Abstract
Development of a vascularized tissue is one of the key challenges for the successful clinical application of tissue engineered constructs. Despite the significant efforts over the last few decades, establishing a gold standard to develop three dimensional (3D) vascularized tissues has still remained far from reality. Recent advances in the application of microfluidic platforms to the field of tissue engineering have greatly accelerated the progress toward the development of viable vascularized tissue constructs. Numerous techniques have emerged to induce the formation of vascular structure within tissues which can be broadly classified into two distinct categories, namely (1) prevascularization-based techniques and (2) vasculogenesis and angiogenesis-based techniques. This review presents an overview of the recent advancements in the vascularization techniques using both approaches for generating 3D vascular structure on microfluidic platforms.
1. Introduction
Development of three dimensional (3D) vascularized tissue has been a major challenge hindering the widespread clinical application of tissue engineering [1, 2]. Due to a lack of proper vascularization methods, the current techniques of tissue engineering have encountered severe limitations when applied to the development of vascularized complex 3D tissues, particularly those intended for large vital organs such as the liver, kidney, and heart [3, 4]. Adequate vascularization of tissue structures is, therefore, crucial for improving survival rate and function of implanted tissue engineered constructs [5]. The ability to induce and control vascularization of tissues will also help in advancing the clinical utility of therapeutic angiogenesis such as in healing of tissues affected by chronic wounds associated with diabetic ulcers, myocardial ischemia and peripheral arterial ischemia [6–9].
In addition, in vitro models of blood vessel will pave the path for the development of various vascular disease models that could revolutionize the new therapeutics for atherosclerosis, hypertension, cardiac arrest, stroke, cancer and many other diseases [10–12]. Furthermore, the progress of patient specific smart diagnostics and personalized medicine will greatly benefit from the development of functional healthy or diseased models of blood vessels on microfluidic platforms. Thus, the ability to form functional blood vessels within engineered tissues, ranging from tens of microns to several millimeters, will greatly expand the application of tissue engineering. However, despite the extensive research on vascularization and 3D blood vessels, the formation of a fully functional tissue engineered blood vessel and/or a 3D fully functional vascular network have remained elusive.
Microscale technologies and microfluidic systems have emerged as useful tools to overcome the challenges of developing an artificial microvasculature [2, 5, 13]. The merger of hydrogels and microfabrication techniques has further led to the development of microfluidic platforms[14] that offer precise control over various aspects of the cellular microenvironment [15] including fluid flow [16, 17], chemical gradients [18, 19], localized extracellular matrix (ECM) [20–23] as well as the microenvironmental cues such as mechanical properties (e.g. stiffness) [24], chemical properties (e.g. ligand density and orientation) and topographic features (e.g. patterning of surfaces with substances having different cell-substrate affinity) [25, 26]. All of these advantages can be used to facilitate the formation of biomimicking in vitro blood vessel models and vascularized networks in 3D engineered tissue constructs [27].
In this review we highlight the recent advancements in vascularization techniques for the fabrication of in vitro blood vessel models and vascularized networks in 3D engineered tissue constructs. We briefly describe the different types of vascular structures, the natural processes involved in their formation in vivo and the design criteria for their in vitro fabrication. Next, we discuss different techniques developed so far for fabricating vascular structures. The scope of the review is limited to the techniques for fabrication of microscale vasculatures in the size ranges of tens of microns to hundreds of microns, even though some of the presented techniques might also be suitable for fabrication of medium to large size vascular structures for in vitro blood vessel models. The two main types of techniques namely the prevascularization and the vasculogenesis/angiogenesis-based ones have been discussed with a particular focus on their use in conjunction with microfluidic platforms.
2. Biology of Native Blood Vessels
The successful development of biomimetic in vitro fabricated vascularized tissue requires an in-depth understanding of the biology of native blood vessels as it is important to replicate the anatomical, physiological and functional aspects of the native vasculatures at different length scales [28]. In the context of tissue engineering, the development of vascularized tissues has two distinct aims: 1) to carry the necessary oxygen and nutrients and remove the waste products from the surrounding cells in an implantable tissue engineered construct, and 2) to replace a damaged native blood vessel, or to be used as in vitro blood vessel models for ex-vivo studies of vascular biology, cardiovascular pathology, as well as pharmacological modelling, drug testing and connecting multiple organs in body-on-a-chip applications [29]. For both of these aims it is important to understand the processes of native blood vessel formation in vivo which we discuss here in brief.
2.1. In vivo Formation of Native Blood Vessels: Vasculogenesis and Angiogenesis
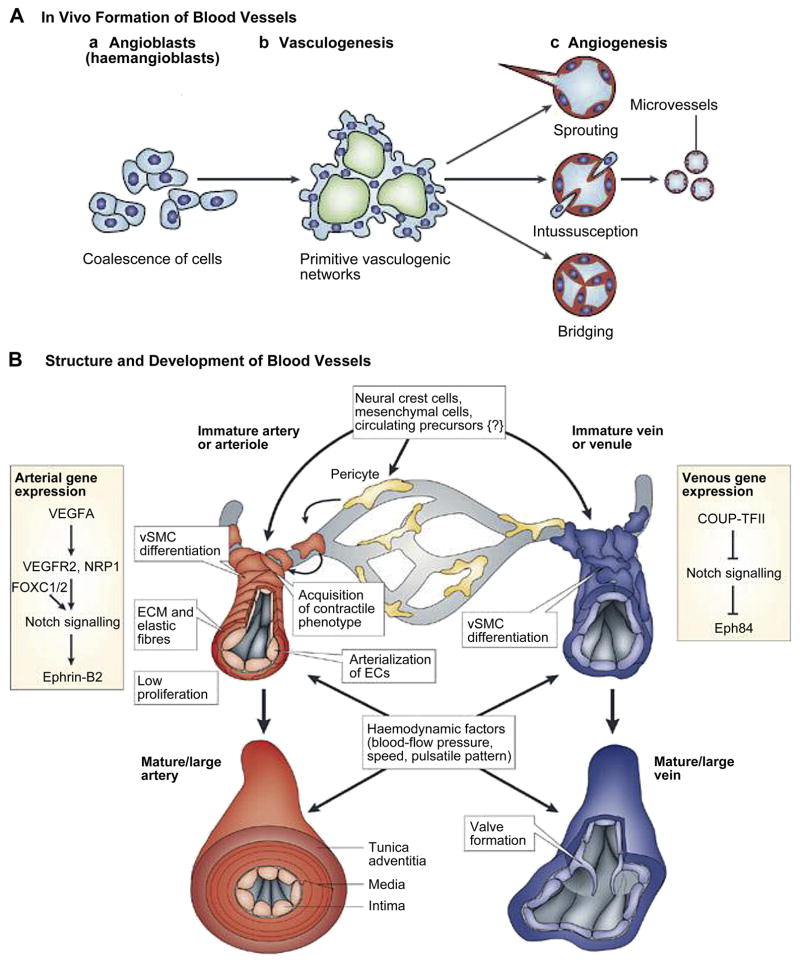
The mechanisms of in vivo formation of native vasculature can be classified into two types, namely, vasculogenesis and angiogenesis, Fig. 1a [30]. Vasculogenesis gives rise to the first vascular plexus and heart during embryo formation [31]. It is the process whereby new blood vessels are formed from endothelial progenitor cells (EPCs). At first the mesodermal stem cells are differentiated into EPCs, also known as angioblasts. The EPCs migrate to different regions forming discrete blood islands which eventually fuse together to form a vascular plexus and endothelial cells (ECs). The ECs migrate and organize themselves into nascent endothelial tubes and form capillaries [32]. Vascular endothelial growth factor (VEGF) expression is required for angioblasts’ differentiation [33]. The recruitment of pericytes, smooth muscle cells (SMCs) and fibroblast layers around the endothelial tubes turn them into more matured and larger blood vessels such as arterioles, arteries, venioles, and veins [34]. Even though vasculogenesis is mainly a developmental event, it can also occur in adult mammalians in the case of revascularization following extensive damage or during tumor growth [14].
Figure 1.
Schematic representation for mechanisms of in vivo blood vessels formation and their structure, (A) vasculogenesis and angiogenesis: two predominant mechanisms of blood vessel formation. Vasculogenesis gives rise to the primitive vascular plexus during embryogenesis. Angiogenesis remodels and expands the vascular network through biochemical cues including growth factor gradient, budding tumors, and hypoxia conditions [30]. (B) Schematic diagrams for structure of blood vessels: Cross-section of arteries with their major components and maturation steps [39]. Figures reproduced from [30] and [39] with permissions from Nature publishing group.
Angiogenesis is the expansion and remodeling of the vascular network through the sprouting of ECs from existing vessels. It results from a sequence of events influenced by cell-cell and cell-ECM interactions [35]. The process starts with the sprouting of ECs and continues with the stabilization of the formed capillaries by pericytes. Angiogenesis can also happen through intussusception which is the formation of new blood vessels via splitting of an existing one, in which case it is also commonly referred to as splitting angiogenesis [35].
2.2. Structure of Native Blood Vessels at Different Length Scales
The native blood vessels have complex unique structures at different length scales [10, 36]. The inner diameter of blood vessels ranges from microscopic size, 5 μm for the smallest capillaries, to 25 mm, for the largest artery (aorta). The blood vessels on the arterial side of the capillaries can be divided into elastic arteries, muscular arteries and arterioles while those on the venus side are divided into veins and venioles [37]. The walls of the large vessels, namely elastic arteries, muscular arteries and veins have three distinct layers starting from the vessel lumen: intima, tunica media and tunica adventitia respectively [38], Fig. 1b [39]. Intima, the innermost layer is a thromboresistent confluent monolayer of ECs which is attached to a basement membrane (40–120 nm) [40]. Media, the middle layer, is comprised of a dense population of concentrically organized SMCs with bands or fibers of elastic tissues, and adventitia, the outermost layer is a collagenous ECM containing mainly fibroblasts and perivascular nerves. In arterioles (diameter ~30 μm) some of these layers might be less obvious or absent [41] while the smallest vessels, capillaries, are only composed of a single monolayer of ECs, basement membrane and pericytes. An internal elastic lamina and an external elastic lamina separate the intima from the media, and the media from the adventitia respectively [42].
The nutrition for the vascular wall itself is supplied by small vasculatures existing throughout the adventitia. The adventitia also contains innervations [43]. The stress, both longitudinal and lateral, due to the pulsatile nature of the blood flow is mainly born by tunica media [44]. There is a gradient of physical properties from central to peripheral vascular tree, i.e. arteries that are closer to the heart are thicker and more compliant whereas further along the vascular tree arteries are considerably thinner and stiffer. As the pressure goes down and the arteries give way to arterioles elastic properties of tunica media and the presence of tunica adventitia become less prominent [45].
The ECs are adherent to the luminal surface of the vessels [46]. They form a continuous monolayer, and are in direct control of blood homeostasis, interaction with immune system cells, and regulation of the molecular exchange to and from the blood and the activities of the surrounding SMCs. The most distinguishing property of ECs is their wide range of strong cell to cell junctions [47]. They react to shear stress by increasing their surface area through spreading. In vivo, they are elongated in the direction of the blood flow [48]. A healthy endothelium prevents initiation of the coagulation cascade. Under normal conditions ECs secrete the anticoagulant thrombomodulin [49], but in case of an injury they start to express pro-platelet adhesive proteins such as selectins. The endothelial lining also directs the behavior of SMCs and white blood cells by secreting various signaling molecules. One of these secreted bioactive molecules that have important roles in homeostasis of the vasculature is nitric oxide (NO). It is a free radical with a wide range of functions including inhibition of vasoconstrictor signals during vasodilatation, prevention of platelet adhesion, and exhibition of anti-inflammatory and anti-proliferative effects [50]. It is important to control the EC phenotypes for a tissue engineered blood vessel, i.e. the endothelial lining should be in a phenotypic state in which it prevents blood coagulation. The absence of an intact endothelium induces conversion to a synthetic phenotype for SMCs [51].
SMCs are in the quiescent, contractile state under normal conditions. Following injury, they convert to a more synthetic phenotype resulting in cell proliferation, MMP mediated enzymatic degradation of ECM and vessel wall remodeling via newly secreted ECM. This is possible due to the phenotypic plasticity of SMCs, which enables them to switch between a wide range of phenotypes with distinct characteristics; defined as contractile and synthetic phenotypes for the two extreme conditions [52]. SMCs are very sensitive to strain, and dynamic culture experiments have shown that SMCs become more oriented under controlled strain. Cell proliferation is also affected by the application of strain. These effects are sensed by SMCs through the interaction of their integrin mediated connections with the ECM [53]. In vivo, tunica media, populated by SMCs, is responsible for vascular tone/diameter and in this sense highly differentiated SMCs are responsible for the dilatation and constriction of vessels [54].
Fibroblasts, the predominant cell type found in adventitia layer, are mainly responsible for the secretion of highly collagenous ECM [55]. Even though in the past, adventitia and the fibroblasts were considered as passive supporting components; recent studies have shown the involvement of fibroblasts in response to injury and in extensive interaction with SMCs in tunica media [56]. Under normal conditions, they are static fibroblasts but triggers such as injury can convert them to myofibroblasts. In tissue engineering they have been commonly used in scaffold-free vessel production methodologies due to their extensive ECM secretion [57].
2.3. Functions of Blood Vessel
Although carrying the oxygenated blood from the heart to different parts of the body and returning the deoxygenated blood from various parts to the heart are among the most important functions of blood vessels, these are not their only functions. For instance, maintaining proper solute to water balance in the blood and tissues, shielding the parenchymal cells from interstitial fluid shear, and providing appropriate physical and chemical signals to the surrounding tissues are just few of examples of the many functions of blood vessels [58–61]. For successful vascularization of tissue engineered constructs and formation of model blood vessels it is therefore important to understand and replicate the biological and physical functionalities of different elements of blood vessels [62].
The main physical properties of blood vessels include their elasticity and burst strength, as they need to go through considerable deformations and stress fluctuations as part of their function [63]. Tunica media is a complex fiber reinforced composite with interspersed SMCs that exhibit non-linear viscoelastic behavior [64]. The mechanical properties of the blood vessels are related to the abundance and orientation of its ECM components. The main components present in the ECM of blood vessels, e.g. collagen, proteoglycans and elastin all have distinct functions [65]. Collagen is the load bearing constituent. The most abundant collagen types in blood vessels are collagen type III and type I. It is present in the form of self-assembled fibrils whose particular orientation is the main reason of tissues’ anisotropic mechanical properties. Elastin, as its name suggests, is a highly elastic protein. 3D networks of elastin are the reasons for vessel elasticity [66]. The elastin network gets oriented in response to stress and as a result store potential energy. The vessels can go through significant deformations and exhibit viscoelastic properties, which are related to the presence and activities of proteoglycans, another type of ECM components [67].
The mechanical properties of the vessels have some specific characteristics such as anisotropic response to stress/strain conditions, non-linear stiffening and the dependence of the final strength to the strain rate [68]. The stress induced by the blood flow causes approximately 10–20% strain. The response of the blood vessels to stress happens in three phases. The initial phase is a linear, isotropic response due to elastin fiber stretching. In the second phase, the response becomes nonlinear due to the straightening of collagen fibers which resists the stress. The collagen fibres are completely elongated in the third phase and the vessel becomes stiffer with the increase in stress [69]. In designing and fabrication of vascular structures it will be beneficial to mimic these behaviors.
Moreover, the most important mechanical parameter for arteries is the burst strength, which depends on the materials, vessel diameter and thickness. Vessel compliance is also an important parameter [70]. In vivo, neointimal hyperplasia can occur due to compliance mismatch between the transplanted vessel and the target. The capacity to recoil and being extensible are indispensable properties of the vessel walls. This enables the SMC mediated constriction and dilation of the vessels [71]. If an artificial vessel lacks elasticity, it can dilate and be weakened by creep [72]. These effects can be mimicked in on-chip artificial blood vessel models. Such models would also provide a method to monitor the constructs under highly controlled microenvironments that can mimic in vivo stress/strain conditions [10].
The function of EC monolayer is to provide selective barrier functions to different constituents of blood, control the blood flow and the tone of vessel walls, ensure the thrombo-resistance of the luminal surface [73] and to modulate the adhesion of leukocytes [74]. The EC monolayer is also important for regulating the gaseous and molecular (oxygen and nutrients) exchange, as well as the signaling to the muscular component of the vessel wall [66]. The SMCs in the middle layer (media) have a specialized contractile function and the fibroblast cells in the outer layer are mostly responsible for secreting and remodeling of ECM molecules. Thus ensuring the formation of a continuous EC monolayer as well as the presence and appropriate phenotype of SMCs and fibroblast cells are important for proper functioning of a tissue engineered vascular structure [10].
3. In Vitro Techniques for Vascularization
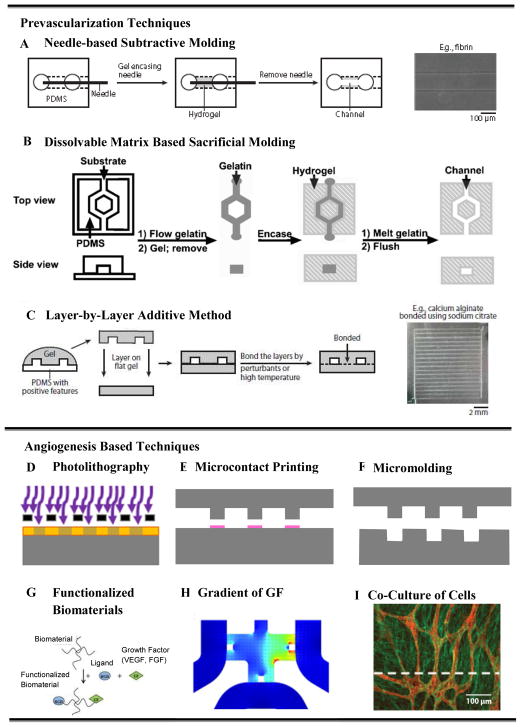
Early work on vascularization of tissues involved generating two dimensional (2D) vascularized constructs of non-biodegradable, e.g. silicon, pyrex [75], and PDMS [76] channels using photolithography and soft lithography techniques. The non-biodegradability and lack of biocompatibility of these systems made them unsuitable for implantation. The subsequent studies employed biodegradable materials such as poly(lactic-co-glycolic) acid (PLGA) [77], poly(glycerol-sebasate) (PGS) [78], polycaprolactone (PCL) [79], and silk fibroin protein [80]. Even though these materials are biodegradable, their poor interaction with cells still remained as the main concern. The early 3D vascularized tissue formations were based on stacking up 2D vascularized polymer films into large 3D structures. For example, a PLGA network by King et al. [77], and a PGS network by Bettinger et al. [81]. However stacking of 2D layers to build a 3D vascular network is a cumbersome process [5]. The more modern techniques involve 3D hydrogel structures, which offer a number of advantages including the ability to engineer biomimetic microenvironments with tunable physical (e.g. biodegradability), mechanical (strength and elasticity), and biological properties (e.g. cell adhesion, spreading, migration and proliferation). Examples include but are not limited to the calcium alginate [82, 83], agarose [84], collagen and fibrin [85], poly(ethylene glycol) diacrylate (PEGDA), and methacrylated gelatin (GelMA) [86] hydrogels. A schematic overview of various recently developed techniques for vascularization in 3D hydrogels is presented in Fig. 2. The methods include prevascularization-based methods such as needle-based molding (Fig. 2A), sacrificial molding (Fig. 2B), and additive bonding of prevascularized hydrogel slabs (Fig. 2C); and vasculogenesis/angiogenesis-based methods that use microfabrication, such as, photolithography (Fig. 2D), microcontact printing (Fig. 2E) and micromolding (Fig. 2F), as well as vasculogenesis/angiogenesis-based methods that use functionalization of biomaterials (Fig. 2G), gradients of growth factors (Fig. 2H), and co-culture of multiple cells (Fig. 2I). These modern approaches of vascularization can be broadly classified under two main categories, (1) prevascularization-based techniques, and (2) vasculogenesis and angiogenesis based techniques.
Figure 2.
Schematic diagrams of different types of in vitro vascularization techniques: (A–D) various prevascularization techniques, (A–B) subtractive methods, (A) stainless steel needle- based molding, (B) dissolvable-network-based sacrificial molding, (C) additive method - soft lithography/PDMS stamping-based micromolding and layer-by-layer stacking, (D) hybrid method – Bioprinting, (E–I) various vasculogenesis and angiogenesis-based techniques, (E) photolithography, (F) microcontact printing, (G) functionalization of scaffold material, (H) gradient of growth factor in a microfluidic device, and (I) co-culture of multiple cells. Figures adopted and modified from [83, 85, 87, 98, 160, 161] with permissions from American Chemical Society, Royal Society of Chemistry and Elsevier Science.
3.1. Prevascularization-based Techniques
Prevascularization of engineered tissue constructs is a relatively recent and fast growing approach that has drawn tremendous attention lately. A major advantage of these approaches lie in that they allow immediate perfusion of the constructs helping the proliferation and growth of the cells from the very beginning. Moreover, the delivery of oxygen and nutrients, and the removal of metabolic wastes can be performed continuously in a biomimetic manner. Various prevascularization methods that have been used so far can be grouped under (i) subtractive methods, (ii) additive methods, and (iii) hybrid methods.
3.1.1. Subtractive Methods
In subtractive methods, a hollow structure, i.e. a single channel or a network of channels is formed by removing a sacrificial material from a hydrogel. Examples include removal of a sacrificial template either by extraction of a cylindrical object or by dissolving a pre-formed network of a sacrificial material. Hence the subtractive approach used by various researchers can be grouped into (i) needle-based molding method, and (ii) dissolvable network-based sacrificial molding.
3.1.1.1. Needle-Based Molding Method
When the purpose of making blood vessels is to build experimental models for in vitro studies of vascular functions, a single channel might be sufficient instead of a branched vascular network, and a simple method can be used by pre-inserting a needle, wire or other cylindrical structures in a pre-polymer solution followed by removal of the cylindrical object after cross linking of the gel. Joe Tien and colleagues [87, 88] developed this “engineered” approach using stainless steel needle-removal-based subtractive method. In this approach of Chrobak et al. [87] and Price et al. [88], a cylindrical channel was formed in collagen type I hydrogel by casting the hydrogel around a stainless steel needle. After the gel was formed, the needle was removed, creating a cylindrical channel that could be readily perfused. ECs were seeded into these channels forming a confluent EC monolayer that exhibited tight EC junctions, Fig. 3A, strong barrier functions, resistance to the adhesion of leukocytes, and appropriate reactions to the tested inflammatory cytokines.
Figure 3.
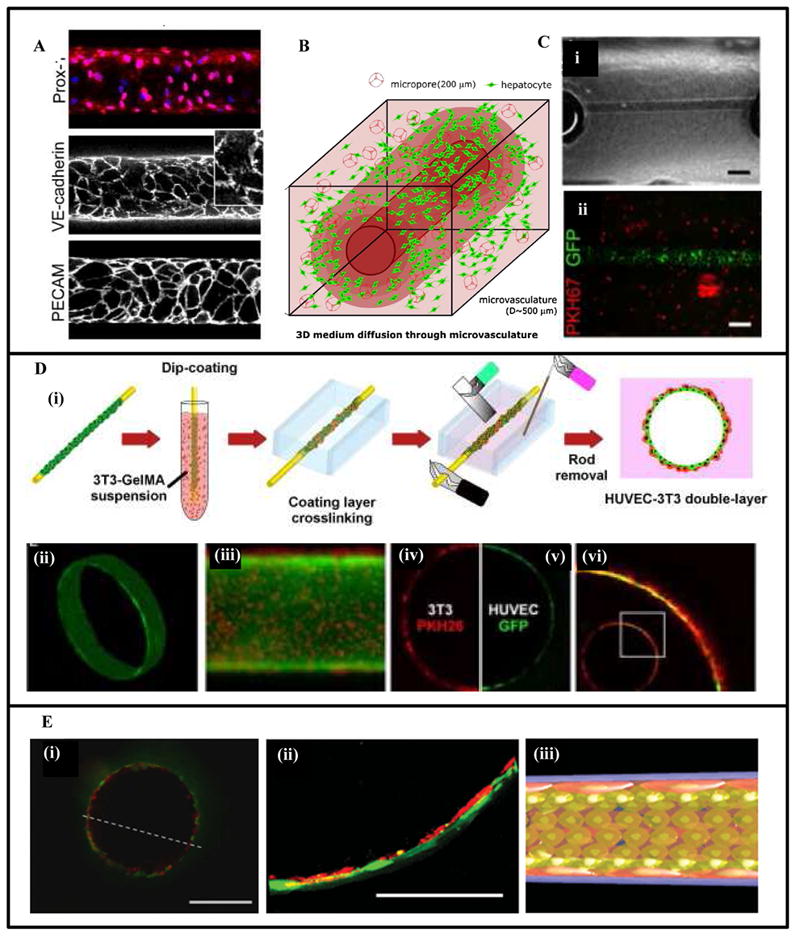
Fabrication of single vascular-like structures in hydrogels using needle-based subtractive prevascularization technique. (A) Microvascular tubes were formed in type I collagen hydrogel by extracting a pre-inserted 120-μm-diameter stainless steel needle. Removal of the needle generated cylindrical channels that were seeded with microvascular ECs. The EC monolayer exhibited strong cell-cell junctions as shown by expression of Prox-1, junctional markers VE-cadherin and PECAM [162]. (B) A cylindrical channel was formed in a cell-laden hydrogel using extraction of a stainless steel microneedle [89]. (C) A 300 μm microchannel in cell-laden GelMA hydrogels seeded in the lumen with GFP-labeled-HUVECs [86]. (D) Fabrication of a vascular-like structure in cell-laden GelMA using cell transfer technique. A peptide chemically adsorbed on a gold wire was used to adsorb/capture cells that were transferred to a hydrogel construct by embedding the rod in hydrogel and applying an electrical voltage. The gold was then removed leaving a channel seeded with HUVECs. The multilayer structure was generated by an additional dip-coating step in GelMA solution enriched with 3T3 cells, (i) schematic representation of the dip-coating-cell-transfer technique, (ii) a 3D continuous monolayer of ECs in the microvasculature, (iii) the bilayer structure of 3T3-HUVECs, (iv–vi) confocal fluorescence image of the cross section, red: 3T3 cells, green: HUVECs [90]. (E) A multilayered capillary structure fabricated using a hierarchical cell deposition technique in a prevascularized polyglycolic acid (PGA) scaffold. A uniaxial microchannel was formed inside the scaffold by extraction of a silica tube. Layers of SMCs and ECs were formed sequentially inside the channel using a hierarchical cell deposition [91], (i) confocal fluorescence image of the fabricated bilayer capillary structure, green: SMCs, red: HUVECs, (ii) a magnified image of (i), (iii) schematic representation of the bilayer structure. Scale bar: 200 μm in C(ii), 400 μm in E (i), and 100 μm in E (ii). Figures modified from [87] [89] [86] [90] and [91] with permissions from Elsevier Ltd. Wiley Periodicals Inc. and Wiley-VCH Verlag GMbh & Co. respectively.
Khademhosseini and colleagues also used the needle-based approach to show that perfusable microchannels with EC-seeded lumen can be formed in cell-laden hydrogels, Figs. 3B [89], C [86]. Sadr et al. [90] combined a self-assembled-monolayer (SAM)-based cell transfer technique with the needle-based prevascularization method to obtain a free standing multilayered blood vessel with a hollow channel and controlled geometrical design. They used 600 μm diameter gold sputtered rods modified with SAM-oligopeptides coating. Layers of human umbilical vein endothelial cells (HUVECs) and 3T3-fibroblast cells were formed on these rods by dipping them in suspensions of 3T3 cells and HUVECs in GelMA pre-polymer, followed by UV-cross-linking. The rod was then removed using an electrical stimulation after transferring the cell layers in a GelMA matrix, Fig. 3D.
Yoshida et al. [91] used a hierarchical cell manipulation technique in combination with a needle- based prevascularization method to form a bilayered blood capillary mimetic comprising a SMC layer and an EC layer. The authors fabricated uniaxial microchannels in γ-PGA-SS (poly (γ-glutamic acid) with di-sulfide linkage) hydrogels by extracting 620 μm diameter silica capillary tubes. Layers of umbilical artery smooth muscle cells (UASMCs), fibronectin-gelatin (FN-G) thin membrane and HUVECs were formed inside the luminal surface by a sequential deposition to obtain a bilayer vascular structure of UASMC and HUVECs, Fig. 3E. The vascular construct exhibited strong barrier functions similar to native blood capillaries.
Despite its numerous merits, which include its ease of fabrication, the needle-based method, however, is not suitable for forming complex networks of interconnected channels as is required for vascularization of large tissues.
3.1.1.2. Dissolvable Network-Based Sacrificial Molding
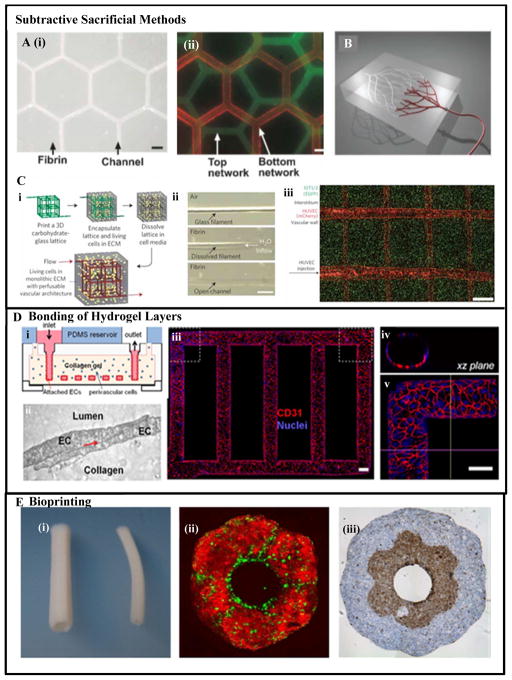
In the dissolvable-network-based sacrificial molding, at first a 2D or 3D network of a negative mold is formed using an easily dissolvable gel or solid material. The pre-formed mesh or network is then encapsulated in a 3D hydrogel. Finally, the sacrificial mesh or network is dissolved or melted and flushed out from the gel matrix, leaving behind interconnected channels in the hydrogel structure. Andrew et al. [85] used this technique employing micromolded meshes of gelatin as the sacrificial material in collagen and fibrin hydrogels, Figs. 4A(i–ii). Others employed 3D printing technology for forming the dissolvable-network of sacrificial material [92], Fig. 4B.
Figure 4.
Vascularization using various prevascularization techniques: (A) Sacrificial molding by dissolving a gelatin mesh in fibrin and collagen hydrogels [85], (i) overlaid phase contrast images of a hexagonal vascular network in fibrin gel perfused with fluorescent microspheres, (ii) perfusable collagen gel with multiplanar networks shown with an overlay of suspensions of green and red fluorescent beads, respectively; (B) Prevascularization using omnidirectional printing of 3D microvascular networks with a fugitive ink into a physical gel reservoir. The technique allows full control of channel hierarchical and branching patterns. Post photocrosslinking the ink (shown in red) is liquefied and removed slowly under pressure exposing the microvascular channels [96]. (C) A rapid casting-based prevascularization technique (i) schematic overview of generating 3D vascular network: an open, perfusable vascular network was generated using 3D printing of an interconnected sacrificial carbohydrate network able to support cell-laden hydrogels [96], (ii) a single carbohydrate-glass fiber approximately 200 μm is encapsulated in a fibrin gel showing the dissolution of the carbohydrates within hydrogel post-crosslinking, (iii) viable cells constitutively expressing enhanced green fluorescent protein (eGFP) imaged with confocal microscopy z-stack to visualize two intersecting channels demonstrating endothelialization of channel walls and across the intervessel junctions. (D) A microfluidic vessel networks (μVNs) fabricated using micromolding method followed by additive stacking and bonding of two hydrogel slabs [99]: (i) schematic cross-sectional view of the microfluidic collagen construct after fabrication, (ii) cell-cell junction of EC seeded inside the channels, (iii) Z-stack projection of horizontal confocal sections of endothelialized microfluidic vessels, (iv) view of xz plane and (v) view of a corner. Red: CD31, blue: nuclei. (Scale bar: 100 μm) [99]. (E) Vascular tubes fabricated using bioprinting technique [110], (i) the tubular constructs after fusing the cylindrical bioink, (ii) a bilayer tubular construct fabricated using spheroids of ECs (green) and SMCs (red), (iii) a bilayer construct fabricated using building blocks of fibroblast cells (outer layer) and SMCs (inner layer) representing the adventitia and media of a blood vessel. Figures reproduced and modified from [85, 92, 96, 99, 110] with permissions from the Royal Society of Chemistry, Wiley-VCH Verlag GMbh & Co. Nature Publishing, PNAS and IOP Publishing respectively.
Recently, more complex structural motifs have been generated for vascularization of tissue constructs with methods such as the direct ink writing (DIW), allowing for better replication of 3D microvascular structures while retaining relatively simple fabrication under benign conditions [93–95]. In the DIW method a fugitive organic ink is used to create uniform microchannels interconnected into a 3D network. However, architectural motives that could be fabricated with DIW were limited. A solution to overcome this challenge has been developed that creates more representative biomimetic 3D interpenetrating microvascular networks by using vertical printing combined with dual fugitive inks [93]. However, a more advanced method for fully unconstrained vascular network printing is based on omnidirectional printing (ODP) method, where fugitive ink networks are printed within a reservoir of a photocrosslinkable gel that provides physical support to the designed patterns and allows for unconstrained printing of motifs [96].
Using a carbohydrate sacrificial material in combination with 3D printing and cell infusions Chen and colleagues employed a rapid prototyping technique for developing vascular networks. They created a 3D perfusable vascular-like network architecture surrounded by ECM mimetic hydrogels. Prior to adding hydrogels with encapsulated cells, a 3D carbohydrate-based backbone was printed using 3D printing technology, acting as a support during hydrogel crosslinking. Once the gels were solidified the carbohydrate glass network could easily be dissolved in water leaving behind a perfusable, hollow, cylindrical channel network mimicking vascular-like structures and architecture of more complex tissues, Fig. 4C. The group then infused and seeded the hollow network with HUVECs. This led to the formation of an endothelialized channel network that behaved similar to native microvessels. Spontaneous formation of HUVEC sprouts was observed. The authors demonstrated the functionality of the fabricated network and showed that culturing under dynamic conditions could sustain cell viability deep inside the scaffold, facilitating nutrient delivery and waste removal within the construct.
Additional advantages of this approach lie in the fabrication method. In general, the technique is highly versatile allowing selection of an array of cell types that can be encapsulated within a range of ECM mimic hydrogel that act as support. Specifically, utilizing carbohydrate glass as a sacrificial material adds and improves to cytocompatibility. Next, the approach allows the control of channel diameters that compose the network. Printed filaments of different diameters can be seamlessly connected and controlled by varying the translational velocity of the extrusion nozzle. More importantly, the printed 3D channel structures can support their own weight, paving the way for designing highly complex interconnected structures available through 3D printer technology. Finally, carbohydrate glass-based materials offer optimal optical properties that do not interfere with photocrosslinking and enable cellular fluorescence and light imaging.
Miller et al. showed that emerging technologies such as 3D printing can provide highly versatile approaches in recreating the vasculature needed in the successful engineering of thick tissues. They clearly demonstrated a functionally perfusable hollow tubular network lined with HUVECs. The developed structure sustained the viability and proliferation of encapsulated cells and demonstrated the formation of spontaneous intrinsic vascular sprouts.
3.1.2. Additive Method- Bonding of Pre-formed Hydrogel Slabs
In the additive methods, a 3D network of interconnected channels is formed by layer–by-layer stacking and bonding of pre-formed planer hydrogel slabs. The slabs contain network of channels featured in 2D planes, pre-formed using micropatterning techniques such as photomask lithography or micromolding in such a way that when stacked together they result in a 3D network of channels. The adjacent layers are bonded together irreversibly using partial melting or fusion of hydrogel at the interfaces thereby resulting in a 3D tightly sealed interconnected perfusable network. One such method uses the micromolding technique, in which a master mold with desired microstructural features is created on a silicon wafer using microfabrication processes. A transfer mold is prepared by casting and curing an elastomeric polymer solution on the master mold. The transfer mold with the desired microstructural features is either bonded to a flat substrate resulting in microfluidic channels or networks, or is used as a stamp for imprinting the micropatterns to a flat hydrogel. Bonding of two adjacent flat slabs of hydrogels to ensure a leak-proof perfusable system have been successfully demonstrated [97] through partially dissolving the gel interface by chelating calcium (in case of calcium alginate hydrogel) [83], depolymerizing the gel interface using chaotropes (for natural gels such as collagen and fibrin) [98], and transient melting of the interface (e.g. silk fibronin) [80] and agarose gels [84].
Zheng et al. [99] recently developed a microfluidic tubular network using the additive bonding of a micropatterned hydrogel slab over a flat hydrogel layer, Fig. 4D. A silicone mold was used to cast PDMS stamps that imprinted micropatterns of a vascular network to a collagen casting gel. The biomimetic gel slab containing microscale features was then bonded to a flat collagen substrate forming a microvascular network. The authors demonstrated the formation of a smooth and functional endothelium layer without any leakage that also promoted angiogenesis. The developed microvascular network demonstrated various complicated angiogenic and thrombogenic processes such as vessel sprouting, interaction of ECs with mural cells, bioactivation of ECs in response to various biochemical agents, anti-thrombotic behavior of the EC monolayer and its expression of a pro-thrombotic behavior in presence of inflammatory signals. This model can be of immense value in designing a more complicated network, for understanding the blood-vessel function, interaction of blood vessels with tumors, and neoangiogenesis from circulating stem cells.
3.1.3. Hybrid Method- Bioprinting
Bioprinting is the process of printing living cells in a 3D space for the construction of a biological structure using computer-aided design and layer-by-layer deposition of cell-laden-matrices. It is relatively a new method and is a growing area of research. Currently, several groups are focused on generating 3D vascular structures and blood vessel mimics with the aid of bioprinting technique. As the method employs both the principles of additive layer-by-layer deposition and the subtractive sacrificial removal of filler material, we classify it as a hybrid method.
Two predominant approaches have arisen from the bioprinting technology, specifically those based on inkjet printing [100–104] and mechanical extruders [105, 106]. Inkjet printing is a versatile, robust and a cost effective method, as it relies on direct printing of individual or pockets of cells. However, substantial challenges remain with this approach, most notably the limitation in cell density that the inkjet method can achieve, especially considering cell densities meaningful for structures meant for fabrication of biomimetic organs. This barrier is further compounded by the observation that the high speed of cell deposition can lead to damage and lower cell survival. These challenges in addition to attaining appropriate structural and functional organization with scalability, resolution, and repeatability required for construction of 3D vascular networks still remain to be solved.
Bioprinting can be utilized for generating small and intermediate diameter blood vessels using mechanical extruder-based techniques [105, 106]. In these approaches the exact purpose of the mechanical extruders is to place the ‘bio-ink’ or multicellular aggregated particles (that could be made of multiple cell types) with a defined composition into a supporting structure or ‘bio-paper’. High consistency is achieved by computer-generated templates in order to mimic the desired biological topology [107–109]. This way, layer-by-layer complex structures are fabricated.
In general, the formation of organ-like structures is made from fusion and sorting of bio-ink particles once the printing process is complete, [110], Fig. 4E. Several advantages are obvious in generating 3D tissue constructs by employing these methods. Specifically it is beneficial that the cells find themselves in a physiologically relevant microenvironment both structurally for support and biologically for promoting adhesive cellular contacts allowing molecular signaling. These methods do require high initial cost for the necessary bioprinting instrumentation [109]. However, current research efforts are geared towards integrating intrinsic self-assembly principles from biology in the design of next generation of constructs, that perhaps could make these approaches more feasible and cost effective. Furthermore, tubular and vascular-like-structure can be built with various compositions and distribution of ECs and SMCs in which fundamental principles of blood vessel formation could be studied. It is noteworthy that both the inkjet and extruder bioprinting can be made compatible with rapid prototyping.
3.2. Vasculogenesis and Angiogenesis-Based Techniques
Vasculogenesis, as explained earlier, is the process in which new blood vessels are formed from EPCs through the formation of vascular plexus, while angiogenesis is the process whereby groups of ECs sprout, migrate and organize to form new tubular structures eventually forming blood vessels. The mechanisms of vasculogenesis and angiogenesis can be utilized to promote the formation of vascular networks in 3D hydrogels in a controlled and regulated manner. In these approaches, the neovascularization in engineered tissue constructs is promoted by providing biomimetic microenvironments to the cells, i.e. through integrated use of biophysical and biochemical cues. Several techniques have been developed and utilized under this category, including (i) micropatterning for vascular morphogenesis, (ii) use of functionalized biomaterials for promoting vasculogenesis and angiogenesis, (iii) gradient of growth factors for vasculogenesis and angiogenesis and (iv) controlling cell-cell interactions for vasculogenesis and angiogenesis using co-culture of multiple cell types.
3.2.1. Micropatterning to promote tubulogenesis and vascular morphogenesis
There has been a rapid development in the area of micropatterning technologies for applications in promoting the formation of vascular networks in engineered tissue constructs [14, 25]. Micropatterning has broadened the scope to develop cell micropatterned vascularized tissue constructs [111]. The techniques mainly include photolithography, micromolding, microcontact printing, laser photolithography, nanoprinting and UV light-based chemistry.
3.2.1.1. Photolithography
In photolithography technique, usually a photosensitive pre-polymer solution of hydrogel is exposed to the light of certain wavelength through a photomask. The areas that are exposed to the light wave are crosslinked while the rest of the material remains uncrosslinked and is washed out afterward, leaving a hydrogel structure with desired micropattern on the substrate. The hydrogel is formed by a crosslinking process which is initiated by the free radical-based chemical reactions between the photo-initiator and the photosensitive prepolymer. In tissue engineering applications, these light-sensitive biopolymer materials are often acrylated or methacrylated to form hydrogels with desired porosity and mechanical properties [86, 112]. Recently, Nikkhah et al. investigated the application of photopatterning to develop 3D endothelial cord-like structures of varied dimensions within micropatterned GelMA hydrogels [113]. The results confirmed that the behavior of ECs can be altered by varying the geometrical features of the patterned structures, Figs. 5A, B. This method can serve as a tool to form blood vessel-like structures for organized vasculatures. In another study, the use of GelMA was investigated for generating vascular networks using ECFCs (endothelial colony-forming cells) derived from human blood. [114]. GelMA was reported to promote microcapillaries of blood vessels in vitro, when combined with co-cultures of EPCs and mesenchymal stem cells (MSCs). The vessels demonstrated lumen-like structures, resulted from a morphogenic fusion process involving the intracellular vacuoles. The in vivo performance of the construct was confirmed using immunodeficient mice by subcutaneous implantation. 7 days post-transplantation data demonstrates the presence of lumen throughout the GelMA constructs. The majority of the lumens contained murine erythrocytes, which confirmed the functional connections with the host vasculature. Other groups used collagen, fibronectin and poly (ethylene glycol) - diacrylate (PEGDA) hydrogels to promote vasculogenesis [115–118]. It was reported that dense collagen matrices, with concentration ranging between 10 to 20 mg/ml, are mechanically strong, and retain the ability to be restructured by cells using microfabrication techniques [119].
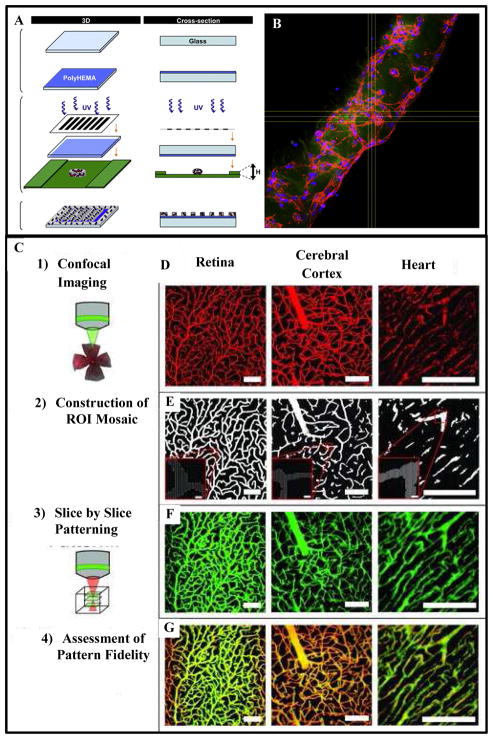
Figure 5.
Formation of vascular structure using photolithography-based micropatterning technique: (A) schematic representation of the micropatterning process. Cell encapsulated photosensitive hydrogel prepolymer suspension placed between two spacers and covered with a glass is exposed to UV light through a photomask. The areas exposed to UV light gets crosslinked while the uncrosslinked region is washed out following the crosslinking step. (B) Confocal fluorescence image of a microconstruct showing the organization of cytoskeletal actin fibers (red) of HUVECs, encapsulated in GelMa hydrogel, leading to the formation of an endothelial cord. The cord was imaged after 5 days of culture [113]. Scale bar represents 100 μm. (C) Micropatterning using confocal-imaging-of-native-tissue-based templates and two- photon laser lithography technique: Confocal microscopy was used to image labeled tissues in 3D. Each optical section was then processed to reconstruct the cross-sectional structure of the tissue using a mosaic of regions of interest (ROIs). Next the ROIs were used to control precise scanning of a laser scanning microscope. To pattern a 3D structure, the mosaic of ROIs for each axial cross section was utilized to sequentially pattern each corresponding plane of the hydrogel [123]. (D) Projections of imaged vasculature from the retina, cerebral cortex, and heart. (E) ROI mosaics reconstructing the vasculature of various tissues at individual cross-sectional planes. (F) 3D projections of hydrogels with fluorescently labeled PEG-RGD patterned to mimic the vasculature from various tissues. (G) A merge of the imaged vasculature with the imaged hydrogels, with yellow indicating excellent overlap between vessels and patterns. Scale bars = 100 μm (5 μm for insets). Figures reproduced from [113] and [123] with permissions from Elsevier Ltd. and Wiley-VCH Verlag GMbh & Co. respectively.
Apart from varying the biomaterial composition, different kinds of cells have also been studied for this purpose. In particular, human pluripotent stem cell derived co-differentiated early vascular cells [116] and EPCs, which play a pivotal role in wound healing, have been successfully used in vitro and in vivo [115]. Microfluidic micropatterning of EPC produced elongated EC-like morphology with aligned actin fibers. The EPCs also rapidly deposited and remodeled the ECM without exogenous factors. In other similar studies, fibronectin patterned and nanofabricated substrates were shown to promote the organization of EPCs into well-defined vascular tube-like structures in vitro compared to plain surfaces [115, 120].
3.2.1.2. Three dimensional laser photolithography
Laser photolithography technique uses laser beams to selectively photopolymerize biomolecules to form 3D hydrogel structures. EC encapsulated micropatterned hydrogel; covalently crosslinked with RGD peptide molecules, pro-angiogenic VEGF and matrix metalloproteinase (MMP), was fabricated using multi-photon laser photolithography technique. ECs demonstrated accelerated and guided tubulogenesis and directional sprouting in the micropatterned hydrogel [26, 121, 122]. In another study, Culver et al. [123] used confocal images from native tissue samples as templates for developing micropatterned structures based on two-photon laser lithography. The group encapsulated HUVECs and messenchymal progenitor cells in MMP-sensitive photocrosslinkable PEG hydrogels modified with RGD peptide. Image-guided laser lithography technique was used to immobilize the RGD conjugated PEG (PEG-RGD) on the cell-encapsulated hydrogel. For this, complex micropatterns obtained from microvasculature images of retina, cerebral cortex and heart were used, Fig. 5C. The results affirmed the potential for application of this technique in fabrication of biomimetic tissue constructs with detailed features at a microscopic scale. The ongoing research on this technique is focused on how to integrate the technique with microfluidic systems to generate 3D microfluidic vascularized tissue networks for bottom-up tissue engineering and other applications [124, 125].
3.2.1.3. Microcontact Printing
The need to fabricate patterns on material surfaces at micro-and nano-scales for controlling cell adhesion geometry and shape, has led to the development of microcontact printing. It is a simple and flexible microfabrication technique for generating a large spectrum of structural variations on the substrate at micro-and nano-scales [126]. The quality of produced patterns depends critically on the quality of the contact between the stamp and the substrate. This technique is mainly used to fabricate surfaces with self-assembled monolayer (SAM) regions with different physical and chemical properties. The method has been used to study the polymerization of actin and phosphorylation of tyrosine in patterned vascular ECs subjected to uniaxial and cyclic strains using fibronectin microdots [127].
3.2.1.4. Micromolding
Micromolding is another widely used method for micropatterning cell-laden hydrogels [128, 129]. In micromolding, the shape of a preformed master pattern is transferred onto a prepolymer solution through direct contact, during which the prepolymer undergoes crosslinking. PDMS is the most commonly used material for master mold. This method can be used to design constructs containing spatially patterned EC tubular structure with varied geometrical properties [99, 130, 131]. Micromolding and similar techniques have also been used to promote capillary tubule formation by spatially patterning ECs using long tubes of collagen hydrogel [78, 92, 132]. The size of the lumen varied based on both microgel size and the concentration of collagen. This approach has the potential for application in developing complex blood vessel-like structures for investigating the fundamental behavior of ECs in initiation of tubulogenesis.
The micropatterning techniques are simple and convenient for patterning complex structures of cell-laden hydrogels and elastomers on diverse substrates. They can be used to make complex patterned network over a wide range of scale in 2D planes and can be useful for detailed study of cell behavior under diverse microarchitectural cues. However, their applicability is mostly limited to 2D, and as of now they cannot be used to develop 3D perfusable endothelial cords or vascular structures.
3.2.2. Vascularization using functionalized biomaterials
The formation of vascular network can be promoted by functionalizing the scaffold materials with various angiogenic biomolecular cues. It has been found that the cell migration, penetration, organization and matrix remodeling can be guided by functionalizing the scaffold materials [133], e.g. by immobilizing angiogenic growth factors (GFs), ECM proteins, peptides, or other biomacromolecules within the scaffolds, thereby promoting angiogenesis and formation of vascular networks.
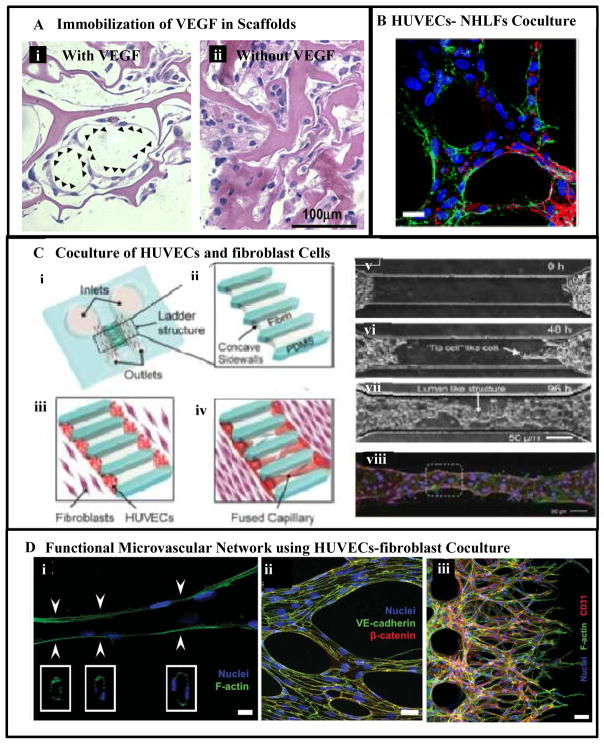
For example, genetically modified VEGF, where N-terminal cysteine was chemically crosslinked to a fibrin-based structure via thiol-directed cross-linker, significantly improved angiogenesis [134]. Similarly covalent immobilization of VEGF in collagen hydrogel exhibited improved vascularization compared to collagen hydrogel without any VEGF, Fig. 6A. Additionally, concomitant addition of VEGF and angiopoietin-1 (ANG1) to collagen via EDC crosslinking resulted in higher tube formation by ECs compared to the collagen scaffolds with individual components [135]. Heparin, which interacts with VEGF, has also been immobilized in the scaffold, leading to indirect immobilization of VEGF and thus resulting in a prolonged VEGF release and improved angiogenic effect [136].
Figure 6.
Vasculogenesis and angiogenesis-based approaches for vascularization: (A) promoting vascularization using functionalization of collagen hydrogel with covalently immobilized VEGF [135]. The organization and morphology of cells after a 7- day in vitro cultivation are shown using hematoxylin and eosin staining (arrowheads indicate vascular like structures). (B) Formation of microvascular network using coculture of HUVECs and NHLFS [149]. (C) Formation of vascular capillaries using co-culture of EC and fibroblast cells in a microfluidic device [150], (i) schematic of the microfluidic device consisting of two main channels connected with a group of bridge channels arranged in a ladder like structure. (ii) Enlarged view of ladder structure filled with fibrin gel forming concave sidewalls. (iii) HUVECs loaded and attached on the concave sidewalls and fibroblasts loaded through the two main inlet channels after attachment of HUVECs to the fibrin gel. (iv) Formation of self-organized capillaries by HUVECs grown from both sides. (v–vii) Image of HUVECs sprouts from both sides and formation of capillaries through fusion of the sprouts’ tips over time. (viii) Imunostaining of perfusable capillaries. Blood vessels were stained with Hoechst 33342 for the nucleus in blue and Phalloidin for F-actin in green and ZO-1 for tight junction in red. (D) Formation of a perfusable, functional microvascular network in a versatile microfluidic device [151]: (i) a representative microvessel formed, (ii) The cells were able to form tight cell-junction connections visualized by VE cadherin and β-catenin. (iii) aberrant morphology and frequent branching of immature tubules within 48h of culture as shown by multiple tip cells visualized by F-actin and CD31 staining. Figures adapted and modified from [135, 149–151] with permissions from Elsevier Ltd., Federation of American Society for Experimental Biology and Royal Society of Chemistry.
Functionalization of biomaterials can also be achieved via non-chemical immobilization instead of chemical immobilization (e.g. by promoting non-covalent-interaction mediated immobilization or by the addition of enzyme sensitive moieties for highly controlled immobilization) [137]. For example, artificial ECM molecules which are designed to interact with several GFs for their non-covalent immobilization via coiled-coil interactions have been used to fabricate tissue engineering scaffolds and shown to promote angiogenesis [138]. Advantages of this strategy include mimicking the in vivo microenvironment of angiogenesis, maintaining the angiogenic factor activities for a prolonged period of time by protecting the compounds against fast metabolism through routes such as endocytosis and encapsulating and delivering angiogenic compounds in a temporally and spatially controlled manner [139].
3.2.3. Vascularization using gradient of growth factors
Among the most extensively investigated methods for generating vascular network by promoting angiogenesis is the use of concentration-gradients of common angiogenic growth factors in a cell laden or cell-seeded hydrogel, often in a microfluidic device. In this method, when a concentration gradient of GFs is established in the vicinity of ECs, either in a hydrogel construct or in a microfluidic channel, the ECs tend to migrate from the region of low-GF-concentration toward the region of high GF concentration, thereby aligning themselves into well-organized structures and enhancing the capillary-like tubular structure formation [3]. The evaluated GFs include VEGFs, angiopoeitins, the transforming growth factors (TGFs), the fibroblast growth factors (FGFs) and the platelet derived growth factors (PDGFs) [3]. These GFs can be incorporated within the scaffolds to ensure a sustained release over time thereby creating local concentration gradients or can be delivered through the growth media flowing through microfluidic channels. Also a single GF can be used as well as a number of GFs simultaneously.
Upon incorporation of a single GF in a scaffold, angiogenesis with increased capillary densities, as well as more matured capillaries were obtained compared to scaffolds without any GF. For example, VEGF-loaded PLGA scaffolds resulted in double as many blood vessel capillaries after implantation in an area of irradiated osseous defects compared to the control scaffolds without VEGF [140]. Perets et al. [141] reported the development of hybrid scaffold system combining basic FGF (bFGF) encapsulated PLGA microspheres within alginate gel. The in vivo results revealed that after 21 days of implantation, a significantly higher number of capillaries (70 ± 7 capillaries/mm2) was observed with the involvement of bFGF compared to the scaffolds without bFGF (18 ± 5 capillaries/mm2) [141].
Recent research has revealed that combining multiple types of GFs enhances the formation of EC tubes and their stabilization. For instance, Hao et al. [142] developed an alginate-based system for the co-delivery of VEGF and PDGF. It was found that after 4 weeks of hydrogel injection into a rat myocardial infarction model, the scaffolds with both VEGF and PDGF resulted in a vessel density of about 40 capillaries/mm2 whereas scaffolds with either VEGF or PDGF had a resultant vessel density of about 30 capillaries/mm2, indicating that the sequential delivery of VEGF and PDGF may be able to facilitate the formation of capillary vessels [142]. In addition, Nillesen et al. [143] evaluated the individual and combined effects of VEGF and FGF2 for blood vessel formation. The results showed that the system loaded with a combination of GFs displayed a two-fold increase in the formation of mature blood vessels compared to the system with a single GF, confirming the synergistic effect of multiple GFs.
GF gradient has been found to regulate the angiogenesis. For example, Barkefors et al. [144] studied the role of VEGF gradient in migration of ECs. The authors designed a microfluidic device with three inlets to generate a concentration gradient by three parallel fluid streams. The VEGF gradient was tunable by adjusting the solution flow rates. The results showed that the ECs migrated towards the high concentration of VEGF. These results also indicated that the steep gradients induced faster cell migration from 0 – 50 ng/ml, but no obvious migration from 50–100 ng/ml probably due to receptors saturation [144].
Silvia et al. [145] further investigated the spatial and temporal effects of GFs on angiogenesis. They studied the temporal effect of VEGF concentration on angiogenesis using alginate hydrogel models and analyzed the impact of the spatial distribution of VEGF using murine hind limb ischemia models. A profile with a high initial concentration of VEGF (50 ng/ml/day) and a programmed concentration decrease was able to develop more EC sprouts compared to a constant dose of VEGF, the total amount being the same. It was also found that a higher level of perfusion and better vascularization performance were obtained by distributing a VEGF dose (0.1 μg/g tissue) into two streams and delivering from two different locations in a murine hindlimb ischemia model. These findings suggest that control and regulation of the spatial and temporal distribution of VEGF may be effective in inducing angiogenesis in vivo [145].
Thus, it is clear that controlled GF release, right combination of GFs, correct dosage and proper exposure time are among key factors for inducing the formation and growth of functional vasculature and establishing appropriate vessel architecture and stability [146].
3.2.4. Vascularization using co-culture of different cells
Co-culturing different suitable cell types under proper microenvironmental cues can induce spontaneous alignment of the vascular cells and generate vascular networks. This technique of promoting vascularization through angiogenesis has been widely investigated lately. Some examples of these studies have reported capillary/microvascular tubules formation in tissue-engineered scaffolds containing ECs or their progenitor cells. Cells co-cultured with ECs or their progenitor cells to facilitate angiogenesis include fibroblasts, SMCs and various stem cells.
For example, Tremblay et al. [147] engineered vascularized human skin by co-culturing HUVECs, dermal fibroblasts, and keratinocytes on 3D porous chitosan-collagen scaffolds. After 15 days of culturing, capillary tubes were observed clearly in the co-culture system but not in the monoculture system. The formation of 3D capillary network could be attributed to the cell-matrix interactions and the cell-cell interactions of HUVECs with fibroblasts. Specifically, it was attributed to the fibroblasts which are able to produce ECM in large amounts when co-cultured with VEGF-producing keratinocytes [147]. The angiogenic effect of cell co-culture was also investigated by Sudo et al. [148] who implemented a microfluidic platform made of a micropatterned PDMS with two parallel microfluidic channels connected using an intervening 3D collagen gel to analyze the angiogenesis using primary rat hepatocytes and microvascular ECs (rMVECs). The vascular and hepatic cells were cultured separately on the sidewalls of the collagen hydrogel bridging two parallel channels in a microfluidic device. The results indicated that the 3D capillary-like structures formed by rMVECs could extend across the intervening hydrogel to the hepatic tissue in the co-culture system while only 2D sheet-like structures were observed in the rMVEC monoculture [148]. This may be attributed to the hepatocytes-secreted hepatocyte growth factor which could stimulate EC motility and growth. In another study Chen et al. [149] used a similar microfluidic device with coculture of HUVECs and normal human lungs fibroblasts (NHLFs) resulting in a well-developed microvascular network, Fig. 6B. In addition, Yeon et al. [150] reported the development and characterization of perfusable capillary networks formed by HUVECs and fibroblasts in vitro in a microfluidic device. This device contained two large channels, connected with eight small bridging channels (Fig. 6C i), which were completely filled with fibrin gel (Fig. 6C ii). Following gel formation, HUVECs were seeded on opposite ends of the fibrin gel (Fig. 6C iii) while fibroblast cells were seeded in the channels adjacent to the gel. After 3 – 4 days, HUVECs migrated into the fibrin gel from the opposite ends and connected/merged with each other, forming a connective vessel expressing tight junction proteins (e. g., ZO-1), which are characteristic of post-capillary venioles (Figs. 6.C. iv–vi). This capillary network formation was facilitated via the recruitment of fibroblasts which were found to express essential matrix proteins (e.g., collagen I), supporting EC lumen formation. Kim et al. [151] continued further investigations and reported development of a more versatile, perfusable and functional microvascular-like network where the results demonstrated formation and morphogenesis of interconnected microvessels (Fig. 6Di). Furthermore, the cell-cell interactions facilitated continuous hollow lumen formation enclosed by ECs throughout the length of the vessels. The cells were able to form tight cell-junction connections visualized by VE cadherin and β-catenin (Fig. 6D-ii). The authors also demonstrated that the microfluidic platform that they employed could also be used to model angiogenic sprout formation induced by cancer cell secreted growth factors. They observed aberrant morphology and frequent branching of immature tubules within 48h of culture as shown by multiple tip cells (Fig. 6Diii) visualized by F-actin and CD31 staining.
In addition, the impact of ECs on the proliferation, spreading and differentiation of SMCs was studied by Liu et al. [152] utilizing a hybrid hydrogel prepared from gelatin modified with methacrylamide, and dextran-graft-lysine modified through methacrylation. With the co-culture of ECs, the SMC proliferation, capillary network formation as well as elastin synthesis were promoted within the 3D hydrogel, suggesting that co-culture of ECs and SMCs is a promising method for constructing functional vasculature in vitro.
Among different cell types, stem cells, particularly, have demonstrated a functional role in EC networks on in vitro 3D culture, i.e., to stabilize the developed EC networks. This may be due to the molecular machinery contained in stem cells that is also expected in perivascular progenitor cells. For example, Moon et al. [153] synthesized a PEG polymer with MMP-sensitive and RGD peptide sequences to mimic the natural provisional ECM. When cultured in this system, HUVECs spontaneously formed capillary-like structures. These structures were stabilized by a lineage of SMCs (differentiated from messenchymal progenitor cells) which deposited laminin and collagen IV. Boyd et al. [154] have also shown that the co-culture of HUVECs with multi-potent MSCs derived from human embryonic stem cells increased the degree of branching of EC networks and maintained the network integrity for up to 6 days compared to HUVECs alone in the 3D collagen I-fibronectin scaffolds. More recently, Chen et al. [114] found that the co-culturing of endothelial colony-forming cells (ECFCs) derived from human blood with MSCs derived from bone marrow generated networks of capillary-like structures when encapsulated in 3D GelMA hydrogels, whereas in the absence of MSCs, no capillary-like network formation from ECFCs was observed [114]. It was also reported that the presence of MSCs increased the overall survival of ECFCs and enhanced the formation of capillary networks from ECFCs. Furthermore, in vivo implantation of this prevascularized construct into immune-deficient mice resulted in a fast development of functional anastomoses between the native vasculature of mouse and the engineered vascular network. Therefore, incorporating appropriate cell types in a pre-designed matrix may be an effective and safe strategy for vascularizing tissue engineering constructs [155].
Thus, a growing body of evidence suggests that co-culture of multiple cell types under proper microenvironmental cues can result in perfusable vascular network. However, all methods have their inherent merits and demerits. Future approaches to vascularization are, therefore, likely to be a combination of multiple techniques such as prevascularization or microfabrication using functionalized biomaterials in combination with multiple growth factors and co-culture of different cells [156].
6. Conclusions and Future Directions
Vascularization is one of the biggest challenges for the widespread clinical application of tissue engineering. For fabrication of implantable thick, large and complex tissue engineered constructs, or repair and regeneration of living tissues in general, the issue of vascularization must be resolved. In this review, we have provided an overview of the techniques available for vascularization of engineered tissue constructs on microfluidic platforms. In general, the techniques developed to date can be classified into (i) prevascularization techniques and (ii) vasculogenesis and angiogenesis-based techniques. The prevascularization techniques can be divided into (a) subtractive methods, (b) additive methods, and (c) hybrid methods, all of which are based on engineering readily available perfusable channels in tissue engineered constructs. Examples of the subtractive methods include needle-based molding and dissolvable sacrificial network-based molding, while those of the additive and hybrid methods include layer-by-layer assembly of micropatterned or micromolded planer hydrogels and bioprinting respectively. The vasculogenesis and angiogenesis-based techniques rely on modulating cell-cell interactions to achieve a vascularized construct. Commonly used vasculogenesis and angiogenesis-based techniques are (a) microfabrication of cellular networks for tubulogenesis and vascular morphogenesis, (b) functionalization of biomaterials with proangiogenic agents to promote angiogenesis, (c) utilizing either a single or multiple proangiogenic growth factors to induce angiogenesis and (d) promoting spontaneous vascularization by co-culture of two or more different cell types including EC, SMC, fibroblasts, progenitor cells or various stem cells. The various types of microfabrication or micropatterning techniques that have been used so far include but are not limited to photolithography, microcontact printing, and micromolding. The microfluidic platforms have great potential to provide the necessary microenvironmental cues for promoting vascularization leading to formation of viable thick tissue constructs [76–78, 83, 86]. Hence the practice of incorporating microfluidics into tissue engineering, thereby generating vascularized constructs has been steadily gaining ground.
Each of the vascularization methods has its own advantages and limitations. While the prevascularization techniques provide readily available channels allowing immediate perfusion of growth media or blood, and are perhaps suitable for fabrication of larger blood vessels, they are not suitable for vascular microcapillary beds with cascading bifurcations down to few micron sizes. The vasculogenesis and angiogenesis-based approaches, on the other hand, provide very limited control over the temporal and spatial factors, require days to weeks before cells can organize and give rise to perfusable lumens, and are to date not suitable for formation of vascular structures in the size ranges suitable for suturing and anastomosis with the host vasculatures. Since the successful implantation of vascularized tissue constructs lies in their ability to be functionally integrated with the host tissue, creating upon implantation anastomosis contacts between the microvessels of the implanted tissue constructs and the host’s blood vessel system, the ultimate solution for proper vascularization may lie in methods combining more than one technique.
Moreover, while many of the publications have demonstrated the proof-of-principles and feasibility studies for different vascularization techniques, many remaining challenges are yet to be solved. For instance, a better understanding is required about factors that determine and guide precise temporal and spatial emergence of biochemical cues and their effects on the formation of stable, functional microvasculature suitable for clinical application. Amongst other factors that require further attention is the role of mechanical stability of the constructs as more complex vascular network structures are integrated. Furthermore, shear stress resulting from different perfusion and flow rates and the behavior of ECs under such conditions including their attachment and proliferations needs to be carefully evaluated as critical design concepts in developing vascularized scaffolds. Other challenges include how essentially 2D methods such as microfabrication can be translated into a 3D vascularized tissue construct, and how to integrate these techniques as the channel scale dimensions increase from microns to millimeters requiring channel integration across layers.
Beyond the engineering challenges, understanding the long term biological effects is crucial for the future application of vascularized tissue constructs. Of particular importance are the effects of vascularized tissue constructs on the surrounding organ, cell/tissue differentiation and vascular remodeling post implantation. It is important to ensure that these vascularized constructs do not impede full vascular maturation, leading to crucial physiological functions in response to biochemical or vasoactive stimuli. Additionally, it is necessary to ensure proper cellular phenotypes, as well as functional and biologically relevant cell-cell communications and signaling in addition to ECM proteins and cytokines secretions. Such challenges need to be addressed while incorporating, particularly for larger blood vessels, multilayered vascular walls within vascularized constructs with layers of fibroblasts and SMCs, in addition to the EC monolayer.
When intended for applications in brain and ocular tissues, the engineered vasculatures must exhibit the required specialized physiological effects such as the blood-brain-barrier and blood-retina-barriers, showing that tight junction permeability is not compromised due to the implantation of the tissue engineered constructs. Furthermore, effects of mechanical stimuli and shear stress have to be better understood in a biological and biochemical context. Polarity of ECs must be investigated and their ability to form future instructive physical and biochemical gradients necessary for downstream signaling and proper function. These functions are essential for capillary morphogenesis dependent on both spatial and temporal control of both biochemical and mechanical cues. Hence, each scaffold should at least be neutral if not instructive for these functions [157–159]. Finally, these vascularized tissue constructs should allow for better modeling and aid in the study of physiology and physiopathologies of the native vasculature and perhaps provide clues to more complex cellular processes.
The future direction is clear; to create implantable thick vascularized tissue constructs that could serve as artificial organs or aid in their repair and/or regeneration. However, in order to translate the basic science discoveries into clinically relevant transplants and therapies, issues down the road such as standardization has to be resolved beyond the basic engineering. With the advancement in various microfabrication techniques, such as photolithography and micromolding techniques, it is necessary now to establish methods and criteria that will evaluate the performance and clinical relevance of cell-laden vascularized constructs. This will aid in generating a systematic approach that will assess if the intended organ application requirements have been met. Furthermore, there is a void in the development of tools for real-time monitoring of growing tissues on microfluidic chips. With the advances in incorporation of micro- and nano-sensors it would be beneficial to integrate them into microfluidic tissue constructs for real-time monitoring. These sensors could be applied in a wide variety of fashions including assessing in real-time cell viability and function. Such approaches could lead to the development of functional materials that could bring the necessary revolution to solve the current issues and challenges.
To summarize, even though significant progress has been made toward vascularization of engineered tissue constructs during the past decade, there is still plenty of work to be done. Even though the clinical goals are clear, the potential paths to lead toward the goals offer multiple alternatives. Ultimately, most likely, a combination of multiple approaches taking the best of what microfluidics, biosensing, vasculogenesis and angiogenesis-based bottom-up approaches and prevascularization-based top-down approaches have to offer, might lead to the achievement of the clinical goals of fully functional and adequately vascularized implantable complex tissues.
Acknowledgments
Anwarul Hasan acknowledges the start up grant from American University of Beirut, Lebanon. Arghya Paul acknowledges postdoctoral award from FRQS (Fonds de recherché du Quebec-Sante) Quebec, Canada). Adnan Memic thanks the Strategic Technologies Program of King Abdulaziz City for Science and Technology (KACST), grant number 12-MED3096-03 for their support and funding. Nihal Engin Vrana acknowledges funding from EuroTransBio “BiMoT” project (ETB-2012-32). Ali Khademhosseini acknowledges funding from the National Science Foundation CAREER Award (DMR 0847287), the office of Naval Research Young National Investigator Award, the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392), and the Presidential Early Career Award for Scientists and Engineers (PECASE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bae H, Puranik AS, Gauvin R, Edalat F, Carrillo-Conde B, Peppas NA, et al. Building vascular networks. Sci Trans Med. 2012;4:1–4. doi: 10.1126/scitranslmed.3003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Y, Cropek D, Mofrad MRK, Weinberg EJ, Khademhosseinil A, Borenstein J. Microfluidic systems for engineering vascularized tissue constructs. In: Tian W-C, Finehout E, editors. Microfluidics for Biological Applications. New York: Springer; 2008. [Google Scholar]
- 3.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. Vascularization - the conduit to viable engineered tissues. Tissue Eng Pt B: Rev. 2009;15:159–69. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 4.Hasan A, Ragaert K, Swieszkowski W, Selimović Š, Paul A, Camci-Unal G, et al. Biomechanical properties of native and tissue engineered heart valve constructs. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.09.023. In press. [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci U S A. 2008;105:9522–7. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–6. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RN, Yu J, Torres PB, Schickedanz AC, Park JK, Mueller MD, et al. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod Sci. 2009;16:140–6. doi: 10.1177/1933719108324893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polverini PJ. Angiogenesis and wound healing: basic discoveries, clinical implications and therapeutic opportunities. Endod Top. 2012;24:130–45. [Google Scholar]
- 9.Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. In: Moncada S, Higgs A, editors. Handbook of Experimental Pharmacology: The Vascular Endothelium II. Berlin Heidelberg: Springer-Verlag; 2006. pp. 157–212. [DOI] [PubMed] [Google Scholar]
- 10.Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, et al. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2013;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–18. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gailloud P, Muster M, Piotin M, Mottu F, Murphy KJ, Fasel JHD, et al. In vitro models of intracranial arteriovenous fistulas for the evaluation of new endovascular treatment materials. Am J Neuroradiol. 1999;20:291–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Paul A, Hasan A, Rodes L, Sangaralingam M, Prakash S. Bioengineered baculoviruses as new class of therapeutics using micro and nanotechnologies: Principles, prospects and challenges. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.01.004. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu Y-H, Moya ML, Hughes CCW, George SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab Chip. 2013;13:2990–8. doi: 10.1039/c3lc50424g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–6. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Zhu X, Futai N, Cho BS, Takayama S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc Natl Acad Sci U S A. 2004;101:15861–6. doi: 10.1073/pnas.0404353101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamloo A, Heilshorn SC. Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients. Lab Chip. 2010;10:3061–8. doi: 10.1039/c005069e. [DOI] [PubMed] [Google Scholar]
- 19.Mosadegh B, Huango C, Park JW, Shin HS, Chung BG, Hwang SK, et al. Generation of stable complex gradients across two-dimensional surfaces and three-dimensional gels. Langmuir. 2007;23:10910–2. doi: 10.1021/la7026835. [DOI] [PubMed] [Google Scholar]
- 20.Vickerman V, Blundo J, Chung S, Kamm R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip. 2008;8:1468–77. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JW, Bazou D, Munn LL. Anastomosis of endothelial sprouts forms new vessels in a tissue analogue of angiogenesis. Integr Biol. 2012;4:857–62. doi: 10.1039/c2ib20061a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim HY, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;9:1740–8. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaw KC, Manimaran M, Tay FEH, Swaminathan S. Matrigel coated polydimethylsiloxane based microfluidic devices for studying metastatic and non-metastatic cancer cell invasion and migration. Biomed Microdevices. 2007;9:597–602. doi: 10.1007/s10544-007-9071-5. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B, Waller TM, Larson JC, Appel AA, Brey EM. Fibrin-loaded porous poly(Ethylene Glycol) hydrogels as scaffold materials for vascularized tissue formation. Tissue Eng Pt A. 2013;19:224–34. doi: 10.1089/ten.tea.2012.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 2012;33:5230–46. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JJ, Hahn MS, Kim I, Nsiah BA, West JL. Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng Pt A. 2009;15:579–85. doi: 10.1089/ten.tea.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuchiara MP, Gould DJ, McHale MK, Dickinson ME, West JL. Integration of self-assembled microvascular networks with microfabricated PEG-based hydrogels. Adv Funct Mater. 2012;22:4511–8. doi: 10.1002/adfm.201200976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542–50. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson A, Singh S, Fernekorn U, Schober A. The future of the patient-specific Body-on-a-chip. Lab Chip. 2013;13:3471–80. doi: 10.1039/c3lc50237f. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix MJC, Seftor EA, Hess AR, Seftor REB. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 31.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein BM. What guides early embryonic blood vessel formation? Dev Dynam. 1999;215:2–11. doi: 10.1002/(SICI)1097-0177(199905)215:1<2::AID-DVDY2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neuro-Oncology. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- 34.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000;190:292–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Rhodin J. Architecture of the vessel walls. In: Bohr D, Somlyo A, Sparks H, editors. Handbook of Physiology: The Cardiovascular System. Bethesda, Maryland: American physiological society; 1980. pp. 1–31. [Google Scholar]
- 38.Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med. 2003;5:1–39. doi: 10.1017/S1462399403006628. [DOI] [PubMed] [Google Scholar]
- 39.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 40.McGuigan AP, Sefton MV. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials. 2007;28:2547–71. doi: 10.1016/j.biomaterials.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratcliffe A. Tissue engineering of vascular grafts. Matrix Biol. 2000;19:353–7. doi: 10.1016/s0945-053x(00)00080-9. [DOI] [PubMed] [Google Scholar]
- 42.Daamen WF, Veerkamp JH, van Hest JCM, van Kuppevelt TH. Elastin as a biomaterial for tissue engineering. Biomaterials. 2007;28:4378–98. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 43.Monos E, Lóránt M, Fehér E. Influence of long-term experimental orthostatic body position on innervation density in extremity vessels. Am J Physiol-Heart C. 2001;281:H1606–H12. doi: 10.1152/ajpheart.2001.281.4.H1606. [DOI] [PubMed] [Google Scholar]
- 44.Shadwick RE. Mechanical design in arteries. J Exp Biol. 1999;202:3305–13. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 45.Zhang WJ, Liu W, Cui L, Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11:945–57. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–62. doi: 10.1016/j.jvs.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 47.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 48.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Card. 2008;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stroncek JD, Xue Y, Haque N, Lawson JH, Reichert WM. In vitro functional testing of endothelial progenitor cells that overexpress thrombomodulin. Tissue Eng Pt A. 2011;17:2091–100. doi: 10.1089/ten.tea.2010.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–5. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986;58:427–44. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- 52.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 53.Rensen SSM, Doevendans PAFM, Eys GJJM. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–8. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, et al. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003;22:6027–34. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling from innocent bystander to active participant. Circ Res. 2001;89:1111–21. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 56.McGrath JC, Deighan C, Briones AM, Shafaroudi MM, McBride M, Adler J, et al. New aspects of vascular remodelling: the involvement of all vascular cell types. Exp physiol. 2005;90:469–75. doi: 10.1113/expphysiol.2005.030130. [DOI] [PubMed] [Google Scholar]
- 57.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nature Medicine. 2006;12:361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oberleithner H, Riethmüller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104:16281–6. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 2006;12:905–15. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 60.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, et al. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscl Throm Vas Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 61.Epstein FH, Vane JR, Änggård EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 62.Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the holy grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–54. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 63.Fung YC, Liu SQ. Determination of the mechanical properties of the different layers of blood vessels in vivo. Proc Natl Acad Sci U S A. 1995;92:2169–73. doi: 10.1073/pnas.92.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holzapfel GA, Gasser TC, Stadler M. A structural model for the viscoelastic behavior of arterial walls: Continuum formulation and finite element analysis. Eur J Mech A-Solid. 2002;21:441–63. [Google Scholar]
- 65.Heydarkhan-Hagvall S, Esguerra M, Helenius G, Söderberg R, Johansson BR, Risberg B. Production of extracellular matrix components in tissue-engineered blood vessels. Tissue Eng. 2006;12:831–42. doi: 10.1089/ten.2006.12.831. [DOI] [PubMed] [Google Scholar]
- 66.Stegemann JP, Kaszuba SN, Rowe SL. Review: Advances in vascular tissue engineering using protein-based Biomaterials. Tissue Eng. 2007;13:2601–13. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–8. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 68.Ku DN. Blood flow in arteries. Ann Rev Fluid Mech. 1997;29:399–434. [Google Scholar]
- 69.Holzapfel GA. The handbook of materials behavior models. Boston: Academic Press; 2001. Biomechanics of soft tissue. [Google Scholar]
- 70.Mitchell SL, Niklason LE. Requirements for growing tissue-engineered vascular grafts. Cardiovasc Pathol. 2003;12:59–64. doi: 10.1016/s1054-8807(02)00183-7. [DOI] [PubMed] [Google Scholar]
- 71.Seliktar D, Black R, Vito R, Nerem R. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351–62. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 72.Lillie MA, Gosline JM. Limits to the durability of arterial elastic tissue. Biomaterials. 2007;28:2021–31. doi: 10.1016/j.biomaterials.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 73.Archipoff G, Beretz A, Bartha K, Brisson C, de la Salle C, Froget-Léon C, et al. Role of cyclic AMP in promoting the thromboresistance of human endothelial cells by enhancing thrombomodulin and decreasing tissue factor activities. Brit J Pharmacol. 1993;109:18–28. doi: 10.1111/j.1476-5381.1993.tb13526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seifalian AM, Tiwari A, Hamilton G, Salacinski HJ. Improving the clinical patency of prosthetic vascular and coronary bypass grafts: The role of seeding and tissue engineering. Artificial Organs. 2002;26:307–20. doi: 10.1046/j.1525-1594.2002.06841.x. [DOI] [PubMed] [Google Scholar]
- 75.Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, et al. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6:105–17. doi: 10.1089/107632700320739. [DOI] [PubMed] [Google Scholar]
- 76.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication technology for vascularized tissue engineering. Biomed Microdevices. 2002;4:167–75. [Google Scholar]
- 77.King KR, Wang CCJ, Kazempour-Mofrad MR, Vacanti JP, Borenstein JT. Biodegradable microfluidics. Adv Mat. 2004;16:2007–12. [Google Scholar]
- 78.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302–9. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 79.Sarkar S, Lee GY, Wong JY, Desai TA. Development and characterization of a porous micro-patterned scaffold for vascular tissue engineering applications. Biomaterials. 2006;27:4775–82. doi: 10.1016/j.biomaterials.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 80.Bettinger CJ, Cyr KM, Matsumoto A, Langer R, Borenstein JT, Kaplan DL. Silk fibroin microfluidic devices. Adv Mat. 2007;19:2847–50. doi: 10.1002/adma.200602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang Y, Borenstein JT, et al. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv Mat. 2006;18:165–9. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–15. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 83.Cabodi M, Choi NW, Gleghorn JP, Lee CSD, Bonassar LJ, Stroock AD. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788–9. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 84.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp JM, et al. A cell-laden microfluidic hydrogel. Lab Chip. 2007;7:756–62. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 85.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–5. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 86.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–96. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Price GM, Wong KHK, Truslow JG, Leung AD, Acharya C, Tien J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials. 2010;31:6182–9. doi: 10.1016/j.biomaterials.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park JH, Chung BG, Lee WG, Kim J, Brigham MD, Shim J, et al. Microporous cell-laden hydrogels for engineered tissue constructs. Biotechnol Bioeng. 2009;106:138–48. doi: 10.1002/bit.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sadr N, Zhu M, Osaki T, Kakegawa T, Yang Y, Moretti M, et al. SAM-based cell transfer to photopatterned hydrogels for microengineering vascular-like structures. Biomaterials. 2011;32:7479–90. doi: 10.1016/j.biomaterials.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshida H, Matsusaki M, Akashi M. Multilayered blood capillary analogs in biodegradable hydrogels for in vitro drug permeability assays. Adv Funct Mater. 2012;23:1736–42. [Google Scholar]
- 92.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansen NS, Ferguson TE, Panels JE, Park A-HA, Joo YL. Inorganic nanofibers with tailored placement of nanocatalysts for hydrogen production via alkaline hydrolysis of glucose. Nanotechnology. 2011;22:1–13. doi: 10.1088/0957-4484/22/32/325302. 325302. [DOI] [PubMed] [Google Scholar]
- 94.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat Mater. 2003;2:265–71. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 95.Toohey KS, Hansen CJ, Lewis JA, White SR, Sottos NR. Delivery of two-part self-healing chemistry via microvascular networks. Adv Funct Mater. 2009;19:1399–405. [Google Scholar]
- 96.Wu W, DeConinck A, Lewis JA. Omnidirectional printing of 3D microvascular networks. Adv Mater. 2011;23:H178–H83. doi: 10.1002/adma.201004625. [DOI] [PubMed] [Google Scholar]
- 97.Wong KHK, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Ann Rev Biomed Eng. 2012;14:205–30. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- 98.Price GM, Chu KK, Truslow JG, Tang-Schomer MD, Golden AP, Mertz J, et al. Bonding of macromolecular hydrogels using perturbants. J Am Chem Soc. 2008;130:6664–5. doi: 10.1021/ja711340d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther. 2007;7:1123–7. doi: 10.1517/14712598.7.8.1123. [DOI] [PubMed] [Google Scholar]
- 101.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells. 2008;26:127–34. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 102.Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008;29:193–203. doi: 10.1016/j.biomaterials.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 103.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, et al. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–8. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 104.Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26:93–9. doi: 10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 105.Smith CM, Christian JJ, Warren WL, Williams SK. Characterizing environmental factors that impact the viability of tissue-engineered constructs fabricated by a direct-write bioassembly tool. Tissue Eng. 2007;13:373–83. doi: 10.1089/ten.2006.0101. [DOI] [PubMed] [Google Scholar]
- 106.Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, Kachurin AM, et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;10:1566–76. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 107.Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101:2864–9. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jakab K, Norotte C, Damon B, Marga F, Neagu A, Besch-Williford CL, et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Pt A. 2008;14:413–21. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 109.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson DE, Hinds MT. Endothelial cell micropatterning: methods, effects, and applications. Ann Biomed Eng. 2011;39:2329–45. doi: 10.1007/s10439-011-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–92. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 113.Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials. 2012;33:9009–18. doi: 10.1016/j.biomaterials.2012.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y-C, Lin R-Z, Qi H, Yang Y, Bae H, Melero-Martin JM, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22:2027–39. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dickinson LE, Moura ME, Gerecht S. Guiding endothelial progenitor cell tube formation using patterned fibronectin surfaces. Soft Matter. 2010;6:5109–19. [Google Scholar]
- 116.Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:12601–6. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–24. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 118.Turturro MV, Christenson MC, Larson JC, Young DA, Brey EM, Papavasiliou G. MMP-sensitive PEG diacrylate hydrogels with spatial variations in matrix properties stimulate directional vascular sprout formation. PLoS ONE. 2013;8:e58897. doi: 10.1371/journal.pone.0058897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bettinger CJ, Zhang Z, Gerecht S, Borenstein JT, Langer R. Enhancement of in vitro capillary tube formation by substrate nanotopography. Adv Mat. 2008;20:99–103. doi: 10.1002/adma.200702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials. 2011;32:5782–9. doi: 10.1016/j.biomaterials.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 122.Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 2008;29:2962–8. doi: 10.1016/j.biomaterials.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Culver JC, Hoffmann JC, Poche RA, Slater JH, West JL, Dickinson ME. Three-dimensional biomimetic patterning in hydrogels to guide cellular organization. Adv Mat. 2012;24:2344–8. doi: 10.1002/adma.201200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He J, Mao M, Liu Y, Zhu L, Li D. Bottom-up fabrication of 3D cell-laden microfluidic constructs. Mater Lett. 2013;90:93–6. [Google Scholar]
- 125.Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol Adv. 2013;31:669–87. doi: 10.1016/j.biotechadv.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruiz SA, Chen CS. Microcontact printing: A tool to pattern. Soft Matter. 2007;3:168–77. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- 127.Katanosaka Y, Bao JH, Komatsu T, Suemori T, Yamada A, Mohri S, et al. Analysis of cyclic-stretching responses using cell-adhesion-patterned cells. J Biotechnol. 2008;133:82–9. doi: 10.1016/j.jbiotec.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 128.Koroleva A, Gittard S, Schlie S, Deiwick A, Jockenhoevel S, Chichkov B. Fabrication of fibrin scaffolds with controlled microscale architecture by a two-photon polymerization-micromolding technique. Biofabrication. 2012;4:015001. doi: 10.1088/1758-5082/4/1/015001. [DOI] [PubMed] [Google Scholar]
- 129.He J, Wang Y, Liu Y, Li D, Jin Z. Layer-by-layer micromolding of natural biopolymer scaffolds with intrinsic microfluidic networks. Biofabrication. 2013;5:025002. doi: 10.1088/1758-5082/5/2/025002. [DOI] [PubMed] [Google Scholar]
- 130.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Pt A. 2010;16:2255–63. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takei T, Sakai S, Ono T, Ijima H, Kawakami K. Fabrication of endothelialized tube in collagen gel as starting point for self-developing capillary-like network to construct three-dimensional organs in vitro. Biotechnol Bioeng. 2006;95:1–7. doi: 10.1002/bit.20903. [DOI] [PubMed] [Google Scholar]
- 132.Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, et al. Endothelialized networks with a vascular geometry in microfabricated poly(dimethyl siloxane) Biomed Microdevices. 2004;6:269–78. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- 133.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–80. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 134.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF–fibrin matrices for endothelialization. J Control Release. 2001;72:101–13. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 135.Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–41. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 136.Singh S, Wu BM, Dunn JCY. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials. 2011;32:2059–69. doi: 10.1016/j.biomaterials.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ito Y, Tada S. Bio-orthogonal and combinatorial approaches for the design of binding growth factors. Biomaterials. 2013;34:7565–74. doi: 10.1016/j.biomaterials.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 138.Assal Y, Mie M, Kobatake E. The promotion of angiogenesis by growth factors integrated with ECM proteins through coiled-coil structures. Biomaterials. 2013;34:3315–23. doi: 10.1016/j.biomaterials.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 139.Naderi H, Matin MM, Bahrami AR. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J biomater appl. 2011;26:383–417. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- 140.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–44. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 141.Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65A:489–97. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 142.Hao X, Silva EA, Månsson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–85. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 143.Nillesen STM, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–31. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 144.Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, Johansson H, et al. Endothelial cell migration in stable gradients of vascular endothelial growth factor a and fibroblast growth factor 2 - Effects on chemotaxis and chemokinesis. J Biol Chem. 2008;283:13905–12. doi: 10.1074/jbc.M704917200. [DOI] [PubMed] [Google Scholar]
- 145.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31:1235–41. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hopkins SP, Bulgrin JP, Sims RL, Bowman B, Donovan DL, Schmidt SP. Controlled delivery of vascular endothelial growth factor promotes neovascularization and maintains limb function in a rabbit model of ischemia. J Vasc Surg. 1998;27:886–95. doi: 10.1016/s0741-5214(98)70269-1. [DOI] [PubMed] [Google Scholar]
- 147.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transp. 2005;5:1002–10. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 148.Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, et al. Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J. 2009;23:2155–64. doi: 10.1096/fj.08-122820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen MB, Whisler JA, Jeon JS, Kamm RD. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr Biol. 2013;5:1262–71. doi: 10.1039/c3ib40149a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip. 2012;12:2815–22. doi: 10.1039/c2lc40131b. [DOI] [PubMed] [Google Scholar]
- 151.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 152.Liu Y, Rayatpisheh S, Chew SY, Chan-Park MB. Impact of endothelial cells on 3D cultured smooth muscle cells in a biomimetic hydrogel. ACS Appl Mater Interfaces. 2012;4:1378–87. doi: 10.1021/am201648f. [DOI] [PubMed] [Google Scholar]
- 153.Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, et al. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31:3840–7. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boyd NL, Nunes SS, Jokinen JD, Krishnan L, Chen YL, Smith KH, et al. Microvascular mural cell functionality of human embryonic stem cell-derived mesenchymal cells. Tissue Eng Pt A. 2011;17:1537–48. doi: 10.1089/ten.tea.2010.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 156.Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J. 2008;22:2949–56. doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–84. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 158.Helm CLE, Fleury ME, Zisch AH, Boschetti F, Swartz MA. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci U S A. 2005;102:15779–84. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Inoguchi H, Tanaka T, Maehara Y, Matsuda T. The effect of gradually graded shear stress on the morphological integrity of a huvec-seeded compliant small-diameter vascular graft. Biomaterials. 2007;28:486–95. doi: 10.1016/j.biomaterials.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 160.Kothapalli CR, Van Veen E, De Valence S, Chung S, Zervantonakis IK, Gertler FB, et al. A high-throughput microfluidic assay to study neurite response to growth factor gradients. Lab Chip. 2011;11:497–507. doi: 10.1039/c0lc00240b. [DOI] [PubMed] [Google Scholar]
- 161.Tsuda Y, Shimizu T, Yamato M, Kikuchi A, Sasagawa T, Sekiya S, et al. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28:4939–46. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 162.Price GM, Chrobak KM, Tien J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res. 2008;76:46–51. doi: 10.1016/j.mvr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]