Abstract
Objectives:
Application of vitamin K to the skin has been used for suppression of pigmentation and resolution of bruising. However, in rats, no study was reported on its effect regarding wound healing. Thus, the present study was designed to examine the healing effects of creams prepared from vitamin K1 on full-thickness wound in rats.
Materials and Methods:
For inducing full-thickness wound in rats, the excisional wound model was used. Five groups consisting of 8 rats each were used. Vitamin K cream (1% and 2%, w/w) was prepared in eucerin base and applied on the wound once a day until complete healing had occurred. Healing was defined by decreased wound margin (wound contraction), re-epithelialization, tensile strength and hydroxyproline content. Histopathological examination was also done.
Results:
The effects produced by the topical vitamin K showed significant (P < 0.01) healing when compared with control group in parameters such as wound contraction, epithelialization period, hydroxyproline content and tensile strength. Histopathological studies also showed improvement with vitamin K.
Conclusions:
Topical vitamin K demonstrates wound healing potential in full-thickness wound model.
KEY WORDS: Excision, rat, vitamin K1, wound healing
Introduction
Wound is defined as a loss or break of cellular, anatomic or functional continuity of living tissues. Wound healing is an essential process involved in repair and regeneration of tissue structure and function disrupted by various biological, physical and chemical insults. Coagulation, epithelization, granulation, collegenation and tissue remodeling are the phases of wound healing.[1] Coagulation is the immediate response to initial injury. Thrombin generation leads to aggregation of a platelet and fibrin mass that fills and stabilizes the wound by preventing further blood loss. Fibroblasts line along the fibrin matrix and begin to form connective tissue, and the endothelial sprouts begin to revascularize the site, converting the wound into granulation tissue. During this, the epithelial cells migrate and proliferate across the surface of the wound, displacing the scab, until the wound is closed (re-epithelization).
Vitamin K (vitK), an essential micronutrient present in low concentration in the blood,[2] is required for the γ-carboxylation of specific glutamyl residues in several hepatic and extra-hepatic proteins.[3] These proteins are present virtually in every tissue and is important to cellular processes including proliferation,[4] bone mineralization,[5] arterial calcification,[6] apoptosis, phagocytosis,[7,8] growth control, chemotaxis[9] and signal transduction.[10] Furthermore, vitK also has redox properties and has been shown to alter cellular metabolism in a manner which might confer anti-inflammatory properties.[11]
Topical application of vitK has been used for the prevention of vascular manifestations of aging, suppression of pigmentation and for the resolution of bruising. VitK may facilitate the removal of extravascular blood from the skin, which accounts for its effectiveness in hastening the clearing of bruising and reducing its severity after laser treatment.[12]
In view of the above mentioned actions, it was thought worthwhile to investigate the action of vitK in wound healing. Hence, the aim of this study was to evaluate the healing effect of vitK on full thickness model of wound in rats.
Materials and Methods
Preparation of formulation
Vitamin K1 (phylloquinone) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). It was mixed with eucerin for preparing 1% and 2% creams (w/w). Phenytoin (positive control) purchased from Darupakkhsh Co., Tehran, Iran was incorporated in eucerin for preparing 1% cream (w/w). The doses of phenytoin and vitK for topical administration were selected based on previous reports[13,14] and pilot experiments in our laboratory.
Animals
Adult male albino rats (150-170 g) were used for the study. They were kept for acclimatization for a period of 7 days before start of the experiment, housed at controlled temperature (22 ± 2°C) and allowed free access to feed and clean drinking water during the period. Testing took place in the middle of the light period of a 12 h light/dark cycle. Animal procedures were in accordance with the guidelines for animal care prepared by Committee on Care and Use of Laboratory Animal resources, National Research Council, USA, and were approved by the Animal Ethics Committee of AJUMS. Every effort was made to minimize the animal suffering and reduce the number of animals used.
Experimental groups
The study was done in 5 groups of 8 rats each (lesioned) i.e., group 1 receiving no treatment, group 2 receiving eucerin as negative control, group 3 receiving 1% vitK cream in eucerin base, group 4 receiving 2% vitK cream and group 5 receiving 1% phenytoin in eucerin base as a standard healing agent.
Excision wound model
The animals were anesthetized with xylazine (20 mg/kg, i.p.) and ketamine (100 mg/kg, i.p.). They were then placed in the prone position and the dorsal midline of the cervical region was sterilized with 0.1% iodine alcohol. An excision wound was inflicted by cutting a 225 mm2 full thickness of skin from a pre-determined shaved area in the dorsal interscapular region. To remove the skin, subcutaneous tissue, fascia, and fleshy panniculus, a scalpel and straight scissors and Adson forceps were used.[15] The wounds were left undressed to the open environment. Eucerin, standard drug (1% phenytoin) and vitK (1 and 2%, w/w) were applied topically once a day to the control group, standard group and treated groups, respectively, till the wound was completely healed.
Measurement of wound contraction
The margin of excision wound was traced by following the progressive changes in wound area planimetrically, beginning on the day of wound healing. The size of wounds was traced on a transparent paper, throughout the monitoring period, which was then shifted to graph paper from which the wound surface area was evaluated.[16] The extent of wound closure was expressed as the ratio of wound area (each day after wound healing process) to the original wound size.
Epithelialization period
It was evaluated by noting the number of days required for the scar to fall off from the wound surface exclusive of leaving a raw wound behind.[17]
Tensile strength
The tensile strength of a wound represents the degree of tissue integrity. The tensile strength increment indicates better wound healing stimulation by the applied drug. At the end of healing period, a strip of repaired tissue measuring 20 × 5 mm was isolated and the tensile strength was measured with an aid of tensiometer. Tensile strength was calculated using the following formula:[15]
Tensile strength = Breaking strength (g)/Cross-sectional area of skin (mm2)
Hydroxyproline estimation
Hydroxyproline is an uncommon amino acid present in the collagen fibers of granulation tissues. Its estimation helps clinically to understand progress rate at which the healing process is undergoes in the connective tissue of the wound. After complete healing, a piece of skin (100 mg) from the healed wound area was collected and analyzed for hydroxyproline content, which is a basic constituent of collagen. Hydroxyproline was measured by the method used by Edwards and his colleague.[18] Tissues were hydrolyzed in 6M HCl for 18 hours at 110°C. Samples were dried in a hot air oven at 60-70°C to constant weight and then washed thrice with distilled water. The acid-free samples were reconstituted in 2.0 ml of acetate-citrate buffer [1.2% sodium acetate trihydrate, 5% citric acid, 12% sodium acetate, 3.4% sodium hydroxide. (pH 6.0)]. 500 ml of 0.05 M chloramine-T was added to 1 ml of sample, after which the samples were incubated for 20 minutes at room temperature, followed by the addition of 0.5 ml of 15% perchloric acid and 15% 4-dimethyl aminobenzaldehydein 1-propanol. After incubation for 15 minutes at 60°C, each sample was transferred to a microtiter plate and absorbance was read at 550 nm using ultraviolet-visible (UV/Vis) spectrophotometer. Hydroxyproline concentrations of the unknown samples were calculated from a linear standard curve and presented as μg/mg dry tissue weight.
Histopathological examination
On the 7th day of treatment, two samples each were prepared from control, standard and treated groups for histopathological examination. Tissue samples from the wound area were fixed in 10% formalin then embedded in paraffin and 5 μm slices was prepared using microtome slicer. Samples were stained by hematoxylin-eosin and were observed for the changes such as fibroblast proliferation, collagen formation and angiogenesis.
Statistical analysis
All experimental results are given as the mean ± SEM. The statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test unless otherwise stated. A two-way ANOVA followed by Bonferroni's test was carried out for the time-course effect of vitK. Data analysis was performed using the Prism 5.0 (San Diego, CA, USA) statistical package program. P values less than 0.05 were considered to be statistically significant.
Results
There was no significant difference in wound contraction between the non-treatment group and eucerin-treated group throughout the study period. Application of vitK improved wound healing at all times beginning on the 3rd day with statistical significance (P < 0.05) achieved from 7th day as compared with the eucerin-treated group [Figure 1]. However, no significant difference was observed between vitK 1% and 2%. Furthermore, results showed that phenytoin treatment was significant from the 10th day onwards.
Figure 1.
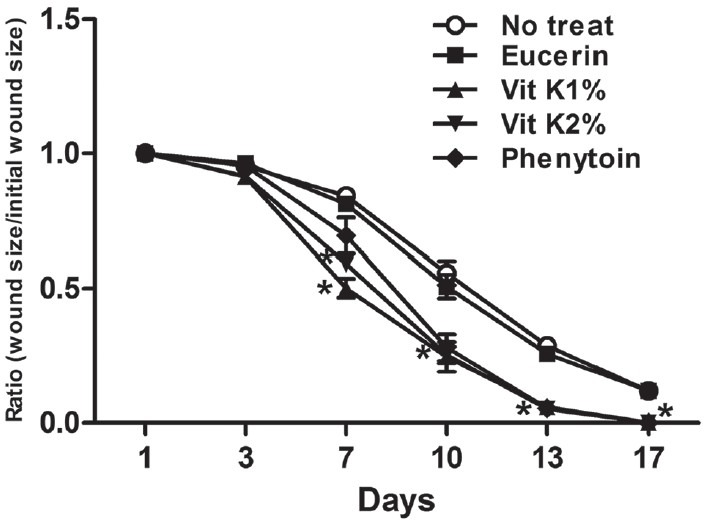
Effect of topical application of vitamin K1 (vitK) on wound healing (wound contraction). Time course of healing effect of vitK on contraction of wound. Each group represents the mean of 8 animals, and the vertical lines indicate the SEM. *P < 0.05 compared with eucerin-treated group
The time taken for complete healing i.e., epithelialization time in eucerin group (21.7 ± 0.5 days) was comparable to non-treated group (22 ± 0.8 days). A statistical significant decrease in epithelialization time in vitK 1%, vitK 2% (P < 0.01) and 1% phenytoin-treated (P < 0.05) groups (14.7 ± 0.8, 15 ± 0.7 and 16.7 ± 1.5 days, respectively) was observed as compared with eucerin-treated group.
The tensile strength of the group treated with eucerin (987 ± 33 g/cm2) was comparable with the non-treatment group (980 ± 36 g/cm2). Tensile strength for vitK 1% (1200 ± 40 g/cm2), vitK 2% (1170 ± 34 g/cm2) and 1% phenytoin (1229 ± 60 g/cm2) at the end of healing period was found to be significantly higher (P < 0.05) as compared to eucerin-treated rats. Moreover, an increase in hydroxyproline content in vitK 1%, vitK 2% and 1% phenytoin-treated (P < 0.05) groups (1100 ± 40, 1074 ± 34 and 1239 ± 38 μg/g tissue, respectively) was observed to be significantly higher (P < 0.05) than eucerin and non-treatment groups (878 ± 18 and 868 ± 3 μg/g tissue, respectively).
Histopathological examination
Promotion of wound healing by vitK was confirmed by histological investigation. Fibroblast cells, collagen fibers and blood vessels are prominently present in phenytoin and vitK-treated group as compared with eucerin-treated group [Figure 2a–d].
Figure 2.
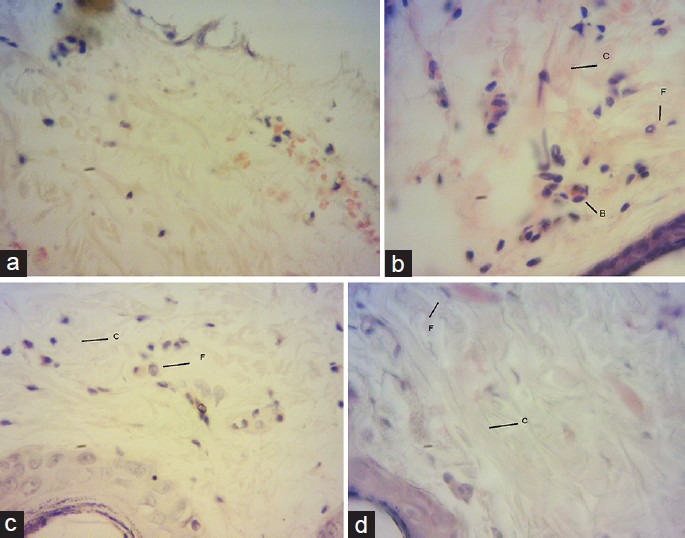
Histopathological characteristics of skin on the 7th day treatment. Eucerin-treated group shows poor fibroblast cells and blood vessels in wound (a). Phenytoin, vitK 1% and 2% treated groups shows increased fibroblasts cells (F), collagen fibers (C) and blood vessels (B) in wound (b-d respectively). Sections were stained with hematoxylin and eosin, magnification ×40
Discussion
Wound healing is a complex and dynamic process of restoring structure in damaged tissues as closely as possible to its normal state. Skin is a complex tissue and thus a ‘full thickness’ wound results in damage to many structures including: the epidermal keratinocyte layer, the basement membrane that underlies the epidermis, and the dermis which is an intricate structure that consists of fibroblasts, extracellular matrix, nerves, and blood and lymphatic vessels. In addition, a wound also causes damage at the level of individual cells.[19] The present study for the first time demonstrated that vitK1 may promote wound healing activity in rats, probably due to its ability to significantly increase the rate of wound contraction, enhancement of epithelialization period, formation of fibroblasts cells, collagen fibers and blood vessels, and increment in hydroxyproline content. The results indicate that the topical application of vit K1 hastened the process of wound healing significantly in the full thickness excision model.
The mechanism of this action could be attributed to various reasons. It has been shown that the two vitK-dependent proteins, Gas6 (Growth Arrest Specific gene 6) and protein S, are major regulators for cell proliferation and cell growth.[20] In our study, vitK increased cellular proliferation and collagen synthesis at the wound site as evidenced by increase in total hydroxyproline content of granulation tissues. Furthermore, the observed increase in tensile strength by vitK could be due to increased collagen synthesis as well as its proper deposition and alignment. Sharaev et al. (1976) showed that in rats with avitaminosis K, the collagen content in the skin was reduced and the rate of hydrolysis of collagen increased.[21] Hence, the healing effect of topical vitK could be attributed to its effect on collagen stability. Angiogenesis, which is integral to successful wound repair, refers to the in growth of new vessels into a wound from the surrounding tissue.[22] Wang et al. (2005) showed that the blood coagulation system can be a tool to coordinate angiogenesis and vascular development.[23] Since vitK has a well-known effect on γ-carboxylation modification of certain coagulation factors, the wound healing action of vitK may be due to its effect on blood coagulation system.
Molecular oxygen plays a central role in the pathogenesis and therapy of chronic wounds. Overproduction of reactive oxygen species (ROS) results in oxidative stress thereby causing cytotoxicity and delayed wound healing. Therefore, elimination of ROS could be an important strategy in healing of chronic wounds.[24] Several studies have showed that vitK is considered as a potent antioxidant.[25] Therefore, vitK could also improve the wound healing based on its antioxidant properties. However, further studies to elucidate the exact mechanism of action of vitamin K in wound healing will be required for confirmation.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Ayello EA. What does the wound say? Why determining etiology is essential for appropriate wound care. Adv Skin Wound Care. 2005;18:98–109. doi: 10.1097/00129334-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Hodges SJ, Pilkington MJ, Shearer MJ, Bitensky L, Chayen J. Age-related changes in the circulating levels of congeners of vitamin K2, menaquinone-7 and menaquinone-8. Clin Sci. 1990;78:63–6. doi: 10.1042/cs0780063. [DOI] [PubMed] [Google Scholar]
- 3.Vermeer C. g-Carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990;266:625–36. doi: 10.1042/bj2660625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goruppi S, Ruaro E, Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12:471–80. [PubMed] [Google Scholar]
- 5.Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2009;297:1358–67. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- 6.Fodor D, Albu A, Poantă L, Porojan M. Vitamin K and vascular calcifications. Acta Physiol Hung. 2010;97:256–66. doi: 10.1556/APhysiol.97.2010.3.2. [DOI] [PubMed] [Google Scholar]
- 7.Hall MO, Obin MS, Prieto AL, Burgess BL, Abrams TA. Gas6 binding to photoreceptor outer segments requires gammacarboxyglutamic acid (Gla) and Ca (2+) and is required for OS phagocytosis by RPE cells in vitro. Exp Eye Res. 2002;75:391–400. [PubMed] [Google Scholar]
- 8.Li R, Chen J, Hammonds G, Phillips H, Armanini M, Wood P, et al. Identification of Gas6 as a growth factor for human Schwann cells. J Neurosci. 1996;16:2012–9. doi: 10.1523/JNEUROSCI.16-06-02012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridell YW, Villa JR, Attar EC, Liu ET. GAS6 induces Axl-mediated chemotaxis of vascular smooth muscle cells. J Biol Chem. 1998;273:7123–6. doi: 10.1074/jbc.273.12.7123. [DOI] [PubMed] [Google Scholar]
- 10.Varnum BC, Young C, Elliott G, Garcia A, Bartley TD, Fridell YW, et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth- arrest-specific gene 6. Nature (Lond) 1995;373:623–6. doi: 10.1038/373623a0. [DOI] [PubMed] [Google Scholar]
- 11.Hanck A, Weiser H. Physiological and pharmacological effects of vitamin K. Int J Vitam Nutr Res. 1983;24:155–70. [PubMed] [Google Scholar]
- 12.Elson ML, Nacht S. Treatment of a periorbital hyperpigmentation with topical vitamin K/vitamin A. Cosmet Dermatol. 1999;12:27–127. [Google Scholar]
- 13.Lou W, Quintana A, Geronemus R, Grossman M. Effects of topicalvitamin K and retinol on laser-induced purpura on nonlesionalskin. Dermatol Surg. 1999;25:942–4. doi: 10.1046/j.1524-4725.1999.99145.x. [DOI] [PubMed] [Google Scholar]
- 14.Elson M. Topical phytonadione (vitamin K1) in the treatment of actinic and traumatic purpura. Cosmet Dermatol. 1995;8:25–7. [Google Scholar]
- 15.Saha K, Mukherjee PK, Das J, Pal M, Saha BP. Wound healing activity of Leucas lavandulaefolia Rees. J Ethnopharmacol. 1997;56:139–44. doi: 10.1016/s0378-8741(97)01522-5. [DOI] [PubMed] [Google Scholar]
- 16.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid-ul-Zafar Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107:161–3. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Rashed AN, Afifi FU, Disi AM. Simple evaluation of wound healing activity of a crude extract of Portuloca oleracea Linn. (growing in Jordan) in Mus musculus JVI-1. J Ethanopharmacol. 2003;88:131–6. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 18.Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–7. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 19.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 20.Gely-Pernot A, Coronas V, Harnois T, Prestoz L, Mandairon N, Didier A, et al. An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche. Stem Cells. 2012;30:719–31. doi: 10.1002/stem.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharaev PN, Bogdanov NG, Yamaldinov RN. Effect of body vitamin K level on collagen metabolism in the skin. B Exp Biol Med. 1976;81:822–3. [PubMed] [Google Scholar]
- 22.Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg. 2005;39:293–306. doi: 10.1177/153857440503900401. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhen Y, Shi Y, Chen J, Zhang C, Wang X, et al. Vitamin k epoxide reductase: A protein involved in angiogenesis. Mol Cancer Res. 2005;3:317–23. doi: 10.1158/1541-7786.MCR-04-0221. [DOI] [PubMed] [Google Scholar]
- 24.Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacol Physiol. 2011;24:113–26. doi: 10.1159/000322643. [DOI] [PubMed] [Google Scholar]
- 25.Vervoort LM, Ronden JE, Thijssen HH. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem Pharmacol. 1997;54:871–6. doi: 10.1016/s0006-2952(97)00254-2. [DOI] [PubMed] [Google Scholar]


