Abstract
Donor factors influence hepatitis C virus (HCV) disease severity in liver transplant (LT) recipients. Living donors, because they are typically young and have short cold ischemic times, may be advantageous for HCV-infected patients. Among HCV-infected patients in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) surviving >90 days and followed for a median 4.7 years, advanced fibrosis (Ishak stage ≥3) and graft loss were determined. The 5-year cumulative risk of advanced fibrosis was 44% and 37% in living donor LT (LDLT) and deceased donor LT (DDLT) patients (P = 0.16), respectively. Aspartate aminotransferase (AST) activity at LT (hazard ratio [HR] = 1.38 for doubling of AST, P = 0.005) and biliary strictures (HR = 2.68, P = 0.0001) were associated with advanced fibrosis, but LDLT was not (HR = 1.11, 95% confidence interval [CI] 0.73-1.69, P = 0.63). The 5-year unadjusted patient and graft survival probabilities were 79% and 78% in LDLT, and 77% and 75% in DDLT (P = 0.43 and 0.32), with 27% and 20% of LDLT and DDLT graft losses due to HCV (P = 0.45). Biliary strictures (HR = 2.25, P = 0.0006), creatinine at LT (HR = 1.74 for doubling of creatinine, P = 0.0004), and AST at LT (HR = 1.36 for doubling of AST, P = 0.004) were associated with graft loss, but LDLT was not (HR = 0.76, 95% CI: 0.49-1.18, P = 0.23).
Conclusion
Donor type does not affect the probability of advanced fibrosis or patient and graft survival in HCV-infected recipients. Thus, while LDLT offers the advantage of shorter wait times, there is no apparent benefit for HCV disease progression. Biliary strictures have a negative effect on HCV fibrosis severity and graft survival, and a high AST at LT may be an important predictor of fibrosis risk post-LT.
Liver transplant (LT) recipients with chronic hepatitis C virus (HCV) infection have a lower graft survival than patients with non-HCV indications for transplantation.1 Many of these graft losses are related to recurrent HCV disease. Over the past 2 decades, studies have focused on identifying the recipient, donor, and transplant-related factors that are associated with a high risk of severe and progressive HCV disease. Donor liver quality, older donor age, prolonged warm and cold ischemia times, donation after cardiac death, and donors with high donor risk index have been associated with lower graft survival and higher rates of advanced fibrosis.2 Whether the type of donor—living versus deceased—influences the risk of severity of HCV disease remains controversial, with conflicting reports.3-8
Living donor LT (LDLT) is an important means of expanding the donor pool, and receipt of an LDLT is associated with a decreased wait-list mortality compared with waiting for deceased donor LT (DDLT).9 For wait-list patients with HCV, living donors may provide additional advantages depending on the severity of HCV disease, as living donors are typically younger and cold ischemia time typically shorter than with DDLT. Most single-center studies suggest HCV disease severity among LDLT recipients is worse or similar to DDLT but not superior.3-5,7,10-14 However, these studies were of limited sample size and follow-up of only 1-3 years. Additionally, most studies included the center's early experience with LDLT despite the fact that graft survival is influenced by center experience with LDLT.15 A recent systematic review concluded the recurrence rates were equivalent in LDLT and DDLT recipients, but statistically significant heterogeneity between studies and limited follow-up (1 or 3 years) limits confidence in the findings.16
The Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) is a multicenter study designed to allow comparisons of outcomes between LDLT and DDLT recipients, including severity of HCV-related liver disease. In our previous study, we showed that survival between LDLT and DDLT were similar once centers had sufficient experience with LDLT; however, our ability to evaluate differences in HCV disease severity and predictors of that outcome were limited by short histologic follow-up (median 12 months) and a small number of patients with advanced disease.15 The current study, with a median follow-up of nearly 5 years posttransplant, examines the association between LDLT and HCV disease severity posttransplantation and the factors predictive of advanced disease. We hypothesized that LDLT recipients, because their donors are younger and cold ischemia times shorter than DDLT, would have less severe HCV disease and a lower risk of advanced fibrosis with longer-term follow-up.
Materials and Methods
Study Population
The A2ALL cohort of HCV-infected recipients included adult patients who had a potential living donor evaluated between January 1 1998 and August 31 2009. Among the 513 potentially eligible candidates with pretransplant HCV infection, 138 were excluded from the current analysis (Fig. 1). Fifteen patients had their LT procedure aborted, received deceased donor split liver transplantation, or had domino liver transplant, and 18 patients achieved sustained virologic response pretransplant. Since graft loss due to recurrent HCV is rare in the early post-transplant period, patients whose grafts failed within 90 days posttransplant (n = 32) were excluded, as were the first 20 LDLT cases for each center (n = 73) while experience with the procedure was being developed. The remaining 195 LDLT and 180 whole DDLT recipients were compared.
Fig. 1.
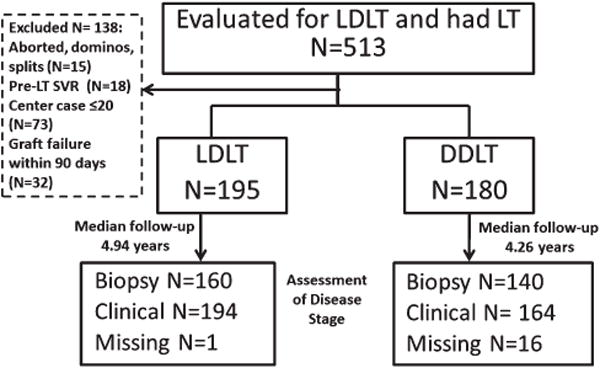
Patient Disposition. A total of 513 patients were evaluated for LDLT during the study period and underwent liver transplantation. A total of 195 LDLT recipients and 180 DDLT recipients met inclusion criteria. Stage of liver disease was assessed using clinical and biopsy criteria. A total of 1 LDLT and 16 DDLT patients did not have clinical or biopsy data to determine stage of disease.
Data were abstracted from the clinical records at each site. For specific variables, including date of death, information from the Scientific Registry of Transplant Recipients (SRTR) was used to augment the available data. Immunosuppression protocols, treatment of rejection, and treatment of recurrent HCV infection were not standardized across centers. The laboratory Model for Endstage Liver Disease (MELD) score was calculated at the time of transplantation, and values were capped at 40. Cold ischemia time was defined as the interval from the donor cross clamp to graft removal from ice. Acute rejection was defined by the requirement for antirejection treatment whether or not rejection was biopsy-proven. Biliary complications included leaks and strictures identified by operative, endoscopic, or radiological studies.
The study protocol included the use of liver biopsies to assess disease severity. The intended frequency was annually with additional “for cause” biopsies done as dictated by center practices. All available liver biopsies were assessed locally using the modified Knodell Hepatitis Activity Index (HAI) to measure necroinflammatory activity and the Ishak score to assess fibrosis. Biopsies were also read centrally by two pathologists who were unaware of patient outcomes or type of transplant. Central reads for Ishak fibrosis scores were performed independently to assess for interobserver variability of fibrosis scores. Central pathology reads were used for analysis. Some patients in the study cohort consented for chart review only and not acquisition of prior biopsy slides (n = 45), and for these patients the local pathology fibrosis stage was used. For patients who achieved sustained viral clearance with antiviral therapy pretreatment biopsies were used to assess disease severity. For patients who did not have a liver biopsy performed within the last year of follow-up, the site investigator completed a standardized supplemental “Advanced Disease” form indicating whether there was clinical, radiologic, or laboratory evidence of advanced liver disease. The intent of this form was to capture patients who had cirrhosis (with or without decompensation), as it was recognized that such patients infrequently had biopsies performed. The Advanced Disease form was designed to be highly specific for the diagnosis of cirrhosis, but patients with early cirrhosis, without radiologic, clinical, or laboratory evidence of portal hypertension or impaired hepatic synthetic function may have been missed.
The primary study endpoint was advanced fibrosis defined by an Ishak fibrosis score (range 0-6) greater than or equal to 3.17 Since the two sets of Ishak scores from central readings reached 96% concordance in determining advanced fibrosis, only one set of central reading was used in analyses. For patients without liver biopsies, the presence or absence of advanced disease (i.e., cirrhosis) was derived from the Advanced Disease form. In analyses of the time to advanced disease, biopsies occurring less than 30 days after transplant were excluded. Secondary endpoints included patient and graft survival, and all causes of graft loss were included; death was regarded as a graft loss.
The study was approved by the Institutional Review Boards and Privacy Boards of each of the nine participating transplant centers and the University of Michigan Data Coordinating Center.
Statistical Analysis
Descriptive statistics included median, interquartile range (IQR), and proportions as appropriate. Comparisons of recipient characteristics, donor characteristics, immunosuppression, and posttransplant complications between LDLT and DDLT recipients were performed using chi-square tests for categorical variables and Wilcoxon rank-sum test for continuous variables. Time to graft and patient survival were estimated by the Kaplan-Meier method, and unadjusted comparisons were made using the logrank test. For advanced fibrosis, analogous methods were used for this interval censored outcome (Turnbull estimate of the distribution function18 and logrank test for interval censored data19). For graft and patient survival, Cox regression was used to adjust for the effects of potentially confounding variables. For advanced fibrosis, an exponential survival model that allowed for interval censored data was used after first confirming that this model provided an appropriate fit. However, when testing time-dependent covariates, Cox regression was used, assuming the advanced fibrosis events occurred on the date of biopsy or clinical confirmation of advanced fibrosis.
Analyses of graft and patient survival were limited to patients with the initial grafts surviving beyond the first 90 days posttransplantation, and comparisons were made between later cases of LDLT patients (LDLT case number >20 at each center) and DDLT patients. The predictor variable of primary interest was donor type (LDLT versus DDLT). Potential variables tested in the models for graft and patient survival and advanced fibrosis were donor age, donor sex, donor race/ethnicity, recipient age, recipient sex, recipient race/ethnicity, recipient body mass index (BMI), recipient weight, recipient diabetes, hepatocellular carcinoma (HCC) diagnosis, pretransplant lab MELD score, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), warm ischemia time, cold ischemia time, pretransplant antiviral therapy, and the immunosuppressive therapy used at 6 months (conditional on survival to 6 months; therapies included tacrolimus, cyclosporine, steroid, sirolimus, and mycophenolate mofetil). HCV genotypes were not routinely collected on A2ALL participants. Pretransplant HCV viral load data were only available in 56 patients. Donor age was evaluated as a continuous variable and by using specific cutoffs (e.g., ≤50 versus >50). The results were similar. Age was entered as a continuous variable (reported per 10 years) in final models. Due to skewed distributions of creatinine and AST, the log-transformation (base 2) was also considered and found to have a better fit for all models. In addition, the following variables were entered and tested in the Cox model as time-varying covariates: treated acute rejection, posttransplant antiviral therapy, and biliary complications (leaks and strictures). Selected interactions were evaluated.
For the model of advanced fibrosis, a sensitivity analysis was conducted to assess the effect of donor type among patients with and without biliary strictures. In addition, given the importance of donor risk index (DRI) in graft outcomes among DDLT recipients, a subanalysis was conducted to determine if there was a DRI cutoff above which LDLT was more advantageous to the recipient in terms of fibrosis severity or graft survival. DRI by category was evaluated in the Cox models of advanced fibrosis and graft loss, with LDLT used as the reference group.
All analyses were performed using SAS v. 9.2 (SAS Institute, Cary NC) and R (icfit and ictest functions) statistical software.
Results
The 375 HCV-infected patients in the A2ALL Cohort Study meeting eligibility criteria (195 LDLT recipients and 180 DDLT recipients) were followed for a median of 4.7 years (IQR 2.7-6.6). The median age of HCV recipients was 53.1 years, 70% were male, and 85% Caucasian race. HCC was present in 23% of the patients; 52% of these patients were within Milan criteria and 60% within University of California San Francisco (UCSF) criteria.20,21 The characteristics of the LDLT and DDLT recipients are shown in Table 1. LDLT recipients had younger donors who were more likely to be non-Hispanic white; less frequently had HCC as their indication for LT; had lower laboratory MELD scores at the time of transplantation; had shorter cold ischemia times; and post-LT, had a higher frequency of biliary complications compared with DDLT recipients. Initial tacrolimus use was more frequent in LDLT compared with DDLT recipients, but use of steroids was similar. Forty percent of HCV patients were treated for acute rejection, with no difference between LDLT and DDLT in rates of rejection or time to first episode of rejection. Half of the cohort received antiviral therapy post-LT, and 31% of those treated achieved a sustained virologic response, with no differences in median time to antiviral treatment, treatment frequency, or outcomes between groups.
Table 1. LDLT and DDLT Recipient Characteristics.
| LDLT (n = 195) | DDLT (n = 180] | P Value* | |
|---|---|---|---|
|
| |||
| Median [IQR] or n (%) | Median [IQR] or n (%) | ||
| Donor Characteristics | |||
| Age (years) | 37 [27-45] | 43 [26-51] | 0.01 |
| Age >50 years old | 26 (13%) | 53 (29%) | <0.0001 |
| Male | 104 (53%) | 100 (56%) | 0.42 |
| Race/ethnicity | <0.0001 | ||
| White | 164 (84%) | 109 (61%) | |
| African American | 10 (5%) | 31 (17%) | |
| Asian | 6 (3%) | 6 (3%) | |
| Hispanic/Latino | 15 (8%) | 33 (18%) | |
| Warm ischemia time (minutes) | 36 [30-45] | 40 [35-46] | 0.004 |
| Cold ischemia time (minutes) | 41 [31-60] | 423 [300-560] | <0.0001 |
| Recipient Characteristics | |||
| Age (years) | 53 [49-57] | 54 [49-58] | 0.18 |
| Male | 134 (69%) | 130 (72%) | 0.46 |
| Race | 0.31 | ||
| White | 170 (87%) | 147 (82%) | |
| African American | 7 (4%) | 11 (6%) | |
| Other | 18 (9%) | 22 (12%) | |
| Pretransplant and Peritransplant | |||
| BMI (kg/m2) | 26.8 [24.2-30.2] | 26.6 [23.9-30.2] | 0.62 |
| HCC diagnosis | 35 (18%) | 54 (30%) | 0.006 |
| Diabetes | 30 (15%) | 39 (22%) | 0.12 |
| Transplant in the MELD Era | 110 (56%) | 108 (60%) | 0.48 |
| Lab MELD (max 40) | 15 [12-18] | 19 [14-25] | <0.0001 |
| Albumin (g/dL) | 2.8 [2.4-3.2] | 2.7 [2.4-3.2] | 0.52 |
| Alkaline phosphatase (IU/L) | 124 [92-169] | 117 [84-154] | 0.27 |
| ALT (IU/L) | 56 [33-89] | 46 [30-76] | 0.07 |
| AST (IU/L) | 89 [64-136] | 79 [56-121] | 0.17 |
| Bilirubin (mg/dL) | 2.5 [1.5-4.0] | 3.5 [1.8-6.7] | 0.0001 |
| INR | 1.5 [1.3-1.7] | 1.6 [1.3-2.1] | 0.0006 |
| Creatinine (mg/dL) | 0.9 [0.7-1.2] | 1.1 [0.8-1.5] | 0.0007 |
| Immunosuppressive Therapy | |||
| At Month 6† | |||
| Tacrolimus | 92 (87%) | 61 (66%) | 0.0004 |
| Steroid | 55 (52%) | 51 (55%) | 0.68 |
| Cyclosporine | 8 (5%) | 21 (23%) | 0.002 |
| MMF | 48 (45%) | 52 (56%) | 0.13 |
| Sirolimus | 9 (8%) | 16 (17%) | 0.06 |
| Ever used Sirolimus | 59 (30%) | 54 (30%) | 0.96 |
| Post-Transplant‡ | |||
| HCV treatment | 77 (55%) | 61 (48%) | 0.27 |
| SVR | 18 (35%) | 10 (23%) | 0.58 |
| Treatment of acute rejection | 71 (39%) | 64 (41%) | 0.80 |
| Biliary complication | 84 (46%) | 43 (35%) | <0.0001 |
| Bile leak | 42 (23%) | 17 (10%) | 0.001 |
| Biliary stricture | 64 (36%) | 31 (19%) | 0.0014 |
| Follow-up (years) | 4.94 [2.82-6.93] | 4.26 [2.54-6.03] | 0.05 |
Chi-square tests for proportions were used for categorical variables, Wilcoxon rank-sum tests were used for continuous variables, log-rank tests for post-transplant variables.
Data available for 106 LDLTs and 93 DDLTs.
Percentages were calculated using Kaplan-Meier estimation.
Abbreviations: MMF, mycophenolate mofetil; SVR, sustained virologic response.
Fibrosis Severity in LDLT and DDLT
A total of 665 biopsies were evaluated for HCV fibrosis severity, and clinically advanced disease status was assessed in all but 17 patients (Fig. 1). The proportion of patients with Ishak fibrosis stage 3 or higher on biopsy was 14% at year 1 and 35% at year 5 (Supporting Fig. 1a). There was no difference in time to advanced fibrosis by donor type (P = 0.32) based on histology alone (Fig. 2A) or based on histology plus clinically advanced disease status (P = 0.16) (Fig. 2B). This lack of association between donor type and advanced fibrosis was unchanged by restriction of the analysis to centrally read biopsies only (data not shown). Moreover, in multivariate analysis, controlling for potential confounders including donor age and cold ischemia time, LDLT was not associated with less advanced disease compared with DDLT (hazard ratio [HR] = 1.11, 95% confidence interval [CI] 0.73-1.69, P = 0.63) (Table 2). The only factors associated with advanced fibrosis were recipient AST at transplant (HR = 1.38 for a doubling of AST, 95% CI 1.10-1.74, P = 0.005) and posttransplant biliary stricture (HR = 2.68, 95% CI 1.75-4.09, P = 0.0001). In a sensitivity analysis, LDLT was tested for its association with advanced fibrosis both prior to and after biliary stricture (the former including patients who never had biliary stricture and the time prior to biliary stricture among patients who had one, and the latter including the time after biliary stricture among patients who had one). Adjusted for AST and donor age, LDLT (versus DDLT) was not associated with advanced fibrosis either prior to or following biliary stricture (HR = 1.19, P = 0.50 and HR 0.86, P = 0.69, respectively). Donor age was not significantly associated with advanced fibrosis in the overall study population (HR = 1.15 per 10 years, 95% CI 0.98-1.35, P = 0.10) nor in the population without biliary strictures (HR = 1.13 per 10 years, 95% CI 0.92-1.38, P = 0.24). Interactions between graft type and both donor age and cold ischemia time were tested but were not significant. Analysis of the outcome of Ishak stage 5-6 (or clinical cirrhosis reported on the Advanced Disease form) yielded similar results (Supporting Table 2, Supporting Fig. 2a,b). Finally, there was no threshold of DDLT DRI after which LDLT was more advantageous to the recipient with respect to advanced fibrosis (Supporting Table 3a).
Fig. 2.
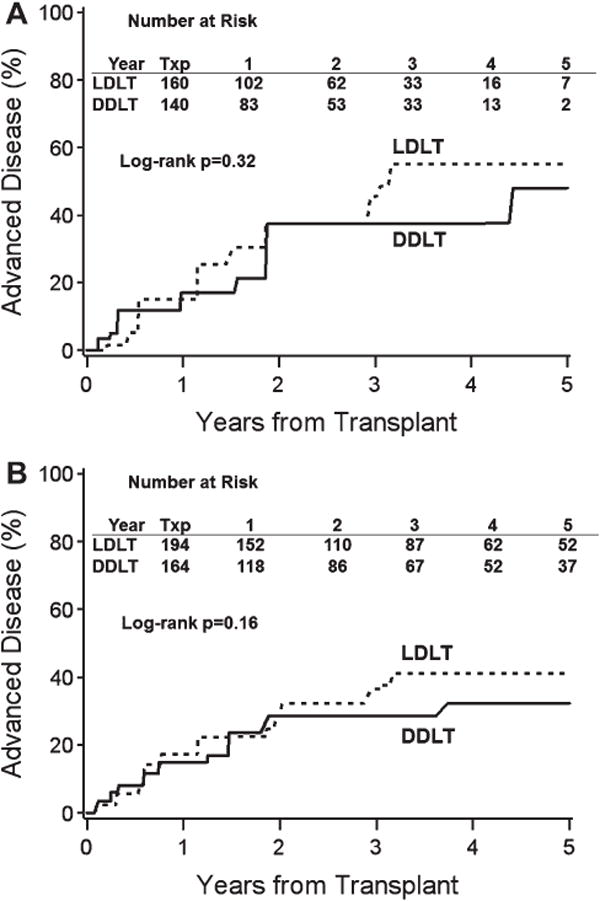
Cumulative Risk of Advanced Disease by Donor Type. The unadjusted cumulative risk of advanced disease by donor type based on (A) histological data and (B) histological and clinical data was not significantly different in LDLT (dashed line) and DDLT (solid line) groups (P > 0.05 for both comparisons by log-rank test).
Table 2. Predictors of Advanced Fibrosis (Using Histological and Clinical Data).
| Predictors | Single Variable Models | Multivariable Model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| LDLT vs. DDLT | 1.29 | 0.87-1.91 | 0.20 | 1.11 | 0.73-1.69 | 0.63 |
| AST at transplant (log2 IU/L) | 1.28 | 1.03-1.59 | 0.0247 | 1.38 | 1.10-1.74 | 0.005 |
| Biliary stricture complication* | 2.68 | 1.78-4.03 | 0.0001 | 2.68 | 1.75-4.09 | 0.0001 |
| Donor age (per 10 years) | 1.07 | 0.92-1.25 | 0.37 | 1.15 | 0.98-1.35 | 0.10 |
| Albumin (g/dL) | 0.74 | 0.53-1.03 | 0.07 | - | - | - |
| LAB MELD (per 5 units) | 0.85 | 0.74-0.99 | 0.04 | - | - | - |
As a time-varying covariate.
Other predictors were tested and were not significant: recipient sex, recipient age, recipient race, recipient ethnicity, recipient BMI, recipient weight, donor sex, donor race, warm ischemia time, cold ischemia time, alkaline phosphatase, ALT, bilirubin, INR, creatinine, HCC diagnosis, diabetes status, steroid and tacrolimus use at transplant or within 10 days posttransplant, and HCV treatment, treatment for acute rejection, and biliary leak complication as time-varying covariates.
Graft and Patient Survival
Recipients of LDLT versus DDLT did not differ with respect to unadjusted patient (P = 0.43; Fig. 3A) or graft survival (P = 0.32; Fig. 3B). The cumulative patient survival probabilities at 3, 5, and 7 years were 88%, 79%, and 71% in LDLT and 83%, 77%, and 70% in DDLT recipients, respectively. Recurrent HCV was the primary cause of graft loss or death in 27% of LDLT and 20% of DDLT recipients (P = 0.45) (Supporting Table 1).
Fig. 3.
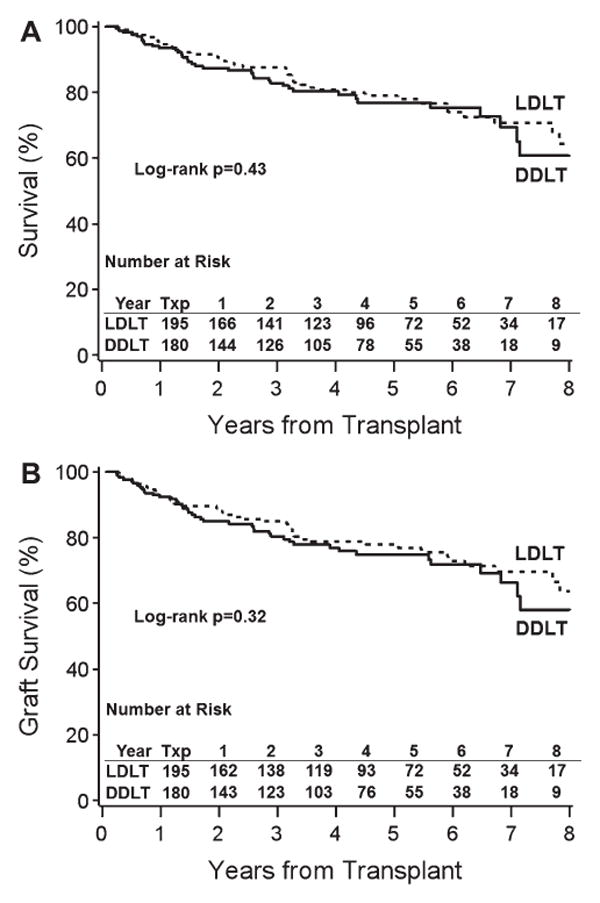
Cumulative Patient and Graft Survival by Donor Type. The unadjusted cumulative risk of (A) patient (P = 0.43) and (B) graft survival (P = 0.32) did not differ between recipients of LDLT versus DDLT.
In the Cox regression analysis, controlling for potential confounders, LDLT was not found to be associated with graft loss (HR = 0.76, 95% CI 0.49-1.18, P = 0.23) (Table 3). The only factors associated with graft loss were AST at transplant (HR = 1.36 for a doubling of AST, 95% CI 1.1-1.68, P = 0.004), biliary stricture complication (HR = 2.25, 95% CI 1.41-3.57, P = 0.0006), and creatinine at transplant (HR = 1.74, 95% CI 1.28-2.36, P = 0.0004). If graft failures or deaths caused by HCC recurrence were censored, the HR for LDLT in association with graft loss/death was similar (HR = 0.67, P = 0.11). Donor age was not significantly associated with graft loss or death. Similarly, treated acute rejection and HCV treatment were not predictive of graft loss, and adjustment for these variables did not change the association between donor type and graft outcome. Interactions between graft type and both donor age and cold ischemia time were tested but were not significant.
Table 3. Predictors of Graft Loss.
| Predictors | Single Variable Models | Multivariable Model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| LDLT vs. DDLT | 0.81 | 0.53-1.23 | 0.32 | 0.76 | 0.49-1.18 | 0.23 |
| Creatinine at transplant (log2 mg/dL) | 1.68 | 1.26-2.24 | 0.0004 | 1.74 | 1.28-2.36 | 0.0004 |
| AST at transplant (log2 IU/L) | 1.28 | 1.02-1.6 | 0.03 | 1.36 | 1.10-1.68 | 0.004 |
| Biliary stricture complication* | 2.44 | 1.57-3.79 | 0.0001 | 2.25 | 1.41-3.57 | 0.0006 |
| Bilirubin (per 10 mg/dL) | 1.25 | 0.98-1.58 | 0.07 | - | - | - |
As a time-varying covariate.
Other predictors were tested and were not significant: recipient sex, recipient age, recipient race, recipient ethnicity, recipient BMI, recipient weight, donor sex, donor race, donor age, warm ischemia time, cold ischemia time, albumin, alkaline phosphatase, ALT, INR, MELD, HCC diagnosis, diabetes status, steroid and tacrolimus use at transplant or within 10 days posttransplant, and HCV treatment, treatment for acute rejection, biliary leak complication as time-varying covariates.
In an analysis of graft loss among DDLT defined by DRI quartiles and using LDLT as the reference group, there was no significant difference in graft loss between LDLT and DDLT recipients until the DRI for DDLT was ≥1.8 (HR = 2.49, 95% CI 1.26-4.92, P = 0.01, Supporting Table 3b).
Discussion
Receipt of LDLT offers a survival advantage to the wait-listed patient compared with DDLT.22 With the increasing numbers of patients in need of organs, LDLT offers an important means of expanding the donor pool. While initial studies suggested that outcomes in liver transplant patients with HCV were worse for LDLT recipients than their DDLT counterparts, these findings were likely confounded by the “learning curve” for surgical and postoperative management of LDLT recipients. We have previously shown that in the A2ALL cohort of HCV-infected patients, short-term graft survival rates were similar between LDLT and DDLT when transplant outcomes were restricted to LDLT beyond the first 20 cases at a given site.15 However, the earlier study was limited by a median follow-up of only 3 years, a histologic follow-up of only 12 months, and lack of information on liver disease severity in survivors with initial grafts. In the current study, the largest cohort of HCV-infected LDLT recipients reported to date, we confirm that there is no difference in graft survival between LDLT and DDLT with a median follow-up of nearly 5 years (up to maximum of 10.9 years).
While overall graft survival is of key importance in assessing the viability of LDLT for HCV-infected patients, the issue of whether the natural history of HCV disease is influenced by the source of the donor was of primary interest in this study Recognizing the critical importance of donor age in HCV disease progression and the risk of cirrhosis, we hypothesized that live donors may confer improved outcomes compared with deceased donors. Additionally, other factors linked to poor graft survival as reflected in the donor risk index (prolonged cold ischemia time, nontraumatic cause of death) were also absent in LDLT However, despite these theoretical benefits of LDLT, we did not find that LDLT was associated with less severe fibrosis. Indeed, even after adjustment for many of the factors recognized to influence the risk of advanced fibrosis (including treated acute rejection, HCV treatment, cold ischemia time, and donor age), LDLT was still unassociated with advanced disease. On the one hand, these results are reassuring to the HCV-infected LDLT recipient, as no increase in risk of severe disease was evident, but on the other hand, the lack of benefit was somewhat surprising.
We found that biliary strictures were strongly associated with both fibrosis severity and graft loss, after adjustment for potential confounders. These results conflict with a study from two centers that examined HCV and non-HCV recipients with and without biliary complications and found that, while biliary complications affect early graft loss, the combination of biliary complications and HCV had no impact on fibrosis rates on 1-year protocol biopsies.23 However, that study focused on early outcomes, included both biliary leaks and strictures, and the histological assessment at 1 year may have been insufficient to capture the more long-term effects of biliary complications on advanced fibrosis risk in HCV-infected patients. Another single-center study reported higher rates of fibrosis among HCV patients with versus without late strictures (>30 days post-LT) but without use of a time-varying covariate analysis.24 Our results, in which biliary complications were evaluated as a time-varying covariate, showed that the development of biliary strictures places the HCV-infected patient at significant risk of subsequent advanced fibrosis. Given the higher rates of biliary strictures in LDLT compared with DDLT recipients, biliary strictures will more frequently be a factor influencing fibrosis progression in LDLT recipients. Of note, the rate of biopsy among patients with biliary complications was not significantly different from those without biliary complications, minimizing the likelihood of a measurement bias.
Serum AST levels at the time of LT were associated with risk of advanced fibrosis and graft loss in unadjusted and adjusted analysis. To our knowledge, no prior study has specifically evaluated pretransplant serum aminotransferase levels as a predictor of post-transplant outcomes. This may represent a simple and novel predictor of risk and serve to identify patients who may warrant close follow-up biopsies to monitor disease progression. The mechanisms underlying this association are entirely speculative, but higher AST levels, possibly reflecting a genetic or immunological predilection to higher HCV necroinflammatory activity pre-LT, may persist post-LT There was no relationship between viral load and AST, and pretransplant antiviral therapy did not modify the association between AST and disease severity or graft loss (data not shown). In a recently published study of IL28B polymorphisms and disease activity post-LT, the GG allele (rs8099917) was associated with higher AST and ALT levels.25
A lack of interleukin-28B genotype data of donors and recipients is a limitation of our study. Overall, the less favorable genotypes are overrepresented among transplant recipients, and any differences in the distribution of genotypes among recipients of LDLT and DDLT are likely to be small. Another limitation of our study is lack of complete biopsy information at all timepoints posttransplant. Missed biopsies were due to several factors including the patient being on antiviral therapy, presence of relative contraindication (thrombocytopenia), and presence of cirrhosis based on clinical/radiological criteria. To ensure that those with advanced disease were “counted,” we had each patient without a biopsy assessed for advanced disease based on laboratory, clinical, or radiological criteria. These criteria used typical findings of cirrhosis and so only captured the more severe end of the fibrosis spectrum. The results of our analysis comparing LDLT and DDLT were similar when we used histology alone versus histology plus clinical criteria. However, it is acknowledged that some cases of advanced fibrosis (without clinical cirrhosis) were missed. We also acknowledge that a lack of central read on all biopsies may introduce measurement bias; however, our results were similar when analysis was limited to biopsies read centrally, regardless of which set of central results was used. Moreover, this multicenter study includes the most comprehensive longitudinal evaluation of histology and graft outcomes among LDLT to date.
In summary, in this large prospective U.S. study of LDLT recipients, HCV disease progression is not affected by donor type, and graft loss occurs at similar rates in LDLT and DDLT recipients with a median follow-up of ∼5 years. Our study supports the use of LDLT in patients with HCV and highlights the need to develop interventions to prevent or halt HCV disease progression posttransplantation.
Supplementary Material
Acknowledgments
This study was presented in part at the 12th annual meeting of the American Transplant Congress, Boston, MA, June 2-6, 2012. This is publication number 23 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study. The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions as follows: Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, M.D.; Co-Is: Robert S. Brown, Jr., M.D., M.P.H., James Guarrera, M.D., FACS, Martin R. Prince, M.D., Ph.D., Benjamin Samstein, M.D., Elizabeth Verna, M.D., M.S.; Study Coordinators: Taruna Chawla, M.D., Scott Heese, M.P.H., Theresa Lukose, Pharm.D., Rudina Odeh-Ramadan, Pharm.D., Jonah Zaretsky, B.S. Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, M.D., M.B.A.; Co-Is: Talia Baker, M.D., Laura M. Kulik, M.D., Daniela P. Ladner, M.D.; Study Coordinator: Patrice Al-Saden, R.N., CCRC University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, M.D.; Co-I: Ronald W. Busuttil, M.D., Ph.D.; Study Coordinator: Janet Mooney, R.N., B.S.N. University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, M.D., FACS; Co-I: Norah A. Terrault, M.D., M.P.H.; Study Coordinator: Dulce MacLeod, R.N. University of Colorado Denver, Aurora, CO (DK62536): PI: James R. Burton, Jr., M.D.; Co-Is: Gregory T. Everson, M.D., FACP, Igal Kam, M.D., James Trotter, M.D.; Study Coordinators: Carlos Garcia, R.N., B.S., Anastasia Krajec, R.N. University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, M.D., FACS; DCC Staff: Mary Akagi, M.S., CCRP, Douglas R. Armstrong, B.S.N., M.S., Abby Brithinee, B.A., Margaret Hill-Callahan, B.S., L.S.W., Lisa Holloway, B.S., CCRC, Terese A. Howell, B.S., CCRC, Brenda W. Gillespie, Ph.D., Beth Golden, B.Sc.N., Anna S.F. Lok, M.D., Monique Lowe, M.S.I., Akinlolu O. Ojo, M.D., Ph.D., Samia Shaw, AAIT, Abigail Smith, M.S., Robert A. Wolfe, Ph.D. University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, M.D., M.P.H.; Study Coordinator: Tracy Russell, M.A. University of Pennsylvania, Philadelphia, PA (DK62494): PI: Abraham Shaked, M.D., Ph.D.; Co-Is: Kim M. Olthoff, M.D., FACS, K. Rajender Reddy, M.D., Mark A. Rosen, M.D., Ph.D.; Study Coordinators: Brian Conboy, P.A., M.B.A., Mary Kaminski, P.A.-C., Debra McCorriston, R.N., Mary Shaw, R.N., B.B.A. University of Virginia (DK62484): PI: Carl L. Berg, M.D.; Co-I: Timothy L. Pruett, M.D.; Study Coordinator: Jaye Davis, R.N. Virginia Commonwealth University - Medical College of Virginia, Richmond, VA (DK62531): PI: Robert A. Fisher, M.D., FACS; Co-Is: Adrian Cotterell, M.D., FACS, Mitchell L. Shiffman, M.D., R. Todd Stravitz, M.D., FACP; Study Coordinators: April Ashworth, R.N., B.S.N, Joanne Davis, R.N., Ede Fenick, R.N., Charlotte Hofmann, R.N., Sarah Hubbard, Andrea Lassiter, B.S., Cheryl Rodgers, R.N., Jose Rodriguez, M.P.H., Luke Wolfe, M.S. National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, M.D.: Edward Doo, M.D., James E. Everhart, M.D., M.P.H., Jay H. Hoofnagle, M.D., Stephen James, M.D., Patricia R. Robuck, Ph.D., Leonard B. Seeff, M.D., Rebecca J. Torrance, R.N., M.S.
Supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, and U01-DK62536). Additional support was provided by the Health Resources and Services Administration (HRSA) and the American Society of Transplant Surgeons (ASTS).
Abbreviations
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Cohort Study
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- DDLT
deceased donor liver transplant
- HAI
Knodell Hepatitis Activity Index
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IOR
interquartile range
- LDLT
living donor liver transplant
- LT
liver transplant
- MELD
Model for Endstage Liver Disease
- SRTR
Scientific Registry of Transplant Recipients
- UCSF
University of California San Francisco
Footnotes
Potential conflict of interest: Dr. Saab consults for, advises, is on the speakers' bureau for, and owns stock in Bristol-Myers Squibb and Gilead. He consults for, advises, and is on the speakers' bureau for Genentech, Merck, and Vertex. He consults for and advises Janssen and Boehringer Ingelheim. Dr. Brown consults for and received grants from Salix. He received grants from Gilead, Bristol-Myers Squibb, Roche, Vertex, and Schering Dr. Kulik received grants from Gilead.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Thuluvath P, Guidinger M, Fung J, Johnson L, Rayhill S, Pelletier S. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer M, Schuppan D. Progression of liver fibrosis in posttransplant hepatitis C: Mechanisms, assessment and treatment. J Hepatol. 2013;58:1028–1041. doi: 10.1016/j.jhep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, et al. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778–1786. doi: 10.1002/lt.21598. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Retortillo M, Forns X, Llovet J, Navasa M, Feliu A, Massaguer A, et al. Hepatitis C recurrence is more severe after living donor compared to cadaveric liver transplantation. Hepatology. 2004;40:699–707. doi: 10.1002/hep.20357. [DOI] [PubMed] [Google Scholar]
- 5.Schmeding M, Neumann UP, Puhl G, Bahra M, Neuhaus R, Neuhaus P. Hepatitis C recurrence and fibrosis progression are not increased after living donor liver transplantation: a single-center study of 289 patients. Liver Transpl. 2007;13:687–692. doi: 10.1002/lt.21138. [DOI] [PubMed] [Google Scholar]
- 6.Russo M, Galanko J, Beavers K, Fried M, Shrestha R. Patient and graft survival in hepatitis C recipients after adult living donor liver transplantation in the United States. Liver Transpl. 2004;10:340–346. doi: 10.1002/lt.20090. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Singhal A, Kashyap R, Safadjou S, Ryan CK, Orloff MS. Comparative analysis of hepatitis C recurrence and fibrosis progression between deceased-donor and living-donor liver transplantation: 8-year longitudinal follow-up. Transplantation. 2011;92:453–460. doi: 10.1097/TP.0b013e3182259282. [DOI] [PubMed] [Google Scholar]
- 8.Sher L, Jennings L, Rudich S, Alexopoulos SP, Netto G, Teperman L, et al. Results of live donor liver transplantation in patients with hepatitis C virus infection: the HCV 3 trial experience. Clin Transplant. Multicenter Study Randomized Controlled Trial Research Support, Non-US Gov't. 2012;26:502–509. doi: 10.1111/j.1399-0012.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 9.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaglio P, Malireddy S, Levitt B, Lapointe-Rudow D, Lefkowitch J, Kinkhabwala M, et al. Increased risk of cholestatic hepatitis C in recipients of grafts from living versus cadaveric liver donors. Liver Transpl. 2003;9:1028–1035. doi: 10.1053/jlts.2003.50211. [DOI] [PubMed] [Google Scholar]
- 11.Bozorgzadeh A, Jain A, Ryan C, Ornt D, Zand M, Mantry P, et al. Impact of hepatitis C viral infection in primary cadaveric liver allograft versus primary living-donor allograft in 100 consecutive liver transplant recipients receiving tacrolimus. Transplantation. 2004;77:1066–1070. doi: 10.1097/01.tp.0000122142.00818.9e. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman M, Stravitz R, Contos M, Mills A, Sterling R, Luketic V, et al. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl. 2004;10:1248–1255. doi: 10.1002/lt.20232. [DOI] [PubMed] [Google Scholar]
- 13.Guo L, Orrego M, Rodriguez-Luna H, Balan V, Byrne T, Chopra K, et al. Living donor liver transplantation for hepatitis C-related cirrhosis: no difference in histological recurrence when compared to deceased donor liver transplantation recipients. Liver Transpl. 2006;12:560–565. doi: 10.1002/lt.20660. [DOI] [PubMed] [Google Scholar]
- 14.Humar A, Beissel J, Crotteau S, Kandaswamy R, Lake J, Payne W. Whole liver versus split liver versus living donor in the adult recipient: an analysis of outcomes by graft type. Transplantation. 2008;85:1420–1424. doi: 10.1097/TP.0b013e31816de1a3. [DOI] [PubMed] [Google Scholar]
- 15.Terrault NA, Shiffman ML, Lok AS, Saab S, Tong L, Brown RS, Jr, et al. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transpl. 2007;13:122–129. doi: 10.1002/lt.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu A, Liang W, Zheng Z, Guo Z, He X. Living donor vs. deceased donor liver transplantation for patients with hepatitis C virus-related diseases. J Hepatol [Research Support, Non-US Gov't] 2012;57:1228–1243. doi: 10.1016/j.jhep.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. JR Stat Soc B. 1976;38:290–295. [Google Scholar]
- 19.Sun J. A non-parametric test for interval censored failure time data with applications to AIDS studies. Stat Med. 1996;15:1387–1395. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1387::AID-SIM268>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 21.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 22.Berg CL, Merion RM, Shearon TH, Olthoff KM, Brown RS, Jr, Baker TB, et al. Liver transplant recipient survival benefit with living donation in the MELD allocation era. HEPATOLOGY. 2011;54:1313–1321. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verna EC, De Martin E, Burra P, Neri D, Gaglio PJ, Emond JC, et al. The impact of hepatitis C and biliary complications on patient and graft survival following liver transplantation. Am J Transplant. 2009;9:1398–1405. doi: 10.1111/j.1600-6143.2009.02649.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujita S, Fujikawa T, Mizuno S, Reed AI, Kim RD, Howard RJ, et al. Is early recurrence of hepatitis C associated with biliary anastomotic stricture after liver transplantation? Transplantation. 2007;84:1631–1635. doi: 10.1097/01.tp.0000295983.55088.96. [DOI] [PubMed] [Google Scholar]
- 25.Eurich D, Boas-Knoop S, Ruehl M, Schulz M, Carrillo ED, Berg T, et al. Relationship between the interleukin-28b gene polymorphism and the histological severity of hepatitis C virus-induced graft inflammation and the response to antiviral therapy after liver transplantation. Liver Transpl. 2011;17:289–298. doi: 10.1002/lt.22235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


