soluble IL-2 (411 ± 19.9 pg/ml) as those containing no cells (362.8 ± 0.36 pg/ml). In comparison, natural T-regs consumed almost half of the IL-2 (218 ± 1.3 pg/ml) over the 72-h period.
To determine whether IL-2 production by naive CD4+ responders was suppressed in the presence of TCDD-CD4+ cells, IL-2 mRNA transcript levels were measured at 14 h in suppression assay cultures containing titrated numbers of TCDD-CD4+ cells or natural T-regs. As shown in Fig. 2C, compared with naive CD4+ T cells, IL-2 transcript levels were reduced >90% in the presence of natural T-regs and barely detectable in the presence of TCDD-CD4+ cells. Taken together, these data show TCDD-CD4+ cells produce little IL-2, do not sequester or deplete the culture of IL-2, and suppress early IL-2 production by CD4+ responders at the level of transcription. These findings, in part, account for the reduced proliferation of CD4+ responders cocultured with TCDD-CD4+ cells.
TCDD-CD4+ cells are not anergic to stimuli in culture
Previous studies have shown that TCDD-CD4+ cells are anergic in culture in response to anti-CD3 Ab stimulation without the addition of exogenous IL-2 (9). Unexpectedly however, more recent studies using CFSE dilution to measure proliferation show that TCDD-CD4+ cells continue to proliferate in culture in the presence of various stimuli. Specifically, at the time the donor cells were harvested from the host mice (48 h into the GvH response), the majority had undergone 2–4 divisions, but after 40 h of additional culturing with anti-CD3 Ab or IL-2, the majority of cells had undergone 6–9 divisions (Fig. 3A). The previous studies of TCDD-CD4+ anergy measured [3H]TdR incorporation after 72 h of anti-CD3 Ab stimulation (9). We found, however, that the viability of TCDD-CD4+ cells (and VEH-CD4+ cells) stimulated with anti-CD3 Ab can drop to <20% after 72 h, which may explain the original findings. Thus, in the suppression assay that included anti-CD3 Ab as the stimulus, TCDD-CD4+ cells continue to divide while suppressing the activation and proliferation of naive CD4+ T cells. The results from these CFSE studies demonstrating that TCDD-CD4+ cells are not anergic in vitro are consistent with our observations that TCDD-CD4+ cells continue to expand if left in vivo through 72 h after adoptive transfer (9).
FIGURE 3.
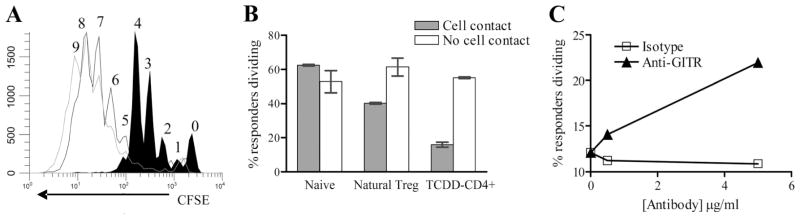
TCDD-CD4+ cells proliferate in culture and require cell contact for suppressive function that is released by ligating GITR. A, TCDD-CD4+ cells were magnetically purified from pooled F1 host spleens (filled histogram) and cultured for 40 h with 50 U/ml IL-2 (black line histogram) or plate-bound anti-CD3 Ab (5 μg/ml) (gray line histogram). Cell divisions are numbered as per CFSE dilution, and are representative of three separate experiments. B, For cell-contact studies, TCDD-CD4+ cells or B6 natural T-regs were magnetically purified and then cultured with anti-CD3 Ab (0.5 μg/ml) and irradiated ACs together with (“Cell contact”) or separated from (“No cell contact”) CFSE-labeled naive CD4+ responders by using a 96-well Transwell system for 72 h; “Naive” indicates wells that contained no suppressors. C, TCDD-CD4+ cells were cocultured with CFSE-labeled CD4+CD25− T cells, anti-CD3 Ab (0.25 μg/ml) and irradiated ACs for 72 h with 0, 0.5, or 5 μg/ml anti-GITR (clone DTA-1) or rat IgG2b isotype control Abs.
Suppressive functions of TCDD-CD4+ cells require contact with responder cells but are relieved by costimulation through GITR
Natural T-regs require contact/close proximity with the cells they suppress in vitro (33, 34). To determine whether cellular contact was also required for the ex vivo suppressive function(s) of TCDD-CD4+ cells, the TCDD-CD4+ cells were separated from CFSE-labeled responder CD4+ T cells using a Transwell system with a 0.4-μm membrane to prohibit cell contact but still allow transfer of soluble molecules. We found that when separated by the membrane, the proliferation of the responder T cells was not suppressed by TCDD-CD4+ cells (Fig. 3B), suggesting that the suppressive mechanism(s) of TCDD-CD4+ cells require contact with the target responder cells in vitro. It has also been demonstrated that costimulatory signals abrogate suppression by natural T-regs, including stimulation through GITR (35, 36). Because TCDD-CD4+ cells also express high levels of GITR (9), we added an agonistic anti-GITR Ab to the suppression assay culture and saw a dose-dependent reduction in the suppression of responder T cell proliferation by TCDD-CD4+ cells (Fig. 3C). Thus, similar to natural T-regs, the absence of cell contact or the presence of costimulation are both capable of abrogating the suppressive activity of TCDD-CD4+ cells in culture.
Allostimulation also discriminates TCDD-CD4+ cells from VEH-CD4+ cells
Previous studies used anti-CD3 Ab/accessory cell stimulation of TCDD-CD4+ cells to measure their functional activity ex vivo. TCDD-CD4+ cells, however, were generated under conditions of allostimulation in vivo; thus we were interested to see whether continued allostimulation in vitro might discriminate TCDD-CD4+cytokine production and function from VEH-CD4+ cells. F1 host spleens were harvested 48 h after adoptive transfer of donor T cells, and the splenocytes were cultured as a mixture of donor and host cells (n = 3 mice). Cytokines were measured in the culture supernatants after 18 h. Under these conditions, there was significantly more IL-10 detected in the supernatants from TCDD-treated mice (328.3 ± 34 pg/ml) compared with vehicle-treated mice (179.6 ± 48.3 pg/ml; p < 0.01) (Fig. 4A). In contrast, the supernatants collected from the vehicle-treated splenocytes contained significantly more IL-2 (445.3 ± 84.8 pg/ml) than those from TCDD-treated splenocytes (130.5 ± 15 pg/ml; p < 0.01) (Fig. 4B). The concentration of IL-2 in the supernatants from naive nonstimulated splenocytes was not significantly different from that in the supernatants from TCDD-treated splenocytes, supporting our earlier observations that TCDD-CD4+ cells produce little IL-2. There were, however, no significant differences in the concentrations of IFN-γ in the supernatants (data not shown).
FIGURE 4.
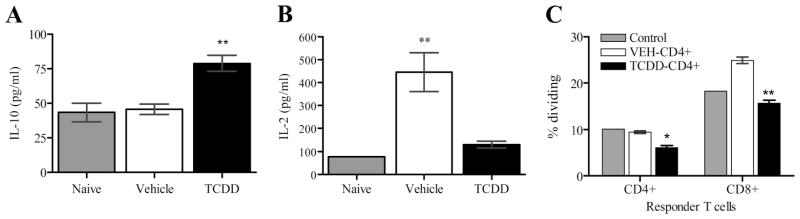
Allostimulation discriminates TCDD-CD4+ and VEH-CD4+ cytokine production and suppressive function. A and B, Splenocytes were harvested from either naive B6 mice, TCDD-treated, or vehicle-treated F1 host mice 48 h after adoptive transfer of B6 T cells (n = 3 mice) and then cultured at 1 × 107/ml for 24 h before the harvest of supernatants and assaying for cytokines. C, Magnetically purified TCDD-CD4+ cells or VEH-CD4+cells (1 × 105) were cultured for 72 h with 2 × 105 CFSE-labeled responder T cells (combination of CD4+ and CD8+) and 3.2 × 104 LPS-matured bone marrow-derived F1 DCs; the percentage of responder T cells dividing was measured by CFSE dilution. Control indicates no donor cells added (adjusted for cell density). Statistical significance compared with vehicle is indicated (t test; *, p < 0.05; ** < 0.005).
To compare the suppressive function of VEH-CD4+ cells and TCDD-CD4+ cells under conditions of allostimulation, the cells were titrated into cultures containing LPS-matured F1 bone marrow-derived DCs (allo-DCs) and CFSE-labeled responder T cells (both CD4+ and CD8+) purified from naive B6 mice. Results showed significantly more suppression of proliferation of both CD4+ and CD8+ responder T cells in the presence of TCDD-CD4+ cells compared with VEH-CD4+ cells (Fig. 4C). Taken together these results demonstrate that TCDD-CD4+ cells have suppressive function in culture in response to both anti-CD3 Ab and allo-DC stimuli and that they suppress proliferation of both CD4+ and CD8+ effector T cells.
TCDD-CD4+ cells produce significant amounts of IL-10
Because increased concentrations of IL-10 were detected in the mixed donor and F1 host splenocyte cultures from TCDD-treated mice, it was important to determine whether TCDD-CD4+ cells were producing it. Thus, IL-10, along with other cytokines of interest, was measured in supernatants harvested from sort-purified, actively dividing (according to CFSE dilution) TCDD-CD4+ cells and VEH-CD4+ cells after 72 h of anti-CD3 Ab stimulation. As with allostimulation, anti-CD3 Ab-stimulated TCDD-CD4+ cells produced significantly more IL-10 than VEH-CD4+ cells (Fig. 5A). IL-10 was not detected in cultures containing anti-CD3 Ab-stimulated natural T-regs or naive CD4+ T cells. When TCDD-CD4+ cells were titrated into a suppression assay with naive CD4+ responders, the IL-10 concentrations in the supernatants were significantly increased (p < 0.005) compared with cultures containing VEH-CD4+ cells (Fig. 5B). Neutralization of IL-10 with Ab had no effect on the proliferation of responder T cells in the presence of TCDD-CD4+ cells (data not shown), as has also been reported for natural T-regs in vitro (33, 34).
FIGURE 5.
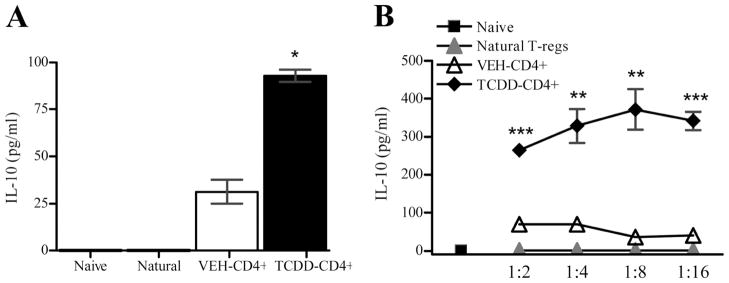
TCDD-CD4+ cells secrete significant levels of IL-10. Naive CD4+ responders, natural T-regs, VEH-CD4+ cells or TCDD-CD4+ cells were cultured separately with irradiated ACs and anti-CD3 Ab (5 μg/ml plate-bound) (A) or titrated in with CD4+ responders (B); supernatants were harvested at 72 h and assayed for IL-10 (triplicates). Statistically significant differences are indicated in comparison to VEH-CD4+ cells (t tests; *, p < 0.05; **, p< 0.005; ***, p < 0.0005).
TGF-β1, another cytokine implicated in T-reg generation and/or effector function, was not detected in soluble form in TCDD-CD4+ or VEH-CD4+ supernatants; however, expression of cell surface-bound TGF-β1, as has been reported on natural T-regs (37), was not analyzed. A soluble TGF-β type II receptor was added to culture to sequester TGF-β1/TGF-β3, which had no effect on responder proliferation in the presence of TCDD-CD4+ cells (data not shown) as has been reported for natural T-regs (33, 34). Other soluble cytokines measured, including IFN-γ, IL-4, and IL-17A, were not detected.
Significant changes in gene expression occur in TCDD-CD4+ cells compared with VEH-CD4+ cells
The expressions of genes associated with different T cell differentiation pathways, including cytokines, transcription factors, activation markers and other immune-response-related genes, were examined in purified TCDD-CD4+ cells and VEH-CD4+ cells (n = 3 per treatment) using a commercially available PCR array (SuperArray Bioscience). As shown in Table I, 15 of the 84 genes in the array were significantly up-regulated in TCDD-CD4+ cells. The greatest change in expression was seen for TGF-β3, which was up-regulated 13-fold compared with VEH-CD4+ cells. Genes associated with T-reg function that were significantly increased in TCDD-CD4+ cells included Ctla-4, IL-2Ra (CD25), and IL-10. Also significantly increased >1.3-fold over VEH-CD4+ cells were IL-12Rb2, Ccr4, Stat4, Ccr5, Socs3, Tnfrsf8 (CD30), Bcl-3, Gata-3, Icos, CD28, and Jak2. In addition, TCDD exposure resulted in the significant down-regulation of some genes compared with vehicle (>1.3-fold) including Tnfsf4 (OX40-L), IL-13Ra, CD86, Bcl-6, IL-5, Nfkb-1, and Ccl5 (Table I).
Table I.
Significant changes in gene expression in TCDD-CD4+ cells relative to VEH-CD4+ cells after 48 h of acute GVH responsea
| Gene | Average Fold Difference (TCDD vs Vehicle) |
|---|---|
| Tgf-b3 | 13.1 |
| IL-12rb2 | 9.8 |
| Ccr4 | 4.7 |
| Stat4 | 3.3 |
| Ccr5 | 3.0 |
| Socs3 | 2.7 |
| Cd30 | 2.6 |
| Bcl3 | 2.4 |
| Ctla4 | 2.3 |
| Cd25 | 1.9 |
| IL-10 | 1.8 |
| Gata3 | 1.8 |
| Icos | 1.5 |
| Cd28 | 1.4 |
| Jak2 | 1.3 |
| Ox40L | −2.9 |
| IL13-ra | −2.3 |
| Cd86 | −2.2 |
| Bcl6 | −1.5 |
| IL-5 | −1.4 |
| Nfkb1 | −1.4 |
| Ccl5 | −1.4 |
Using the same RNA samples, relative expression levels of Cyp1a1 and AhR repressor (Ahrr), two genes known to be regulated by TCDD-mediated activation of the AhR, were measured by qPCR. The Cyp1a1 message was detected in TCDD-CD4+ cells but not in VEH-CD4+ cells and the Ahrr message was increased 21-fold in TCDD-CD4+ cells compared with VEH-CD4+ cells. Expression of two target genes specifically altered by activation of the AhR had been appropriately up-regulated in TCDD-CD4+ cells, allowing us to correlate other gene expression changes with the effects of TCDD-mediated activation of AhR. The relative expression levels of 11 other genes originally identified on a DNA microarray chip were also measured (Fig. 6). Transcripts that were expressed in TCDD-CD4+cells >3-fold over VEH-CD4+ cells included Ccr9, granzyme B, and Blimp-1 (Fig. 6). However, the remaining genes, including Ador2b, Bach2, Entpd1, Ncoa1, Nfe212, Nr3c1, Pdcd4, and Ppp3cb were not changed compared with VEH-CD4+ cells.
FIGURE 6.
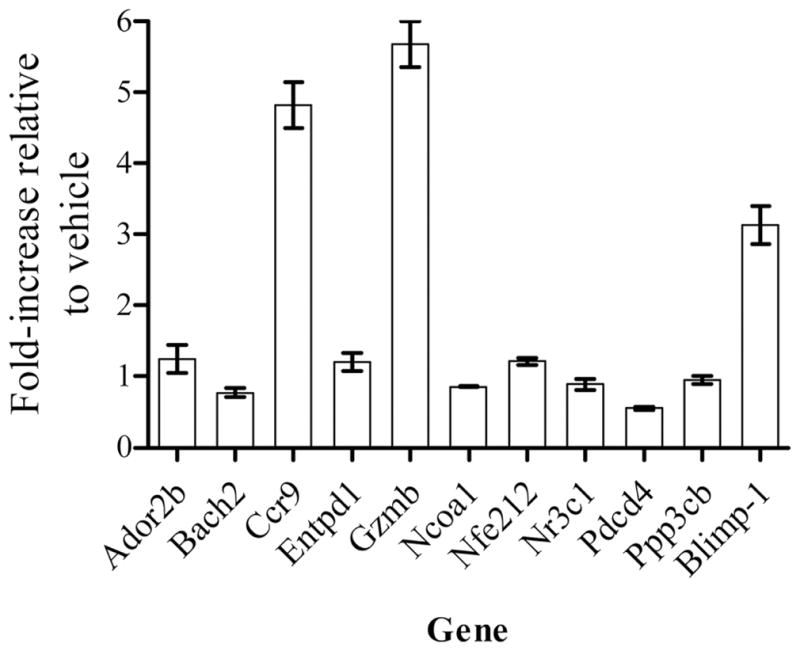
qPCR validation of gene expression in TCDD-CD4+ cells. Up-regulated genes of interest identified on a DNA microarray chip were validated by qPCR using gene-specific primers and normalized for β-actin expression. Results are expressed as fold increase in gene expression in TCDD-CD4+ cells relative to VEH-CD4+ cells; n= 3 per treatment (n = 2 pooled mouse spleens).
Protein changes in/on TCDD-CD4+ cells consistent with gene expression changes
Several of the genes increased at the transcript level in the PCR array also showed increased expression at the protein level. Increased expression of CTLA-4 and CD25 on donor CD4+ cells from TCDD-treated mice was reported previously using flow cytometry (9), and increased levels of IL-10 had been identified in the supernatants of cultured TCDD-CD4+ cells (Figs. 4A and 5A). Unexpectedly, the IL-12Rb2 message, along with other components of the IL-12R signaling pathway, Stat4, Jak2, and Socs3 were up-regulated in TCDD-CD4+ cells compared with VEH-CD4+ cells. To determine whether the IL-12R pathway was more active in TCDD-CD4+ cells, levels of phosphorylated STAT4 protein were measured in TCDD-CD4+ cells and VEH-CD4+ cells immediately after harvest from F1 host mice 48 h after adoptive transfer of the donor T cells. The percentage of cells expressing phosphorylated STAT4 in the TCDD-CD4+ population was markedly increased compared with VEH-CD4+ cells (Fig. 7A; time 0), suggesting an enhanced signaling of the IL-12R signaling pathway in TCDD-CD4+ cells.
FIGURE 7.
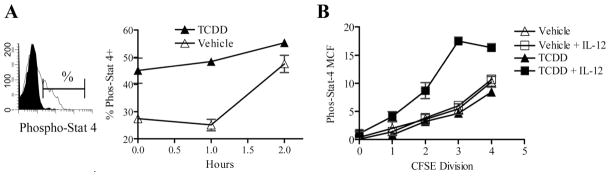
TCDD-CD4+ cells express enhanced STAT4 phosphorylation and responsiveness to IL-12. A, Splenocytes harvested from vehicle- or TCDD-treated F1 hosts (n = 2) at 48 h after the adoptive transfer of donor cells were cultured in cRPMI with rIL-12 (2 ng/ml) at room temperature (duplicate cultures). The percentage of donor CD4+ cells expressing phosphorylated STAT4 was measured by flow cytometry (left) immediately after harvest from the mice (0) (representative of three separate experiments) or after 1–2 h of culture (right). B, STAT4 phosphorylation (MCF, mean channel fluorescence) was measured in VEH-CD4+ cells or TCDD-CD4+ cells per cell division (indicated by CFSE dilution) after 1 h of culture at room temperature with or without IL-12 (2 ng/ml).
To examine the responsiveness of TCDD-CD4+ cells and VEH-CD4+ cells to IL-12, the cells were incubated with IL-12 for 1 or 2 h at room temperature and the percentage of cells expressing phosphorylated STAT4 was measured (Fig. 7A, inset). Results showed a time-dependent increase in the percent of TCDD-CD4+cells expressing phosphorylated STAT4; only after 2 h did the percentage of VEH-CD4+ cells expressing phosphorylated STAT4 attain that of TCDD-CD4+ cells (Fig. 7A). The mean channel fluorescence values for phosphorylated-STAT4 also increased per cell division, with the greatest levels expressed by TCDD-CD4+cells incubated with IL-12 (Fig. 7B). Together, the results suggest that TCDD-CD4+ cells are more responsive to IL-12 than VEH-CD4+ cells, both as a percentage of total cells and on a per cell basis, thus validating the functional significance of increased IL-12Rβ2 and STAT4 expression in TCDD-CD4+ cells.
The gene with the greatest fold increase in TCDD-CD4+ cells was an isoform of TGF-β, TGF-β3. However, soluble TGF-β3 protein was not detected in TCDD-CD4+ or VEH-CD4+ culture supernatants by ELISA. Whether surface-bound or intracellular levels were present is not yet known. Also identified on the DNA microarray and validated by qPCR was granzyme B, increased almost 6-fold in TCDD-CD4+ cells (Fig. 6). Soluble granzyme B protein was measured at picogram levels in culture supernatants by ELISA, but levels were not different between TCDD-CD4+ cells and VEH-CD4+ cells stimulated with anti-CD3 Ab or alloantigen (data not shown). Intracellular granzyme B expression as measured by flow cytometry was also not altered by TCDD (data not shown). We were also unable to confirm increased CD30, increased CCR5, or decreased CD86 cell surface protein expression at 48 h by flow cytometry on TCDD-CD4+ cells. Table II summarizes protein expression that has been measured in/on TCDD-CD4+ cells at 48 h and indicates the changes in expression relative to VEH-CD4+ cells. Measurement of protein expression for other genes affected by TCDD exposure and their functional significance are still under investigation.
Table II.
Summary of protein expression changes in/on TCDD-CD4+ cells relative to VEH-CD4+ cells after 48 h of acute GVH response
| Proteina | Change in Expression Relative to Vehicleb |
|---|---|
| 4-1BB | ND |
| CCR5 | ND |
| CD5 | Decrease (MCF) |
| CD25 | Increase |
| CD28 | ND |
| CD30 | ND |
| CD62L | Decrease |
| CD86 | ND |
| CD103 | ND |
| CTLA-4 | Increase |
| FasL | ND |
| Foxp3 | Decrease (%) |
| GITR | Increase |
| Granzyme B | ND |
| Phosphorylated STAT4 | Increase |
Discussion
We previously reported that the profound suppression of an acute GVH response in TCDD-treated mice was associated with the generation of donor-derived CD4+CD25+ T-reg cells that was dependent upon activation of the AhR (9). However, these studies did not address the mechanisms of their suppressive function or identify changes in gene expression associated with AhR activation that may play a role in the generation or the effector function of these cells (TCDD-CD4+cells). Here, we have further characterized TCDD-CD4+ cells by comparing and contrasting them ex vivo with natural T-regs as well as identified and validated changes in gene expression that correlate with the activation of AhR by TCDD.
Using natural T-regs as a control in our assays allowed us to identify the characteristics that were shared between them and TCDD-CD4+ cells. Results showed that even though TCDD-CD4+ cells do not express Foxp3, they do share in vitro characteristics with natural T-regs, including the requirement for contact/close proximity with the effector T cells they suppress and the abrogation of suppression by costimulation through GITR. It is not yet known whether the GITR costimulatory signal acts on TCDD-CD4+ cells, CD4+ responders, or both to ultimately abrogate suppression. Furthermore, the effects of enhanced costimulation on TCDD-CD4+ cells in vivo and the ability of this stimulation to relieve the suppression of the allo-CTL response have not yet been studied. The finding that TCDD-CD4+ cells can suppress the proliferation of both CD4+ and CD8+ allo-responders in culture suggests they could also suppress alloreactive CTL during the GVH response.
Potential suppressive effector molecules used by TCDD-CD4+ cells were also examined. We found a significant increase in the amount of IL-10 produced both at the mRNA transcript and protein levels by TCDD-CD4+ cells. When TCDD-CD4+ cells were titrated into a suppression assay with CD4+ responders, the levels of soluble IL-10 in the supernatants increased, suggesting that either TCDD-CD4+ cells induced the responders to secrete IL-10 or the responders provided TCDD-CD4+ cells with some factor(s) that enhanced TCDD-CD4+ production of IL-10. Additionally, we found that TGF-β3 was increased 13-fold in TCDD-CD4+ cells compared with VEH-CD4+ cells. An increase in TGF-β3 has also been observed in previous studies of mouse thymocytes exposed to TCDD (38); however the immunological relevance of this more recently identified TGF-β isoform, primarily studied in processes of cell differentiation and development (39, 40) is not well known. We did not detect TGF-β1 or TGF-β3 protein in supernatants isolated from cultured TCDD-CD4+ cells; however, it is possible they are expressed on the cellular membrane, because surface-bound TGF-β is a mechanism of suppression used by natural T-regs (37). Recently, Quintana et al. reported that the transfer of CD4+ T cells from TCDD-treated mice offered some protection from the development of experimental autoimmune encephalomyelitis (EAE) that was not observed when transferred into mice expressing a T cell-restricted deficient TGF-β receptor II (41). The authors interpreted this to mean that T-regs induced by the activation of AhR by TCDD controlled EAE by a TGF-β1-dependent mechanism; however, TGF-β3 also binds this receptor. Neutralization of IL-10 or TGF-β in the in vitro suppression assay was unable to abrogate the suppression by TCDD-CD4+ cells, however, the importance of these effector molecules for in vivo suppression of the GvH response in TCDD-treated mice remains to be determined.
Although the collective mechanisms of suppression used by natural T-regs and the identification of the most important of these has not been fully elucidated, the ultimate result is suppressed production of IL-2 by target responder T cells in the presence of natural T-regs in vitro (34). This same observation for TCDD-CD4+cells helps explain the suppressed proliferation of effector T cells when they are cocultured in the suppression assay. Decreased IL-2 in the cultures did not appear to be due to sequestering by TCDD-CD4+ cells, although the CD25 expressed by TCDD-CD4+ cells is functional as measured by increased STAT5 phosphorylation after the addition of exogenous IL-2 (C. J. Funatake, and N. I. Kerkvliet, manuscript in preparation). TCDD-CD4+ cells, like natural T-regs, expressed little to no IL-2 in response to anti-CD3 Ab or alloantigen stimuli. Dioxin response elements distal to the IL-2 gene promoter have been identified that, when bound by liganded AhR, enhance IL-2 gene expression (42). Although we observed a lack of IL-2 expression by TCDD-CD4+ cells at 48 h, enhanced IL-2 secretion by TCDD-CD4+ cells appears to occur early in the GVH response (C. J. Funatake, and N. I. Kerkvliet, manuscript in preparation). Neutralization of IL-2 with Ab during the GVH response did not decrease CD25 expression on TCDD-CD4+ cells (D. Farrer, L. B. Steppan, and N. I. Kerkvliet, unpublished observations), suggesting that CD25 expression could be driven independently of IL-2 production at 48 h.
Analysis of changes in gene expression in TCDD-CD4+ cells led to the unexpected finding that genes involved in the IL-12R signaling pathway were up-regulated. IL-12Rb2 and Stat4 were up-regulated >9-fold and >3-fold over vehicle control, respectively. Jak2, the kinase that phosphorylates tyrosine residues on the activated IL-12R and on STAT4 was also up-regulated. At the protein level, the percentage of TCDD-CD4+ cells expressing phosphorylated STAT4 was increased compared with that of the VEH-CD4+ cells, and ex vivo stimulation with IL-12 validated an enhanced IL-12R signaling capacity of TCDD-CD4+ cells with enhanced phosphorylation levels of STAT4 both as a percentage of total cells and on a per cell basis. The implications of enhanced signaling of the IL-12Rb2 pathway on the function or generation of TCDD-CD4+ cells is currently under investigation. Because IL-12-responsive elements can cause chromatin remodeling at the CD25 gene locus and increase CD25 expression (43), IL-12 signaling may be an important pathway for up-regulating CD25 on TCDD-CD4+ cells. The JAK2/STAT4 pathway is also important in the promotion of IFN-γ production; however increased expression of IFN-γ at the transcript or protein level in TCDD-CD4+ cells was not found. This may suggest a breakdown in the signaling pathway distal to JAK/STAT phosphorylation or additional constraints imposed on IFN-γ gene expression by TCDD exposure. For example, Gata-3 transcript that is up-regulated almost 2-fold in TCDD-CD4+ cells can also induce IL-10 expression (44) and inhibit IFN-γ production mediated by STAT4 (45).
We were surprised to find the granzyme B message increased almost 6-fold in TCDD-CD4+ cells compared with VEH-CD4+ cells and that granzymes A and B were some of the most highly expressed genes in TCDD-CD4+ cells according to the microarray gene chip. We explored the significance of these findings given that granzymes have been identified as T-reg effector molecules (46, 47). Soluble and intracellular granzyme B levels were measured but were not different between TCDD-CD4+ cells and VEH-CD4+ cells stimulated with anti-CD3 Ab or alloantigen. Granzymes, which induce apoptosis, are an unlikely in vitro suppressive mechanism used by TCDD-CD4+ cells, as there is no additional decrease in the viability of CD4+ responders cocultured with them compared with those cocultured with VEH-CD4+ cells (N. B. Marshall and N. I. Kerkvliet, unpublished observations). The significance of granzyme production by TCDD-CD4+cells in vivo, however, is still not known.
There were a few additional changes in gene expression that we found particularly interesting with respect to the effects of TCDD on T cells. Blimp-1, which is up-regulated ~3-fold at the transcript level in TCDD-CD4+ cells, is a transcriptional repressor that is postulated to play a role in effector T cell differentiation (48). It has been shown that Blimp-1-deficient CD4+ T cells produce excess IL-2 and IFN-γ but reduced IL-10 after TCR stimulation (49). Additional findings have suggested a negative feedback loop exists wherein IL-2 inhibits its own production through induction of Blimp-1 (50). These data support our observations that TCDD-CD4+ cells produce little IL-2 and IFN-γ but significantly more IL-10. However, we have not yet validated increased Blimp-1 expression at the protein level. Also, the >2-fold increase in CD30 transcript expression in TCDD-CD4+ cells is potentially important, as CD30 increases on T-regs exposed to alloantigen and is critical for protection against acute GvH disease (51). We were unable to validate increased CD30 protein expression at 48 h, which could suggest that expression increases at a different time point during the response. A significant down-regulation (>2-fold) of IL-13Ra and CD86 gene transcripts was also observed and was consistent with a microarray analysis of genes expressed in PBMCs collected from humans exposed to TCDD (52). Surface CD86 protein expression however, was not different between TCDD-CD4+ cells and VEH-CD4+ cells at 48 h.
We do not yet know the fate of TCDD-CD4+ cells in vivo, as the cells start to disappear from the F1 host spleen at 96 h. The up-regulation of chemokine receptor transcripts associated with T cell homing suggests the cells may be trafficking to other tissues. CCR4, a chemokine receptor associated with homing to allograft tissues and the skin (53, 54), is expressed by natural T-regs (55) and is up-regulated almost 5-fold at the transcript level in TCDD-CD4+ cells. The CCR5 transcript, increased 3-fold in TCDD-CD4+ cells, is a chemokine receptor normally associated with Th1-polarized T cells and yet its expression by T-regs is required for suppression of GVH disease (56). CCR9, expressed by leukocytes that home to the small intestine, is also up-regulated almost 5-fold at the transcript-level in TCDD-CD4+ cells. Determining whether or where TCDD-CD4+ cells traffic in vivo is currently being studied and could help to identify where the generation of similar T-reg like cells occurs in other models with TCDD exposure.
In this GVH model we found no evidence for the expansion or de novo generation of Foxp3+ cells in either the donor or host CD4+ T cell populations exposed to TCDD. Furthermore, depletion of CD25+ cells from the donor inoculum does not alter the T-reg phenotype of TCDD-CD4+ cells (9). Thus, because the phenotype and suppressive function of TCDD-CD4+ cells appear to be independent of Foxp3 expression, it is possible that ligand-activated AhR acts as an alternative to Foxp3 in naive T cells exposed to TCDD during activation and differentiation into effectors. Although Foxp3 does not appear to be involved in T-reg generation during GVH, a recent report links suppression of EAE by TCDD treatment with increased Foxp3+ T-regs (41). Similarly, recent data from our laboratory links suppression of diabetes in NOD mice treated with TCDD to an expanded population of Foxp3+ CD4+ cells (N. I. Kerkvliet, L. B. Steppan, W. R. Vorachek, S. Oda, C. Wong, D. Pham, and D. V. Mourich, manuscript in preparation). Thus the role of AhR activation by TCDD in T-reg development appears to depend on the conditions of T cell activation; in some conditions it may drive de novo induction of adaptive T-regs, or in other conditions it may support Foxp3+ T-reg expansion.
One of the questions that needs to be answered is this: if the normal pattern of gene expression required for activation-induced differentiation of a naive T cell into an effector T cell is interrupted, is the default differentiation into a regulatory T cell? Furthermore, what are the specific genetic changes that may cause this? For example, a deacetylase inhibitor has been shown to promote the generation and function of T-regs (57) as a result of interrupting normal chromatin remodeling during T cell activation. Similarly, perhaps the inappropriate activation by TCDD of a transcription factor such as AhR during T cell activation causes a default differentiation into a T-reg, as the “normal” gene expression pattern is altered. Thus, it is important to further identify the epigenetic changes that occur in T cells exposed to TCDD to understand how the AhR pathway may provide new insight into regulatory T cell induction. Ultimately, this may introduce a novel therapeutic role for the AhR and certain other agonist AhR ligands.
Acknowledgments
We thank Dr. Scott Menn and the Oregon State University (OSU) Radiation Center, Julie Oughton and Danielle King of the Cell and Tissue Analysis Facilities and Service Core of the Environmental Health Sciences Center at OSU, and the OSU Center for Genome Research and Biocomputing for technical assistance. We also thank Dr. Castle Funatake and Dr. Dave Farrer for technical assistance and helpful discussion.
Footnotes
This work was supported by National Institutes of Health Grants P01ES00040, P30ES00210, and T32ES07060.
Abbreviations used in this paper: TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; AC, accessory cell; AhR, aryl hydrocarbon receptor; B6, C57BL/6 (mice); DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis; F1, B6D2F1 (mice); GITR, glucocorticoid-induced TNFR; GVH, graft-vs-host; qPCR, semiquantitative PCR; TCCD-CD4+, TCDD-treated CD4+ T cell; T-reg, regulatory T cell; VEH-CD4+, vehicle-treated CD4+ T cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Okey AB, Riddick DS, Harper PA. The Ah receptor: mediator of the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Toxicol Lett. 1994;70:1–22. doi: 10.1016/0378-4274(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 2.Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- 3.Yao EF, Denison MS. DNA sequence determinants for binding of transformed Ah receptor to a dioxin-responsive enhancer. Biochemistry. 1992;31:5060–5067. doi: 10.1021/bi00136a019. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Lai ZW, Pineau T, Esser C. Identification of dioxin-responsive elements (DREs) in the 5′ regions of putative dioxin-inducible genes. Chem Biol Interact. 1996;100:97–112. doi: 10.1016/0009-2797(96)03691-5. [DOI] [PubMed] [Google Scholar]
- 6.Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 2004;32:4512–4523. doi: 10.1093/nar/gkh782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerkvliet NI, Shepherd DM, Baecher-Steppan L. T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol Appl Pharmacol. 2002;185:146–152. doi: 10.1006/taap.2002.9537. [DOI] [PubMed] [Google Scholar]
- 8.Funatake CJ, Dearstyne EA, Steppan LB, Shepherd DM, Spanjaard ES, Marshak-Rothstein A, Kerkvliet NI. Early consequences of 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on the activation and survival of antigen-specific T cells. Toxicol Sci. 2004;82:129–142. doi: 10.1093/toxsci/kfh245. [DOI] [PubMed] [Google Scholar]
- 9.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) renders influenza virus-specific CD8+ T cells hyporesponsive to antigen. Toxicol Sci. 2003;74:74–84. doi: 10.1093/toxsci/kfg110. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence BP, Roberts AD, Neumiller JJ, Cundiff JA, Woodland DL. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J Immunol. 2006;177:5819–5828. doi: 10.4049/jimmunol.177.9.5819. [DOI] [PubMed] [Google Scholar]
- 12.Camacho IA, Hassuneh MR, Nagarkatti M, Nagarkatti PS. Enhanced activation-induced cell death as a mechanism of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced immunotoxicity in peripheral T cells. Toxicology. 2001;165:51–63. doi: 10.1016/s0300-483x(01)00391-2. [DOI] [PubMed] [Google Scholar]
- 13.Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-κB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol. 2005;175:90–103. doi: 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108:586–590. [PubMed] [Google Scholar]
- 15.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 16.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 18.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 20.Hansen W, Westendorf AM, Reinwald S, Bruder D, Deppenmeier S, Groebe L, Probst-Kepper M, Gruber AD, Geffers R, Buer J. Chronic antigen stimulation in vivo induces a distinct population of antigen-specific Foxp3 CD25 regulatory T cells. J Immunol. 2007;179:8059–8068. doi: 10.4049/jimmunol.179.12.8059. [DOI] [PubMed] [Google Scholar]
- 21.Chen TC, Cobbold SP, Fairchild PJ, Waldmann H. Generation of anergic and regulatory T cells following prolonged exposure to a harmless antigen. J Immunol. 2004;172:5900–5907. doi: 10.4049/jimmunol.172.10.5900. [DOI] [PubMed] [Google Scholar]
- 22.Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-β-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 24.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 26.Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, Clarkson MR, Sayegh MH, Khoury SJ. Regulatory functions of CD8+CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 29.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 30.Durakovic N, Radojcic V, Powell J, Luznik L. Rapamycin promotes emergence of IL-10-secreting donor lymphocyte infusion-derived T cells without compromising their graft-versus-leukemia reactivity. Transplantation. 2007;83:631–640. doi: 10.1097/01.tp.0000256158.84418.ff. [DOI] [PubMed] [Google Scholar]
- 31.Proudnikov D, Yuferov V, Zhou Y, LaForge KS, Ho A, Kreek MJ. Optimizing primer: probe design for fluorescent PCR. J Neurosci Methods. 2003;123:31–45. doi: 10.1016/s0165-0270(02)00325-4. [DOI] [PubMed] [Google Scholar]
- 32.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 34.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 36.Shevach EM, Stephens GL. The GITR-GITRL interaction: costimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai ZW, Hundeiker C, Gleichmann E, Esser C. Cytokine gene expression during ontogeny in murine thymus on activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1997;52:30–37. doi: 10.1124/mol.52.1.30. [DOI] [PubMed] [Google Scholar]
- 39.Kaartinen V, Cui XM, Heisterkamp N, Groffen J, Shuler CF. Transforming growth factor-β3 regulates transdifferentiation of medial edge epithelium during palatal fusion and associated degradation of the basement membrane. Dev Dyn. 1997;209:255–260. doi: 10.1002/(SICI)1097-0177(199707)209:3<255::AID-AJA1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT, Li W. A “traffic control” role for TGFβ3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093–1105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 42.Jeon MS, Esser C. The murine IL-2 promoter contains distal regulatory elements responsive to the Ah receptor, a member of the evolutionarily conserved bHLH-PAS transcription factor family. J Immunol. 2000;165:6975–6983. doi: 10.4049/jimmunol.165.12.6975. [DOI] [PubMed] [Google Scholar]
- 43.O’Sullivan A, Chang HC, Yu Q, Kaplan MH. STAT4 is required for interleukin-12-induced chromatin remodeling of the CD25 locus. J Biol Chem. 2004;279:7339–7345. doi: 10.1074/jbc.M309979200. [DOI] [PubMed] [Google Scholar]
- 44.Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, Radbruch A. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol. 2007;37:807–817. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- 45.Mendoza L. A network model for the control of the differentiation process in Th cells. BioSystems. 2006;84:101–114. doi: 10.1016/j.biosystems.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 47.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Kallies A, Hawkins ED, Belz GT, Metcalf D, Hommel M, Corcoran LM, Hodgkin PD, Nutt SL. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 49.Martins GA, Cimmino L, Shapiro-Shelef M, Szabolcs M, Herron A, Magnusdottir E, Calame K. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 50.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 51.Zeiser R, Nguyen VH, Hou JZ, Beilhack A, Zambricki E, Buess M, Contag CH, Negrin RS. Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft-versus-host disease. Blood. 2007;109:2225–2233. doi: 10.1182/blood-2006-07-038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McHale CM, Zhang L, Hubbard AE, Zhao X, Baccarelli A, Pesatori AC, Smith MT, Landi MT. Microarray analysis of gene expression in peripheral blood mononuclear cells from dioxin-exposed human subjects. Toxicology. 2007;229:101–113. doi: 10.1016/j.tox.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell JJ, O’Connell DJ, Wurbel MA. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 56.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR, Serody JS. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]