Abstract
Objectives:
The study aimed at examining the role of oxidative stress in cadioprotective effects of oleuropein in a rat model of simultaneous type 2 diabetes and renal hypertension.
Materials and Methods:
Five groups of male Sprague-Dawley rats including a control group, a diabetic-hypertensive group receiving vehicle, and three diabetic-hypertensive groups receiving oleuropein at 20, 40, or 60 mg/kg/day were used. Blood pressure and glucose, serum malondialdehyde, and erythrocyte superoxide dismutase were measured, and animal's hearts with ischemia/reperfusion injuries were used using Langendorff technique.
Results:
Blood pressure, blood glucose, serum malondialdehyde, infarct size, coronary effluent creatine kinase-MB, and coronary resistance of diabetic-hypertensive group were significantly higher than those of the control group, while those of the oleuropein-receiving groups were significantly lower than those of the diabetic hypertensive group receiving the vehicle. Erythrocyte superoxide dismutase, left ventricular developed pressure, and rate of rise and rate of decrease of ventricular pressure of diabetic-hypertensive group were significantly lower than those of the control group. These parameters as well as heart rate of oleuropein-receiving groups were significantly higher than those of the diabetic-hypertensive group.
Conclusion:
The findings indicate that oleuropein offered cardioprotection, which might be partly mediated by its antioxidant properties.
KEY WORDS: Cardioprotection, hypertension, oleuropein, type 2 diabetes
Introduction
A sizable number of reports indicate that Mediterranean diets are associated with longevity and low cardiovascular risk.[1] Olive products such as olive oil, leaves, and fruits constitute a main component of Mediterranean diets.[2] Oleuropein is one of the most important phenolic compounds of olive leaves and is believed to mediate most of the beneficial pharmacological properties of olive oil or leaves.[3]
Previous studies have shown that oleuropein has beneficial cardiovascular effects. Oleuropein was shown to possess hypotensive[4] as well as coronary vasodilator[5] activities. It also decreased myocardial infarct size in isolated healthy rat hearts subjected to ischemia/reperfusion.[6] Moreover, it reduced infarct size in healthy rabbit hearts,[7] and hearts of rats with doxorubicin-induced cardiotoxicity.[8] In addition, oleuropein decreased plasma glucose in animal models of alloxan[9] and streptozotocin_ induced diabetes.[10]
As far as the literature is concerned, the effects of oleuropein on cardiac functions in experimental models of simultaneous renal hypertension and type 2 diabetes have not been investigated. Therefore, the present study was designed to examine such effects using Langendorff technique. It was also aimed at examining whether antioxidant properties of oleuropein could underlie its possible beneficial effects.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 200-250 g obtained from Laboratory Animal Breeding Center, Shiraz University of Medical Science, Shiraz, Iran. They were kept under standard condition (12 h light/12 h dark with 25-35% humidity and 22-28°C) with standard food and water ad libitum. All animal procedures were approved by Institutional Committee for Care and Use of Animals.
Materials
Oleuropein was obtained from the Indofine Chemical Company, Inc. (Hillsborough, NJ). Streptozotocin (Zanosar®) was obtained from Teva Parenteral Medicine Inc. (Irvine, CA). Nicotinamide (NA) was purchased from Sigma-Aldrich Chemical Co. (Germany). Penicillin G powder was obtained from Jaberebne Hayyan Pharmaceutical Company. Streptozotocin (STZ) and NA were dissolved in 0.9% sodium chloride.
Experimental Design
Animals were randomized into five groups including a control (n = 8), a simultaneous type 2 diabetes (DM2) and renal hypertension (HTN) group assigned to receive vehicle (DM2+HTN-Veh) (n = 8), and three simultaneous DM2 and HTN groups assigned to receive oleuropein at 20 mg/kg/day (DM2+HTN-Ole20) (n = 7), 40 mg/kg/day (DM2+HTN-Ole40) (n = 6), or 60 mg/kg/day (DM2+HTN-Ole60) (n = 8).
Experimental Protocol
Induction of diabetes and renal hypertension
Animals were given single intraperitoneal injections of NA (110 mg/kg) 15 min before single intraperitoneal injections of STZ (60 mg/kg). Seven days later, blood levels of glucose were determined using a glucometer (Accu-check® active, Germany). Animals with fasting blood glucose (FBG) levels lower than 126 mg/dl were considered non diabetic, and excluded from the study.[11]
Six weeks after the induction of diabetes, two-kidney, one clip renal hypertension was induced as described previously.[12,13] Briefly, under Ketamine (60 mg/kg) and Xylazine (8 mg/kg) anesthesia an incision was made in the left flank, and left renal arteries were exposed and dissected away from renal veins. Solid Plexiglass clips (internal diameter of 0.2-0.22 mm) were then placed on the arteries. Antibiotic (Penicillin G powder) was then applied to the wounds, and abdominal wall and skin incisions were closed using absorbable (catgut 3/0) and non-absorbable (silk 3/0) suture materials, respectively. Sham-operated animals were subjected to a similar procedure but no clip was placed around renal arteries.[13]
Starting from the next day after the operation, animals assigned to receive daily administrations of distilled water as vehicle (control and (DM2+HTN-Veh groups) or oleuropein at 20 mg/kg/day (DM2+HTN-Ole20), 40 mg/kg/day (DM2+HTN-Ole40) or 60 mg/kg/day (DM2+HTN-Ole60) in the same volume as vehicle for four weeks. The vehicle or oleuropein was administered by oral gavage.
Systolic blood pressure (SBP) was measured four weeks later using tail-cuff method (Chart 5.0 software, PowerLab 4/30, AD Instruments Inc., MA, Australia). Measurements which were not different by more than 5 mmHg in each case were considered valid. The mean of the three measurements was then recorded and reported as the value of SBP.[13] Afterwards, FBG was determined using the glucometer, and blood samples were obtained for the measurement of malondialdehyde (MDA) and superoxide dismutase (SOD). The blood samples were allowed to clot for 30 min, centrifuged at 3000 rpm for 20 min, and their serums were separated and stored at -80°C until analysis. The animals were then sacrificed, and their hearts were removed and used for isolated heart studies using Langendorff apparatus.
Isolated Heart Study
The hearts were mounted, via aorta, on a Langendorff apparatus (model: LE05200; Spain, PanLab) and perfused retrogradely with Krebs-Henseleit buffer with a pH of 7.4 and following composition in mmol/L: NaCl 118.0, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2 PO4 1.2, NaHCO3 25.0 and glucose 11.0. The buffer was kept at 37°C and bubbled constantly with 95% O2 and 5% CO2 at constant flow. A latex balloon was placed in the left ventricle through the left atrium. The balloon's catheter was connected to a PowerLab 8/30 data acquisition system (Chart 5.0 software, PowerLab 8/30, AD Instruments Inc., MA, Australia) via a pressure transducer for continuous recording of cardiac function. The balloon was inflated to an end-diastolic pressure of 5-10 mmHg. The Langendorff apparatus was then switched to constant pressure (60 mmHg) for the rest of the experiment.[13]
After an equilibration of 30 min, baseline measurement of left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), rate of increase (+dp/dt) and decrease (–dp/dt) of ventricular pressure (as the indices of cardiac contraction and relaxation performances), heart rate (HR), coronary flow (CF), were performed. The hearts were then subjected to 20 min global ischemia (zero-flow) and 60 min reperfusion, during the first minute of which coronary artery effluent was collected for the measurement of creatine kinase-MB (CK-MB). The above cardiac hemodynamic parameters were measured every 30 min during reperfusion. At the conclusion of reperfusion, the hearts were used for the measurement of infarct size.[13]
Determination of Cardiac Infarct Size
The hearts were cut into 2-mm-thick slices, which were incubated in TTC solution (1%) at 37°C for 20 min. The slices were then incubated with formalin10% for 24 h. Afterwards, they were digitalized (Digital camera PSG1, Canon) and the infarct areas were quantified as the percentage of total area of slices using image analysis software (NIH Image pro. 1.16).[13]
Biochemical Measurements
Coronary artery effluent levels of CK-MB and serum MDA were determined using Pars Azmun commercial kits (Pars Azmun Co, INC, Karaj, Iran) and Rat MDA ELISA (Cusabio Biotech., LTD) kits, respectively. Erythrocyte SOD activity was determined using Randox SOD kit.
Calculations and Statistical Analysis
Left ventricular developed pressure (LVDP) was calculated as the difference between left ventricular systolic pressure and left ventricular end-diastolic pressure. Coronary resistance (CR) was calculated as the coronary perfusion pressure divided by the coronary flow. Data, presented as mean ± SEM, were compared using One-way Analysis of Variance (ANOVA). When a significant difference was found with ANOVA, the source of difference was located using Duncan's Multiple Range test. A P ≤ 0.05 was considered statistically significant. The data were analyzed and plotted using SigmaStat (version 3.0) and SigmaPlot (version 8.0) statistical software, respectively.
Results
Blood Pressure and Blood Glucose
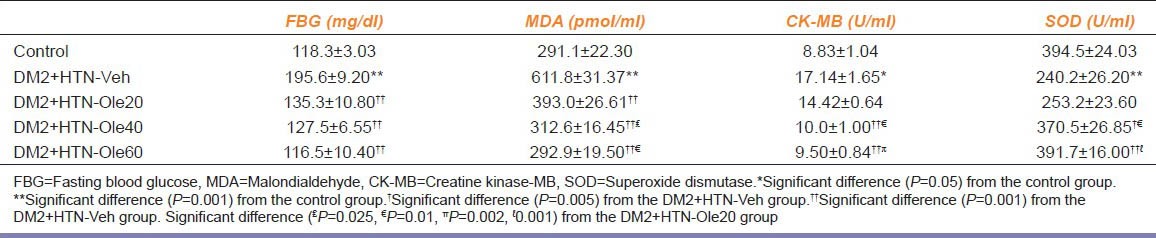
Four weeks after the induction of hypertension and treatment with vehicle or oleuropein, SBP of DM2+HTN-Vehgroup (178.5 ± 1.4 mmHg) was significantly higher than that of the control group (118.3 ± 2.9 mmHg). However, SBP of DM2+HTN-Ole20 (131.8 ± 2.1 mmHg), DM2+HTN-Ole40 (132.6 ± 1.7 mmHg), and DM2+HTN-Ole60 (125.0 ± 2.6 mmHg) were significantly lower than that of the DM2+HTN-Veh group. There was no significant difference between SBP of DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups. FBG of DM2+HTN-Vehgroup was significantly higher than that of the control group [Table 1]. FBG of DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups were significantly lower than that of the DM2+HTN-Vehgroup [Table 1]. There was no significant difference between FBG of DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups [Table 1].
Table 1.
Biochemical parameters (mean±SEM) of control group (n=8), simultaneous type 2 diabetes and renal hypertension group receiving vehicle (DM2+HTN-Veh) (n=8), simultaneous type 2 diabetes and renal hypertension groups receiving oleuropein at 20 mg/kg/ day (DM2+HTN-Ole20) (n=7), 40 mg/kg/day (DM2+HTN-Ole40) (n=6), or 60 mg/kg/day (DM2+HTN-Ole60) (n=8) at the 4th week of treatment
Isolated Heart Studies
Preischemia (baseline) LVDP of DM2+HTN-Veh group was significantly lower than that of the control group [Figure 1a]. However, LVDP of DM2 + HTN-Ole40 and DM2+HTN-Ole60, but not DM2+HTN-Ole20, groups were significantly higher than that of the DM2+HTN-Veh group. A similar pattern of difference was found at 30 and 60 min of reperfusion [Figure 1a]. Moreover, LVDP of DM2+HTN-Ole40 and DM2+HTN-Ole60 at preischemia and 60 min of reperfusion, but not 30 min of reperfusion, were significantly higher than that of DM2+HTN-Ole20 group, while those of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups were not significantly different [Figure 1a].
Figure 1.
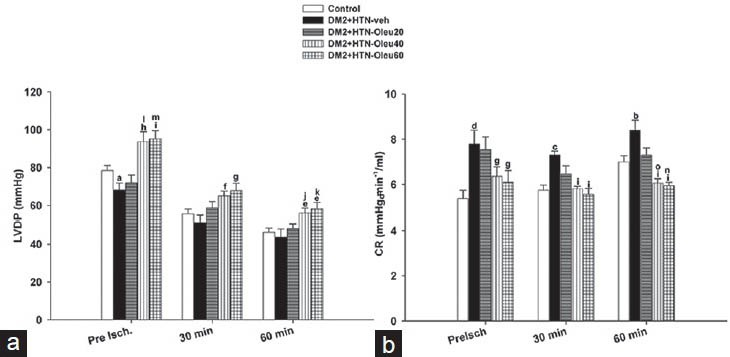
(a) Left ventricular developed pressure (LVDP) and (b) coronary resistance (CR) from control group (n = 8), simultaneous type 2 diabetes and renal hypertension group receiving vehicle (DM2 + HTN-Veh) (n = 8), simultaneous type 2 diabetes and renal hypertension groups receiving oleuropein at 20 mg/kg/day (DM2 + HTN-Ole20) (n = 7), 40 mg/kg/day (DM2 + HTN-Ole40) (n = 6), or 60 mg/kg/day (DM2 + HTN-Ole60) (n = 8) at preischemia (preisch), and at 30 and 60 min of reperfusion. Data are presented as mean ± SEM. *Significant difference (P = 0.05) from the control. Significant difference from the control (a; P = 0.03, b; P = 0.02, c; P = 0.01, d; P = 0.001). Significant difference from the DM2 + HTN-Veh (e; P = 0.05, f; P = 0.02, g; P = 0.01, h; P = 0.003, I; P = 0.001). Significant difference from the DM2 + HTN-Ole20 (J; P = 0.05, k; P = 0.03, l; P = 0.009, o; P = 0.005, m; P = 0.003, n; P = 0.001)
Preischemia (baseline) CR of DM2+HTN-Vehgroup were significantly higher than that of the control group [Figure 1b]. However, CR of DM2+HTN-Ole40 and DM2+HTN-Ole60, but not DM2+HTN-Ole20, groups were significantly lower than that of the DM2+HTN-Veh group. At 30 and 60 min of reperfusion, CR in DM2+HTN-Veh was significantly higher than that of the control group, but CRs of oleuropein-treated groups (DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2 + HTN-Ole60) were significantly lower than that of DM2+HTN-Veh [Figure 1b]. There was no significant difference between CR of DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups at preischemia or 30 min of reperfusion. However, CR of DM2+HTN-Ole40 and DM2+HTN-Ole60 at 60 min of reperfusion were significantly higher than that of DM2+HTN-Ole20 group, while those of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups were not significantly different [Figure 1b].
Preischemia (baseline) +dp/dt and −dp/dt of DM2+HTN-Veh was significantly lower that of the control group [Figures 2a and b]. However, +dp/dt and −dp/dt of oleuropein-treated groups (DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60) were significantly higher than those of the DM2+HTN-Veh group. A similar pattern of difference was found at 30 and 60 min of reperfusion [Figures 2a and b]. Moreover, +dp/dt of DM2+HTN-Ole40 and DM2+HTN-Ole60 at preischemia and 60 min of reperfusion, but not 30 min of reperfusion, were significantly higher than that of DM2+HTN-Ole20 group, while those of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups were not significantly different [Figure 2a]. In addition, there was no significant difference between the −dp/dt of DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups at preischemia or 30 or 60 minutes of reperfusion [Figure 2b].
Figure 2.
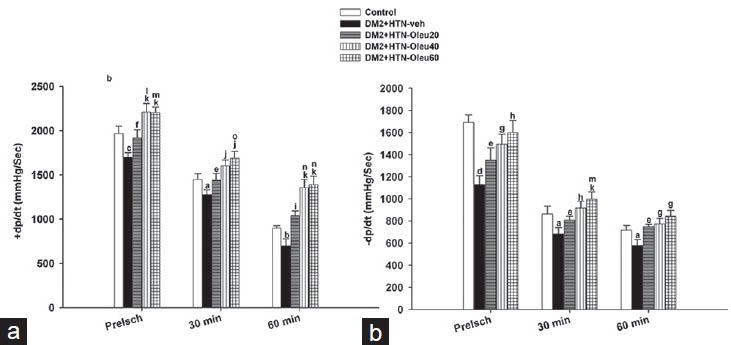
(a) Rate of rise of ventricular pressure (+dp/dt, mmHg/min) and (b) rate of decrease of ventricular pressure (−dp/dt, mmHg/min) from control group (n = 8), simultaneous type 2 diabetes and renal hypertension group receiving vehicle (DM2 + HTN-Veh) (n = 8), and simultaneous type 2 diabetes and renal hypertension groups receiving oleuropein at 20 mg/kg/day (DM2 + HTN-Ole20) (n = 7), 40 mg/kg/day (DM2 + HTN-Ole40) (n = 6) or 60 mg/kg/day (DM2 + HTN-Ole60) (n = 8) at the preischemia (preisch), and at 30 and 60 min of reperfusion. Data are presented as mean ± SEM. Significant difference from the control (a; P = 0.05, b; P = 0.04, c; P = 0.02, d; P = 0.001). Significant difference from the DM2 + HTN-Veh (e = P = 0.05, f; P = 0.04, g; P = 0.01, h; P = 0.009, I; P = 0.005, J; P =0.003, k; P = 0.001). Significant difference from DM2 + HTN-Ole20 (l; P = 0.05, o; P = 0.04, m; P = 0.03, n; P = 0.01)
There was no significant difference between preischmia (baseline) HRs of the control, DM2+HTN-Veh, DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups [Figure 3a]. Moreover, the HRs of such groups were not significantly different at 30 min of reperfusion. However, at 60 min of reperfusion, HR of DM2+HTN-Veh group was significantly lower than that of the control group, and HRs of DM2+HTN-Ole40 and DM2+HTN-Ole60, but not that of DM2+HTN-Ole20, groups were significantly higher than that of DM2+HTN-Veh [Figure 3a].
Figure 3.
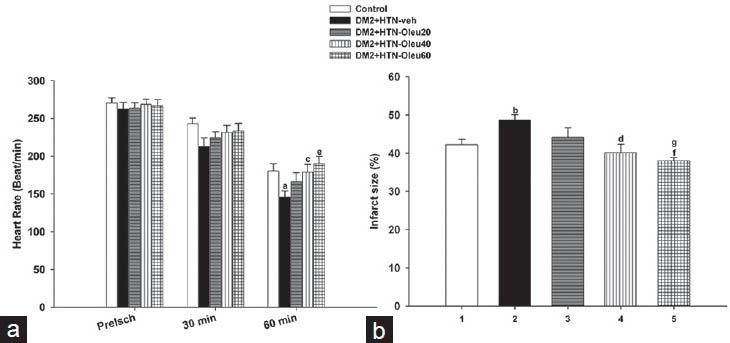
(a) Heart rate of control group (n = 8), simultaneous type 2 diabetes and renal hypertension group receiving vehicle (DM2 + HTN-Veh) (n = 8), and simultaneous type 2 diabetes and renal hypertension groups receiving oleuropein at 20 mg/kg/day (DM2 + HTN-Ole20) (n = 7), 40 mg/kg/day (DM2 + HTN-Ole40) (n = 6) or 60 mg/kg/day (DM2 + HTN-Ole60) (n = 8) at the preischemia (preisch), and at 30 and 60 min of reperfusion. (b) Myocardial infarct size (as percentage of left ventricle) of the same groups at the end of ischemia/reperfusion studies. Data are presented as mean ± SEM. Significant difference from the control (a; P = 0.02, b; P = 0.009). Significant difference from the DM2 + HTN-Veh (c; P = 0.03, d; P = 0.008, e; P = 0.005, f; P = 0.001). Significant difference from DM2 + HTN-Ole20 (g; P = 0.04)
In addition, there was no significant difference between the HR of DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60 groups at preischemia or 30 or 60 min of reperfusion [Figure 3a].
Infarct Size
Infarct size of DM2+HTN-Veh group was significantly higher than that of the control group [Figure 3b]. The infarct sizes of DM2+HTN-Ole40 and DM2+HTN-Ole60, but not DM2+HTN-Ole20, groups were significantly lower than that of the DM2+HTN-Veh group [Figure 3b]. There was significant difference between the infarct size of DM2+HTN-Ole60 and DM2+HTN-Ole20 groups; however, there was no significant difference between the infarct sizes of DM2+HTN-Ole20 and DM2+HTN-Ole40 or DM2+HTN-Ole40 and DM2+HTN-Ole60 groups [Figure 3b].
Biochemical Assay
Serum level of MDA of DM2+HTN-Veh group was significantly higher than that of the control group [Table 1]. However, serum levels of MDA of oleuropein-treated groups (DM2+HTN-Ole20, DM2+HTN-Ole40, and DM2+HTN-Ole60) were significantly lower than that of the DM2+HTN-Veh group [Table 1]. Serum levels of MDA of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups were significantly lower than that of DM2+HTN-Ole20, but there was no significant difference between those of DM2+ HTN-Ole40 and DM2+HTN-Ole60 groups [Table 1]. Erythrocyte level of SOD of DM2+HTN-Veh group was significantly lower than that of the control group [Table 1]. However, erythrocyte levels of SOD of DM2+HTN-Ole40 and DM2+HTN-Ole60, but not that of DM2+HTN-Ole20, groups were significantly higher than that of the DM2+HTN-Veh group [Table 1]. Erythrocyte levels of SOD of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups were significantly lower than that of DM2+HTN-Ole20, but there was no significant difference between those of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups [Table 1]. The concentration of CK-MB of DM2+HTN-Veh group was significantly higher than that of the control group [Table 1]. The concentration of CK-MB of DM2+HTN-Ole40 and DM2+HTN-Ole60, but not DM2+HTN-Ole20, groups were significantly lower than that of the DM2+HTN-Veh group [Table 1]. The concentrations of CK-MB of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups were significantly lower than that of DM2+HTN-Ole20, but there was no significant difference between those of DM2+HTN-Ole40 and DM2+HTN-Ole60 groups [Table 1].
Discussion
The present study showed that 4-week administration of oleuropein to rats with simultaneous renal hypertension and type 2 diabetes demonstrated antihypertensive, antidiabetic, and cardioprotective effects characterized by decreased blood pressure and FBG, improved contractility, and decreased cardiac damages. The study also shows that such beneficial effects might be related to oleuropein's antioxidant activity.
The findings of the study indicate that the present model of simultaneous renal hypertension and type 2 diabetes was associated with increased SBP and FBG, and impaired cardiac function. These findings are similar to those of our previous[13] and others’[14,15] reports.
In the present study, oleuropein prevented the increase of blood pressure in rats with simultaneous renal hypertension and type 2 diabetes. This finding is in agreement with previous reports that olive leaves extract or oleuropein decreased blood pressure.[16,17] The antihypertensive effects of oleuropein have been attributed to the inhibition of angiotensin-converting enzyme,[17] calcium channel blockade, direct vasodilator and diuretic,[5,17] and antioxidant activities.[18] The administration of oleuropein in the present study was also associated with increased antioxidant activity characterized by increased erythrocyte levels of SOD, which may partly explain oleuropein's antihypertensive activity.
Oleuropein, at the doses administered, decreased FBG in rats with simultaneous renal hypertension and type 2 diabetes. Such a finding is in agreement with earlier reports that olive leaf extract[10,19] or oleuropein[9,20] reduced blood glucose in experimental models of diabetes. Blood glucose-lowering activity of olive leaves or oleuropein has been attributed to the regulation of glucose transporter-4 (GLUT4).[20] Potentiation of glucose-induced insulin release and/or increased peripheral uptake of glucose,[21] and a modulatory role on redox homeostasis and maintenance of beta-cell physiology against oxidative stress.[20] The blood glucose lowering effects of oleuropein was associated with the reduction of indices of oxidative stress; therefore, the observed antioxidant activity of oleuropein may partly be responsible for such a finding.
The present study showed that oleuropein increased indices of cardiac contraction (+dp/dt) and relaxation (−dp/dt), and decreased coronary resistance during preischemia period of isolated heart studies. These findings suggest that oleuropein has demonstrated cardioprotective effects. These findings are in agreement with those of previous reports that oleuropein showed cardioprotective effects in isolated hearts from rabbits with doxorubicin-induced cardiotoxicity[8] and hypercholesterolemia.[7] The conclusion that oleuropein had cardioprotective effects is strengthened by the findings that oleuropein decreased myocardial infarct size and coronary effluent CK-MB in the present study. This conclusion receives support from previous studies showing similar effects by oleuropein in isolated hearts from rabbits with doxorubicin-induced cardiotoxicity[11] and hypercholesterolemia.[7] The cardioprotection offered by oleuropein has been attributed to its ability to prevent life threatening and malignant arrhythmias such as ventricular fibrillation, and anti-inflammatory.[22] and antioxidant[1] activities. Therefore, increased antioxidant activity of oleuropein, characterized by decreased serum MDA and increased erythrocyte SOD levels, might partly explain its cardioprotective effects in the present study.
The findings of the present study indicate that no clear pattern of dose-dependency in effects of oleuropein. This might be due to the way that the doses of oleuropein were selected, or due to the nature of measured parameters. Earlier studies had shown that oleuropein at 20 mg/kg/day for 3[7] or 16 weeks[20] had antioxidative stress activities. We used these studies as a basis to select 20 mg/kg/day as the lowest dose and to examine the effects of oleuropein at a wider dose range; the two higher doses (40 and 60 mg/kg/day) were selected as well. The measured parameters are varied, and this diversity precludes any expectation that oleuropein shows a clear pattern of dose-dependency for all parameters.
Taken together, the findings of the study suggest that oleuropein prevented diabetes-induced cardiomyopathy. This finding in agreement with an earlier study[23] reporting that coronary heart disease was not a major indicator of mortality in men living in areas where Mediterranean diet constituted the major portions of their diet. It also receives support from an earlier finding[24] that the occurrence of fatal and nonfatal myocardial infarction was less in areas consuming Mediterranean diet. This is because olive products constitute a major portion of the Mediterranean diet.[25]
In conclusion, the findings of the present study show that oleuropein decreased blood pressure and blood glucose in rats with simultaneous renal hypertension and type 2 diabetes, and offered cardioprotection in Langendorff studies on the hearts from such rats. They also show that such effects might be partly related to oleuropein's antioxidant activity.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Czerwińska M, Kiss AK, Naruszewicz M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012;131:940–7. [Google Scholar]
- 2.Boccardi V, Esposito A, Rizzo MR, Marfella R, Barbieri M, Paolisso G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS One. 2013;8:e62781. doi: 10.1371/journal.pone.0062781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Morittu VM, et al. Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J Pharmacol Exp Ther. 2011;339:859–69. doi: 10.1124/jpet.111.182808. [DOI] [PubMed] [Google Scholar]
- 4.Khayyal MT, el-Ghazaly MA, Abdallah DM, Nassar NN, Okpanyi SN, Kreuter MH. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittelforschung. 2002;52:797–802. doi: 10.1055/s-0031-1299970. [DOI] [PubMed] [Google Scholar]
- 5.Zarzuelo A, Duarte J, Jiménez J, González M, Utrilla MP. Vasodilator effect of olive leaf. Planta Med. 1991;57:417–9. doi: 10.1055/s-2006-960138. [DOI] [PubMed] [Google Scholar]
- 6.Manna C, Migliardi V, Golino P, Scognamiglio A, Galletti P, Chiariello M, et al. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J Nutr Biochem. 2004;15:461–6. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Andreadou I, Iliodromitis EK, Mikros E, Constantinou M, Agalias A, Magiatis P. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J Nutr. 2006;136:2213–9. doi: 10.1093/jn/136.8.2213. [DOI] [PubMed] [Google Scholar]
- 8.Andreadou I, Papaefthimiou M, Zira A, Constantinou M, Skaltsounis L, Iliodromitis EK, et al. Oleuropein restores the pathological metabolic pathways induced by doxorubicin's cardiotoxicity. An NMR based metabonomic study. J Mol Cell Cardiol. 2007;42:S168–9. [Google Scholar]
- 9.Jemai H, El Feki A, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. 2009;57:8798–804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- 10.Eidi A, Eidi M, Darzi R. Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytother Res. 2009;23:347–50. doi: 10.1002/ptr.2629. [DOI] [PubMed] [Google Scholar]
- 11.Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol. 2006;107:285–90. doi: 10.1016/j.jep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Nekooeian A, Mashhoodi T. Solid plexiglass clips to induce reproducible renal hypertension in the rat. Indian J Pharmacol. 2007;39:25–6. [Google Scholar]
- 13.Nekooeian AA, Khalili A, Khosravi MB. Effects of short-term renovascular hypertension and type 2 diabetes on cardiac functions in rats. Iran J Med Sci. 2014;39:51–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Di Filippo C, Marfella R, Cuzzocrea S, Piegari E, Petronella P, Giugliano D, et al. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes. 2005;54:803–10. doi: 10.2337/diabetes.54.3.803. [DOI] [PubMed] [Google Scholar]
- 15.Kain V, Kumar S, Puranik AS, Sitasawad SL. Azelnidipine protects myocardium in hyperglycemia-induced cardiac damage. Cardiovasc Diabetol. 2010;9:82. doi: 10.1186/1475-2840-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekooeian AA, Dehghani GA, Mostafavi H, Khalili A. The effect of hydroalcoholic extract of olive leaves on blood pressure in rat model of two-kidney, one-clip goldblatt hypertension. Int Cardiovasc Res J. 2011;5:1–6. [Google Scholar]
- 17.Kiss AK, Mańk M, Melzig MF. Dual inhibition of metallopeptidases ACE and NEP by extracts, and iridoids from Ligustrum vulgare L. J Ethnopharmacol. 2008;120:220–5. doi: 10.1016/j.jep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Oliveras-López MJ, Molina JJ, Mir MV, Rey EF, Martín F, de la Serrana HL. Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalized elderly humans. Arch Gerontol Geriatr. 2013;57:234–42. doi: 10.1016/j.archger.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Kadan S, Saad B, Sasson Y, Zaid H. In Vitro evaluations of cytotoxicity of eight antidiabetic medicinal plants and their effect on GLUT4 translocation. Evid Based Complement Alternat Med 2013. 2013:549345. doi: 10.1155/2013/549345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Azzawie HF, Alhamdani MS. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006;78:1371–7. doi: 10.1016/j.lfs.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez M, Zarzuelo A, Gamez MJ, Utrilla MP, Jimenez J, Osuna I. Hypoglycemic activity of olive leaf. Planta Med. 1992;58:513–5. doi: 10.1055/s-2006-961538. [DOI] [PubMed] [Google Scholar]
- 22.Dell’Agli M1, Di Lorenzo C, Badea M, Sangiovanni E, Dima L, Bosisio E, et al. Plant food supplements with anti-inflammatory properties: A systematic review (I) Crit Rev Food Sci Nutr. 2013;53:403–13. doi: 10.1080/10408398.2012.682123. [DOI] [PubMed] [Google Scholar]
- 23.Menotti A, Blackburn H, Kromhout D, Nissinen A, Fidanza F, Giampaoli S, et al. Changes in population cholesterol levels and coronary heart disease deaths in seven countries. Eur Heart J. 1997;18:566–71. doi: 10.1093/oxfordjournals.eurheartj.a015298. [DOI] [PubMed] [Google Scholar]
- 24.Keys A. Cambridge: Harvard University Press; 1990. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; p. 392. [Google Scholar]
- 25.Huang CL, Sumpio BE. Olive oil, the mediterranean diet, and cardiovascular health. J Am Coll Surg. 2008;207:407–16. doi: 10.1016/j.jamcollsurg.2008.02.018. [DOI] [PubMed] [Google Scholar]


