Abstract
Objectives:
To investigate the antiplatelet activity of alpha-lipoic acid (α-LA) and dihydroquercetin (DHQ).
Materials and
Methods: Antiplatelet activity of the α-LA and DHQ was evaluated in rich platelet plasma of rat. The platelet aggregation was induced by adenosine diphosphate (ADP) in concentration of 4 × 10-5 M.
Results:
α-LA and DHQ inhibited platelet aggregation in concentration-dependent manner. The antiplatelet activity of α-LA was more pronounced than DHQ. DHQ also increased the antiplatelet activity of α-LA by 1.4 times.
Conclusion:
Combined simultaneous use of α-LA and DHQ possessed the high antiplatelet activity, and DHQ potentiated the activity of α-LA.
KEY WORDS: Alpha-lipoic acid, antioxidants, antiplatelet activity, dihydroquercetin, platelet aggregation
Introduction
Thrombosis is a widespread pathology and can cause serious complications including death.[1] Platelet aggregation plays a critical role in the initiation and pathogenesis of thrombosis.[2] In recent years, there has been an increased resistance to antiplatelet drugs widely used in the treatment of vascular diseases.[3] Thus, effective and less toxic drugs are necessary. It has been documented that some natural compounds in the diet can inhibit the platelet activation.[4]
Alpha-lipoic acid (α-LA) is a naturally occurring compound, synthesized in small amounts by plants and animals, including humans. Currently, α-LA is used as a dietary supplement for preventing and treating vascular, neurodegenerative, and endocrine diseases.[5,6] Studies have reported that α-LA inhibit platelet aggregation in vitro and in type I diabetic patients.[7]
Dihydroquercetin (DHQ) is a flavonoid founded in Larix dahurica, Larix sibirica, and Pseudotsuga taxifolia.[8] Despite the fact that dihydroquercetin possesses antioxidative, anti-inflammatory, hepatoprotective, and anticancer activities, it is not widely used.[9,10] Moreoever, antiplatelet activity of DHQ has been observed by Kubatiev et al.[11,12] Thus, the objective of the study was to investigate the antiplatelet activity of α-LA and DHQ and their combined use.
Materials and Methods
Animals
Males Wistar rats weighing 250–300 g were used for the study. The rats were kept in the vivarium (at temperature 22 ± 2°C, relative humidity of 55 ± 15%, 12/12 h light-dark cycle). Food for conventional laboratory small rodents (CJSC «Assortment-AGRO», Russia) and water was freely available. The food contained antioxidants: Vitamins A (30,000 IU/kg), E (130,000 IU/kg), C (78.7 mg/kg), and selenium (0.07 mg/kg). Rats were fasted for 12 hours preceding the experiment, but had access to water. Permission for experiments was received from the local ethics committee of the E.D.Goldberg Institute of Pharmacology, Siberian Branch, Russian Academy of Medical Sciences (Tomsk, Russia). All experimental procedures with animals were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (March 18, 1986; Strasburg; ETS 123) and the Requirement of Public Health Ministry of Russian Federation Order No. 267 (Moscow, 2003).
Reagents
Diethyl ether (“Kuzbassorgchem” Ltd, Russia), DHQ (“Ametis” Ltd, Russia), α-LA (“Marbiofarm” Ltd, Russia), dimethylsulfoxide (“Marbiofarm” Ltd, Russia), Na3C6H5O7 (“Sigma” Co Ltd, the USA), adenosine diphosphate (ADP; “Sigma” Co Ltd, the USA) were used.
Preparation of Platelet-Rich and Platelet-Poor Plasma
Wistar rats were anesthetized with diethyl ether, and blood was collected from the common carotid artery. Blood was anticoagulated with sodium citrate (3.8%; 1:9, v/v) and centrifuged at 400 g for 10 min at room temperature, platelet-rich plasma (PRP) was obtained from the resulting supernatant. The residue was centrifuged at 1,500 g for another 15 min to produce platelet-poor plasma (PPP).[13] PRP was diluted with PPP to a platelet concentration of 4 × 105 cells/ml before using in the aggregation assays.
Platelet Aggregation Assay In Vitro
α-LA and DHQ were dissolved in dimethylsulfoxide (DMSO) and added to platelet suspension correspondingly to the final concentration of 0.3, 0.6, 1.2, and 0.6, 2.5, 5.0 mM 2 min before addition of ADP. Equivolume quantity of DMSO (the final concentration of 0.6 mM) was added in the control samples. Aggregation was stimulated by ADP at a final concentration of 4 × 10−5 M and measured with a platelet aggregometer (AT-2, “NPF” Ltd, Russia).[14]
Statistical Analysis
Data were expressed as the means ± standard error of the mean (SEM). P < 0.05 was considered statistically significant. Comparisons between groups were performed by Mann-Whitney U test. IC50 was determined by probit analysis. Statistical analysis was performed using Statistica 6.0 (Stat Soft Inc., USA).
Results
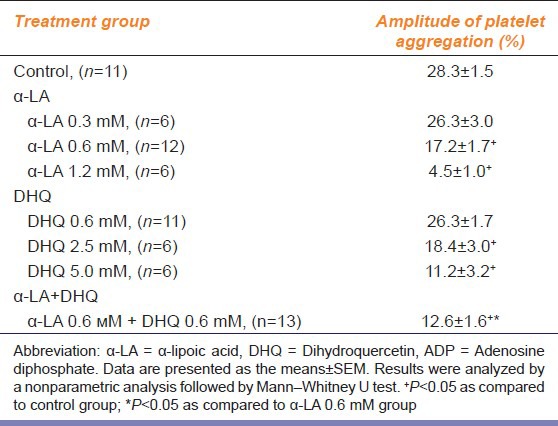
α-LA and DHQ inhibited platelet aggregation induced by ADP in a concentration-dependent manner in vitro [Table 1]. However, incubation of PRP with α-LA (0.3 mM) and DHQ (0.6 mM) did not change the aggregation amplitude and was comparable to control groups. Aggregation amplitudes in α-LA group (0.6 mM) and DHQ group (2.5 mM) were less than control group by 1.6 and 1.5 times, respectively. The antiplatelet activity of α-LA at 1.2 mM was more significant (P < 0.05) and pronounced than that of DHQ at 5.0 mM [Table 1]. While combination of α-LA and DHQ in equal concentration of 0.6 mM reduced the aggregation amplitude as compared to the control group by 2.2 times (P < 0.05).
Table 1.
Effect of α-LA and DHQ on ADP-induced platelet aggregation
Discussion
ADP contributes to platelet activation occurring both during protective hemostasis (formation of the initial platelet monolayer) and during formation of occlusive platelet-rich thrombi.[15] Two G protein-coupled receptors are known to be largely responsible for platelet responses to ADP: P2Y1, which activates phospholipase C and mobilization of Ca2+, and P2Y12, which suppresses cAMP formation so that activation can occur.[16]
Recent studies showed that α-LA and DHQ possessed nonselective antiplatelet activity.[7,12] α-LA (0.3-0.8 mM) dose-dependently inhibited platelet aggregation induced by collagen, ADP, or arachidonic acid.[7] Investigators link the antiplatelet activity of α-LA with an elevation of cyclic adenosine monophosphate (cAMP) formation, involving the subsequent inhibition of Thromboxane A2 (TXA2), cyclooxygenase-1, protein kinase Cα-mediated pathways, peroxisome proliferator-activated receptors (PPARγ), Ca2+ mobilization and inhibition of reactive oxygen species formation, and the increase of platelet membrane fluidity.[7,17]
DHQ (1-5 mM) is able to inhibit platelet aggregation induced by ADP and thrombin. Kubatiev et al., showed that DHQ produced the decrease in the levels of both base and ADP- or thrombin-induced cytoplasmic Ca2+ levels and elevated cAMP and cyclic guanosine monophosphate (cGMP) contents in native and thrombin-activated platelets.[11,12]
The present study demonstrates the inhibition of platelet aggregation by combined use of α-LA and DHQ in vitro. Our study showed that α-LA and DHQ possess antiplatelet activity in the concentration range reported by other authors.[7,11,12,16] α-LA had a higher antiplatelet activity than DHQ. From the data presented in the Table 1, IC50 values of 0.69 ± 0.03 and 3.76 ± 0.42 mM for α-LA and DHQ were calculated. Secondly, DHQ also increased the antiplatelet activity of α-LA by 1.4 times [Table 1]. However, the mechanisms underlying DHQ-mediated potentiation of platelet aggregation needs further research.
On the basis of the facts mentioned above, α-LA and DHQ have some common mechanisms of antiplatelet activity (via cAMP, and its influence on Ca2+ mobilization).
Moreover, antiplatelet effect of combination of the effect being observed from α-LA and DHQ use was greater than the sum of α-LA and DHQ effects then used separately. Enhanced antiplatelet activity of α-LA and DHQ simultaneous use may be determined by the ability of DHQ to raise the level of cGMP and as a result increase phosphodiesterase type 3 (PDE3) activity.
PDE3 has a relatively high affinity for both cAMP and cGMP, but a much lower efficacy of hydrolysis for cGMP, behaving essentially as a pure cAMP PDE; it has also been referred to as cGMP-inhibited cAMP PDE because cGMP may competitively inhibit ρAMP hydrolysis.[18,19]
In conclusion, our study demonstrated that α-LA and DHQ possessed the high antiplatelet activity and DHQ increased the activity of α-LA.
Footnotes
Source of Support: Nill
Conflict of Interest: No
References
- 1.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44:62–9. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston FE, Greaves M. Thrombotic vascular disease. Br J Hosp Med. 1985;34:46–50. [PubMed] [Google Scholar]
- 3.Kumar A, Kao J. Platelet resistance to antiplatelet drugs. Recent Pat Cardiovasc Drug Discov. 2009;4:98–108. doi: 10.2174/157489009788452959. [DOI] [PubMed] [Google Scholar]
- 4.Gadi D, Bnouham M, Aziz M, Ziyyat A, Legssyer A, Bruel A, et al. Flavonoids purified from parsley inhibit human blood platelet aggregation and adhesion to collagen under flow. J Complement Integr Med. 2012;9 doi: 10.1515/1553-3840.1579. >Article 19. [DOI] [PubMed] [Google Scholar]
- 5.Bilska A, Włodek L. Lipoic acid-the drug of the future? Pharmacol Rep. 2005;57:570–7. [PubMed] [Google Scholar]
- 6.Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic acid. Front Pharmacol. 2011;2:69. doi: 10.3389/fphar.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai YS, Shih CY, Huang YF, Chou TC. Antiplatelet activity of alpha-lipoic acid. J Agric Food Chem. 2010;58:8596–603. doi: 10.1021/jf101518p. [DOI] [PubMed] [Google Scholar]
- 8.Khutoryansky VA, Bazhenov BN, Saybotalov MY, Tyukavkina NA. A method of producing dihydroquercetin. Patent of the Russian Federation 2091076. 1997 [Google Scholar]
- 9.Lee CW, Park NH, Kim JW, Um BH, Shpatov AV, Shults EE, et al. Study of skin anti-ageing and anti-inflammatory effects of dihydroquercetin, natural triterpenoids, and their synthetic derivatives. Bioorg Khim. 2012;38:374–81. doi: 10.1134/s1068162012030028. [DOI] [PubMed] [Google Scholar]
- 10.Oi N, Chen H, Ok Kim M, Lubet RA, Bode AM, Dong Z. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer Prev Res (Phila) 2012;5:1103–14. doi: 10.1158/1940-6207.CAPR-11-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubatiev AA, Yadigarova ZT, Rud’ko IA, Bykov VA, Tyukavkina NA. Effect of diquertin on platelet content of cyclic nucleotides. Bull Exp Biol Med. 1999;128:890–91. [Google Scholar]
- 12.Kubatiev AA, Yadigarova ZT, Rud’ko IA, Tyukavkina NA, Bykov VA. Diquertin suppresses ADP- and thrombin-induced accumulation of cytoplasmic calcium in human thrombocytes. Pharm Chem J. 1999;33:629–30. [Google Scholar]
- 13.Aster RH, Enright SE. A platelet and granulocyte membrane defect in paroxysmal nocturnal hemoglobinuria: Usefulness for the detection of platelet antibodies. J Clin Invest. 1969;48:1199–210. doi: 10.1172/JCI106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–9. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 15.Jennings LK. Mechanisms of platelet activation: Need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–57. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 16.Woulfe D, Yang J, Brass L. ADP and platelets: The end of the beginning. J Clin Invest. 2001;107:1503–5. doi: 10.1172/JCI13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC, Shih CY, Chen YT. Inhibitory effect of α-lipoic acid on platelet aggregation is mediated by PPARs. J Agric Food Chem. 2011;59:3050–9. doi: 10.1021/jf103940u. [DOI] [PubMed] [Google Scholar]
- 18.Grant PG, Colman RW. Purification and characterization of a human platelet cyclic nucleotide phosphodiesterase. Biochemistry. 1984;23:1801–7. doi: 10.1021/bi00303a034. [DOI] [PubMed] [Google Scholar]
- 19.Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: Phosphodiesterase inhibitors. Br J Clin Pharmacol. 2011;72:634–46. doi: 10.1111/j.1365-2125.2011.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


