Abstract
Background:
Information regarding early predictive factors for mortality and morbidity in sepsis is limited from developing countries.
Methods:
A prospective observational study was conducted to determine the clinical outcome and predictors of mortality in children with sepsis, severe sepsis, and septic shock. Children aged 1 month to 14 years admitted to a tertiary care pediatric intensive care unit (PICU) with a diagnosis of sepsis, severe sepsis, or septic shock were enrolled in the study. Hemodynamic and laboratory parameters which discriminate survivors from nonsurvivors were evaluated.
Results:
A total of 50 patients (30 [60%] males) were enrolled in the study, of whom 21 (42%) were discharged (survivors) and rest 29 (58%) expired (nonsurvivor). Median (interquartile range) age of enrolled patients were 18 (6, 60) months. Mortality was not significantly predicted individually by any factor including age (odds ratio [OR] [95% confidence interval [CI]]: 0.96 [0.91-1.01], P = 0.17), duration of PICU stay (OR [95% CI]: 1.18 [0.99-1.25], P = 0.054), time lag to PICU transfer (OR [95% CI]: 1.02 [0.93-1.12], P = 0.63), Pediatric Risk of Mortality (PRISM) score at admission (OR [95% CI]: 0.71 [0.47-1.04], P = 0.07) and number of organ dysfunction (OR [95% CI]: 0.03 [0.01-1.53], P = 0.08).
Conclusion:
Mortality among children with sepsis, severe sepsis, and septic shock were not predicted by any individual factors including the time lag to PICU transfer, duration of PICU stay, presence of multiorgan dysfunction, and PRISM score at admission.
Keywords: Multiorgan dysfunction, pediatric risk of mortality scoring, sepsis, septic shock
Introduction
Sepsis in children is a significant cause of morbidity and mortality worldwide.[1] The mortality rate of sepsis in children from pediatric intensive care unit (PICU) of developing countries is higher than 50%.[2] World Health Organization statistics have shown that 80% of death in children <4 years can be classified as sepsis-related deaths.[3] Assessment of severity of illness at admission is important for effective patient management, prognostication, and optimum utilization of resources.[4] Patient's outcome in PICU of developing country is affected by not only by clinical diagnosis at admission but also by demographic characteristics of the population, available infrastructure, and admission policies of PICU.
Simple interventions such as early rapid fluid administration, early antibiotics therapy, oxygen supplementation, and early use of inotropes through peripheral intravenous access have shown to improve the outcome of pediatric sepsis.[5] Although sepsis is one of the leading cause of mortality in hospitalized patients, information regarding predictive factors for mortality and morbidity are limited, especially in developing countries.[6,7,8,9,10] Hence, the present prospective study was designed to assess the predictors of mortality among septic patients admitted to PICU in India.
Methods
This study was a prospective observational study conducted in PICU of a tertiary care government sponsored teaching hospital in Rohtak District, Haryana, India. The patients were recruited from November 2012 to February 2013. The patients seeking treatment in this hospital belong to the urban area of city and surrounding rural districts of Haryana.
The PICU is eight bedded closed unit with two junior resident doctors, two senior resident doctors, two consultants along with nine nursing staff. The patient care is delivered by pediatric residents under supervision of attending consultants who are board certified in pediatric critical care medicine. There are on an average yearly more than 430 admissions in our PICU.
Study participants
All children aged 1 month to 14 years admitted to PICU who met definition of pediatric sepsis guidelines (2010) for diagnosis of sepsis, severe sepsis, or septic shock were enrolled consecutively in the study.[11] Children who had PICU stay of <6 h, trauma patients, surgical patients, patients transferred from other center were excluded from the study. Children who left against medical advice whose final outcome could not be ascertained were also excluded. An informed consent was obtained from the parents, and institutional ethical permission was obtained.
Baseline clinical data
Detailed history of presenting complaints with duration was elicited. The baseline demographic and clinical data were recorded: Age, gender, diagnosis at PICU transfer, type of admission (emergency or elective). Anthropometric parameters were obtained at the time of admission. Laboratory parameters including hemoglobin, total leukocyte count, differential leukocyte count, serum C-reactive protein, blood urea, serum creatinine, prothrombin time, partial thromboplastin time with international normalized ratio, serum lactate, blood pH, serum bicarbonate, arterial oxygen (PaO2 ), and carbon dioxide content (PaCO2 ) were obtained. Blood cultures were collected prior to initiation of antibiotics. In addition, culture of urine, cerebrospinal fluid and tip of the endotracheal tube were also obtained. Culture proven sepsis was defined as one or more positive blood culture. Pediatric Risk of Mortality III (PRISM III) scoring and number of organ dysfunction was recorded in all enrolled patients.
Follow-up
At the time of discharge/death following data was recorded: Duration of PICU stay, duration of mechanical ventilation, number, and duration of inotropes required. The final outcome was recorded in terms of “survivor” and “nonsurvivor” at the time of discharge from PICU.
Management
The therapeutic interventions were started from the time diagnosis was suspected and included early aggressive fluid resuscitation, early administration of empiric antibiotics, oxygen supplementation, and early infusion of inotropic agent. A central venous line was inserted among all children admitted with septic shock in pediatric intensive care. All patients were managed as per standard clinical guidelines for management of sepsis in children.[11] The management includes placement of central venous catheter, appropriate fluid resuscitation, use of inotropes, empirical antibiotics, and blood component transfusion.
Analysis
Data were entered into Microsoft Excel sheets by two investigators (GK and JSK) and any inconsistency was cross checked with original data. The data were analyzed using SPSS Version 15.0, Chicago, SPSS Inc. Categorical variables were expressed as proportions and continuous variables as mean (standard deviation [SD]) or median (interquartile range [IQR]). All quantitative variables (between the groups of survivors and nonsurvivors) were compared by unpaired t-test; categorical variables were compared by Chi-square test or Fisher's exact test. Nonparametric tests were adopted for the skewed distribution. P < 0.05 was considered as significant. Multivariate logistic regressions were used to model the independent predictors of mortality (nonsurvivors) among children with sepsis.
Results
Clinical details of 50 patients were finally analyzed (60 patients enrolled; 6 expired within 2 h of PICU admission; 4 refused consent). Of the 50 patients (30 males {60%}) enrolled, 21 (42%) survived, and 29 (58%) expired (nonsurvivors). The median (IQR) age (in months) of enrolled patients was comparable among the nonsurvivors (27 {6, 64.5}) and survivors (11 {5, 37.5}) (P = 0.26) [Table 1].
Table 1.
Depicting baseline demographic and clinical characteristics of nonsurvivors and survivors among patients with sepsis
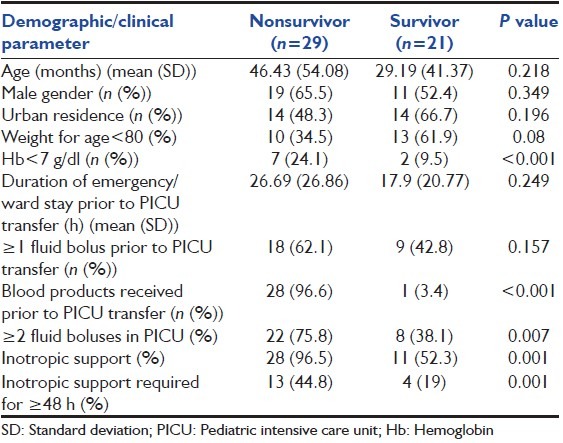
Following clinical features were observed among the enrolled septic children: Respiratory distress (42%), shock (30%), worsening sensorium with refractory status epilepticus (12%), acute kidney injury (2%), and congestive heart failure (4%) and rest 10% had a combination of them. They required larger number of fluid boluses (P = 0.007), prolonged inotropic support (P = 0.001), and need of steroids (P < 0.001) [Table 1].
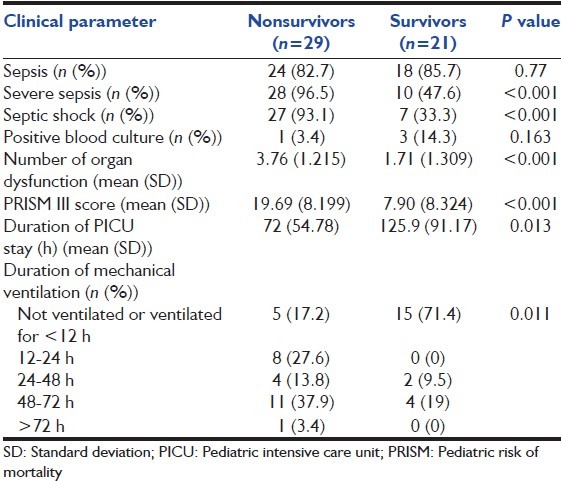
All patients enrolled had systemic inflammatory response syndrome, but severe sepsis, septic shock, and the number of organ dysfunctions was more with nonsurvivor group (P < 0.001) [Table 2]. The mean (SD) PRISM score was significantly higher among nonsurvivors (19.69 {8.19}) as compared with survivors (7.90 {8.32}) (P < 0.001) [Table 2]. Similarly, nonsurvivors had significantly more number of organ dysfunction (3.76 {1.215} and 1.71 {1.309}) (P < 0.001) [Table 2]. However, multivariate logistic regression revealed that mortality is not significantly predicted by age (odds ratio [OR] [95% confidence interval [CI]]: 0.96 [0.91-1.01], P = 0.17), duration of PICU stay (OR {95% CI}: 1.18 [0.99-1.25], P = 0.054), time lag to PICU transfer (OR [95% CI]: 1.02 {0.93-1.12], P = 0.63), PRISM score at admission (OR [95% CI]: 0.71 {0.47-1.04}, P = 0.07) and number of organ dysfunction (OR [95% CI]: 0.03 [0.01-1.53], P = 0.08).
Table 2.
Depicting clinical characteristics of nonsurvivors and survivors during PICU stay
Discussion
This study revealed that factors including the time lag to PICU transfer, duration of PICU stay, presence of multiorgan dysfunction and PRISM score at admission did not independently affect the mortality among children with sepsis. Prediction of patient outcome at admission to PICU is essential not only for counseling parents about the expected outcome, but also for the optimum utilization of limited resources. There are limited studies analyzing the outcome of children with sepsis based on admission criteria.[6,8] The present prospective study provides further insight on the predictors of outcome of pediatric sepsis in a PICU setting of a developing country.
Pediatric Risk of Mortality scoring is a reliable prognostic marker for prediction of mortality in children admitted to PICU. The scoring system is adopted in many Indian PICU settings irrespective of diagnosis at admission. However, studies have raised the concern of poor validity of the scoring in children with sepsis from developing country.[7] Utility of scoring system at admission for prediction of the final outcome in a disease is also determined by natural course of the disease. There are a large number of pediatric illnesses, which have slow progressive deterioration, and hence possibly the mortality in such illnesses is under predicted by lower PRISM scores at admission. However, sepsis in children is rapidly progressive illness and scoring at the time of admission could well-predict the final outcome. We observed a higher PRISM scoring among the nonsurvivors, but failed to independently predict the mortality. These finding are consistent with previous studies from Delhi.[7,9]
Sepsis contributes to almost two-third of admission to PICU. We observed a mortality rate of 42%, consistent with previous studies (overall mortality 35%).[7] There are a large number of factors that determine the outcome of children with sepsis in India. We believe some of them could be generated by delay in seeking medical attention, treatment by local quacks, use of irrational combinations of antibiotics and routine use of steroids by few untrained local practitioners. These findings are reinforced by our observation of higher PRISM score at admission and shorter duration of stay in PICU among the nonsurvivors. Moreover, six children in our study had expired within 2 h of PICU admission, depicting severity of illness at admission and hence a poor outcome of patients despite the best of management.
Management of sepsis in PICU setting would largely depend on infrastructure availability, presence of committed staff, patient to doctor and patient to staff nurse ratio. This could probably influence differing outcome of patients admitted to government centers in comparison to those of corporate hospitals in India. Moreover, majority of patient admission to our PICU were emergency medical indications. Surgical patients, elective preoperative and postoperative patients are managed at surgical ICUs, and the factors affecting the morbidity and mortality among surgical patients needs further exploration.
As anticipated, our study revealed that nonsurvivors had significantly marked derangement of renal, hepatic and coagulation profile; higher requirement of inotropes and the larger number of organ dysfunction, but none of them independently influenced the overall mortality. Similar observations have been reported by other authors from India and abroad.[6,8,9,12] In a study by Khilnani et al., it was observed that almost half of children who expired in tertiary care PICU had multiorgan dysfunction.[13,14] Although each one of parameters including the number of organ dysfunction, PRISM score, duration of PICU stay could influence final outcome; however, our study revealed that none of them could independently predict mortality among children with sepsis. Surprisingly, we did not find any significance of malnutrition to the final outcome in contrast to findings of others from India. This could probably generate from the fact that we defined malnutrition on basis of weight for age alone, rather than weight for height and height for age.
The major limitations of the study include small sample size and short duration of the study. Small sample size of 50 cases could additionally influence the utility of multivariate logistic regression using five variables. Hence, it is acknowledged that lack of association in our study could be due to small sample size. It has to be kept in mind that the clinical judgment of the severity of a disease process is subjective and is related to the experience and clinical ability of a physician. Strengths of our study include prospective data collection and true representation of Indian children with sepsis. This argument is proposed considering that our center is prime referral center for sick patients from surrounding districts of Haryana. The patient population caters to all strata of socioeconomic status and representation of both rural and urban districts of Haryana.
Conclusion
Sepsis in Indian children is associated with high mortality. The multiorgan dysfunction syndrome, high PRISM score and need for multiple inotropes, deranged hematological and biochemical variables are important risk factors for mortality in combination although we are yet to identify a single independent predictor for overall mortality. However, larger multicenter prospective studies should be done on the basis of new sepsis definitions and clinical practice guidelines to evaluate the true burden and outcome of sepsis in the developing countries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Carcillo JA. Reducing the global burden of sepsis in infants and children: A clinical practice research agenda. Pediatr Crit Care Med. 2005;6:S157–64. doi: 10.1097/01.PCC.0000161574.36857.CA. [DOI] [PubMed] [Google Scholar]
- 2.Sarthi M, Lodha R, Vivekanandhan S, Arora NK. Adrenal status in children with septic shock using low-dose stimulation test. Pediatr Crit Care Med. 2007;8:23–8. doi: 10.1097/01.pcc.0000256622.63135.90. [DOI] [PubMed] [Google Scholar]
- 3.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO child health epidemiology reference group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 4.Pollack MM, Ruttimann UE, Getson PR. Accurate prediction of the outcome of pediatric intensive care. A new quantitative method. N Engl J Med. 1987;316:134–9. doi: 10.1056/NEJM198701153160304. [DOI] [PubMed] [Google Scholar]
- 5.Bertolini G, Ripamonti D, Cattaneo A, Apolone G. Pediatric risk of mortality: An assessment of its performance in a sample of 26 Italian intensive care units. Crit Care Med. 1998;26:1427–32. doi: 10.1097/00003246-199808000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Shrestha P, Mohan A, Sharma S, Guleria R, Vikram N, Wig N, et al. To Determine the Predictors of Mortality and Morbidity of Sepsis in Medical ICU of All India Institute of Medical Sciences (AIIMS) New Delhi, India: Chest; 2012. [Last accessed on 2013 June 13]. p. 142. Available from: Http://www journal.publications.chestnet.org/article.aspx?articleid=1376342#tab1 . [Google Scholar]
- 7.Thukral A, Lodha R, Irshad M, Arora NK. Performance of Pediatric Risk of Mortality (PRISM), Pediatric Index of Mortality (PIM), and PIM2 in a pediatric intensive care unit in a developing country. Pediatr Crit Care Med. 2006;7:356–61. doi: 10.1097/01.PCC.0000227105.20897.89. [DOI] [PubMed] [Google Scholar]
- 8.Khan MR, Maheshwari PK, Masood K, Qamar FN, Haque AU. Epidemiology and outcome of sepsis in a tertiary care PICU of Pakistan. Indian J Pediatr. 2012;79:1454–8. doi: 10.1007/s12098-012-0706-z. [DOI] [PubMed] [Google Scholar]
- 9.Singhal D, Kumar N, Puliyel JM, Singh SK, Srinivas V. Prediction of mortality by application of PRISM score in intensive care unit. Indian Pediatr. 2001;38:714–9. [PubMed] [Google Scholar]
- 10.Bellad R, Rao S, Patil VD, Mahantshetti NS. Outcome of intensive care unit patients using Pediatric Risk of Mortality (PRISM) score. Indian Pediatr. 2009;46:1091–2. [PubMed] [Google Scholar]
- 11.Khilnani P, Singhi S, Lodha R, Santhanam I, Sachdev A, Chugh K, et al. Pediatric Sepsis Guidelines: Summary for resource-limited countries. Indian J Crit Care Med. 2010;14:41–52. doi: 10.4103/0972-5229.63029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna U, Joshi SP, Modh M. An evaluation of serial blood lactate measurement as an early predictor of shock and its outcome in patients of trauma or sepsis. Indian J Crit Care Med. 2009;13:66–73. doi: 10.4103/0972-5229.56051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khilnani P, Sarma D, Zimmerman J. Epidemiology and peculiarities of pediatric multiple organ dysfunction syndrome in New Delhi, India. Intensive Care Med. 2006;32:1856–62. doi: 10.1007/s00134-006-0373-5. [DOI] [PubMed] [Google Scholar]
- 14.Khilnani P, Sarma D, Singh R, Uttam R, Rajdev S, Makkar A, et al. Demographic profile and outcome analysis of a tertiary level pediatric intensive care unit. Indian J Pediatr. 2004;71:587–91. doi: 10.1007/BF02724117. [DOI] [PMC free article] [PubMed] [Google Scholar]


