Abstract
Interleukin (IL)-15, is a cytokine that is important for the maintenance of long-lasting, high-avidity T cell response to invading pathogens and has, therefore, been used in vaccine and therapeutic platforms as an adjuvant. In addition to pure protein delivery, plasmids encoding the IL-15 gene have been utilized. However, it is critical to determine the appropriate dose to maximize the adjuvanting effects. We immunized rhesus macaques with different doses of IL-15 expressing plasmid in an influenza non-human primate immunogenicity model. We found that co-immunization of rhesus macaques with a Flu DNA-based vaccine and low doses of plasmid encoding macaque IL-15 enhanced the production of IFN-γ (0.5 mg) and the proliferation of CD4+ and CD8+ T cells, as well as TCM levels in proliferating CD8+ T cells (0.25 mg). Whereas, high doses of IL-15 (4 mg) decrease the production of IFN-γ and the proliferation of CD4+ and CD8+ T cells and TCM levels in the proliferating CD4+ and CD8+ T cells. In addition, the data of hemagglutination inhibition (HI) antibody titer suggest that although not significantly different, there appears to be a slight increase in antibodies at lower doses of IL-15. Importantly, however, the higher doses of IL-15 decrease the antibody levels significantly. This study demonstrates the importance of optimizing DNA-based cytokine adjuvants.
Keywords: Influenza, Vaccines, IL-15
Introduction
Studies have shown that strong immune responses help contain HIV replication by specific CD4+ and CD8+ T cell proliferation (Halwani et al., 2008). CD4+ T cells help promote memory CD8+ T cell proliferation which results in increased effector responses, including secretion of IFN-γ and cytokine molecules, such as IL-15, which share the γ-receptor chain subunit and have a role in generating and maintaining memory T cells (Kutzler et al., 2005; Chong et al., 2007). Because of the need for a vaccine to be able to produce a long-term memory response and IL-15’s role in the stimulation, proliferation, and survival of memory CD8+T cells (Ku et al., 2000; Becker et al., 2002; Moore et al., 2002; Oh et al., 2003; Sprent, 2003); we sought to find an optimal dose for IL-15 cytokine to be used as an adjuvant in vaccine and therapeutic platforms. In rhesus macaques chronically infected with SIVmac251, pharmacological doses of IL-15 have been shown to increase the proliferation of effector CD4+ and CD8+ T cells (Mueller et al., 2005; Picker et al., 2006). In uninfected macaques immunized with tetanus toxoid or a live influenza vaccine, IL-15 increased long-term memory response (Villinger et al., 2004). Our previous study has demonstrated that an optimized IL-15 immune adjuvant delivered with a DNA vaccine can impact the cellular immune profile in non-human primates and lead to an enhanced suppression of viral replication against SHIV (Boyer et al., 2007). We further assessed the long-term immune protection and mechanism induced by IL-15 in HIV DNA vaccine. In doing so, we were able to demonstrate that DNA vaccine plus IL-15 could induce high levels of central memory T cells and T cell responses (Yin et al., 2008).
DNA vaccine therapies are a relatively novel method of vaccination with the goal to induce broad cellular immunity and humoral responses in a multitude of infectious disease models. Both viral and non-viral vaccines have been tested in pre-clinical and clinical models with variable success (Luxembourg and Lindhoff-Last, 2007) (Prud’homme, 2006). Furthermore, the developments of new delivery methods, such as electroporation (EP) (Abdulhaqq and Weiner, 2008; Kutzler and Weiner, 2008), as well as the use of agents that improve antigen uptake or presentation, and optimization of the transgene sequences are overcoming historical drawbacks. Many studies have shown that the use of EP enhances plasmid uptake and immune responses by a factor of 5 to 1000 in function of the specific model (Bodles-Brakhop and Draghia-Akli, 2008). Nevertheless, doses of plasmids used in many DNA vaccine studies have been determined empirically, or based on data generated in small animal experiments, which do not rigorously translate to large animal models and humans; this is moreover true for plasmids encoding for molecular adjuvants, such as IL-15.
Finding the best possible dose for DNA vaccination that will promote long-lasting memory cell immunity upon challenge with a pathogen is needed in order to develop an effective vaccine. In this manuscript, we present data from a dose finding study for the IL-15 DNA molecular adjuvant delivered in conjunction with an optimized consensus influenza DNA vaccine (Laddy et al., 2008) and identify an optimum dose for the IL-15 DNA adjuvant, while illustrating the use of a new IL-15 molecule.
Results
DNA plasmid delivery with electroporation induces robust cellular immune responses
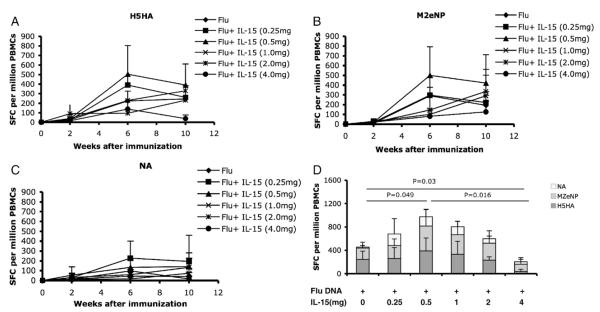
Six groups of macaques were each immunized by electroporation (Table 1). We evaluated the induction of a cellular immune response in each animal by IFN-γ ELISA-linked immunospot (ELISpot) assay. PBMCs isolated 2 weeks after each immunization were assayed for influenza specific IFN-γ production. After the second immunization, animals co-immunized with DNA + 0.5 mg IL-15 induced the highest H5HA-specific IFN-γ response (the average of 503 SFCs per million PBMCs), followed by 0.25 mg IL-15, 1 mg IL-15 and Flu DNA alone group (control) (Fig. 1A). On the contrary, the high dose IL-15 groups, 2 mg IL-15 and 4 mg IL-15, induced the lowest IFN-γ response to H5HA (the average of 95 SFCs per million PBMCs and 138 SFCs per million PBMCs, respectively) (Fig. 1A). Although a small change in the order of the groups occurred after the third immunization, the 0.5 mg IL-15 group still induced the highest H5HA-specific IFN-γ response (390 SFCs per million PBMCs) and the 4 mg IL-15 group induced the lowest IFN-γ response (38 SFCs per million PBMCs) (Fig. 1A).
Table 1.
Immunization schedule.
| Immunization | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
|---|---|---|---|---|---|---|
| Week 0 | Flu DNA | Flu DNA | Flu DNA | Flu DNA | Flu DNA | Flu DNA |
| pmac IL-15 | pmac IL-15 | pmac IL-15 | pmac IL-15 | pmac IL-15 | ||
| 0.25 mg | 0.5 mg | 1.0 mg | 2.0 mg | 4.0 mg | ||
| Week 4 | Flu DNA | Flu DNA | Flu DNA | Flu DNA | Flu DNA | Flu DNA |
| pmac IL-15 | pmac IL-15 | pmac IL-15 | pmac IL-15 | pmac IL-15 | ||
| 0.25 mg | 0.5 mg | 1.0 mg | 2.0 mg | 4.0 mg | ||
| Week 8 | Flu DNA | Flu DNA | Flu DNA | Flu DNA | Flu DNA | Flu DNA |
| pmac IL-15 | pmac IL-15 | pmac IL-15 | pmac IL-15 | pmac IL-15 | ||
| 0.25 mg | 0.5 mg | 1.0 mg | 2.0 mg | 4.0 mg |
Fig. 1.
IFN-γ-production after immunization. Samples were taken 2 weeks after each immunization. Peripheral blood mononuclear cells (PBMCs) were isolated by a standard percoll separation technique and assessed for H5HA antigen- (A) M2eNP antigen- (B) and NA antigen- (C) specific responses by ELISpot. (D) Average SFCs, spot-forming cell levels of IFN-γ-producing cells after third stimulation with H5HA, M2eNP, or NA antigen.
Similar results occurred in the M2eNP-specific IFN-γ response after every immunization compared to the IFN-γ response to H5HA (Figs. 1A and B). After the second and third immunizations, 0.5 mg IL-15 induced the highest IFN-γ-producing cells to M2eNP (499 SFCs per million PBMCs), while the 4 mg IL-15 group consistently induced the lowest IFN-γ-producing cells to M2eNP (80 SFCs per million PBMCs) (Fig. 1B). However, the results obtained for IFN-γ response to NA antigen showed that 0.25 mg IL-15 produced the most IFN-γ-secreted cells and the high dosage IL-15 groups, 2 mg and 4 mg, induced few IFN-γ-secreted cells (Fig. 1C).
In summary, the animals that received Flu DNA plus a low dose of IL-15, 0.5 mg, induced the higher antigen-specific IFN-γ response after the third immunization (Fig. 1D), which is the average of 953 SFCs per million PBMCs. The 4 mg IL-15 dose induced the lowest IFN-γ response after the third immunization (Fig. 1D). In all, these data showed that high doses of IL-15, 4 mg, inhibited the IFN-γ response and a low dose, 0.5 mg, gave a robust IFN-γ response after immunization.
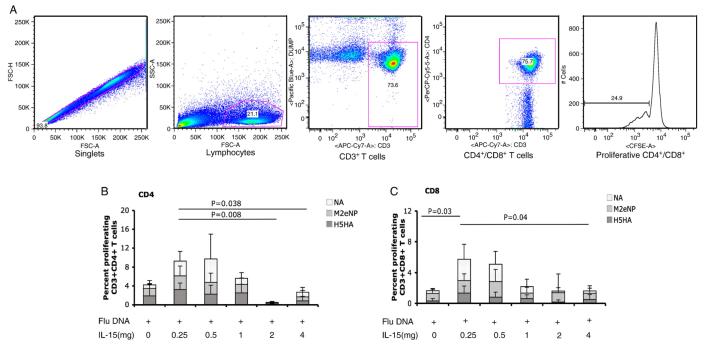
Low dose of IL-15 enhances CD8+ T cell proliferation in rhesus macaques
We next assessed the proliferative capacity of the vaccine to induce CD4+ and CD8+ T cell responses using the CFSE proliferation assay. PBMCs isolated from macaques 2 weeks after the third immunization were incubated with CFSE, washed, and stimulated for 5 days with growth media, and antigens: H5HA, M2eNP, NA, or concanavalin A (ConA). Following stimulation, T-cell proliferation was measured by flow cytometry. We found that the proliferative responses of CD4+ cells (Fig. 2B) were higher in the low dose of IL-15 (0.25 mg) group than in two high dose (2 mg and 4 mg) of IL-15 groups (P=0.008 and P=0.038, respectively). In the proliferation of CD8+ cells (Fig. 2C), low doses of IL-15 (0.25 mg) also induced significantly higher responses than the high dose group (4 mg) (P=0.04). Moreover, low dose of IL-15 (0.25 mg) plus DNA induced higher CD8+ T cell proliferative response than DNA alone group (P=0.03). These results demonstrate that the proliferative capacity of CD8+ T cells is notably increased in macaques co-immunized with the Flu DNA plus low doses of IL-15. On the other hand, compared to low doses of IL-15, high dose can decrease CD4+ and CD8+ T cell proliferative responses in macaques.
Fig. 2.
Proliferative capacity of antigen-specific T cells. Peripheral blood mononuclear cells (PBMCs) from immunized macaques are stained with CFSE and stimulated with growth medium H5HA, M2eNP, NA peptides, or concanavalin A (ConA) for 5 days. Following stimulation, cells were stained for phenotypic markers and analyzed by flow cytometry. Panels A) is gating strategy, B) and C) are CD4 and CD8 average proliferative capacity of CD4+ and CD8+ T cells, respectively, among the six groups.
Lower doses of IL-15 increase frequency of central memory T cells in proliferating T cells
We then compared the frequency of proliferating memory T cell subsets among the six groups of macaques 2 weeks after third immunization. Proliferating memory T cells were divided into two subsets by surface marker expression: central memory T cells (TCM) were defined as CD28+CD95+ and effector memory T cells (TEM) were defined as CD28−CD95+ (Fig. 3A). When we evaluated the frequency of TCMs and TEMs in proliferating CD4+ cells (Fig. 3B, left panels), we detected a significantly lower percentage of TCMs in the 4 mg and 2 mg dosage IL-15 groups (average 22.9% and 10%, respectively) compared to the 0.25 mg IL-15 groups (average 65.5%) (Fig. 3B, upper left panel). Among the six groups, there was no difference in the percentages of TEMs (Fig. 3B, lower right panel).
Fig. 3.
Percent of central memory and effector memory CD4+ and CD8+ T cells in peripheral blood of macaques after third immunization. (A) Gating strategy shows central memory and effector memory T cells which were defined as CD28+CD95+ and CD28−CD95+, respectively. (B) An average for all primates is presented for CD4+ or CD8+ TCM and TEM cells. Data were acquired on an LSRI instrument and analyzed with FlowJo software.
We also evaluated the frequency of TCMs and TEMs in proliferating CD8+ T cells. The percentages of both TCMs and TEMs were higher in the 0.25 mg IL-15 group than in the Flu DNA alone group (P=0.018 and P=0.039, respectively) (Fig. 3B, right panels). However, high dose of IL-15 groups (4 mg and 2 mg) induced lower TCMs and TEMs than the low dose group (0.25 mg) (Fig. 3B, right panels), though the difference did not reach statistical significance. Taken together, the data demonstrate that a low dose of IL-15 (0.25 mg) can induce higher levels of TCMs in both proliferating CD4+ and CD8+ T cells as well as higher proliferation of T in CD8+ EMs T cells (Fig. 3B). In addition, co-immunization with Flu DNA and high dose IL-15 results in fewer TCMs in proliferating CD4+ T cells compared to co-immunization with Flu DNA and low dose IL-15 (Fig. 3B, left panel).
High dose IL-15 decreases humoral immune responses in macaques
Hemagglutination inhibition (HI) assays were performed on clade-matched (clade 1) H5N1 viruses to compare the ability of our synthetic vaccine in conjunction with increasing doses of the IL-15 expressing plasmid to induce relevant antibody responses in primates. As shown in Fig. 4, after the initial immunization, there was no significant difference in the antibody titers among the six groups. However, following the third immunization, HI titers were above 1:40 in all macaques and low dose of IL-15 (0.25 mg) induced higher HI titer compared with high dose group (4 mg) (P=0.0034). Nevertheless considerable differences were seen between Flu DNA alone group and any of the IL-15 plasma treated groups: animals that received the flu vaccine combination in conjunction with the 0.5 mg IL-15 expressing plasmid displayed titers of 280±132, median 200. The levels of these titers were more than double compared to the flu vaccine alone group (120±67, median 60), while the group that received the flu vaccine in conjunction with the 4 mg IL-15 expressing plasmid was characterized by an inhibition of responses, with HI titers of 50±10 (median 40). These results suggest that although not significantly different, there appears to be a slight increase in antibodies at lower doses of IL-15.
Fig. 4.
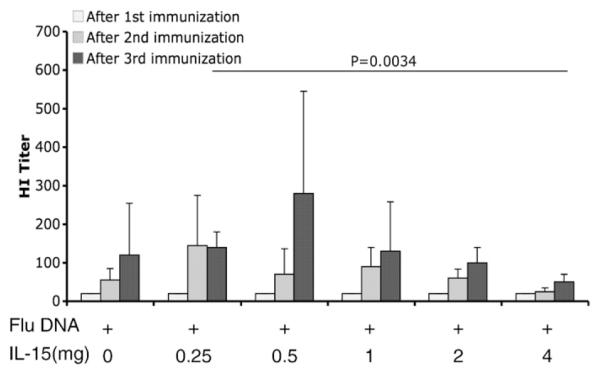
Hemagglutination inhibition (HI) Assay. Sera were collected after each immunization. HI assays were performed in V-bottom 96-well microtiter plates, using four hemagglutinating units (HAU) of virus. Hemagglutination inhibition of RBCs due to an Ag/Ab reaction can be seen when RBCs form a condensed pellet on the bottom of the well. Titer results were then calculated by taking the reciprocal of the last dilution of antiserum that inhibits hemagglutination completely.
IL-15 expression levels in plasma of macaques
Plasma levels of IL-15 were measured on days 0, 2, 4 and 7 after the 3rd immunization and found that the peak levels in plasma varied between 1 and 15 pg/mL (data not shown). Fig. 5 shows that there is no increase in the IL-15 serum levels at the lower IL-15 plasmid doses. At the higher plasmid doses there appears to be an increase in plasma IL-15 concentrations on day 2 after the 3rd immunization. However, the trend disappears on day 4 and day 7 (Fig. 5). The data suggest that there does not need to be a measurable increase in the systemic level of IL-15 for there to be an adjuvant effect.
Fig. 5.

IL-15 expression level in serum. Rhesus IL-15 percent change levels were measured on days 0, 2, 4 and 7 after the third EP using the human IL-15 chemiluminescent immunoassay QuantiGlo kit in all animals.
Discussion
In this study, we demonstrated that co-immunization of rhesus macaques with a Flu DNA-based vaccine and a high dose of plasmid encoding macaque IL-15 inhibits cellular and humoral immune response. Compared to low doses of IL-15, high doses of IL-15 (4 mg and 2 mg) decrease the producing-IFN-γ cells, and CD4+ and CD8+ T cell proliferation, as well as TCM levels in CD4+ T cells. Whereas low doses of IL-15 (0.25 mg and 0.5 mg) boost the IFN-γ response, T cell proliferation, and memory cells in CD4+ and CD8+ T cells. With this, we can conclude that high doses of plasmid IL-15 inhibit immune responses in an influenza non-human primate immunogenicity model.
IL-15, a cytokine that is important for the maintenance of long-lasting, high-avidity T cell responses to invading pathogens, achieves this by supporting the proliferation and survival of CD8+ memory T cells (Zhang et al., 1998; Ku et al., 2000; Marks-Konczalik et al., 2000; Waldmann et al., 2001; Becker et al., 2002; Moore et al., 2002; Oh et al., 2003; Schluns et al., 2004). Because of these properties, IL-15 is often used in vaccine and therapeutic platforms. IL-15 as an adjuvant enhances the function and longevity of CD8+ T cells in mice (Kutzler et al., 2005). Treating PBMCs obtained from HIV-infected patients with IL-15 enhances anti-HIV immune function (Chehimi et al., 1997). IL-15 enhances survival and function of HIV-specific CD8+ T cells in vitro (Mueller et al., 2003). Recently, IL-15 treatment during acute SIV infection was shown to elicit strong SIV-specific CD8+ T cell responses in vivo (Mueller et al., 2008). IL-15’s specificity on CD4+ and CD8+ T cell proliferation, survival, and function increases the ability for enhanced immunity against HIV and as a result makes it a good candidate as an adjuvant for DNA vaccination. Therefore, an optimal dose of IL-15 DNA adjuvant that will favorably affect the immune response and promote long-lasting immunity needs to be determined. However, previous studies have only examined IL-15 dose in small animal models. Our study used non-human primates to demonstrate how the dose of plasmid which expresses IL-15 affects the immune levels post-vaccination. And we have found that a high dose of IL-15 plasmid inhibits immune response. Thus, we propose that a low dose of IL-15, with its capacity to invoke sustainable cellular and humoral responses, is a superior cytokine adjuvant that can be used in the development of an effective vaccine against HIV.
Materials and methods
Animals
Chinese rhesus macaques were housed at Bioqual in Rockville, MD, USA. These facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care International and met the National Institutes of Health standards as set forth in the Guidelines for Care and Use of Laboratory Animals. The University of Pennsylvania Institutional Animal Care and Use Committee (IACUC) reviewed and approved all procedures carried out by Bioqual.
Plasmid design
The DNA vaccine combination used in this study has been previously described in Laddy et al. (2008). For non-human primate (NHP) studies, DNA preparations were made at VGX Pharmaceuticals, Inc. (The Woodlands, TX) as previously described (Hirao et al., 2008) and formulated at 10 mg/mL in water plus 1% w/w poly-l-glutamate sodium salt. In brief, the vaccine consists of a mixture of three consensus optimized plasmids: a plasmid encoding for the avian influenza H5 hemagglutinin (H5HA), a plasmid encoding for neuraminidase 1 (N1NA), and a plasmid encoding for a fusion influenza A matrix 2 and nucleoprotein sequences (M2eNP). Sequences were downloaded from the Los Alamos National Laboratory Influenza Sequence Database, and chosen from geographically diverse locations. MegAlign (DNASTAR, Madison, WI) was used to align the sequences and generate consensus sequences. The constructs were optimized for expression, including codon and RNA optimization (GeneArt, Regensburg, Germany).
The IL-15 plasmid (pmacIL15ECRO) was optimized for high levels of expression. The native IL-15 leader peptide sequence (LSP) was replaced with an IgE leader (Kutzler et al., 2005). Moreover, the codon usage was adapted. It has been determined that the wildtype macaque IL15 gene uses rare codons with a high frequency and the GC content is quite low (35%) which facilitates quick mRNA turnover. Therefore, GC content was increased (56%) to prolong mRNA half-life. The final design of the gene contained 100% congruence with mature form of macaque IL15 with the IgE leader replacing the wild type LSP form.
Immunization
Groups of four Chinese rhesus macaques were immunized three times at weeks 0, 4 and 8, intramuscularly (IM) with 0.5 mg of each of the flu DNA antigens for a total of 1.5 mg of flu DNA. An increasing dose of IL-15-encoding plasmid as listed in Table 1 was co-delivered. All electroporation procedures were performed using the CELLECTRA® device (VGX Pharmaceuticals, Inc., The Woodlands, TX). Three 52 ms pulses were delivered at a constant current of 0.5 A separated by 1-second intervals.
ELISpot assay for IFN-γ
ELISpot assays, using IFN-γ reagents (MabTech, Sweden) and nitrocellulose plates (Millipore, Billerica, MA), were performed according to the manufacturer’s instructions. A positive response was defined as greater than 50 spot-forming cells (SFC) per million peripheral blood mononuclear cells (PBMCs) and two times above background. Data presented in graphs are antigen-specific responses with background deducted. Each sample was performed in triplicate with peptide pools. Peptide pools consist of 15-mer peptides overlapping by 11 amino acids.
T cell proliferation and memory T cell subset assay
PBMCs were incubated with carboxyfluorescein succinimidyl ester (CFSE) (5 μM) for 8 min at 37 °C. Cells were washed and incubated with antigens (H5HA, M2eNP and NA peptide pools) at a concentration of 5 μm/mL for 5 days at 37 °C in 96-well plates. Cultures without peptide were used to determine the background proliferative responses. After the 5 day incubation PBMCs were stained with the following mAbs: anti-CD3 APC-Cy7 (BD-Pharmingen, San Diego, CA), anti-CD4 PerCP-Cy5.5 (BD-Pharmingen, San Diego, CA), anti-CD8 APC (BD-Pharmingen, San Diego, CA), anti-CD28 ECD (Beckman Coulter, Fullerton, CA), and anti-CD95 PE-Cy5 (BD-Pharmingen, San Diego, CA). Central memory and effector memory T cells were defined as CD28+CD95+ and CD28−CD95+, respectively (Pitcher et al., 2002). Stained cells were washed in PBS and fixed (Cell-Fix). Stained and fixed cells were then obtained on an LSRI cytometer device using CellQuest software (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Hemagglutination inhibition (HI) assay
Sera were treated with receptor-destroying enzyme (RDE) by diluting a one part serum with three parts enzyme and incubated overnight in 37 °C water bath. The enzyme was inactivated by 30 min incubation at 56 °C followed by addition of six parts PBS for a final dilution of 1/10. HI assays were performed in V-bottom 96-well microtiter plates, using four hemagglutinating units (HAU) of virus and 1% horse RBC as previously described (Stephenson et al., 2004). Viruses used for the HI assay are reassortant strains obtained from the CDC, influenza branch (Atlanta, GA): clade 1 A/Viet/1203/2004 (H5N1)/PR8-IBCDC-RG.
IL-15 measurement
Rhesus IL-15 was measured on days 0, 2, 4 and 7 after the third EP using the human IL-15 chemiluminescent immunoassay QuantiGlo kit (R&D Systems, No.D1500) in all animals, per manufacturer’s instructions.
Statistical analysis
Statistical analysis of the data was performed using Microsoft Excel package or Prism GraphPad Software. Data analysis was carried out with treatment comparisons using the Wilcoxon Signed Rank Test or one-way ANOVA, where statistically significant results are defined as having a P value of less than 0.05.
Acknowledgments
This research was supported in part by the National Institutes of Health (NIH) Grants N01-AI-50010, P01-A1-071739, R01-A1-071186, and the National Institutes of Health Intramural Research Program. Hanne Anderson at Bioqual for running HI Titers.
References
- Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol. Res. 2008;42:219–232. doi: 10.1007/s12026-008-8076-3. [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodles-Brakhop AM, Draghia-Akli R. DNA vaccination and gene therapy: optimization and delivery for cancer therapy. Expert Rev. Vaccines. 2008;7:1085–1101. doi: 10.1586/14760584.7.7.1085. [DOI] [PubMed] [Google Scholar]
- Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA, Kumar S, Parkinson R, Wu L, Sidhu MK, Pavlakis GN, Felber BK, Brown C, Silvera P, Lewis MG, Monforte J, Waldmann TA, Eldridge J, Weiner DB. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18648–18653. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi J, Marshall JD, Salvucci O, Frank I, Chehimi S, Kawecki S, Bacheller D, Rifat S, Chouaib S. IL-15 enhances immune functions during HIV infection. J. Immunol. 1997;158:5978–5987. [PubMed] [Google Scholar]
- Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, Garcia-Hand D, Montefiori DC, Quiroz J, Rosati M, Schadeck EB, Boyer JD, Pavlakis GN, Weiner DB, Sidhu M, Eldridge JH, Israel ZR. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. 2007;25:4967–4982. doi: 10.1016/j.vaccine.2006.11.070. [DOI] [PubMed] [Google Scholar]
- Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, Parkinson R, WU L, Sidhu MK, Phillipson-Weiner R, Pavlakis GN, Felber BK, Lewis MG, Shen A, Siliciano RF, Weiner DB, Sekaly RP. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J. Immunol. 2008;180:7969–7979. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat. Rev., Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, Ramanathan MP, Parkinson R, Kudchodkar S, Tamura Y, Sidhu M, Roopchand V, Kim JJ, Pavlakis GN, Felber BK, Waldmann TA, Boyer JD, Weiner DB. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J. Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- Laddy DJ, Yan J, Kutzler M, Kobasa D, Kobinger GP, Khan AS, Greenhouse J, Sardesai NY, Draghia-Akli R, Weiner DB. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS ONE. 2008;3:e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxembourg B, Lindhoff-Last E. Genomic diagnosis of thrombophilia in women: clinical relevance. Hamostaseologie. 2007;27:22–31. [PubMed] [Google Scholar]
- Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 2002;76:243–250. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, Altman JD, Katsikis PD. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, Villinger F, Cairns JS, Gracely EJ, Lewis MG, Katsikis PD. Interleukin-15 increases effector memory CD8+ t cells and NK cells in simian immunodeficiency virus-infected macaques. J. Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, Lewis MG, Katsikis PD. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 2008;180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr., Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- Sprent J. Turnover of memory-phenotype CD8+ T cells. Microbes Infect. 2003;5:227–231. doi: 10.1016/s1286-4579(03)00015-7. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–95. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, Sugimoto C, Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- Yin J, Dai A, Kutzler MA, Shen A, Lecureux J, Lewis MG, Waldmann T, Weiner DB, Boyer JD. Sustained suppression of SHIV89.6P replication in macaques by vaccine-induced CD8+ memory T cells. AIDS. 2008;22:1739–1748. doi: 10.1097/QAD.0b013e32830efdae. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]