Abstract
Background and Objectives:
Patients on mechanical ventilation in intensive care unit (ICU) are often uncomfortable because of anxiety, pain, and endotracheal intubation; therefore, require sedation. Alpha-2 agonists are known to produce sedation. We compared clonidine and dexmedetomidine as sole agents for sedation.
Study Design:
Prospective, randomized, controlled open-label study.
Materials and Methods:
A total of 70 patients requiring a minimum of 12 h of mechanical ventilation with concomitant sedation, were randomly allocated into two groups. Group C (n = 35) received intravenous (IV) clonidine (1 μg/kg/h titrated up to 2 μg/kg/h to attain target sedation), and Group D (n = 35) received IV dexmedetomidine for sedation (loading 0.7 μg/kg and maintenance 0.2 μg/kg/h titrated up to 0.7 μg/kg/h to achieve target sedation). A Ramsay Sedation Score of 3-4 was considered as target sedation. Additional sedation with diazepam was given when required to achieve target sedation. The quality of sedation, hemodynamic changes and adverse effects were noted and compared between the two groups.
Results:
Target sedation was achieved in 86% observations in Group D and 62% in Group C (P = 0.04). Additional sedation was needed by more patients in Group C compared with Group D (14 and 8 in Groups C and D, respectively, P = 0.034), mainly due to concomitant hypotension on increasing the dose of clonidine. Hypotension was the most common side-effect in Group C, occurring in 11/35 patients of Group C and 3/35 patients of Group D (P = 0.02). Rebound hypertension was seen in four patients receiving clonidine, but none in receiving dexmedetomidine.
Conclusion:
Both clonidine and dexmedetomidine produced effective sedation; however, the hemodynamic stability provided by dexmedetomidine gives it an edge over clonidine for short-term sedation of ICU patients.
Keywords: Clonidine, dexmedetomidine, intensive care unit sedation
Introduction
As many as 85% of the intensive care unit (ICU) patients experience disorders related to anxiety during their ICU stay.[1] Sedation of mechanically ventilated patients is an essential component of ICU management. It is required to tolerate intubation and other ICU related procedures, to lie down in the same position for a long time, to prevent ventilator dysynchrony, for optimization of oxygenation and for patients safety.[2]
Attaining an optimal level of sedation is a challenging act for the ICU clinician. Both inadequate sedation and oversedation compromise patient's recovery and may prolong ICU stay along with associated complications and increased cost.[3] Many of the currently used agents have specific drawbacks that limit their practical utility along the full spectrum of patients and clinical situations that intensivists face every day. The discovery that clonidine has an opioid sparing property and attenuated withdrawal symptoms, sparked further interest in the use of alpha - 2 (α2) agonists as intravenous (IV) sedatives.[4] A resurgence in the research of α2 agonists for sedation developed after the approval of dexmedetomidine for ICU sedation.
Unlike most other sedative drugs, α2 agonists produce both sedation and analgesia with minimal respiratory depression.[5,6,7] This unique combination makes them highly beneficial especially in the ICUs.[6] We therefore planned this study to compare sedative, analgesic and cardiovascular effects and safety profile of two α2 agonists, clonidine, and dexmedetomidine for patients requiring short-term sedation in ICU.
Materials and Methods
Patients
After approval from the Institutional Review Board and informed written consent, 70 adult patients of either sex were enrolled for this study. The main inclusion criteria were age >18 years, mechanical ventilation with endotracheal intubation and clinical need for light or moderate sedation for <24 h. We excluded pregnant females, patients with a neurological condition, central nervous system trauma, asthma or chronic obstructive pulmonary disease, hemodynamically unstable patients, known cases of conduction defects, cardiac failure, those with a creatinine clearance <30 ml/min, and those requiring neuromuscular blockade and prior use of α2 agonists.
The patients were predominantly postsurgical who were operated for major abdominal, gynecological or urological procedures under general anesthesia on an elective basis. The anesthetic technique was individualized by the anesthetist in-charge; however, fentanyl alone was used for intraoperative analgesia and the dose was recorded. Epidural or spinal technique was not used in any patient. On arrival to the ICU, patients were randomly allocated into two groups, Group C and D, based on computer generated random number tables.
Study drugs
Clonidine was supplied in 1 ml ampoules, containing 150 μg/ml and diluted with normal saline to a concentration of 3 μg/ml. Dexmedetomidine was supplied in 2 ml ampoules that contained 100 μg/ml diluted with normal saline to a concentration of 4 μg/ml.
Monitoring
Physical examination, baseline vitals, electrocardiogram and central venous pressure (CVP) was noted on admission to the ICU. Hematological (complete blood count, coagulation profile) and biochemical profile (electrolytes, glucose, urea, creatinine, and liver function test) were obtained prior to the administration of sedatives and 24 h after the study period. Patients were ventilated with oxygen enriched air to obtain acceptable arterial blood gas (ABG) levels. Temperature and ABG was recorded at regular intervals. Apart from the sedative drugs, all management was according to the ICU protocol. Patients were extubated when clinically indicated.
Heart rate, CVP, noninvasive blood pressure (BP), respiratory rate, and oxygen saturation (measured by pulse oximetry) were monitored continuously over 24 h. Hemodynamic parameters were recorded at 10 min, 30 min after the commencement of sedative infusions and then 2 hourly for the study period. Hemodynamic monitoring continued for 24 h after cessation of the infusions.
Adverse cardiovascular events were defined by hypotension, hypertension, tachycardia, and bradycardia. If systolic BP reduced below 80 mmHg or increased above 180 mmHg, diastolic BP reduced below 50 mmHg or increased above 100 mmHg or heart rate was below 50 or above 120 bpm, they were labeled as adverse cardiovascular events. Any change >30% from the baseline in BP and heart rate were also considered as adverse cardiovascular event.
Protocol for sedation and analgesia
The degree of sedation was assessed by Ramsay Sedation Score (RSS) (1: Patient anxious, agitated or restless, 2: Cooperative, oriented and tranquil, 3: Responds to commands only, 4: Exhibits brisk response to light glabellar tap or loud auditory stimulus, 5: Sluggish response to light glabellar tap or loud auditory sound, 6: No response) obtained on arrival in the ICU, at 10 and 30 min after commencement of the infusion and 2 hourly thereafter for the study period. RSS of 3 - 4 was considered as target sedation and the infusion rates were titrated within their respective range until target sedation was achieved. RSS was also assessed prior to and 10 min after any titration in the study drug infusion rate or the use of additional sedation. Infusion was continued as needed until extubation or for maximum allowable time. Group C patients were administered an IV infusion of clonidine 1 μg/kg/h and titration was achieved with dosage increments up to 2 μg/kg/h. Patients in Group D received dexmedetomidine as a loading dose of 0.7 μg/kg over a period of 10 min followed by maintenance of 0.2 μg/kg/h with dosage increments titrated up to 0.7 μg/kg/h. The infusions rates were maintained to achieve sedation within target range.
Additional sedation with IV diazepam bolus of 0.1 mg/kg was given if the patient did not achieve target sedation on titrating the sedative to the maximum selected dose (2 μg/kg/h for clonidine and 0.7 μg/kg/h for dexmedetomidine) or if the patient experienced side-effects (hypotension) with the drugs. Assessment of pain was by direct communication of the patient and fentanyl was given prior to anticipate noxious stimulus. Inadequate analgesia was treated with IV bolus of 20 μg of fentanyl or infusion if pain persisted.
Statistical analysis
A sample size of minimum 32 patients/group was expected to have an 80% power to detect a 30% reduction in additional sedation requirements (primary endpoint) with a significance level of 5%. All data were recorded and noted on observation charts and were analyzed at the end of the study. Data were expressed as mean ± standard deviation (SD) or as median and interquartile range (IQR) and comparisons made using the unpaired t-test. Medians were quoted for skewed data and were compared using the Mann-Whitney U-test. Nominal or ordinal variables were compared using the Chi-square test. P <<i> 0.05 was considered as significant. Analysis was carried out using the SPSS 18.0 software (IBM ( PASW STATISTICS 18)).
Results
Over a period of 18 months, 70 patients were enrolled in the study to receive sedation with either dexmedetomidine (n = 35) or clonidine (n = 35). These included 59 postsurgical, 7 medical and 4 polytrauma patients evenly distributed in each group [Table 1]. Demographic data and intraoperative details such as operative time, fentanyl requirements, APACHE II scores, and duration of sedative infusions in the ICU were comparable [Table 1].
Table 1.
Demographic and intraoperative details: Median (IQR) or number

Additional sedation with diazepam (primary endpoint) was needed by eight patients in dexmedetomidine treated and by 14 patients in clonidine treated patients (P = 0.034). Of these patients, three patients of Group D and 11 of Group C could not attain target sedation due to development of significant hypotension on increasing infusion rate. Median dose of diazepam required in Group C was significantly higher compared to Group D (15 mg, IQR: 5-22 mg in Group C and 8.5 mg, IQR: 2-10 mg in Group D, P = 0.043). Need for additional sedation was about 43% less in Group D.
The mean ± SD maintenance infusion dose was 0.47 ± 0.27 μg/kg/h for dexmedetomidine and 1.67 ± 8.6 μg/kg/h for clonidine. Median infusion dose was 0.4 μg/kg/h (Group D) and 1.4 μg/kg/h (Group C). A total of 373 observations of RSS were obtained for Group C, of which 235 (62%) observations were in the target sedation range (RSS: 3-4). In Group D, a total of 403 observations were obtained, of which 347 (86%) were in the target sedation range. The proportion of time spent in the target sedation range was greater in Group D (P = 0.04). A score 1-2 was observed on 86 (23%) occasions in Group C and 36 (9%) occasions in Group D (P = 0.047). RSS: 5-6 was achieved in 52 (14%) observations in Group C and 20 (5%) observations in Group D (P = 0.048).
The baseline hemodynamic parameters were comparable in both groups. A significant reduction in systolic and diastolic BP from the baseline (P < 0.05) occurred after bolus infusion in Group D but in none of the patients fall was >30% from baseline. Thereafter, mean values remained well within range throughout study period [Figures 1 and 2]. Mean heart rate also decreased from baseline 2 h after commencement of sedative infusion in Group D, but at none of the observation times fall was significant (P = 0.079) [Figure 3]. In Group C significant fall from baseline values in BP were noted 2 and 4 h after sedative infusion was started; but thereafter, it showed minimal change Figures [1 and 2]. Patients receiving clonidine (Group C) had significantly lower heart rates from baseline (P < 0.05) [Figure 3]. On comparison, the hemodynamic parameters were comparable between the two groups during the study period (P > 0.05).
Figure 1.
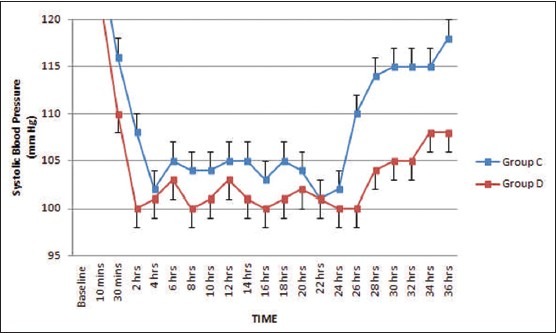
Systolic blood pressure (mean ± standard error of the mean) during dexmedetomidine and clonidine infusion and after discontinuation
Figure 2.
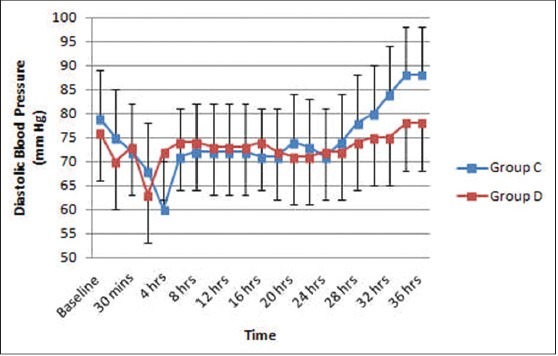
Diastolic blood pressure (mean ± standard error of the mean) during dexmedetomidine and clonidine infusion and after discontinuation
Figure 3.
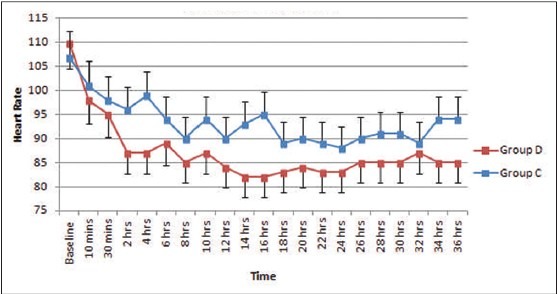
Heart rate (mean ± standard error of the mean) during dexmedetomidine and clonidine infusion and after discontinuation
Bradycardia occurred in 3 of the 35 patients in Group C and 4 of the 35 patients in Group D (P = 0.64). Hypotension occurred in 11 of the 35 patients in Group C (31%) and 3 of the 35 patients in Group D (9%) (P = 0.01). About 50% of the hypotensive episodes occurred within 2-4 h in Group C and at 2 h in Group D. No patient experienced hypotension after 14 h in Group C and after 6 h in Group D. Sustained increase in systolic and diastolic pressure and heart rate occurred after cessation of infusion in Group D, but there were no clinically significant rebound phenomena in any patient. In Group C, rebound hypertension was seen in four patients after cessation of clonidine infusion. In two patients, it increased above 180 mmHg about 2 h after discontinuation of clonidine.
The median 24 h fentanyl requirement was 162 μg (range: 105-175) for Group C and 171 μg (range: 110-185) for Group D (P = 0.73). A median of 3 (range: 2-5) boli of fentanyl was required in both groups over the study period. Most of the boli (81%) were given prior to suctioning and physiotherapy.
Mean time for extubation was similar in both groups, being 19 h (range: 14-30 h) in Group D patients and 18 h (range: 16-32 h) in Group C. There were no adverse respiratory events after extubation in any patient in either group. Biochemical and hematological parameters were not different between two groups at arrival in ICU and 24 h after admission.
Discussion
The chief results of this study showed that target sedation was achieved in more number of patients receiving dexmedetomidine with lesser need for additional sedation. The patients in this group were more stable hemodynamically compared with those receiving clonidine. This study and many previous studies have documented dexmedetomidine to be a safe and effective agent for ICU sedation of postsurgical patients.[8,9]
Although mean cumulative sedation scores over the study period were not significantly different in two groups (3.37 + 1.37 vs. 3.20 + 0.75 in Groups C and D, respectively), percentage of patients who attained target sedation was significantly higher in Group D compared with Group C (86 vs. 62% in Groups D and C, respectively, P = 0.04). Dexmedetomidine is 8 times more specific for α2 receptors than clonidine and the improved specificity for the α2 adrenoreceptors, especially for the 2A subtype may make it to be a much more effective sedative than clonidine.[10] Our finding of dexmedetomidine treated patients is in concurrence with previous studies.[9,11] However, our findings are in contrast with those of Riker et al.[12] who suggested that dexmedetomidine attained target sedation less frequently. They recruited only medical patients, while our most patients were postsurgical. This could possibly be the cause of discrepancy.
A RSS of 1-2 or 5-6 occurred in more number of observations in Group C than in Group D. The short distribution half-life of dexmedetomidine (6 min) makes it an ideal drug for IV titration.[4] This could be the reason for the rapid titration to target sedation and the lesser number of observations pertaining to inadequate sedation in Group D. Although more than 60% patients of both groups attained acceptable sedation, significantly more number of patients in clonidine treated group required additional sedation by diazepam on account of fall of BP on increasing infusion rate to maximum set level. Requirement of additional sedation in this group was about 43% more than dexmedetomidine treated group. In a retrospective analysis of patients receiving clonidine for ICU sedation, Gillison et al.[13] have reported that clonidine reduces requirement of additional sedation and analgesia, but at the cost of higher than routinely prescribed dose. Only 8/35 patients (23%) patients in Group D needed additional sedation which agrees with findings of Martin et al.[14]
There is no consensus on appropriate dose regimen of clonidine during ICU sedation[6] and is extremely variable when given by continuous infusion. However, the usual dose is in the order of 100 μg/h.[15] We used an initial dose of 1 μg/kg/h of clonidine for infusion titrated to 2 μg/kg/h as the maximum dose. The dose of dexmedetomidine for ICU sedation varies greatly ranging between 0.2 and 2.5 μg/kg/h.[9,12,16,17,18] In our study, we used a loading dose of 0.7 μg/kg followed by 0.2-0.7 μg/kg/h. A meta-analysis by Tan and Ho (2010)[19] observed that incidence of bradycardia requiring intervention increased in studies that used both a loading dose and maintenance doses of dexmedetomidine in excess of 0.7 μg/kg/h. Transient hypertensive responses have also been observed with higher doses[20] due to initial stimulation of α2B receptors present in vascular smooth muscles.
Hypotension and bradycardia are the most feared side-effects of α2 agonists. Baseline heart rates which were high in both groups settled to an optimal range over the study period. Hypotension was more commonly seen in Group C compared with Group D. 50% of the hypotensive episodes occurred within 2-4 h in Group C and after bolus infusion and within 2 h after maintenance infusion in Group D, as the steady state plasma concentration of the drugs are achieved at this time duration, causing vasodilatation and hypotension. In general, hemodynamic stability was preserved in most patients receiving dexmedetomidine, a finding in agreement with many previous studies.[9,16,18,21,22] Eleven out of 14 patients in Group C requiring additional sedation to achieve target sedation experienced hypotension on increasing the dose from 1 up to 2 μg/kg/h. This observation was consistent with previous studies of clonidine where adverse hemodynamic effects occurred at doses required for sedation.[5]
Rebound hypertension occurs due to sympathetic activity following discontinuation of the drug.[9,18,21] Previous studies of ICU sedation with dexmedetomidine have found no or minimal increase in heart rate and BP following abrupt cessation,[16,21,23] the finding similar to this study. In Group C, four patients experienced rebound hypertension on cessation of the infusions. It has long been recognized that clonidine can cause rebound hypertension after withdrawal.[24]
There were some limitations of the study that need discussion. A small study group containing patients mostly postsurgical precluded an extensive study on a heterogeneous ICU population. A short study period was considered as dexmedetomidine has been approved by Food and Drug Administration as a sedative in the ICU for patients undergoing mechanical ventilation of < 24 h duration. Therefore the length of ICU stay and outcome could not be studied. Being an open-label study, there is an inherent potential for observer bias. Due to the unavailability of bispectral index (BIS) at our center we restricted our assessment of the degree of sedation to RSS. It is a highly reliable and well-validated sedation scale for use in ICU[17] and has also been shown to have a good correlation with BIS.[25]
Conclusion
Both dexmedetomidine and clonidine can be used as sedative agents for short term ICU sedation of postsurgical patients. On the basis of our study data, we derived that dexmedetomidine has a better cardiovascular safety profile. Further trials with both drugs may define their exact role for sedation of ICU patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 2.Devlin JW. The pharmacology of oversedation in mechanically ventilated adults. Curr Opin Crit Care. 2008;14:403–7. doi: 10.1097/MCC.0b013e32830280b3. [DOI] [PubMed] [Google Scholar]
- 3.Rowe K, Fletcher S. Sedation in the intensive care unit. Contin Educ Anesth Crit Care. 2008;8:50–5. [Google Scholar]
- 4.Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2004;4:CD002024. doi: 10.1002/14651858.CD002024.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Bernard JM, Kick O, Bonnet F. Comparison of intravenous and epidural clonidine for postoperative patient-controlled analgesia. Anesth Analg. 1995;81:706–12. doi: 10.1097/00000539-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: From second- to first-line sedative agents in the critical care setting? J Intensive Care Med. 2012;27:219–37. doi: 10.1177/0885066610396815. [DOI] [PubMed] [Google Scholar]
- 8.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 9.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 10.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 11.Riker RR, Ramsay MA, Prielipp RC, Jorden V. Long-term dexmedetomidine infusion for ICU sedation: A pilot study [Abstract] Anesthesiology. 2001;95:A383. [Google Scholar]
- 12.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 13.Gillison M, Fairbaim J, McDonald K, Zvonar R, Cardinal P. Clonidine use in the intensive care unit of a tertiary care hospital: Retrospective analysis. Can J Hosp Pharm. 2004;57:83–9. [Google Scholar]
- 14.Martin E, Ramsay G, Mantz J, Sum-Ping ST. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003;18:29–41. doi: 10.1177/0885066602239122. [DOI] [PubMed] [Google Scholar]
- 15.Jamadarkhana S, Gopal S. Clonidine in adults as a sedative agent in the intensive care unit. J Anaesthesiol Clin Pharmacol. 2010;26:439–45. [PMC free article] [PubMed] [Google Scholar]
- 16.Venn RM, Ball J, Steele A, Newman PJ, Grounds RM. Dexmedetomidine for sedation in the ICU. Crit Care. 2000;4(Suppl 1):192. [Google Scholar]
- 17.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298:2644–53. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 18.Shehabi Y, Ruettimann U, Adamson H, Innes R, Ickeringill M. Dexmedetomidine infusion for more than 24 hours in critically ill patients: Sedative and cardiovascular effects. Intensive Care Med. 2004;30:2188–96. doi: 10.1007/s00134-004-2417-z. [DOI] [PubMed] [Google Scholar]
- 19.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients; a meta-analysis. Intensive Care Med. 2010 Jun;36:926–39. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 20.Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venn M, Newman J, Grounds M. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29:201–7. doi: 10.1007/s00134-002-1579-9. [DOI] [PubMed] [Google Scholar]
- 22.Takrouri MS, Seraj MA, Channa AB, el-Dawlatly AA, Thallage A, Riad W, et al. Dexmedetomidine in intensive care unit: A study of hemodynamic changes. Middle East J Anesthesiol. 2002;16:587–95. [PubMed] [Google Scholar]
- 23.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Böhrer H, Bach A, Layer M, Werning P. Clonidine as a sedative adjunct in intensive care. Intensive Care Med. 1990;16:265–6. doi: 10.1007/BF01705163. [DOI] [PubMed] [Google Scholar]
- 25.Consales G, Chelazzi C, Rinaldi S, De Gaudio AR. Bispectral Index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol. 2006;72:329–36. [PubMed] [Google Scholar]


