Abstract
Epigenetics encompasses transient and heritable modifications to DNA and nucleosomes in the native chromatin context. For example, enzymatic addition of chemical moieties to the N-terminal “tails” of histones, particularly acetylation and methylation of lysine residues in the histone tails of H3 and H4, plays a key role in regulation of gene transcription. The modified histones, which are physically associated with gene regulatory regions that typically occur within conserved noncoding sequences, play a functional role in active, poised, or repressed gene transcription. The “histone code” defined by these modifications, along with the chromatin-binding acetylases, deacetylases, methylases, demethylases, and other enzymes that direct modifications resulting in specific patterns of histone modification, shows considerable evolutionary conservation from yeast to humans. Direct modifications at the DNA level, such as cytosine methylation at CpG motifs that represses promoter activity, are another highly conserved epigenetic mechanism of gene regulation. Furthermore, epigenetic modifications at the nucleosome or DNA level can also be coupled with higher-order intra- or interchromosomal interactions that influence the location of regulatory elements and that can place them in an environment of specific nucleoprotein complexes associated with transcription. In the mammalian immune system, epigenetic gene regulation is a crucial mechanism for a range of physiological processes, including the innate host immune response to pathogens and T cell differentiation driven by specific patterns of cytokine gene expression. Here, we will review current findings regarding epigenetic regulation of cytokine genes important in innate and/or adaptive immune responses, with a special focus upon the tumor necrosis factor/lymphotoxin locus and cytokine-driven CD4+ T cell differentiation into the Th1, Th2, and Th17 lineages.
1. THE COMPONENTS OF EPIGENETIC TRANSCRIPTIONAL REGULATION
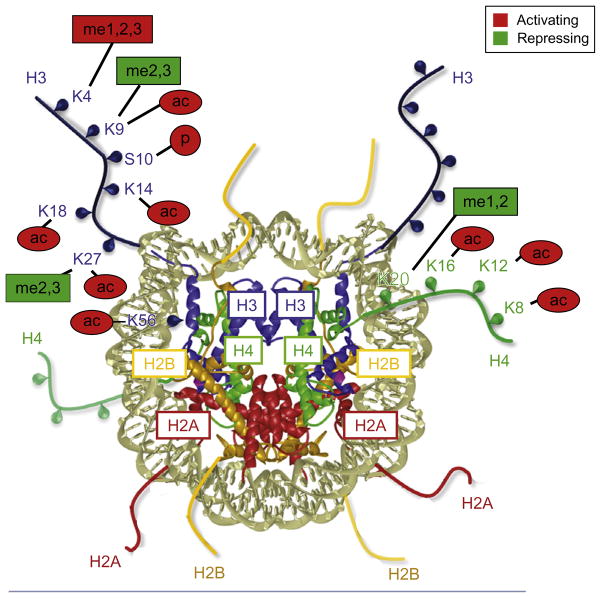
Each human cell, with the exception of enucleated red blood cells, contains roughly 2 m of genomic DNA, which is compacted into a space approximately 10 μm in diameter within the cell’s nucleus. Lengths of genomic DNA are wound tightly around nucleosomes comprised of an octamer of histone proteins (consisting of two molecules each of histone H2A, histone H2B, histone H3, and histone H4; Luger, Dechassa, & Tremethick, 2012; Fig. 2.1). Nuclease digestion and sedimentation gradient assays, respectively, showed that ~145 bp of genomic DNA wraps around each nucleosome, resulting in a nucleoprotein complex of ~206 kD. Cloning the component proteins of the nucleosome revealed that they were members of the highly basic histone family, which is strongly conserved in eukaryotes (Kornberg & Lorch, 1999). Finally, X-ray crystallographic analysis revealed that the nucleosome consists of a disc of histones that is encircled by a left-handed superhelical turn of DNA along its perimeter, such that the relatively unstructured N-terminal ends of the histones are exposed to the outer surface (Luger et al., 1997; Fig. 2.1). This finding that was consistent with biochemical studies, which indicated that the N-terminal “tails” were targets of a range of posttranscriptional modifications (Kornberg & Lorch, 1999).
Figure 2.1.
The structure of the nucleosome. The histone octamer viewed down the superhelical axis of the DNA, illustrating the position of N-terminal histone tails that are targets of posttranslational modifications. Histones H3, H4, H2A, and H2B are shown in blue, green, gold, and red, respectively. Diagram of 2.8 Å resolution structure (Luger, Mader, Richmond, Sargent, & Richmond, 1997) (Protein Data Bank code 1AOI) kindly provided by Karolin Luger.
Nucleosome packaging of DNA presents a physical barrier to the initiation of transcription. When DNA is tightly associated with histones, forming a “closed” nucleosomal configuration, the RNA polymerase complex is prevented from binding to the start site of transcription proximal to the coding region of a gene, and transcription factors are precluded from interacting with their cognate binding sites in gene regulatory regions. However, in response to enzymatic modification of specific histone residues, a nucleosome can adopt an “open” configuration, rendering the DNA accessible to polymerases and transcription factors (Luger et al., 2012). This open nucleosomal conformation is primarily due to electrostatic repulsion between newly acetylated (and thus negatively charged) histone tails and the negatively charged phosphate backbone of DNA (Luger et al., 2012). Histone acetylation is directly coupled to activation of transcription, and a number of general transcription factors (e.g., TFIID) and global coactivator proteins (e.g., CBP and p300) function as histone acetyltransferases (HATs). Conversely, deacetylation of histones, which is mediated by a class of enzymes termed histone deacetylases (HDACs), is coupled to repression of transcription (Medzhitov & Horng, 2009; Wilson, Rowell, & Sekimata, 2009).
An experimental technique that has been instrumental for assaying histone modifications such as acetylation at endogenous genes is chromatin immunoprecipitation, or ChIP (Orlando, Strutt, & Paro, 1997). This technique was initially used for mapping the position, within a gene locus, of histones (Braunstein, Rose, Holmes, Allis, & Broach, 1993; Dedon, Soults, Allis, & Gorovsky, 1991; Hebbes, Thorne, & Crane-Robinson, 1988; Solomon, Larsen, & Varshavsky, 1988; Solomon & Varshavsky, 1985) and other chromosomal proteins (Dedon et al., 1991; Hecht, Strahl-Bolsinger, & Grunstein, 1996; Orlando & Paro, 1993). Later, ChIP was adapted to detect the association of transcription factors with regulatory sequences at endogenous gene loci or in plasmid DNA (Botquin et al., 1998; Falvo, Parekh, Lin, Fraenkel, & Maniatis, 2000; Koipally, Renold, Kim, & Georgopoulos, 1999; Parekh & Maniatis, 1999; Tomotsune, Shoji, Wakamatsu, Kondoh, & Takahashi, 1993). Proteins recruited to regulatory sequences through interactions with DNA-bound transcription factors, such as the coactivator protein CBP, were also detected in ChIP assays (Agalioti et al., 2000; Chen, Lin, Xie, Wilpitz, & Evans, 1999). As antibodies became available for the detection of histones bearing specific post-translational modifications, ChIP was employed to examine how unique histone modifications corresponded to differences in endogenous gene regulation, including responses to various stimuli (Braunstein et al., 1993; Hebbes et al., 1988; Kuo et al., 1996; Parekh & Maniatis, 1999; Solomon et al., 1988). For example, ChIP was used to show that histones H3 and H4 were hyperacetylated in the vicinity of the interferon-β (IFNB1) promoter in HeLa cells following exposure to Sendai virus (Parekh & Maniatis, 1999). ChIP assays have since been adapted to whole-genome analysis, where a “ChIP-on-chip” technique is utilized in which immunoprecipitated DNA is hybridized to a panel of microarray chip-mounted oligonucleotides (Ren et al., 2000). ChIP-on-chip has been applied to the investigation of global histone modifications in yeast (Kurdistani, Tavazoie, & Grunstein, 2004; Vogelauer, Wu, Suka, & Grunstein, 2000) and mammalian cells (Bernstein et al., 2005), revealing broad correlations between specific histone modifications and gene transcriptional activity, depending on the position of the nucleosome relative to the gene (Ong & Corces, 2012; Rowell, Merkenschlager, & Wilson, 2008).
The ChIP assay, in combination with the DNAse I hypersensitivity assay (DHA) and with bioinformatic analyses of comparative sequencing between species, has revealed that conserved noncoding sequences (CNSs) in regulatory regions of cytokine gene loci are associated with inducible and constitutive hypersensitive sites (HSs), or regions of DNA accessibility, and with specific histone modifications. In some cases, these regions are subject to further regulation at the level of DNA modification, specifically by methylation at CpG dinucleotides, which represses transcription, and/or by their organization into higher-order chromatin structures through intra- or intrachromosomal interactions, which can place genes into regions of active or inactive transcription within the nucleus (Amsen, Spilianakis, & Flavell, 2009; Falvo, Tsytsykova, & Goldfeld, 2010; Lee, Kim, Spilianakis, Fields, & Flavell, 2006; Medzhitov & Horng, 2009; Ong & Corces, 2012; Rowell et al., 2008; Williams, Spilianakis, & Flavell, 2010; Wilson et al., 2009). Histone modification, DNA methylation, and higher-order chromatin interactions thus present key mechanisms of epigenetic control, and these will be discussed in the context of specific cytokine loci that play critical roles in the immune response.
1.1. Histone modifications
1.1.1 Covalent modifications
In addition to acetylation, a range of other post-translational histone modifications have been described, including methylation, phosphorylation, and ubiquitylation (Bannister & Kouzarides, 2011; Kouzarides, 2007; Rando, 2012; Tan et al., 2011). The specific combination of these distinct “histone marks” was postulated to mediate distinct patterns of transcriptional regulation, and thereby biological processes; this is known as the “histone code” hypothesis (Jenuwein & Allis, 2001; Strahl & Allis, 2000). While the histone code could theoretically extend to all possible combinations of post-translational modifications in the histone tails, the accumulated data suggests that only a limited number of such combinations occur in nature, and that these are mainly associated with activation or repression of transcription (Rando, 2012). This limitation arises, in part, from the interplay between histone modifications within the same nucleosome, since specific modifications can favor or oppose the occurrence of another modification (Bannister & Kouzarides, 2011; Kouzarides, 2007). Another level of complexity arises, however, from the observation that the positioning of a nucleosome within the context of a gene locus (e.g., enhancer, promoter, coding region, boundary element, or adjacent region) and the transcriptional state of the gene (actively transcribed, recently transcribed or “primed,” “poised” for transcription, or silenced) correspond to characteristic sets of histone marks (Ong & Corces, 2012; Rowell et al., 2008). Thus, the histone code is dynamic and influenced by the activation state of a cell and by the ambient concentration of factors in the nucleus. Cytokine genes, with their tightly controlled expression patterns prior to and in response to cellular stimuli, present a particularly pertinent example of how temporal changes in histone modification state correspond to gene expression.
As outlined above, histone acetylation is normally associated with activation of transcription due to its effect of loosening the DNA-histone interaction within the nucleosome, and it is thus an “activating” or “permissive” histone mark (Table 2.1). Acetylation occurs at the ε-amino groups of specific lysines (i.e., at the terminus of the side chain) in the N-terminal histone tails of histones H3 and H4. For example, certain histone H3 tail acetylation sites are associated with active gene expression, including lysines 9, 14, and 27 (H3K9ac, H3K14ac, and H3K27ac, respectively) (Bannister & Kouzarides, 2011). Lysine 56 of histone H3 (H3K56), which lies in the globular domain of the histone near its interface with the DNA major groove, is also a target of acetylation in yeast (Xu, Zhang, & Grunstein, 2005) and humans (Tjeertes, Miller, & Jackson, 2009). Acetylated histone lysines also provide a docking site for specific protein domains: bromodomains (BRDs), found in a number of HATs and chromatin-remodeling complex proteins, such as Swi2/Snf2 of the SWI/SNF complex; and plant homeodomain (PHD) domains, found in D4 zinc and double PHD fingers family 3b (DPF3b) of the Brg1/Brm-associated factor (BAF) chromatin-remodeling complex (Bannister & Kouzarides, 2011).
Table 2.1.
Histone acetylation, methylation, and phosphorylation marks that are of particular importance in cytokine gene regulation, with an overview of their effects on transcriptional activation/repression. Principal associated gene positions are indicated in bold type.
| Histone modification | Histone net charge affected? | Representative targeted residues | Histone mark abbreviation | Associated gene activity | Associated gene position | Protein interacting domain |
|---|---|---|---|---|---|---|
| Acetylation | Yes | Histone H3: lysines 9, 14, 18, 27 | H3K9ac, H3K14ac, H3K18ac, H3K27ac | Active | Enhancer, promoter, coding region | Bromodomain, PHD domain |
| Histone H4: lysines 5, 8, 12, 16 | H4K5ac, H4K18ac, H4K12ac, H4K16ac | Active | Enhancer, promoter, coding region | |||
| Methylation | No | Histone H3: lysine 4 (monomethylated) | H3K4me1 | Active, poised | Enhancer, coding region, boundary element | Chromodomain, PHD domain, Tudor domain, MBT domain |
| Histone H3: lysine 4 (dimethylated) | H3K4me2 | Active | Downstream of promoter, enhancer, promoter, coding region, boundary element | |||
| Histone H3: lysine 4 (trimethylated) | H3K4me3 | Active, poised | TSS, enhancer, promoter | |||
| Histone H3: lysine 27 (trimethylated) | H3K27me3 | Inactive/ repressed | Enhancer, promoter, coding region, adjacent region | |||
| Histone H3: lysine 27 (dimethylated) | H3K27me2 | Inactive/ repressed | Enhancer, promoter, coding region, adjacent region | |||
| Histone H3: lysine 9 (trimethylated) | H3K9me3 | Inactive/ repressed | Enhancer, promoter, coding region, adjacent region | |||
| Histone H3: lysine 9 (dimethylated) | H3K9me2 | Inactive/ repressed | Enhancer, promoter, coding region, adjacent region | |||
| Phosphorylation | Yes | Histone H3: serine 10 | H3S10 | Active, poised | Enhancer, promoter, coding region | 14-3-3 domain |
The effects of lysine methylation of histones upon gene transcription are more complicated, and depend upon both the lysine residue involved and the number of methyl moieties—one, two, or three—that are coupled to the ε-amino group, which is also predominantly restricted to the N-terminal tails of histones H3 and H4 (Table 2.1). Unlike acetylation, methylation does not change the net charge of the modified histone residue. Rather, methylated histone lysines provide an interaction surface for chromatin-modifying proteins and other regulatory proteins, specifically those containing PHD domains or chromo-like domains (chromodomain, Tudor, MBT and PWWP domains), the latter found in the Tudor “royal” protein family (Bannister & Kouzarides, 2011; Kouzarides, 2007).
Broadly speaking, methylation of lysine 4 of histone H3 (H3K4) correlates with transcriptional activation, while methylation of lysine 9 or lysine 27 of histone H3 (H3K9 and H3K27, respectively) correlates with transcriptional repression (Bannister & Kouzarides, 2011; Kouzarides, 2007; Medzhitov & Horng, 2009; Ong & Corces, 2012; Rowell et al., 2008). Monomethylated H3K4 (H3K4me1) is primarily associated with enhancers that are poised or actively involved in transcriptional activation (Creyghton et al., 2010; Ghisletti et al., 2010; Heintzman et al., 2007; Rada-Iglesias et al., 2011; Zentner, Tesar, & Scacheri, 2011). Trimethylated H3K4 (H3K4me3) is enriched at transcription start sites (TSSs) and is linked to active gene transcription (Ng, Robert, Young, & Struhl, 2003; Santos-Rosa et al., 2002). Furthermore, dimethylated H3K4 (H3K4me2) has been found to recruit HATs to regions downstream of gene promoters, leading to histone acetylation at the linked promoters and, in turn, efficient RNA polymerase II (RNA Pol II) elongation (Kim & Buratowski, 2009). By contrast to the role that some methyl marks play in gene activation, trimethylated H3K27 (H3K27me3) and dimethylated and trimethylated H3K9 (H3K9me2 and H3K9me3, respectively) are typically associated with inactive or repressed gene transcription (Kondo, Shen, & Issa, 2003; Kondo, Shen, Yan, Huang, & Issa, 2004; Okamoto, Otte, Allis, Reinberg, & Heard, 2004; Peters et al., 2003; Plath et al., 2003; Rougeulle et al., 2004). We note, however, that there is some evidence that methylation of H3K9 can also occur within or adjacent to actively transcribed regions (Vakoc, Mandat, Olenchock, & Blobel, 2005). Other N-terminal lysine residues that are methylation targets include lysine 36 of histone H3 (H3K36), which is involved in maintaininghistone integrity in coding regions of actively transcribedgenesand suppressing spurious cryptic transcripts (Carrozza et al., 2005; Joshi & Struhl, 2005; Keogh et al., 2005; Kizer et al., 2005; Li et al., 2002), and lysine 20 of histone H4 (H4K20), which has been associated with active repression of proinflammatory gene expression (Stender et al., 2012). Lysine 79 of histone H3 (H3K79), which is linked to active transcription, lies within the histone globular domain. Methylation of H3K79 only occurs when lysine 120 of histone H2B (H2BK120)—corresponding to lysine 123 of histone H2B (H2BK123) in yeast—is ubiquitylated(Bannister& Kouzarides, 2011; Kim etal., 2009;Lee etal., 2007).
Arginine residues in histones H3 and H4 can also be targets of methylation, and can be monomethyated at the ω-guanidino group, dimethylated symmetrically (via monomethylation of both terminal guanidino nitrogens), or dimethylated asymmetrically (via dimethylation of one of the terminal guanidino nitrogens) (Bannister & Kouzarides, 2011). Histone arginine methylation can influence transcription by promoting or inhibiting interactions between HATs and histone methyltransferases and their targets at nearby residues. For example methylation of arginine 2 of histone H3 (H3R2) inhibits methylation at H3K4, while methylation of arginine 3 of histone H4 (H4R3) can promote acetylation at lysines 8 and 12 of histone H4 (H4K8 and H4K12, respectively) (Arrowsmith, Bountra, Fish, Lee, & Schapira, 2012; Bannister & Kouzarides, 2011). Furthermore, symmetric dimethylation of H3R2 has been associated with active transcription (Migliori et al., 2012), while asymmetric dimethylation of this residue has been linked to transcriptional repression (Guccione et al., 2007; Kirmizis et al., 2007). Methylation of H3R17 has also been linked to gene activation (Selvi et al., 2010), and methylation of H4R3 is associated with both transcriptional activation when present in the asymmetric state (Balint, Gabor, & Nagy, 2005; Balint, Szanto, et al., 2005; Li et al., 2010) and transcriptional repression when present in the symmetric state (Dhar et al., 2012; Zhao et al., 2009).
Histone phosphorylation, another modification linked to gene activation, has been detected at serine, threonine, and tyrosine residues, predominantly within N-terminal tails. Serine, threonine, or tyrosine phosphorylation introduces a negatively charged moiety and thus, like histone acetylation, alters the net charge of the modified residue and disrupts the nucleosome structure. Serine phosphorylation, in particular, provides a recognition motif for regulatory factors of the 14-3-3 protein family (Bannister & Kouzarides, 2011; Kouzarides, 2007). Notably, the phosphorylated serine 10 of histone H3 (H3S10p) mark has been characterized in the greatest detail, and its presence is linked to activation of immediate early response genes (Cheung et al., 2000; Li et al., 2002; Saccani, Pantano, & Natoli, 2002; Thomson, Clayton, & Mahadevan, 2001), including the IL10 gene in human monocytes (Hofmann et al., 2012), as well as chromosome condensation during mitosis (Wei et al., 2009; Table 2.1).
Two lysine residues in the nucleosome, lysine 119 of histone H2A (H2AK119) and lysine 120 of histone H2B (H2BK120), which corresponds to lysine 123 of histone H2B (H2BK123) in yeast, are targets of ubiquitylation. The addition of a molecule of ubiquitin, a 76-amino acid polypeptide, is a much larger covalent modification than those previously mentioned, and it can inhibit or promote the recruitment of other factors, as well as disrupt local and higher-order chromatin compaction (Bannister & Kouzarides, 2011; Kouzarides, 2007; Luger et al., 2012). Monoubiquitylation of H2AK119 (H2AK119ub1) has been linked to repression of expression of several genes (Medzhitov & Horng, 2009; Wang et al., 2004; Zhou et al., 2008), and it appears to rely on prior methylation of H3K27 in the same nucleosome (Cao, Tsukada, & Zhang, 2005). Conversely, monoubiquitylation of H2BK120 (H2BK123ub1) in mammals and H2BK123 (H2BK123ub1) in yeast has been linked to transcriptional activation and, as was mentioned above, is a prerequisite for H3K79 methylation, as well as for H3K4me3 methylation. The disruptive effect of H2BK ubiquitylation upon chromatin compaction may be a broadly conserved mechanism underlying its activating function (Bannister & Kouzarides, 2011; Fierz et al., 2011; Kim et al., 2009; Kouzarides, 2007; Lee et al., 2007).
Sumoylation (from SUMO, small ubiquitin-like modifier) of histones has also been reported. As is the case with ubiquitylation, sumoylation adds a large (~100 amino acids, with some variation in isoforms) covalent modification to lysine residues in histones, and it can potentially have similar effects with respect to steric hindrance or protein recruitment. Although limited data are available, histone sumoylation has been tied to transcriptional repression (Nathan et al., 2006; Shiio & Eisenman, 2003), perhaps due to prevention of acetylation and/or ubiquitylation at lysine residues already occupied by SUMO. While sumoylation of all four component histones occurs in yeast, in mammals this modification has only been detected on histone H4 (Kalocsay, Hiller, & Jentsch, 2009; Nathan et al., 2006; Shiio & Eisenman, 2003). Finally, a range of other histone modifications, including deamination (conversion of arginine to citrulline), addition of β-N-acetylglucosamine to serine and threonine residues, ADP ribosylation of glutamate and arginine residues, biotinylation of lysine residues, and clipping of the N-terminal tail itself, have not been specifically linked to transcriptional regulation (Bannister & Kouzarides, 2011), and the most recently identified histone modification, lysine crotonylation (the crotonyl group is CH3—CH=CH—CO—), marks active transcription of sex chromosome-linked genes in postmeiotic male germ cells (Tan et al., 2011).
1.1.2 Histone modifying enzymes and associated factors
Proteins that regulate histone modifications and link these modifications to changes in chromatin structure can be thought of as belonging to three classes: “writers” that add chemical moieties to histone residues, such as the HATs and methyltransferases; “erasers” that remove these modifications, such as the HDACs and demethylases; and “readers” that recognize specific modifications (Arrowsmith et al., 2012; Ruthenburg, Allis, & Wysocka, 2007).
1.1.2.1 Histone acetyltransferases
Type-A HATs participate in transcriptional regulation, typically through acetylation of the N-terminal tails of histones H3 and H4, while type-B HATs, which are homologous to yeast HAT1, direct transient acetylation of newly translated histones prior to their deposition in nucleosomes (Kleff, Andrulis, Anderson, & Sternglanz, 1995; Kuo et al., 1996; Parthun, Widom, & Gottschling, 1996; Sobel, Cook, Perry, Annunziato, & Allis, 1995; Verreault, Kaufman, Kobayashi, & Stillman, 1996, 1998). Type-A HATs are divided into three categories: the GNAT (Gcn5-related N-acetyltransferase) superfamily, which includes Gcn5 and PCAF; the MYST (MOZ, Ybf2/Sas3, Sas2, and Tip60) family; and the CBP/p300 protein family. HAT activity is exhibited by other factors, including the nuclear receptor coactivators, which modulate the transcriptional response to hormone signals (Chen et al., 1997; Spencer et al., 1997). Subunits of TFIIIC, which directs RNA polymerase III transcription initiation (Hsieh, Kundu, Wang, Kovelman, & Roeder, 1999; Kundu, Wang, & Roeder, 1999), and the largest TBP-associated factor (TAF) that comprises the TFIID complex, TAFII250 (Mizzen et al., 1996), also exhibit HAT activity.
With respect to cytokine gene transcription, the Gcn5/PCAF complex and CBP/p300 are the most relevant HATs. CBP/p300 acetylates H3K14, H3K18, H3K27, H4K5, and H4K8 (as well as H2AK5, H2BK12, and H2BK15), while Gcn5/PCAF acetylates H3K9, H3K14, and H3K18 (Jin et al., 2011; Schiltz et al., 1999; Tie et al., 2009). Gcn5 and p300 have also been implicated in the acetylation of H3K56 (Bannister & Kouzarides, 2011; Das, Lucia, Hansen, & Tyler, 2009; Tjeertes et al., 2009; Table 2.2). Acetylation of histone lysine residues also leads to recruitment of proteins that possess the BRD, which isa conserved four-helixbundle-containing interactionmodule that specifically interacts with ε-N-acetylated lysine residues (Dhalluin et al., 1999; Hassan et al., 2007). The BRD family includes, in addition to Gcn5, PCAF, CBP, and p300 themselves, the bromo and extra terminal (BET) proteins, as well as a number of other transcriptional regulators. In vitro binding studies with acetylated histone peptides indicate that in addition to the above, PCAF interacts with H3K9ac, H3K14ac, H3K36ac, H4K8ac, H4K16ac, and H4K20ac, while GCN5 also interacts with H2AK5ac, H3K9ac, H3K14ac, H3K9ac/K14ac, H4K8ac/K14ac, H4K16ac, and H4K5ac/K8ac/K12ac/K16ac (Dhalluin et al., 1999; Filippakopoulos & Knapp, 2012; Hassan et al., 2007; Hudson, Martinez-Yamout, Dyson, & Wright, 2000; Zeng, Zhang, Gerona-Navarro, Moshkina, & Zhou, 2008; Table 2.2). Gcn5/PCAF-mediated histone acetylation has been specifically linked to recruitment of transcription elongation factors to target genes (Medzhitov & Horng, 2009; Wilson et al., 2009).
Table 2.2.
Histone marks and the modifying enzymes that act to “write” or “erase” these marks
| Histone modification | Histone mark(s) | “Writer” | “Eraser” |
|---|---|---|---|
| Acetylation (H3, H4) | H3K9ac, H3K14ac, H3K18ac | Gcn5, PCAF | HDAC1, HDAC2 |
| H3K14ac, H3K18ac, H4K5ac, H4K8ac | CBP, p300 | HDAC1, HDAC2 | |
| Methylation | H3K4me1 | SET7 | LSD1, JARID1B |
| H3K4me2, H3K4me3 | MLL | LSD1, JARID1A-D | |
| H3K27me2, H3K27me3 | EZH2 | JMJD3, UTX | |
| H3K9me2, H3K9me3 | G9a, SUV39H | LSDI, JMJD2A-D | |
| Phosphorylation | H3S10p | MSK1, MSK2? | unknown |
The BRDs of CBP/p300 interact with acetylated H2BK85, H3K9/K14, H3K14, H3K36, H3K56, H3S10/K14/K18, H4K12, H4K20, and H4K44 (Filippakopoulos & Knapp, 2012; Kouskouti & Talianidis, 2005; Zeng et al., 2008). CBP/p300-mediated histone acetylation, in turn, creates a docking site for histone readers, such as the aforementioned components of the SWI/ SNF and BAF complexes, which promote an open chromatin conformation and stimulate transcription. The BET protein BRD4 also binds with high affinity to diacetylated and tetraaceytlated H4 peptide and diacetylated H3 peptide (Dey, Chitsaz, Abbasi, Misteli, & Ozato, 2003).
1.1.2.2 Histone deacetylases
In 1997, a series of studies in yeast and human cells (Alland et al., 1997; Hassig, Fleischer, Billin, Schreiber, & Ayer, 1997; Heinzel et al., 1997; Kadosh & Struhl, 1997; Laherty et al., 1997; Nagy et al., 1997; Zhang, Iratni, Erdjument-Bromage, Tempst, & Reinberg, 1997) showed that transcription factors that were bound to gene promoters can recruit protein complexes consisting of Sin3 proteins and histone deacetylases 1 and 2 (HDAC1 and HDAC2), or their yeast homolog reduced potassium dependency 3 (Rpd3), leading to transcriptional repression (Pazin & Kadonaga, 1997; Rosenfeld, Lunyak, & Glass, 2006). The general action of HDACs is to counteract HAT-mediated histone acetylation at H3 and H4, serving as the “eraser” counterpart to the HAT “writers,” and to date, a total of eighteen HDACs have been identified in mammals.
HDACs are divided into five classes, which have all been implicated in regulation of cytokine gene transcription (Medzhitov & Horng, 2009; Rajendran, Garva, Krstic-Demonacos, & Demonacos, 2011; Villagra, Sotomayor, & Seto, 2010). Class I is composed of HDACs 1, 2, 3, and 8, which have homology to Rpd3; Class IIa and Class IIbconsist of HDACs 4, 5, 7, and 9 andHDACs6 and 10, respectively, which have homology to yeast histone deacetylase 1 (Hda1); Class III contains sirtuins 1 through 7 (SIRT1-7), homologues of yeast silent information regulator 2 (SIR2), which use NAD+ as a cofactor; and Class IV, which has only one member, HDAC11 (Jüngel et al., 2011; Rajendran et al., 2011; Schneider, Krämer, Schmid, & Saur, 2011; Villagra et al., 2010). Some HDACs target specific lysine residues. For example, SIRT6 interacts with the transactivating nuclear factor κB(NF-κB) subunit p65 (RelA) and specifically deacetylates H3K9 at a subset of NF-κB-dependent genes, resulting in attenuated NF-κB signaling (Kawahara et al., 2009); furthermore, SIRT1 counteracts the p300-mediated acetylation of p65 (Bourguignon, Xia, & Wong, 2009; Finkel, Deng, & Mostoslavsky, 2009; Medzhitov & Horng, 2009; Salminen, Kauppinen, Suuronen, & Kaarniranta, 2008; Yeung et al., 2004). In addition, SIRT2 specifically targets H4K16 for deacetylation (Kouzarides, 2007; Vaquero et al., 2006).
HDAC specificity can also be influenced by the proteins with which they partner to form complexes. For example, HDAC1 and HDAC2 can form complexes with the transcriptional repressors nuclear receptor corepressor (NCoR) and REST corepressor (CoREST). Large-scale binding and transcription profiling has shown that these repressors, in turn, regulate primary response genes, which have GC-rich promoters, but not secondary response genes (Medzhitov & Horng, 2009; Wilson et al., 2009; Table 2.2).
1.1.2.3 Histone methyltransferases and demethylases
Histone lysine methyltransferases and demethylases have a stricter specificity than most of the HAT and deacetylases. Histone lysine methyltransferases include MLL1-5, SET1A, SET1B, and ASH1, which target H3K4; G9a, SUV39H1, SUV39H2, ESET/SETDB1, EuHMTase/GLP, CLL8, and RIZ1, which modify H3K9; SET2, NSD1, and SYMD2, which methylate H3K36; DOT1, which targets H3K79; SET 7/8, SUV420H1, and SUV420H2, which methylate H4K20; and EZH2, which modifies H3K27 (Arrowsmith et al., 2012; Medzhitov & Horng, 2009; Wilson et al., 2009).
Histone lysine demethylases are divided into two classes: the lysine demethylase 1(KDM1) family, which was first described in 2004, and the jumonji C containing protein (JmjC) family, which was discovered in 2006 (Shi et al., 2004; Tsukada et al., 2006). In the KDM1 family, H3K4 is a targeted by KDM1A, KDM1B, KDM2B, and KDM5A-D; H3K9 is demethylated by KDM1A and KDM4A-D; and KDM2A, KDM2B and KDM4A-D target H3K36. In the JmjC family, H3K9 is demethylated by JHDM1D and PHF8; and JHDM1A, UTX, UTY, and JMJD3 target H3K27 (Table 2.2). Another member of the JmjC family, JMJD6, has been reported to be a histone lysine arginine demethylase that demethylates H3R2 and H4R3 (Chang, Chen, Zhao, & Bruick, 2007), although other reports indicate that JMJD6 primarily functions as a lysl hydroxylase, both of nuclear proteins involved in RNA splicing (Webby et al., 2009) and of histones (Unoki et al., 2013).
1.1.2.4 Histone serine kinases
As noted above, phosphorylation of H3S10 is an activating histone mark, functioning through electrostatic disruption of nucleosome structure and recruitment of regulatory proteins of the 14-3-3 family. H3S10 phosphorylation has been shown to depend on the p38 mitogen-activated protein kinase (MAPK) pathway, although it remains to be determined whether H3S10 is a direct substrate for p38 or for a p38-regulated kinase, such as mitogen- and stress-activated kinase 1 (MSK1) or MSK2 (Cano, Hazzalin, Kardalinou, Buckle, & Mahadevan, 1995; Lau & Cheung, 2011; Soloaga et al., 2003; Strelkov & Davie, 2002; Thomson et al., 1999; Table 2.2). H3S10 phosphorylation can also be induced by RSK2 (Kouzarides, 2007; Sassone-Corsi et al., 1999) and by a component of the NF-κB pathway, IκB kinase-α (IKK-α), when that kinase is recruited to gene promoters (Anest et al., 2003; Duncan, Anest, Cogswell, & Baldwin, 2006; Yamamoto, Verma, Prajapati, Kwak, & Gaynor, 2003). The link between NF-κB activation and H3S10 phosphorylation is strengthened by the observation that, following LPS stimulation, H3S10p was detected at the gene promoters of interleukin 6 (IL-6), IL-12p40 and CC-chemokine ligand 2 (CCL2, also known as MCP-1), but not TNF and CCL3 (Saccani et al., 2002). With respect to TNF gene regulation, a recent report did describe enrichment of H3S10p downstream of the TNF promoter early after LPS activation of murine macrophages (Thorne, Ouboussad, & Lefevre, 2012); however, unlike the genes encoding IL-6 and IL-12p40, TNF transcriptional initiation is independent of NF-κB (Falvo et al., 2010), suggesting that a kinase other than IKK phosphorylates H3S10 in this case.
H3S10p is in turn recognized by 14-3-3ζ, which is a member of the 14-3-3 family of regulatory proteins (Macdonald et al., 2005). Notably, it has been reported that 14-3-3ζ, interaction with H3S10p is enhanced in vivo by simultaneous acetylation of H3K9 and/or H3K14 and that this interaction is required for induction of HDAC1 gene expression (Winter et al., 2008). H3S10p has also been implicated in the recruitment of the transcription elongation factor pTEF-b (Ivaldi, Karam, & Corces, 2007; Zippo et al., 2009). Finally, phosphorylation of S10 in histone H3 molecules that possess an adjacent H3K9me2/3 mark displaces heterochromatin protein 1 (HP1) from the genome during mitosis, illustrating a mechanism by which phosphorylation of H3S10 counteracts an epigenetic mark of repression (Fischle et al., 2005).
1.1.2.5 Histone ubiquitylation
H2AK119 ubiquitylation is controlled by the polycomb complex-associated transcriptional repressors Bmi and Ring1A (Cao et al., 2005; Wang et al., 2004) or the U3 ligase 2A-HUB (Zhou et al., 2008). By contrast, H2BK120 ubiquitylation is regulated by the E3 ubiquitin ligase RNF20/ RNF40 in conjunction with WAC and UbcH6 (Zhang & Yu, 2011; Zhu et al., 2005). As noted above, ubiquitylation of H2B at lysine 120 (lysine 123 in yeast) is an activating mark for transcription, as it is required for (tri) methylation of H3K4 and H3K79. Furthermore, it has been implicated in stimulating the function of a histone chaperone and elongation factor, facilitates chromatin transcription (FACT; Pavri et al., 2006). By contrast, H2AK119 ubiquitylation is not conserved in yeast and has been shown to be associated with transcriptional repression of several chemokine genes in mammals, including CCL5, CXC-chemokine ligand 10 (CXCL10) and CXCL2, but not CXCL1, in the mouse RAW 264.7 macrophage cell line (Zhou et al., 2008). Ubiquitylation of H2AK119 by 2A-HUB (also known as DZIP3) blocks FACT recruitment to the gene promoters, suppressing RNA Pol II transcriptional elongation; LPS treatment leads to inhibition of 2A-HUB, and thus to reduced H2A ubiquitylation and concomitant recruitment of FACT (Zhou et al., 2008).
Post-translational histone modifications are thus part of a complex network of factors that write, erase, and read the histone code, and these factors and their interaction partners provide even greater levels of regulation, which result in specific programs of gene transcription. As discussed below, several chromatin-modifying proteins and their associated factors play key roles in the regulation of key cytokine loci and transcription of genes that are key in the innate and adaptive immune response.
1.2. DNA methylation
In addition to methylation at lysine and arginine residues in histones, another epigenetic modification influencing cytokine gene expression is DNA methylation itself. In mammals, DNA methylation occurs on CpG dinucleotides at the 5-carbon position of cytosine, and is directed primarily by three DNA methlytransferases (DNMTs), which transfer a methyl group from S-adenosyl-L-methionine (AdoMet) to cytosine (Bestor & Ingram, 1983; Bestor, Laudano, Mattaliano, & Ingram, 1988; Okano, Xie, & Li, 1998; Turek-Plewa & Jagodziński, 2005; Vaissière, Sawan, & Herceg, 2008). De novo DNA methylation, which consists of incorporation of methyl groups at CpG dinucleotides within regions of unmethylated DNA and is widespread during early embryonic development, is controlled by DNMT3A and DNMT3B. By contrast, maintenance of methylation in somatic cells, particularly during cell division following each round of DNA replication, is directed by DNMT1 (Delcuve, Rastegar, & Davie, 2009; Miranda & Jones, 2007; Turek-Plewa & Jagodzinski, 2005; Vaissière et al., 2008). It has been appreciated for nearly forty years that conversion of cytosine to 5-methylcytosine (m5C) in DNA is associated with control of gene expression (Holliday & Pugh, 1975; Riggs, 1975). CpG methylation has primarily been linked to transcriptional repression, and consistent with this observation, gene promoter regions in particular tend to be devoid of m5C (Bird, 2002; Bird, Taggart, Frommer, Miller, & Macleod, 1985; Gardiner-Garden & Frommer, 1987; Lee, Sahoo, & Im, 2009; Meissner et al., 2008; Vaissière et al., 2008). While 60–90% of CpG sites are methylated across the genome, in CG-rich sequences known as CpG islands, which are present upstream of about 40% of human genes, CpG sites are typically unmethylated (Bird, 2000; Meissner et al., 2008; Miranda & Jones, 2007; Lee, Sahoo, et al., 2009; Turek-Plewa & Jagodziński, 2005).
One major mechanism for transcriptional repression by DNA methylation is occlusion of transcription factor binding sites by CpG methylation. Notably, DNA methylation and demethylation at specific loci, and its linked impact upon the ability of transcriptional activators to bind to regulatory elements, is a key feature of T cell lineage commitment (Barnes, 2011; Lee, Sahoo, et al., 2009; Li, 2002). A second major mechanism of transcriptional repression by DNA methylation involves the recruitment of HDACs to gene promoters by methyl-CpG-binding proteins (MeCPs), including MeCP2 and MBD2 (Feng et al., 2001; Jones et al., 1998; Nan et al., 1998; Ng et al., 1999). MeCPs can also recruit additional factors, including HP1(which recruits several repressive factors including histone methyltransferases) and the histone H3K9 methyltransferases SUV39H1 and SETDB1, which can amplify suppression of gene activation (Feng & Zhang, 2001; Fujita et al., 2003; Ichimura et al., 2005; Jones et al., 1998; Nan et al., 1998; Ng et al., 1999; Vaissière et al., 2008; Zhang et al., 1999). There is also evidence that the acetylation state of adjacent histones can influence DNA methylation. For example, HDAC inhibitors can enhanceDNA methylation, and DNA demethylatingagents like 5-azacytidine and 5-aza-2′-deoxycytidine can, reciprocally, induce histone acetylation (Selker, 1998; Takebayashi et al., 2001; Zhu, Lakshmanan, Beal, & Otterson, 2001). Furthermore, histone H3 hypoacetylation and H3K9 methylation have been observed to precede DNA methylation during gene silencing (Strunnikova et al., 2005; Mutskov & Felsenfeld, 2004); for example, the tumor suppressor gene RASSF1A is progressively inactivated in proliferating human mammary epithelial cells, and this process initially coincides with decreases in histoneH3ac andincreases in H3K9me3 at theRASSF1promoter, and is only linked to DNA methylation of the promoter at later stages (Strunnikova et al., 2005).
1.3. Higher-order chromatin interactions
An important aspect of epigenetic regulation at the chromatin level that has been recently appreciated is the role of higher-order, long-range interactions in modulating gene expression. For many years, it was thought that gene promoters and enhancers operate in cis with TSSs, with regulatory sequences influencing neighboring upstream or downstream genes. This is a straightforward model in the case of promoters, which lie adjacent to the TSS. However, in the case of enhancers that are separated from the TSS by a few thousand base pairs, a prevailing model for their function, advanced by Ptashne based on findings with the bacteriophage lambda model system, was that the intervening DNA would be looped out, bringing enhancer DNA-bound transcriptional activators into close proximity with the transcription machinery at the target gene’s TSS (Ptashne, 1986). A number of experiments with simple enhancer-promoter systems supported this looping model. For example, looping at a distance induced by interaction between the lambda repressor and the bacterial RNA polymerase complex was detected by examining perturbations in the DNA structure using DNase footprinting (Hochschild & Ptashne, 1986), and these loops were observed directly by electron microscopy (Griffith, Hochschild, & Ptashne, 1986). ChIP assays in yeast cells also provided evidence for activation-induced DNA looping, as immunoprecipitation of an enhancer-associated factor in fixed chromatin also pulled down both enhancer and promoter/TSS DNA fragments under conditions of transcriptional competence (de Bruin, Zaman, Liberatore, & Ptashne, 2001).
The ability of an intervening sequence of DNA to loop is constrained by both its length and by the biophysical properties of chromosomal DNA. A loop of naked DNA, for example, requires at least ~0.5 kb to form, while a loop of uninterrupted chromatin fiber requires at least ~10 kb. However, looping of chromosomal DNA can be facilitated by acetylation of histones and by the presence of nucleosome-free regions; thus, chromatin-modifying factors can promote the formation of chromatin loops that are relatively smaller by inducing and/or taking advantage of open chromatin configurations. Such a region of open chromatin configuration can thus be thought of as a “hinge” that permits the formation of tight chromatin loops (Göndör & Ohlsson, 2009; Li, Barkess, & Qian, 2006; Rippe, 2001).
Another mechanism whereby proteins can facilitate transcription via structural changes in DNA involves remodeling upon the binding of “architectural” proteins. For example, proteins of the high mobility group (HMG) box family can facilitate transcription by inducing sharp bends in the DNA between regulatory elements (Alvarez, Rhodes, & Bidwell, 2003; Carey, 1998; Paull, Haykinson, & Johnson, 1993; Pil, Chow, & Lippard, 1993). The HMG box protein lymphoid enhancer-binding factor-1 (LEF-1), in particular, induces a dramatic bend of ~117° over 15 base pairs in its cognate DNA motif, and this promotes enhancer complex formation at the T cell receptor α (TCRα) gene (Giese, Kingsley, Kirshner, & Grosschedl, 1995; Giese, Pagel, & Grosschedl, 1997; Love et al., 1995). Thus, protein-mediated alterations in DNA structure in the context of chromatin can bring distant enhancer complexes into contact with the general transcription machinery. These findings underscore the need to consider the role of distant enhancers when analyzing mechanisms of gene transcription.
As discussed below in the sections reviewing epigenetic control of gene regulation at specific cytokine loci, DNA-looping interactions in the context of chromatin typically involve transcription factors and architectural proteins binding at CNSs that undergo epigenetic modifications at the histone or DNA level, or both. Identifying such regulatory CNSs is not always straightforward, however, as primary DNA sequence does not necessarily reflect the physical proximity or distance of gene regulatory regions and their target genes in vivo. Simply scanning directly upstream or downstream of a TSS for putative regulatory regions disregards the potential role of much more distal regions (Dekker, 2008). Furthermore, multiple upstream and downstream distal enhancers can make long-distance interactions with a specific gene, even, as will be discussed below, if these enhancers lie on different chromosomes.
A straightforward approach for examining long-range looping events at endogenous gene loci, chromosome conformation capture (3C), was introduced by Dekker, Rippe, Dekker, and Kleckner (2002). The basic steps of the 3C assay involve fixation of chromosomal regions that lie in close proximity via formaldehyde-induced protein-DNA crosslinking, digestion with a specific restriction endonuclease, and ligation under dilute conditions to favor intramolecular ligation of crosslinked fragments over random intermolecular ligation. After purification the ligated DNA fragments serve as templates for PCR with primers that recognize widely separated DNA sequences of interest in order to quantify long-range interactions, both intrachromosomal and interchromosomal. Furthermore, addition of an immunoprecipitation step allows for selection of DNA fragments that interact with a specific protein (Dekker, 2003, 2006). To examine long-range interactions on a more global level, and without a priori knowledge of the location of potential interacting sequences, a range of other 3C-based methods have been developed. These extensions of the original 3C protocol allow a more unbiased, quantitative approach to determining interactions between a specific genomic site and sites throughout the genome. One innovation is the ligation of oligonucleotide linkers to immunoprecipitated fragments, followed by sequencing, to identify direct or indirect DNA contact sites of a given protein (Osborne, Ewels, & Young, 2011; Sanyal, Baù, Martí-Renom, & Dekker, 2011; van Steensel & Dekker, 2010). Much as ChIP-on-chip extended the range of the ChIP assay to a genomic scale, interactions between a given locus and the rest of the genome can be determined by 4C (either “circular chromosome conformation capture” or “chromosome conformation capture-on-chip”), which involves ligating a known segment of DNA to an array of purified 3C products, amplifying the resulting population of circular DNA molecules with inverse PCR, and analyzing the PCR products by high-throughput sequencing (Simonis et al., 2006; Würtele & Chartrand, 2006; Zhao et al., 2006).
These approaches have revealed higher-order chromatin interactions at a range of gene loci in mammalian cells that correlate with epigenetic regulation secondary to cellular stimulation and differentiation, including cytokine loci. Within the murine β-globin locus control region (LCR), for example, enhancer regions that lie within the ~200 kb murine β-globin LCR were shown to interact with active, but not inactive, genes within the locus that are located 40–60 kb away. These long-range intrachromosomal looping interactions occur in erythroid cells, which express globin genes, but not in brain tissue, in which β-globin is not expressed (Patrinos et al., 2004; Tolhuis, Palstra, Splinter, Grosveld, & de Laat, 2002). The interactions are both activation-dependent and dynamic, as they change over the course of erythroid differentiation (Palstra et al., 2003). In addition, this spatial re-organization during differentiation was shown to be driven by the transcription factors erythroid Krüppel-like factor (EKLF), CCCTC-binding factor (CTCF), GATA-binding factor 1 (GATA-1), and friend of GATA-1 (FOG-1; Palstra et al., 2003; Splinter et al., 2006; Vakoc, Letting, et al., 2005). These fluctuating, multi-loop structures have been termed “active chromatin hubs,” in which active nucleoprotein complexes are juxtaposed with TSSs, increasing the local concentration of factors to direct transcription (de Laat & Grosveld, 2003; Williams, Spilianakis, & Flavell, 2010).
Long-range interactions also come into play when interactions between transcriptional initiation and termination sites circularize a gene, creating a conformation optimal for re-initiation of transcription. This was first observed in yeast (Ansari & Hampsey, 2005; O’Sullivan et al., 2004) and in studies of mammalian mitochondrial rDNA (Martin, Cho, Cesare, Griffith, & Attardi, 2005), and, as will be described in the following section, was first observed for mRNA transcription in a higher eukaryote at the TNF/ LT locus1 (Tsytsykova, Falvo, et al., 2007). 3C and 4C assays have also lent support to an earlier concept, derived from immunofluorescence-based assays, which postulates that genes physically cluster into subnuclear regions of active transcription known as “transcription factories” (Cook, 2010; Iborra, Pombo, Jackson, & Cook, 1996; Jackson, Hassan, Errington, & Cook, 1993; Osborne et al., 2004; Simonis et al., 2006). Based on 3C and 4C analysis of the maternal allele of the insulin-like growth factor 2 (Igf2)/H19 locus, it also appears that genes can be sequestered away from interaction with active enhancers into “inactive chromatin loops,” a process that requires CTCF (Kurukuti et al., 2006; Ling et al., 2006; Murrell, Heeson, & Reik, 2004; Zhao et al., 2006). High-throughput 3C-based assays have provided global maps of areas where active enhancers colocalize with their target genes (Baù et al., 2011; Sanyal, Lajoie, Jain, & Dekker, 2012), leading to the idea of “neighborhoods” of active and inactive transcription, which can be further grouped into compartments of active and inactive transcription in the nucleus (Sanyal et al., 2011).
These findings have led to a model of the genome as a fractal globule, allowing for dense packing without formation of knots (the classic “nucleosomal beads on a DNA string” further folded into “yarns”). In this model, the genome is partitioned into chromatin interaction domains, termed “topological domains” or “topologically associating domains (TADs)” which can be megabases in length (Dixon et al., 2012; Lieberman-Aiden et al., 2009; Mirny, 2011; Nora et al., 2012; Sanyal et al., 2011, 2012). In the discussion below of how higher-order chromatin organization participates in the regulation of cytokine gene expression, it is helpful to consider how these models inform understanding of the underlying mechanisms that control the rapid and/or persistent three-dimensional association and dissociation of enhancer regions with their target genes.
2. CYTOKINE GENE REGULATION
In the following sections, we will discuss in detail key examples of epigenetic regulationof cytokine gene expression in cells of the innate andadaptive immune systems: (i) the TNF/lymphotoxin (TNF/LT) locus, which encodes factors that are key components of the immediate early innate immune response; and (ii) the interferon-γ (IFNG) locus, Th2 cytokine locus (which includes IL4, IL5, and IL13, which encode interleukin-4, -5, and -13), and the interleukin-17A/interleukin-17F (IL17A/IL17F) locus, which reflect CD4+ T cell differentiation into the Th1, Th2, and Th17 lineages, respectively. Finally, epigenetic modifications that control expression at other loci involved in innate and adaptive immunity will be briefly summarized.
2.1. Innate immunity: The TNF/LT locus
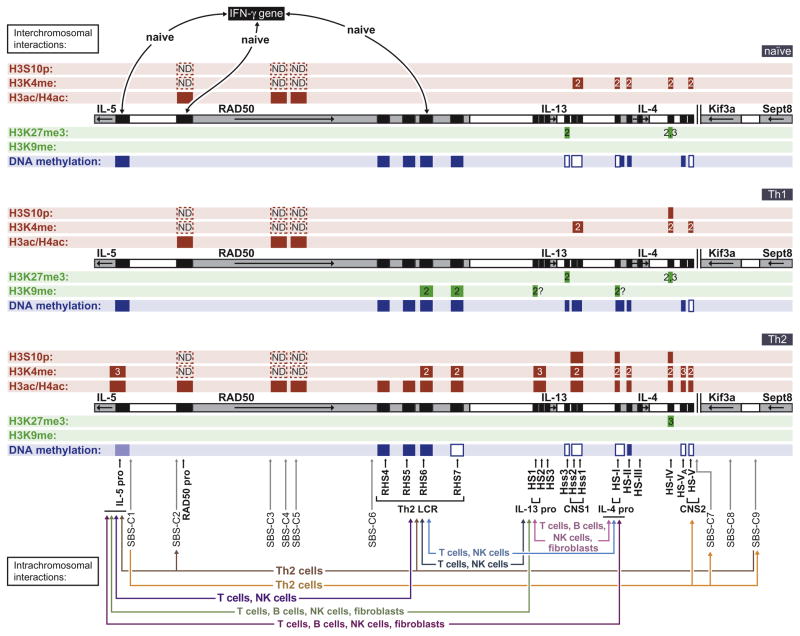
In humans, the coding regions for TNF, LTA, and LTB (encloding the tumor necrosis factor, lymphotoxin-α, and lymphotoxin-β genes, respectively) lie within a ~13 kb region of the TNF/LT locus, which itself occupies ~40 kb within the MHCIII locus on the p arm of chromosome 6 (Browning et al., 1993; Nedospasov et al., 1986; reviewed in Falvo et al., 2010; Shebzukhov & Kuprash, 2011; Fig. 2.2A). The transcriptional orientation of LTB is opposite to that of TNF and LTA, an arrangement that is strikingly conserved in vertebrates, from placentals to the frog (Xenopus tropicalis; diverging ~360 million years ago; Cross et al., 2005; Deakin et al., 2006; Kono et al., 2006). However, in teleost fish (Takifugu rubripes) and zebrafish (Danio rerio), the TNF homologue is in tandem with the TNF/ LT-related gene TNFN (Savan, Kono, Igawa, & Sakai, 2005).
Figure 2.2.
The TNF/LT locus. A. Positions of the LTB, TNF, and LTA genes (numbered exons in dark gray; transcriptional orientation indicated by white arrows) in the murine (top) and human (bottom) TNF/LT loci. Murine HS sites are labeled as in Tsytsykova, Rajsbaum, et al. (2007) and Biglione, Tsytsykova, and Goldfeld (2011) and human HS sites are labeled as in Taylor, Wicks, Vandiedonck, and Knight (2008). The TNF promoter is indicated in yellow, the murine HSS−9/human DHS44500 enhancers in green, the murine enhancer HSS +3 in magenta, and the murine monocyte-specific MAR HSS−7 in cyan. Red bars indicate the position of permissive histone modifications: H3 and H4 histone acetylation, mono-, di-, or trimethylation (1, 2, or 3) at H3K4, and phosphorylation at H3S10, in T cells or monocytes as indicated. Green bars indicate the position of repressive histone modifications: di- or trimethylation (2 or 3) H3K9. Blue bars indicate positions where DNA methylation inversely correlates with TNF gene expression. Arrows between sites in the locus indicate intrachromosomal interactions in the murine (top) and human (bottom) locus (Tsytsykova, Rajsbaum, et al., 2007; Watanabe et al., 2012; Wicks & Knight, 2011). B. Diagram of the higher-order structure of the murine Tnf/Lt locus following T cell activation, adapted from Tsytsykova, Rajsbaum, et al. (2007). Simultaneous interactions between the Tnf promoter and HSS+3, between the Tnf promoter and HSS−9, and between HSS +3 and HSS−9, are depicted, illustrating the facilitation of Tnf transcription by juxtaposition of NFAT-containing nucleoprotein complexes and circularization of the gene to promote reinitiation of transcription.
TNF was initially described as a product of macrophages (Beutler & Cerami, 1986; Rubin et al., 1985), However, later studies established that TNF transcription and TNF expression was also found in T cells, B cells, and fibroblasts (Cuturi et al., 1987; Goldfeld, Doyle, & Maniatis, 1990; Goldfeld & Maniatis, 1989; Goldfeld, Strominger, & Doyle, 1991; Goldfeld et al., 1992; Niitsu et al., 1988; Steffen, Ottmann, & Moore, 1988; Sung, Bjorndahl, Wang, Kao, & Fu, 1988; Sung, Jung, et al., 1988; Turner, Londei, & Feldmann, 1987). Furthermore, it was demonstrated that TNF is immediate early gene, and that it is transcribed within minutes following activation of T and B cells or stimulation of monocytes and macrophages (Goldfeld et al., 1991, 1992; Goldfeld, McCaffrey, Strominger, & Rao, 1993). In T cells, TNF is one of the first genes to be expressed after cellular activation and is one of the few genes that can be induced by signaling through the T cell receptor in the absence of protein synthesis and a CD28 costimulatory signal (Goldfeld et al., 1993). Indeed, calcium influx alone can induce TNF transcription in T cells (Goldfeld et al., 1993). This activation was found to be cyclosporin A-senstive, and, through an early application of a chemical genetics approach, was also found to be dependent upon the phosphatase activity of calcineurin (Goldfeld et al., 1993, 1994). This led to the discovery of the role of the calcineruin-dependent transcription factor family, nuclear factor of activated T cells (NFAT), in the activation of TNF gene transcription in T cells and B cells (Boussiotis, Nadler, Strominger, & Goldfeld, 1994; Goldfeld et al., 1993; McCaffrey, Goldfeld, & Rao, 1994; Tsai, Jain, Pesavento, Rao, & Goldfeld, 1996; Tsai, Yie, Thanos, & Goldfeld, 1996).
The proximal region of the TNF promoter (~200 bp upstream of the TSS) mediates initiation of TNF transcription in response to a wide range of stimuli in multiple cell types, including T cell and B cell activation (Goldfeld et al., 1994; Tsai, Jain, et al., 1996; Tsai, Yie, et al., 1996; Tsytsykova & Goldfeld, 2000, 2002), calcium ionophore (Goldfeld et al., 1993; Goldfeld et al., 1994), LPS (Goldfeld et al., 1990; Tsai et al., 2000), virus infection (Falvo, Uglialoro, et al., 2000; Goldfeld et al., 1990), TNF (Brinkman, Telliez, Schievella, Lin, & Goldfeld, 1999), Mycobacterium tuberculosis (MTb; Barthel et al., 2003), and osmotic stress (Esensten et al., 2005). The proximal TNF promoter is very highly conserved in mammals (Cross et al., 2005; Goldfeld, Leung, Sawyer, & Hartl, 2000; Kuprash et al., 1999; Leung et al., 2000; Shakhov, Collart, Vassalli, Nedospasov, & Jongeneel, 1990) and almost completely conserved in higher primates (Baena et al., 2007; Leung et al., 2000). Depending on cell type and stimulus, discrete sets of transcription factors and coactivators assemble at the proximal TNF promoter to form higher-order nucleoprotein complexes called enhanceosomes, which drive transcription of the gene (Barthel et al., 2003; Falvo, Brinkman, et al., 2000; Falvo et al., 2008; Falvo, Uglialoro, et al., 2000; Tsai et al., 2000; Tsytsykova & Goldfeld, 2002). This cell type- and stimulus-specific activation of TNF gene transcription is also a key feature of epigenetic regulation of the gene (Tsytsykova, Rajsbaum, et al., 2007, Biglione et al., 2011).
A number of constitutive and inducible DNase I hypersensitive sites (HSs), which occur within evolutionarily conserved sequences, have been detected across the TNF/LT locus in a cell type-specific fashion (Barthel & Goldfeld, 2003; Biglione et al., 2011; Ranjbar, Rajsbaum, & Goldfeld, 2006; Taylor et al., 2008; Tsytsykova, Rajsbaum, et al., 2007; Fig. 2.2A). For example, strong HSs are present at the TNF and LTA promoters in multiple cell types. In addition, a number of these sites are enhanced in response to cellular activation, bind to distinct activators, and are cell type-specific (Barthel & Goldfeld, 2003; Biglione et al., 2011; Ranjbar et al., 2006; Tsytsykova, Rajsbaum, et al., 2007).
The activation of TNF transcription is associated with multiple HATs, including the CBP/p300 coactivators (Barthel et al., 2003; Falvo, Brinkman, et al., 2000; Tsai et al., 2000). Notably, CBP is specifically required for TNF gene transcription in response to T cell activation (Falvo, Brinkman, et al., 2000). The first sequence-specific DNA-binding transcription factor to be identified as a HAT, activating transcription factor 2 (ATF-2; Kawasaki et al., 2000), binds to a conserved variant cyclic AMP response element (CRE) in the TNF proximal promoter, which mediates activation of TNF gene expression in many cell types and in response to multiple stimuli (Barthel et al., 2003; Brinkman et al., 1999; Diaz & Lopez-Berestein, 2000; Falvo, Brinkman, et al., 2000; Falvo, Uglialoro, et al., 2000; Newell, Deisseroth, & Lopez-Berestein, 1994; Steer, Kroeger, Abraham, & Joyce, 2000; Tsai et al., 2000; Tsai, Jain, et al., 1996; Tsai, Yie, et al., 1996; Tsytsykova & Goldfeld, 2002). The HATs PCAF and Gcn5 are also critical for TNF gene expression in Jurkat T cells in response to phytohemagglutinin (PHA)/phorbol 12-myristate 13-acetate (PMA) stimulation (Ranjbar et al., 2006). PCAF has also been implicated in TNF expression in THP-1 cells in response to high glucose conditions (Miao, Gonzalo, Lanting, & Natarajan, 2004). By contrast, HDAC1 and HDAC3, as well as the HDAC-recruiting corepressors NCoR and CoREST, associate with the Tnf promoter in unstimulated bone marrow-derived macrophages (BMDMs), and this association is dramatically reduced within an hour of stimulation with LPS (Hargreaves, Horng, & Medzhitov, 2009).
Epigenetic modifications have been characterized at a number of HSs across the TNF/LT locus in human and murine primary cells and cell lines (Biglione et al., 2011; Ranjbar et al., 2006; Tsytsykova et al., 2007; Fig. 2.2A). At the TNF promoter, for example, in Jurkat T cells it was initially shown that acetylation of histone H3 is induced by PHA/PMA, while histone H4 is constitutively acetylated (Ranjbar et al., 2006). Furthermore, in murine primary CD4+ T cells, anti-CD3/CD28 stimulation resulted in increased acetylation of histones H3 and H4 at the Tnf promoter as well as distal enhancers HSS−9 and HSS+3 (sites 9 kb upstream and 3 kb downstream of the Tnf TSS, respectively; Tsytsykova, Rajsbaum, et al., 2007). H3ac and H4ac marks are also enriched at both the Tnf promoter and a novel monocyte-specific matrix attachment region (MAR) at HSS−7 of the Tnf/ Lt locus in the murine J774 monocytic cell line (Biglione et al., 2011). PHA/ PMA stimulation also results in recruitment of the HATs PCAF and Gcn5 to the TNF promoter in Jurkat cells (Ranjbar et al., 2006). As was reported in the same study, the transactivator of transcription (Tat) protein from HIV-1 subtype E (HIV-193TH64Tat) suppresses TNF transcription by, at least in part, inhibiting PCAF and Gcn5 recruitment to the TNF promoter, with concomitant reduction in histone H3 and H4 acetylation (Ranjbar et al., 2006). These studies were confirmed and extended in an analysis of histone modifications at the TNF/LT locus in unstimulated and PMA/ionomycin-stimulated Jurkat cells, which showed a peak of histone H3 and H4 acetylation, as well as H3K4 trimethylation, at an HS within exon 4 of LTB, with other peaks at HSs 3.4 kb upstream of LTA (corresponding to the murine HSS−9 distal enhancer described by Tsytsykova, Rajsbaum, et al., 2007) and at the LTA and TNF promoter regions, as well as at a group of HSs near the 3′ end of the NFKBIL1 gene (Taylor et al., 2008).
These observations of epigenetic modifications at the TNF/LT locus were subsequently supported by a number of other studies of histone acetylation at the TNF promoter. An increase in acetylation of histones H3 and H4 at the TNF promoter correlates with LPS-induced TNF transcription in primary human monocytes and THP-1 cells (Garrett, Dietzmann-Maurer, Song, & Sullivan, 2008; Sullivan, Reddy, et al., 2007) and high glucose-induced TNF gene expression in THP-1 cells (Miao et al., 2004). Enriched H3ac and H4ac levels at the TNF promoter are also associated with maturation of monocytes into macrophages (Lee, Kim, Sanford, & Sullivan, 2003), and with the disease states of diabetes (Miao et al., 2004) and systemic lupus erythematosus (SLE; Sullivan, Suriano, et al., 2007) in primary monocytes. Moreover, IFN-γ treatment of primary human monocytes leads to persistent histone H4 acetylation at the TNF promoter, along with recruitment of ATF-2 and RNA Pol II; this “poised” pre-transcription state results in enhanced histone H3/H4 acetylation and TNF transcription in response to LPS stimulation (Garrett et al., 2008). In addition, the BRD protein Brg1, which interacts with acetylated histones and is an ATPase component of the SWI/SNF chromatin remodeling complex (Euskirchen, Auerbach, & Snyder, 2012) binds to the Tnf promoter in unstimulated murine J774 monocytic cells and BMDMs; however, expression of dominant-negative Brg1, or RNAi-mediated knockdown of Brg1 or the SWI/SNF ATPase Brm, does not impair LPS-induced Tnf transcription in these cells, suggesting that SWI/SNF complexes are dispensable for activation of Tnf gene expression in myeloid cells, and may act in some other capacity (Ramirez-Carrozzi et al., 2006, 2009).
The activating histone marks H3K4me1, H3K4me2, and H3K4me3 are enriched at the TNF promoter following LPS or TNF stimulation of THP-1 cells and PMA/ionomycin stimulation of Jurkat cells (Li et al., 2008; Sullivan, Reddy, et al., 2007; Taylor et al., 2008). In unstimulated murine BMDMs, high levels of H3K4me3 and H3ac (but not H4ac), along with RNA Pol II, TBP, and CBP/p300, are present at the Tnf promoter, consistent with a primary response gene poised for transcription (Hargreaves et al., 2009; Ramirez-Carrozzi et al., 2009). Assembly of this transcriptional complex at the Tnf promoter does not depend on signals mediated through Toll-like receptors (TLRs), as it was observed in macrophages from mice that are deficient in the essential TLR signaling components MyD88 and TRIF (Hargreaves et al., 2009). By contrast, LPS activation of wild-type BMDMs via TLR4 leads to enhanced association of the Tnf promoter with acetylated histone H4, the HATs Gcn5 and PCAF, the p-TEFb components cyclin 11 and cdk9, and the BRD protein Brd4 (Hargreaves et al., 2009). Furthermore, H3K4me2, which is enriched at the TNF promoter and 5′ coding region prior to cellular activation, is lost in response to LPS stimulation of THP-1 cells, while trimethylation of H3K4 increases at the promoter after LPS treatment (Sullivan, Reddy, et al., 2007). By contrast, in LPS-tolerant THP-1 cells, LPS stimulation fails to induce H3K4 methylation, H3K9 demethylation, and HP1 loss at the TNF promoter, which are all events that occur in LPS-responsive cells (El Gazzar et al., 2008; El Gazzar, Yoza, Hu, Cousart, & McCall, 2007). Consistent with the effects of these histone marks upon transcription, inhibition of H3K4 methylation through RNAi-mediated knockdown of either the histone methyltransferase SET7/ 9 or components of the mixed-lineage leukemia (MLL) histone methyl-transferase complex suppresses TNF transcription (Li et al., 2008; Sullivan, Reddy, et al., 2007), while inhibition of H3K9 methylation through RNAi of the histone methyltransferase G9a in LPS-tolerant cells decreases HP1 binding to the TNF promoter and restores TNF transcription (El Gazzar et al., 2008).
The activating histone mark H3S10p has also been observed at the TNF promoter in THP-1 cells, but not in primary human dendritic cells, following LPS stimulation (El Gazzar et al., 2007; Saccani et al., 2002). Infection of murine BMDMs with Toxoplasma gondii, which inhibits LPS-induced TNF expression, results in decreased LPS-mediated H3S10 phosphorylation and histone H3 acetylation at the Tnf promoter (Leng, Butcher, Egan, Abi Abdallah, & Denkers, 2009), while in LPS-tolerant THP-1 cells, H3S10 phosphorylation is reduced at the TNF promoter in comparison to LPS-responsive THP-1 cells (El Gazzar et al., 2007).
Thus, regulation of the TNF gene at its native locus involves a range of specific histone modifications and chromatin-modifying proteins associated with activation and repression. In the T cell lineage, activating histone marks strongly correlate with HSs present at promoter and enhancer regions, including distal enhancers that stimulate TNF transcription. In cells of the monocyte/macrophage lineage, activating histone marks are present at a monocyte-specific HS, and a range of stimuli correlate with the appearance of activating histone marks at the TNF promoter. Furthermore, the TNF promoter exhibits histone marks characteristic of a transcriptionally poised gene prior to activation, while under conditions of LPS tolerance the promoter is associated with histone marks and chromatin-binding proteins that typify a repressed transcriptional state. Taken together, these findings show that HSs at conserved noncoding sequences strongly correspond to the presence of distinct histone modifications, and strongly indicate that epigenetic modification of histones that are associated with TNF regulatory elements play a key role in inducible expression of the gene in the monocyte and T cell lineages.
2.1.1 DNA methylation at the TNF/LT locus
Methylation of DNA at the TNF proximal promoter has also been correlated with regulation of TNF gene transcription. For example, in primary granulocytes, which express TNF but not LT-α, the TNF proximal promoter is unmethylated and the LTA promoter is methylated, while in primary lymphocytes, which express both genes, both promoters are hypomethylated, and in sperm, where neither gene is expressed, both promoters are methylated (Kochanek, Toth, Dehmel, Renz, & Doerfler, 1990). In addition, the TNF coding sequence is hypomethylated in HL-60 (promyelocytic) cells, which actively produce TNF in response to PMA stimulation, and it is also hypomethylated in the RPMI 1788 (B-lymphoblastoid) human cell line, in which PMA induces modest TNF gene expression. By contrast, in the Jurkat human T cell line, which fails to produce TNF in response to PMA treatment, the TNF promoter is heavily methylated (Kochanek et al., 1990). It should be noted that PMA alone is not sufficient to induce TNF expression in a range of cell types (reviewed in Falvo et al., 2010) and selectively induces TNF in T and B cell lines (Goldfeld et al., 1991). In other experiments with primary human monocytes and lymphocytes, where TNF is expressed, the TNF coding sequence and proximal TNF promoter are unmethylated, while in non-TNF-expressing HeLa cells these regions are highly methylated (Kochanek, Radbruch, Tesch, Renz, & Doerfler, 1991). Similarly, in the murine RAW 264.7 macrophage cell line, in which Tnf transcription can be induced by LPS or cycloheximide, the Tnf coding region and 3′ and 5′ UTRs are unmethylated, while in the murine 3T3 fibroblast line, in which Tnf transcription is not activated by either stimulus, these areas of the locus are highly methylated. Moreover, the Tnf gene is highly methylated in hybrid cells created from the fusion of RAW 264.7 and 3T3 cells, and these cells do not express TNF when treated with LPS or cycloheximide (Kruys, Thompson, & Beutler, 1993). More recently, analysis of the TNF promoter region and exon 1 in three human cell lines revealed high levels of DNA methylation in non-TNF-expressing K562 cells and clones of the THP-1 cell line that were selected for lack of TNF expression, and low levels of methylation, especially at the proximal TNF promoter (−200 nt upstream of the TSS), in TNF-expressing HL-60 cells and THP-1 clones (Sullivan, Reddy, et al., 2007). Taken together, these studies indicate that methylation at a number of TNF regions, including the promoter, is associated with transcriptional repression.
Lending further support to this conclusion, demethylation of the TNF gene correlates with cellular differentiation status and increasing competence to express TNF. One study reported that the TNF proximal promoter and first exon are highly methylated in human embryonic stem cells and embryoid bodies, exon 1 is demethylated in hematopoietic stem cells and liver cells, and both the TNF proximal promoter and exon 1 are demethylated in primary monocytes and macrophages, where the gene is readily expressed (Sullivan, Reddy, et al., 2007). Indeed, methylation status at the TNF gene also changes during myeloid commitment, as methylation at two CpG sites flanking the TNF promoter is lower in HL-60 cells than in the more differentiated THP-1 cells (Takei, Fernandez, Redford, & Toyoda, 1996). The TNF proximal promoter is also highly methylated in unrestricted somatic stem cells (USSCs) and in human bone marrow mesenchymal stem cells (BM-MSCs), and after TLR activation of these cells the methylation status of TNF remains unchanged and the gene is not activated to any extent (van den Berk et al., 2009, 2010).
Additional data in support of an important role for DNA methylation in the repression of TNF expression under certain circumstances comes from studies showing that inhibition of DNA methylation at the TNF gene can enhance its transcription. For example, treatment of THP-1 cells with 5-azacytidine, a DNA methyltransferase inhibitor, results in decreased levels of methylation at the TNF promoter and enhanced LPS-mediated TNF expression (Sullivan, Reddy, et al., 2007). In LPS-tolerant THP-1 cells, RNAi-mediated knockdown of the histone methyltransferase G9a inhibits recruitment of DNMT3A and DNMT3B methyltransferases to the TNF gene, and restores TNF transcription (El Gazzar et al., 2008).
The binding of Sp1 to its GC-rich cognate DNA motifs in the TNF promoter is required for TNF gene expression in cells of the monocyte/ macrophage lineage in response to LPS stimulation, Sendai virus infection, or MTb infection (Barthel et al., 2003; Falvo, Uglialoro, et al., 2000; Tsai et al., 2000; Tsytsykova & Goldfeld, 2002). Notably, the relatively high percentage of CpG dinucleotides in the TNF promoter places this gene in a category of primary response genes that are independent of SWI/SWF remodeling after TLR-induced activation, consistent with the findings described above (Ramirez-Carrozzi et al., 2006, 2009). In murine BMDMs for example, the promoters of genes with a similarly high density of CpG islands tend to have a lower affinity for nucleosomes and are usually associated with acetylated histone H3, H3K4me3, RNA Pol II, and TBP in the resting state, poising these genes for transcription (Ramirez-Carrozzi et al., 2009). Furthermore, binding of Sp1 to promoters of this class of primary response genes tends to be essential for RNA Pol II recruitment under basal conditions (Ramirez-Carrozzi et al., 2009). Thus, the cell type- and stimulus-specific binding of Sp1 at the TNF promoter may function in concert with epigenetic modifications that regulate the gene.
2.1.2 The role of intrachromosomal interactions at the TNF/LT locus
In T cells, TNF is one of the first genes expressed upon cellular activation (Goldfeld et al., 1991, 1993). Analysis of the murine Tnf/Lt locus by 3C revealed that, upon T cell activation, intrachromosomal interactions form between the Tnf promoter and two novel, DNase-hypersensitive elements. Specifically, intrachromosomal interactions form between the Tnf promoter and the HSS−9 distal enhancer, between the Tnf promoter and the HSS+3 distal enhancer, and between HSS−9 and HSS+3 (Tsytsykova, Rajsbaum, et al., 2007; Fig. 2.2A). These three pairs of interactions result in a double-loop configuration at the Tnf/Lt locus that brings regulatory regions bound by NFATp into close proximity, creating a higher local concentration of active nucleoprotein complexes (Fig. 2.2B). This higher-order structure is reminiscent of the active chromatin hub observed at the β-globin locus, although instead of directing alternative enhancer-promoter interactions it positions the Tnf gene for optimal transcriptional activation. Specifically, the interaction between the Tnf promoter and HSS+3 circularizes the Tnf gene, potentially facilitating reinitiation of transcription by juxtaposing the transcription initiation and termination sites. In addition, the interaction between the Tnf promoter and HSS−9 sequesters the Lta gene into a discrete loop, placing this gene in a distinct transcriptional environment relative to Tnf and Ltb (Tsytsykova, Rajsbaum, et al., 2007).
Notably, the AT-rich HS 7 kb upstream of the Tnf TSS in the murine Tnf/Lt locus, HSS−7, acts as a MAR. HSS−7 serves as a substrate for topoisomerase II, and treatment of murine monocytic and T cell lines with the topoisomerase II inhibitor etoposide attenuates Tnf mRNA synthesis (Biglione et al., 2011). This presents another level of structural organization of the Tnf/Lt locus. Notably, HSS−7 is only accessible to interact with the nuclear matrix in murine monocytes, suggesting that the Tnf/Lt locus is associated with the matrix in structurally distinct fashions based on cell type (Biglione et al., 2011). It is generally thought that interactions between the nuclear lamina and MARs, along with inter- and intrachromosomal interactions, are major contributing factors to the three-dimensional arrangement of chromosomes in the nucleus (van Steensel & Dekker, 2010). Topoisomerase II may dock at the Tnf/Lt HSS−7 MAR and act to relax positive supercoiling at the locus, which results from transcription of the constitutively expressed upstream Nfkbil1 gene; this ensures efficient transcriptional output at the highly inducible Tnf gene (Biglione et al., 2011). Thus, this finding of cell type-specific epigenetic control of chromatin structure at the Tnf/Lt locus extends previous observations that regulation of TNF gene expression is controlled in a cell type-specific manner at the TNF promoter via distinct factors and regulatory elements (Barthel et al., 2003; Falvo, Uglialoro, et al., 2000; Goldfeld et al., 1993; Tsai et al., 2000; Tsai, Jain, et al., 1996; Tsai, Yie, et al., 1996; Tsytsykova & Goldfeld, 2000, 2002). In summary, these studies indicate that the TNF/LT locus is subject to dynamic structural reconfiguration in response to various stimuli and in a manner that varies based on cell type.
In support of this model, another study found that intrachromosomal interactions among exon 4 of LTB, the LTB promoter, the LTA promoter, and the TNF 3′-UTR occur in unstimulated Jurkat cells and decrease upon PMA/ionomycin stimulation; the intrachromosomal interactions are thus associated with repression of LTB gene transcription (Wicks & Knight, 2011; Fig. 2.2A). This study also found that CTCF binds to LTB exon 4, indicating that CTCF may contribute to the formation of a repressive loop structure (Wicks & Knight, 2011). By contrast, another study in hepatocellular carcinoma cells implicated the formation of an enhancer-containing chromosomal loop, dependent upon CTCF and the cohesin RAD21, in the activation of LTB transcription (Watanabe et al., 2012). CTCF/RAD21 binding sites were characterized within LTB exon 4 (the site being designated TC3), upstream of and within the NFKBIL1 gene (TC1 and TC2, which lie 29.5 and 34.2 kb from TC3, respectively), and upstream of the LST1 gene (TC4, which lies 4.7 kb from TC3; Fig. 2.2A). In the early phase of gene expression following TNF stimulation in these cells, in which expression of both TNF and LTB is favored, TC1–TC4 are physically associated with the TNF, LTA, and LTB promoters and with an NF-κB-dependent enhancer region, TE2, in the 3′-UTR of TNF. By contrast, in the late phase of gene expression, in which LTB transcription is favored, TC3, TE2, and the LTB promoter remain associated, as do TC2 and the TNF and LTA promoters (Watanabe et al., 2012; Fig. 2.2A). Taken together, all these data support a model in which dynamic changes in intrachromosomal interactions within the TNF/LT locus correlate with both activation and repression of specific genes within the locus, most likely through a combination of bringing enhancer regions into close proximity with specific promoters, and by sequestering genes into subnuclear regions of active or inactive transcription.
2.2. CD4+ T cell differentiation: The IFNG locus, Th2 locus, and IL17A/IL17F locus
A central cytokine-regulated process in the establishment of the immune response is the differentiation of naïve CD4+ T cells to helper T cell subsets (Fig. 2.3). Cytokines present in the local environment during antigen presentation strongly influence the differentiation pathway taken by a naïve CD4+ T cell. Th1 cells are primarily involved in host defense to intracellular pathogens, and differentiation of naïve CD4+ T cells to a Th1 phenotype requires the transcription factor T-bet (Szabo et al., 2000). In an elegant model proposed by Schulz et al., Th1 differentiation involves several steps: (i) exposure of the naïve CD4+ T cell to autocrine and/or paracrine IFN-γ during TCR engagement, which induces T-bet expression; (ii) T-bet-mediated expression of the IL-12 receptor subunit β2 once TCR signaling ceases; and (iii) signals transduced by APC-derived IL-12, which drive STAT4 expression and sustained IFN-γ and T-bet synthesis (Schulz, Mariani, Radbruch, & Hofer, 2009). IL-2 is also required for both differentiation and subsequent expansion of the de novo Th1 population (Liao, Lin, Wang, Li, & Leonard, 2011).
Figure 2.3.
CD4+ T helper cell differentiation. Cytokines that polarize a naïve CD4+ T cell to the Th1, Th2, or Th17 lineage; transcription factors that serve as master regulators for the differentiation of each T helper cell lineage; and the effector cytokines expressed by each T helper cell lineage are shown.
Th2 cells are primarily involved in the immune response to extracellular parasites. The master regulator of Th2 differentiation is the transcription factor GATA3 (Zheng & Flavell, 1997). Th2 differentiation is usually considered to rely on IL-4 in the context of TCR ligation and exposure to IL-2, with IL-4 inducing STAT6 and GATA3 expression (Cote-Sierra et al., 2004). However, in vivo mouse studies have found that IL-4 is dispensable for Th2 differentiation, suggesting that IL-4-independent pathways play a role in Th2 development (van Panhuys et al., 2008). As Th2 differentiation proceeds, GATA3 mediates the expression of IL-5 and IL-13, classical Th2 cytokines whose genes share a locus with the IL-4 gene (Kishikawa, Sun, Choi, Miaw, & Ho, 2001; Lavenu-Bombled, Trainor, Makeh, Romeo, & Max-Audit, 2002; Siegel, Zhang, Ray, & Ray, 1995; Yamashita et al., 2002; Zhang, Yang, & Ray, 1998). Initial strength of TCR engagement on naïve CD4+ T cells has also been linked to Th1 versus Th2 differentiation, with weak TCR signaling pushing the cell to a Th2 phenotype and strong TCR signaling pushing the cell to a Th1 phenotype (Constant, Pfeiffer, Woodard, Pasqualini, & Bottomly, 1995; Tao, Constant, Jorritsma, & Bottomly, 1997).
Th17 cells were first described as a distinct CD4+ T helper lineage in 2005 (Harrington et al., 2005). Th17 cells are involved in host defense against extracellular bacteria and fungi, and differentiation of a naïve CD4+ T cell to a Th17 cell is regulated by the transcription factor RORγt (Ivanov et al., 2006). The differentiation of Th17 cells is complex, and a clear picture of the cytokines required for Th17 lineage commitment has not emerged. Both IL-6 and TGF-β have been linked to Th17 differentiation (Bettelli et al., 2006; Gutcher et al., 2011; Li, Wan, & Flavell, 2007; Veldhoen, Hocking, Atkins, Locksley, & Stockinger, 2006; Veldhoen, Hocking, Flavell, & Stockinger, 2006), although a recent report has suggested that TGF-β is not required for in vivo generation of at least some Th17 cells in mice (Ghoreschi et al., 2010). During TCR engagement by MHC Class II/antigen, induction of STAT3 and, in turn, RORγt by IL-6 and TGF-β, as well as other cytokines including IL-1β and IL-23, drives the production of Th17-associated cytokines. These include IL-17A, IL-17F, IL-21, IL-22, and (in humans) IL-26 (Langrish et al., 2005; McGeachy et al., 2009; Wilson et al., 2007). IL-21 may act in an autocrine manner to potentiate Th17 differentiation (Korn et al., 2007; Nurieva et al., 2007; Zhou, Ivanov, et al., 2007).
Dysregulation of Th1 and Th17 responses leads to autoimmune disease states, while dysregulation of Th2 responses leads to atopic conditions (Kanno, Vahedi, Hirahara, Singleton, & O’Shea, 2012; Maddur, Miossec, Kaveri, & Bayry, 2012; Mills, 2011; Oliphant, Barlow, & McKenzie, 2011; Wilson et al., 2009). Below, we discuss epigenetic mechanisms involved in the regulation of gene expression at three specific loci that are associated with the differentiation of Th1, Th2, and Th17 cells, respectively: the IFNG locus, the Th2 locus (which contains the IL4, IL5, and IL13 genes), and the IL17A/IL-17F locus.
2.2.1 The IFNG locus
The IFNG gene is quite isolated in the context of its native locus: the nearest downstream gene coding region lies ~420 and ~500 kb away in mice and humans, respectively, and the nearest upstream gene, which encodes IL-26 in humans and IL-22 in mice, lies ~245 kb away (Kanno et al., 2012). An early study reported that 8.6 kb of the human IFNG locus, containing the coding sequence, 2.3 kb upstream and 1 kb downstream of the IFNG TSS, is sufficient for T cell-specific expression of the gene when integrated into the genome of a transgenic mouse (Young et al., 1989). However, sites outside the IFNG promoter and coding region are essential for proper regulation of the gene’s expression, as transgenic mice bearing the extended human IFNG locus, but not the murine Ifng locus with only the upstream promoter region, exhibited normal IFNG regulation during T helper cell differentiation (Soutto, Zhang, et al., 2002; Soutto, Zhou, & Aune, 2002; Zhu et al., 2001). Indeed, conserved sequences as distant as ~70 kb upstream and ~66 kb downstream of the murine Ifng gene (corresponding to sequences ~63 kb upstream and ~119 kb downstream of the human IFNG gene) contribute to its regulation during T cell lineage commitment (Hadjur et al., 2009; Sekimata et al., 2009). Conserved CNSs that coincide with HSs are spread across the IFNG locus and function as enhancers and/or mediate higher-order chromatin structure (Amsen et al., 2009; Balasubramani, Mukasa, Hatton, & Weaver, 2010; Hatton et al., 2006; Kanno et al., 2012; Lee, Avni, Chen, & Rao, 2004; Shnyreva et al., 2004; Wilson et al., 2009). In the murine Ifng locus, these CNSs include (from 5′ to 3′ with respect to Ifng) CNS−70, CNS−54, CNS−34, CNS−22, CNS−6 (also known as CNS1), Ifng intron 1, CNS+17−19 (also known as CNS2 or CNS+18/20), and CNS+30 (also known as CNS+29), CNS+46, and CNS+66 (Balasubramani, Shibata, et al., 2010; Mukasa et al., 2010; Wilson et al., 2009). The corresponding regions in the human IFNG locus are CNS−63, CNS−31, CNS−22, CNS−18, CNS−4, IFNG intron 1, CNS+22, CNS+40, CNS+80, and CNS+119 (Amsen et al., 2009; Balasubramani, Mukasa, et al., 2010; Barski et al., 2007; Boyle et al., 2008; Rowell et al., 2008; Wang et al., 2008; Wilson et al., 2009; Fig. 2.4).
Figure 2.4.
The IFNG locus. Comparative histone posttranslational modifications, DNA methylation status, and intra- and interchromosomal interactions are shown for the murine Ifng locus in naïve CD4+ T, Th1, Th2, and Th17 cells. Position and transcriptional orientation of the Ifng gene (gray box, arrow) and regulatory elements (black boxes) in the murine Ifng locus are shown, with names of the murine sequences indicated in black at the bottom, and of corresponding elements in the human IFNG locus in gray. Red bars indicate the position of permissive histone modifications: H3 and H4 histone acetylation, and di- or trimethylation (2 or 3) at H3K4. Green bars indicate regions associated with the repressive histone mark H3K27me3. Regions of CpG hypomethylation and methylation are indicated by open and filled blue boxes, respectively. At the bottom, arrows denote intrachromosomal interactions that form within the murine Ifng locus between the Ifng gene and distal CNSs in a Th1-specific fashion or that are present in naïve, Th1, and Th2 cells (Sekimata et al., 2009; Spilianakis, Lalioti, Town, Lee, & Flavell, 2005) as well as intrachromosomal interactions detected among fragments centered at EcoRI sites at positions (relative to the Ifng TSS) −5048, −1248, +229; +6,891, +10,264 and between MARs at positions −7000 and −10,500 in naïve, neutral (Th0), Th1, and Th2 cells (Eivazova & Aune, 2004; Eivazova, Vassetzky, & Aune, 2007; Eivazova et al., 2009). Interchromosomal interactions present in naïve T cells between the Ifng gene and the indicated sites in the Th2 cytokine locus (Spilianakis et al., 2005) are shown by arrows at the top.
A role for histone acetylation in the regulation of the IFNG locus was initially suggested by studies with the HDAC inhibitor sodium butyrate, which enhanced expression of IFN-γ in murine CD4+ T cells (Bird et al., 1998). In naive murine CD4+ T cells, H4ac is enriched at CNS−34 and CNS−22 of the Ifng locus (Hatton et al., 2006). With respect to histone methylation, one study has reported that, in naïve murine CD4+ T cells, low levels of H3K4me1 are present at CNS−34 and CNS−22 (Mukasa et al., 2010), while a second study found that low levels of H3K4me2 are only found at CNS−22 (Schoenborn et al., 2007) and a third study reported that H3K4me2 is present at low levels at CNS−6, the Ifng promoter, and at a region ~13 kb downstream of the Ifng TSS (Hamalainen-Laanaya, Kobie, Chang, & Zeng, 2007). On the other hand, H3K27me3 is modestly enriched at the 3′ end of the Ifng gene and at distal sites ~30–50 kb downstream in naïve murine CD4+ T cells (Mukasa et al., 2010; Schoenborn et al., 2007; Wei et al., 2009), indicating that this repressive mark may counteract any positive activity induced by the methylated H3K4 histones upstream.
Upon differentiation into Th1 cells, there is a marked increase in H3K4me2, H3ac, and H4ac across the IFNG locus, with concomitant removal of H3K27me3 (Agarwal & Rao, 1998a, 1998b; Hatton et al., 2006; Lee et al., 2004; Schoenborn et al., 2007; Shnyreva et al., 2004). In addition, at the early stages of Th1 development the Brg1-containing SWI/SNF remodeling complex is recruited to the murine Ifng promoter in a STAT4-dependent manner and promotes accessibility of the promoter to nuclease digestion (Zhang & Boothby, 2006). Furthermore, the binding of T-bet at multiple enhancers in the locus displaces HDAC-containing complexes (Chen, Osada, Santamaria-Babi, & Kannagi, 2006); T-bet is able to recruit JMJD3 and SET7 to demethylate H3K27me3 and induce dimethylation of H3K4, respectively (Miller, Huang, Miazgowicz, Brassil, & Weinmann, 2008). By contrast, as Th2 differentiation proceeds, H3K27me3 is deposited throughout the Ifng locus (Agarwal & Rao, 1998a; Chang & Aune, 2007; Jones & Chen, 2006; Schoenborn et al., 2007). Notably, some repressive H3K9me2 marks persist at the locus in murine Th1 cells, which may modulate normal expression of the Ifng gene (Berger, 2007; Chang & Aune, 2007). Thus, repressive histone modifications accumulate to silence the IFNG locus in Th2 cells, while primarily activating histone modifications are deposited at enhancers and other conserved regions of the locus as Th1 differentiation proceeds.
Early studies reported that hypomethylation of a CpG island between the CAAAT and TATA boxes in the human IFNG promoter (conserved in mice) corresponds to transcriptional competence for IFN-γ production in human B cells (Pang, Norihisa, Benjamin, Kantor, & Young, 1992), murine Th1 clones, and human CD4+ Th0 clones, but that hypermethylation of this site is found in murine Th2 clones (Young et al., 1994). Furthermore, treatment of a murine Th2 T cell clone with the DNA methylation inhibitor 5-azacytidine promotes IFN-γ production (Young et al., 1994), reminiscent of earlier studies demonstrating that 5-azacytidine restored IFN-γ production in response to IL-2 in a cytotoxic T cell line (Farrar, Ruscetti, & Young, 1985). Progressive demethylation of ten CpG dinucleotides in an HS near Ifng intron 1 occurs as primary murine CD4+ T cells develop into Th1, but not Th2, cells. Moreover, demethylation of DNA at this site ensues after T-bet-mediated chromatin remodeling of the locus and maximal IFN-γ expression occurs, suggesting that the primary function of this modification is to poise the Ifng locus for rapid activation in response to cellular stimulation (Mullen et al., 2002). Similarly, in the human IFNG locus, six CpG sites in the IFNG promoter and seven CpG sites in CNS1 (CNS-4) are hypermethylated in naïve CD4+ cells, and become progressively demethylated during differentiation into Th1 cells, but not Th2 cells (Janson, Marits, Thörn, Ohlsson, & Winqvist, 2008; White et al., 2006).
Detailed analysis of DNA methylation at HSs in the murine Ifng locus revealed that the Ifng promoter and CNS−34, CNS−22, CNS+29, and CNS+46 are demethylated in naïve T cells, and that upon Th2 differentiation CpG methylation is induced at these sites, with a concomitant loss of NFAT binding at the Ifng promoter (Jones & Chen, 2006; Lee et al., 2004; Schoenborn et al., 2007). Notably, methylation of the Ifng promoter does not inhibit the binding and function of T-bet (Tong, Aune, & Boothby, 2005). However, it is interesting to speculate that the limited availability of T-bet in developing Th2 cells most likely precludes any functional impact of T-bet binding to a methylated region of the Ifng locus. The increase of CpG methylation at the Ifng promoter during Th2 differentiation, as well in Th1 cells from Stat4-deficient mice, also correlates with recruitment of DMNT3a (Jones & Chen, 2006; Yu, Thieu, & Kaplan, 2007). Furthermore, in mice lacking DMNT1 or the DNA-binding domain of MBD2 there is an increase in IFN-γ expression not only in naïve and Th1 cells, but also in Th2 cells (Hutchins et al., 2002; Lee, Fitzpatrick, et al., 2001; Makar & Wilson, 2004). Thus, at both the Ifng promoter and at multiple widely separated HSs in the Ifng locus, DNA hypomethylation correlates with the competence of a given T cell subset to express IFN-γ.
Both intra- and interchromosomal interactions have been characterized at the Ifng locus (Amsen et al., 2009; Lee et al., 2006; Williams, Spilianakis, & Flavell, 2010). In primary murine naïve CD4+ T cells, Th1 cells, and Th2 cells, the Ifng gene coding region associates with CNS−6, located ~5 kb upstream of the gene. However, in Th1 cells but not naïve or Th2 cells, the Ifng gene coding region also associates with CNS+18/20, located ~18 to 20 kb downstream of the gene (Spilianakis, Lalioti, Town, Lee, & Flavell, 2005; Fig. 2.4). Another study examined intrachromosomal interactions among fragments generated by EcoRI digestion at positions (relative to the Ifng TSS) −5048 (near CNS1/CNS−6), −1248, +229 (Ifng first intron), +6891, and +10,264, designated sites 1 through 5, respectively (Eivazova & Aune, 2004). Sites 1, 3, and 4 were found to be in close proximity in naïve, Th1, and Th2 cells. Sites 2 and 5 formed relatively weaker associations with sites 1, 3, and 4 in naïve cells, and these interactions were strengthened in Th1 and Th2 cells 5 days after primary stimulation. However, 2 days after secondary stimulation, the interactions with sites 2 and 5 were greatly reduced in Th1 cells (Fig. 2.4). This suggested a more tightly packed, “closed” conformation at the Ifng locus in naïve and Th2 cells, and an “open” conformation in Th1 cells (Eivazova & Aune, 2004). A number of MARs have also been identified in the Ifng locus in unstimulated naïve CD4+ cells as well as in CD4+ cells polarized under neutral, Th1, and Th2 conditions (Eivazova et al., 2007). In CD4+ cells cultured under neutral conditions (Th0) and the murine T cell line EL4, a MAR ~7 kb upstream of the Ifng TSS was observed to form intrachromosomal interactions with a MAR located ~10.5 kb downstream of the Ifng TSS, creating a ~18 kb loop (Eivazova et al., 2009; Eivazova et al., 2007; Fig. 2.4). Thus, distinct intrachromosomal interactions form at the ifng locus depending upon the stage of T cell differentiation.
CTCF and cohesins bind constitutively to the Ifng locus at intron 1 in Th1 cells, in which the CpG motifs are hypomethylated, but they do not bind to the locus in Th2 cells (Parelho et al., 2008; Sekimata et al., 2009). A Th1-specific CTCF/cohesin binding region is also present at CNS+66, located ~66 kb downstream of the murine Ifng gene (corresponding to CNS+119, ~119 kb downstream of the human IFNG gene; Sekimata et al., 2009). Functional consequences of CTCF/cohesin binding at the Ifng locus have been characterized. These studies have shown that a highly conserved hypomethylated element in the mouse locus, CNS−70, which corresponds to CNS−63 of the human IFNG locus, binds to CTCF and cohesin in naïve CD4+, Th1, and Th2 cells; however, it is only in Th1 cells that this element forms intrachromosomal interactions with the Ifng gene and with CNS+66, both of which are able to recruit T-bet (Sekimata et al., 2009). These interactions, as well as IFN-γ expression, are inhibited by shRNA-mediated ablation of CTCF or in a T-bet-deficient genetic background. In addition, Th1-specific CTCF/ T-bet-dependent interactions also occur between the Ifng gene and the CNS−34, CNS−29, and CNS+18/20 regions (Sekimata et al., 2009). Other chromosomal proteins may also play a role in the establishment of intrachromosmal interactions at the Ifng locus: by extending 3C assays using additional immunoprecipitation steps (“ChIP-loop” assays), topoisomerase IIα and MeCP2, but not CTCF and HP1, were implicated as possible mediators of the interaction between the MARs at CNS1/CNS-6 and at −11 kb relative to the Ifng TSS in naïve cells (Eivazova et al., 2009). Notably, MeCP2, implicated as a mediator of structure in naïve cells, binds to methylated CpG sites that correspond with inactive transcription, while CTCF binds to hypomethylated sites associated with active transcription (Eivazova et al., 2009; Sekimata et al., 2009). Thus, CTCF and other mediators of long-range chromosomal interactions direct the higher-order chromatin structure of the IFNG locus, which is further correlated with the level of gene activity and stage of T cell differentiation.
In murine naïve CD4+ T cells, but not Th1 or Th2 cells, the Ifng gene (located on chromosome 10) interacts with the Il5 promoter, the Rad50 promoter, and an HS in the 3′ region of the Rad50 gene (RHS6), all at the Th2 locus on chromosome 11 (Spilianakis et al., 2005). Furthermore, FISH analysis revealed that the Ifng and Th2 loci colocalize in nonheterochromatic regions in naïve CD4+ T cells, and that this interaction is diminished in Th1 and Th2 cells. Moreover, deletion of an HS (RHS7) that lies downstream of RHS6 in the Rad50 gene, which is critical for Th2 locus function and for intrachromosomal interactions between RHS6 and elements within Il4, results in weakened association of the two loci in FISH analysis and in delayed, decreased levels of Ifng transcription in response to anti-CD3/CD28 stimulation (Spilianakis et al., 2005). As Flavell and colleagues proposed based on these structural findings, the Ifng and Th2 loci may participate in the formation of a chromatin hub in naïve CD4+ T cells, primed for rapid initiation of cytokine expression in response to TCR engagement. Only once Th1 or Th2 polarization signals are fully transmitted and one locus is shut down with concomitant upregulation of the other locus does polarization occur, in part due to a transition from interchromosomal to intrachromosomal interactions (Amsen et al., 2009; Lee et al., 2006; Williams, Spilianakis, & Flavell, 2010).
2.2.2 The Th2 cytokine locus: IL4, IL5, and IL13
The Th2 cytokine locus includes the genes that encode the canonical Th2 effector cytokines IL-4, IL-5, and IL-13. In mice, these cytokine genes occupy a ~120 kb region in chromosome 11, and are flanked by the gene encoding the transcription factor IRF-1 at one end and the genes encoding the constitutively expressed kinesin-2 subunit Kif3A and septin 8 (Sept8) at the other (Fig. 2.5; Frazer et al., 1997; Gorham et al., 1996; Lee & Rao, 2004; Loots et al., 2000). The Th2 locus is on the q arm of chromosome 5 in humans. The IL13 and IL4 genes lie in tandem at one end of the locus, downstream and in the same orientation as the gene encoding the DNA repair enzyme Rad50, while IL5 resides ~120 kb telomeric to the IL4 and IL13 genes and in the opposite transcriptional orientation, an arrangement that is conserved in mammals (Frazer et al., 1997; Gorham et al., 1996; Lee & Rao, 2004; Loots et al., 2000). An array of CNSs, constitutive and inducible HSs, and binding sites for the architectural protein special AT-rich sequence binding protein 1 (SATB1) are scattered across the Th2 locus in mice (Fig. 2.5). Specifically, Rad50 hypersensitive sites (RHSs) 1 through 7, each of which contains one to three discrete HSs, lie between Il5 and the 3′ end of Rad50. RHS4, RHS5, RHS6, and RHS7 are clustered near the 3′ end of Rad50, forming the Th2 LCR. This region was classified as an LCR because it drives Il4 and Il13 transcription in Th2 effector cells in transgenic mice in a copy number-dependent fashion and irrespective of integration location (Fields, Lee, Kim, Bartsevich, & Flavell, 2004; Lee, Fields, Griffin, & Flavell, 2003). In addition, three HSs lie within Il13: HS1 (which coincides with a CG-rich element, CGRE), HS2, and HS3. A conserved region between Il13 and Il4 contains HSs as well (named Hss1, Hss2, and Hss3; Hss1 and Hss2 lie within a highly conserved sequence, CNS−1; Loots et al., 2000). The Il4 gene contains HS-I (at the promoter), HS-II (an enhancer in the second intron), and HS-III (Lee, Fields, & Flavell, 2001; Takemoto et al., 1998). Downstream of Il4 lies HS-IV, which coincides with a conserved silencer region, and HS-VA and HS-V, which coincide with a second CNS, CNS−2 (Agarwal, Avni, & Rao, 2000; Tanaka et al., 2006; Fig. 2.5). Il4 transcription is enhanced by the Th2 LCR, Hss1, Hss2, HS-I, HS-II, HS-VA, and HS-V, while Il13 transcription is enhanced by the Th2 LCR, HS1, Hss1, and Hss2; however, these elements do not drive Il5 transcription (Lee, Fields, et al., 2003). Several SATB1-binding sequences, designated SBS-C1 to SBS-C9, lie between Il5 and the Th2 LCR and downstream of Il4. As described below, these are involved in establishing long-range intrachromosomal interactions (Cai et al., 2006; Fig. 2.5).
Figure 2.5.
The Th2 cytokine locus. Comparative histone posttranslational modifications, DNA methylation status, and intra- and interchromosomal interactions are shown for the murine Th2 cytokine locus in naïve CD4+ T, Th1, and Th2 cells. Position and transcriptional orientation of the Il4, Il5, Il13, Rad50, Kif3a, and Sept8 genes (gray boxes, arrows) and regulatory elements (black boxes) are shown; the length of the region containing Kif3a and Sept8 (to the right of the vertical double line) is compressed approximately twofold relative to the rest of the locus. Regulatory sequences and binding sites of the Th2-specific architectural factor SATB1 are labeled with thick and thin vertical arrows, respectively. Red bars indicate the position of permissive histone modifications: H3 and H4 histone acetylation, di- or trimethylation (2 or 3) at H3K4, and phosphorylation at H3S10. Green bars indicate the position of repressive histone modifications: di- or trimethylation (2 or 3) at H3K27 or dimethylation (2) at H3K9. Regions of CpG hypomethylation and methylation are indicated by open and filled blue boxes, respectively. Interchromosomal interactions that form in naïve T cells between sites in the Th2 locus and the Ifng gene are shown at the top. Intrachromosomal interactions within the locus involving the Il5 promoter, the Th2 LCR, the Il13 promoter, and the Il4 promoter, that are present in T cells, B cells, NK cells, and fibroblasts, or only in T cells and NK cells, are shown by thick arrows at the bottom, along with intrachromosomal interactions among SATB1 binding sites in unstimulated Th2 cells, which are shown by thin arrows. Additional SATB1-mediated intrachromosomal interactions that form in activated Th2 cells are described in Cai, Lee, and Kohwi-Shigematsu (2006). ND, not determined in a given cell type; question mark indicates conflicting results in separate studies.
2.2.2.1 Histone modifications
As CD4+ T helper cell differentiation proceeds, DNase I accessibility at HSs in the promoter, intronic, intergenic, and 3′ regions of Il13 and Il4 increases or diminishes depending on the external signals that are received (Agarwal & Rao, 1998b; Takemoto et al., 1998). These findings correspond well with early data that supported an important role for histone acetylation in the regulation of the Th2 locus, which initially came from observations that two pharmacological inhibitors of the HDACs, sodium butyrate and trichostatin A, can derepress IL4 expression in naïve murine CD4+ T cells and in activated human peripheral blood CD4+ T cells (Bird et al., 1998; Valapour et al., 2002). Indeed, mice with T cell-specific loss of HDAC1 exhibit increased inflammatory responses in an in vivo allergic airway inflammation model (Grausenburger et al., 2010). These mice show enhanced production of IL-5, IL-13, and (with PMA/ionomycin stimulation) IL-4 by peripheral lung CD4+ T cells during disease, enhanced production of IL-4, IL-5, IL-13, and IL-10 in Th2-polarized peripheral CD4+ T cells, and higher proliferation of and IL-4 expression by naïve CD4+ T cells that are activated in Th2 conditions. Furthermore, binding of HDAC1 is detected in unstimulated CD4+ T cells at multiple sites in the Th2 cytokine locus, including the Il4 intron 2 enhancer (HS-II), HS-V and HS-VA downstream of Il4, CNS−1/Hss2 in the Il13/IL4 intergenic region, and HS2 in the Il13 promoter (Grausenburger et al., 2010).
In naïve murine CD4+ T cells, low to undetectable levels of H3ac and H4ac are found at the Il5 promoter, the Il13 promoter and first intron, the Il13/Il4 intergenic region CNS−1 (Hss1 and Hss2), the Il4 promoter, the Il4 intron 2 enhancer (HS-II), the Il4 3′ enhancer (HS-VA), the Th2-specific constitutive HS site HS-V (CNS−2), and the common naïve/Th1/Th2 HS site HS-IV, while in Th2 cells all of these regions become persistently hyperacetylated (Avni et al., 2002; Baguet & Bix, 2004; Fields, Kim, & Flavell, 2002; Fields et al., 2004; Grogan et al., 2003; Hatton et al., 2006; Wurster & Pazin, 2008; Yamashita et al., 2002). Th2-specific acetylation of histone H3 also occurs at the murine Th2 LCR (Fields et al., 2004; Wurster & Pazin, 2008). Similarly, in peripheral blood naïve human CD4+ T cells H3ac is not detected at the IL4 promoter, but becomes enriched in Th2-polarized cells relative to Th1-polarized cells (Messi et al., 2003). Histone H3 acetylation at CNS−1, HS-II, HS-III, and HS-VA also correlates with high levels of Il4 expression in murine Th2 clones (Guo et al., 2002). By contrast, histone H3 and H4 are acetylated at roughly equal levels at the murine Rad50 promoter and at two Rad50 intronic regions in Th1 and Th2 cells, albeit at higher levels than at these sites in naïve CD4+ T cells (Fields et al., 2004; Yamashita et al., 2002). Thus, as is found at the IFNG locus, histone acetylation at the Th2 cytokine locus strongly corresponds to the ability of a differentiated CD4+ T helper cell to rapidly synthesize the appropriate array of cytokines in response to activation.
Acetylation during CD4+ T helper cell differentiation appears to be biphasic. After TCR stimulation of murine naïve CD4+ T cells, hyper-acetylation is rapidly induced at the Il4 promoter, HS-II, CNS−1, HS-VA, CNS −2, HS-IV, and the Th2 LCR in both Th1- and Th2-polarized cells; however, with continual exposure to Th2-polarizing conditions, hyperacetylation is sustained at Il4 and gradually lost at Ifng (Avni et al., 2002; Fields et al., 2002). This corresponds to the finding that both IL-4 and IFN-γ are rapidly synthesized after anti-CD3/CD28 stimulation of murine naïve CD4+ T cells under Th2-polarizing conditions, with Ifng expression decreasing as differentiation proceeds (Grogan et al., 2001). The persistence of histone acetylation at the Th2 cytokine locus in mature Th2 cells provides a contrast to the transient histone hyperacetylation that occurs at an innate immune gene like IFNB1 at the time of transcriptional activation (Agalioti, Chen, & Thanos, 2002; Parekh & Maniatis, 1999).
Specific transcription factors, in particular those that regulate Th1/Th2 differentiation, have been linked to histone acetylation at the Th2 locus. Histone H3 and H4 hyperacetyation at the Il4 promoter, the Il4 intron 2 enhancer (HS-II), CNS−1, HS-IV, HS-VA, and HS-V/CNS −2 are decreased under Th2-polarizing conditions and increased under Th1-polarizing conditions in cells from mice lacking STAT6 or STAT4, respectively (Avni et al., 2002; Fields et al., 2002; Yamashita et al., 2002). GATA3, which binds to the Il4 3′ HS-VA enhancer site upon cell stimulation via interaction with NFATp, can induce hyperacetylation at these sites when overexpressed (Avni et al., 2002; Yamashitaet al., 2002, 2004), evenin STAT6-deficient murine Th2 cells (Fields et al., 2002). Moreover, conditional ablation of GATA3 expression in fully differentiated Th2 cells, which reduces IL-4, IL-5 and IL-13 production, also selectively decreases histone acetylation at the Il5 promoter (Yamashita et al., 2004). Deletion of CNS−1 from the endogenous murine Th2 locus abrogates basal levels of H3ac associated with the Il4 and Il13 promoters, and inhibits partitioning of the Il4 gene to heterochromatin in lymph node CD4+ T cells polarized to a Th1 phenotype by Leishmania major infection. Notably, CNS−1 binds to Ikaros, which can recruit histone modifying complexes, and deletion of the cognate Ikaros binding motifs in CNS−1 attenuates CNS−1-mediated enhancement of SV40 promoter-driven reporter gene expression in Jurkat cells (Grogan et al., 2003).
In addition to acetylases and deacetylases, a number of other chromatin-modifying proteins have been linked to Th2 lineage commitment. Mice deficient in thePolycomb group (PcG) ring fingerprotein Mel-18 display impaired Th2 differentiation and expression of IL-4, IL-5, and IL-13 (Kimura et al., 2001). While Mel-18 binds to the mouse Il4 promoter, as well as the Ifng promoter, in an NFAT-dependent manner ( Jacob, Hod-Dvorai, Schif-Zuck, & Avni, 2008; Jacob, Hod-Dvorai, Ben-Mordechai, Boyko, & Avni, 2011) its role in mediating CD4+ T helper cell differentiation remains unclear. Another PcG family member, the H3K27-specific histone methyltransferase EZH2, binds to HS-IV (the Il4 3′ silencer) and Hss3 (in the Il13/Il4 intergenic region) in naïve murine CD4+ T cells, Th1 cells, and Th2 cells. As described below, in the case of naïve and Th1 cells, the binding of EZH2 coincides with H3K27 methylation in the Il13/Il4 region of the locus (Koyanagi et al., 2005). Mice haploinsufficent for the H3K4 methyltransferase MLL exhibit wild-type Th1 differentiation but develop memory Th2 cells that are defective in Il4, Il5, and Il13 gene expression in response to activation, in part due to reduced GATA3 activity; MLL binds to the Th2 locus and Gata3 locus in memory Th2 cells but not in memory Th1 cells or naïve CD4+ T cells (Yamashita et al., 2006). The SWI/SNF ATPase Brg1 also functions at the Th2 cytokine locus; the SWI/SNF complex binds to the Th2 LCR, HS-V (CNS−2), and the Il4 and Il13 proximal promoter regions in Th2 cells, and siRNA-mediated Brg1 knockdown impairs expression of IL-4, IL-5, IL-13, and IL-10 during Th2 differentiation, as well as IL-4, IL-13, and IL-10 expression in differentiated Th2 cells (Wurster & Pazin, 2008).
Both permissive and repressive histone methyl modifications have been characterized at the Th2 cytokine locus. H3K4me2 is present at low levels at CNS−1, the Il4 promoter, and the Il4 intron 2 enhancer in naïve murine CD4+ T cells, is enriched at these sites in Th2 cells, and is absent at these sites in Th1 cells (Ansel et al., 2004; Makar et al., 2003). In comparison, HS-V, which lies within the Il4 3′ enhancer region, is marked by relatively high levels of H3K4me2 in naïve murine CD4+ T cells; H3K4me2 levels at HS-V are dramatically reduced in response to Th1 polarization but only decline modestly in response to Th2 polarization, suggesting that this modification might be important for both early IL-4 expression following TCR engagement and then sustained Th2 locus activity under Th2-polarizing conditions (Baguet & Bix, 2004). Bivalent histone modifications have also been associated with CD4+ helper T cell differentiation. These modifications, which consist of a combination of permissive and repressive marks, have been associated with genes that are either expressed at low levels or that are poised for activation (Bernstein et al., 2006). This is illustrated by HS-IV at the Th2 locus, which is enriched in both H3K4me2 and H3K27me3 in naïve, Th1, and Th2 cells; HS-IV is essential for suppression of IL-4 expression during Th1 lineage commitment, and its deletion skews the differentiation of naïve murine CD4+ T cells to a Th2 phenotype after TCR stimulation under neutral polarizing conditions (Ansel et al., 2004).
The activating mark H3S10p is also enriched at HS-IV in murine Th1 and Th2 clones. However, H3S10p is also enriched at HS-I and CNS−1 at the Th2 locus in the Th2 clone, providing further evidence of distinct histone modification patterns at the Th2 locus in Th1 versus Th2 cells (Baguet & Bix, 2004).
As noted above, the repressive H3K27me3 mark is enriched at HS-IV of the Th2 locus. H3K27me2 is also present at HS-IV, as well as Hss3, in murine naïve CD4+ T cells and Th1-primed cells, but is almost absent in Th2-primed cells (Koyanagi et al., 2005). Little or no enrichment of H3K9me2 or H3K9me3 is found throughout the Il4/Il13 section of the locus in murine Th1 cells, nor at CNS−1, the Il4 promoter, or the Il4 intron 2 enhancer in murine naïve CD4+ T cells (Makar et al., 2003), although an earlier study detected the presence of H3K9me2 at the Il4 and Il13 promoters in a murine Th1 clone (Grogan et al., 2003), and a second study reported that H3K9me2 is enriched in Th1 cells at the Th2 LCR HS sites RHS6 and RHS7, while H3K4me2 levels at these sites are higher in Th2 cells than in Th1 cells, similar to the Il4 promoter (Lee & Rao, 2004). A genome-wide screen also detected H3K27me3 in murine Th1 cells, and H3K4me3 in murine Th2 cells, at the Th2 cytokine locus (Wei et al., 2009). Furthermore, the level of H3K27 meth-ylation at HS-IV correlates with Il4 and Il13 silencing in murine Th1-primed cells; after a second round of Th1 priming H3K27 methylation spreads to neighboring regions of the locus (Koyanagi et al., 2005). Thus, specific regulatory elements in the Th2 cytokine locus are major targets for repressive histone modifications, and maintenance of these silencing marks is critical for proper CD4+ T helper cell differentiation upon antigen recognition.
2.2.2.2 DNA methylation
The first indication that CpG methylation plays a prominent role in regulation of the Th2 locus came from observations that 5-aza-2′-deoxycytidine treatment markedly increases Il4 gene expression by naïve murine CD4+ T cells, and that the Il4 intronic enhancer region (HS-II) and the Il5 promoter are demethylated in murine Th2, but not Th1, clones (Agarwal & Rao, 1998b; Bird et al., 1998). The Il4 proximal promoter and HS-V/ CNS−2 regions are also hypomethylated in naïve murine CD4+ T cells; these regions remain demethylated in mature Th2 cells, where hypomethylation at CNS−1 and Hss3 in the Il13/Il4 intergenic region and extension of demethylation throughout the Il4 gene are also found (Lee, Agarwal, & Rao, 2002; Tykocinski et al., 2005). Demethylation of the GATA3-binding first intron of the Il4 gene, the Il13 promoter, and HS-VA, and partial demethylation of the Il5 promoter, directly correlates with competence for Th2 cytokine secretion in Th2 cells (Guo et al., 2002; Kim, Fields, & Flavell, 2007; Tykocinski et al., 2005). Conversely, HS-V/CNS−2, which is hypomethylated in naïve CD4+ T cells, becomes hypermethylated in Th1 cells (Lee et al., 2002). The Th2 LCR is also subject to DNA methylation-mediated control: RHS7 undergoes Th2-specific demethylation following acetylation of the LCR; however, RHS4, RHS5, and RHS6 remain methylated (Kim et al., 2007).
At the human Th2 locus, while demethylation is clearly evident at the IL13 promoter, IL4 intron 2, and at a CpG motif near the human CNS−1 element in Th2 cells relative to naïve CD4+ T cells and Th1 cells, little difference in methylation state at the IL4 promoter is apparent in these cell types, unlike what is found in the mouse (Santangelo, Cousins, Winkelmann, & Staynov, 2002). Hypomethylation of the IL13 promoter is also modestly enhanced in human Th2 cells as compared to naïve CD4+ T cells, Th1 cells, and Th17 cells (Janson et al., 2011). Further dissection of the human Th2 locus is necessary to determine whether DNA methylation is of greater importance for regulation of Th2 lineage commitment in mice than it is in humans.
Specific DNA methyltransferases have also been implicated in the regulation of cytokine gene expression at the Th2 locus. In single-positive murine CD4+ thymocytes, but not single-positive CD8+ thymocytes, Il4 gene expression is strongly induced in response to TCR stimulation, suggesting that some level of programming at the Th2 locus has already taken place by the time of CD8+ versus CD4+ fate decision in the thymus (Makar et al., 2003). DNMT3B binds to CNS−1 in single-positive CD4+ thymocytes, but is lost in response to TCR stimulation, while DNMT1 is constitutively bound to Il4/Il13 both prior to and after TCR engagement (Makar et al., 2003). DNMT1 recruitment to Il4/Il13 is sustained in naïve CD4+ T cells, even after TCR activation under nonpolarizing conditions; however, under Th2-polarizing conditions DNMT1 presence at Il4/Il13 wanes, followed by demethylation at the Il4 intronic enhancer. Indeed, DNMT1 is actively required for repression of Il4 expression in naïve murine CD4+ T cells, as knockout of DNMT1 results in significantly increased IL-4 production even under nonpolarizing activation conditions (Makar et al., 2003). DNMT1 is also required for suppression of Il10 and, to a lesser extent, Il5 and Il13 expression after TCR engagement under nonpolarizing conditions in both murine CD8+ and murine CD4+ T cells, although exposure to polarizing conditions can partially reverse this skewed profile, consistent with a broad but not exclusive role for DNA methylation in silencing Th2 cytokine expression (Makar & Wilson, 2004). MBD2 also associates with the murine Th2 locus at CNS−1 and the second intron of Il4 in Th1 cells, and Th1 cells from MBD2-deficient mice are competent to express IL-4, suggesting that, upon binding to CpG sites in the locus, MBD2 recruits chromatin modifying factors that are essential for sustained silencing of Il4 expression in Th1 cells (Hutchins et al., 2002). GATA3 overexpression displaces MBD2 from methylated DNA and is critical for demethylation at Il4 intron 2 during Th2 differentiation (Makar & Wilson, 2004; Yamashita et al., 2004). By contrast, GATA3 is not required for demethylation of RHS7 in the murine Th2 LCR (Kim et al., 2007).
2.2.2.3 Higher-order chromatin interactions
The higher-order chromatin structure of the murine Th2 cytokine locus has been extensively investigated in a number of primary cell and cell line systems, revealing several intrachromosomal interactions and, as noted in the previous section, interchromosomal interactions with the Ifng locus (Amsen et al., 2009; Lee et al., 2006; Williams, Spilianakis, & Flavell, 2010). The Th2 locus exhibits constitutive intrachromosomal interactions in T cells, NK cells, B cells, and fibroblasts; these interactions place the Il4, Il5, and Il13 promoters into close proximity and result in looping out of the intervening ~60 kb Rad50 coding region (Spilianakis & Flavell, 2004; Fig. 2.5). The Rad50 gene is constitutively expressed in all of these cell types, and this core configuration of the locus is present regardless of whether the cell expresses IL-4, IL-5, and/or IL-13. It represents a “pre-poised” chromatin conformation that, upon subsequent cell type-specific intrachromosomal and protein-DNA interactions, can become competent for expression of Th2 cytokines. Such interactions are apparent in naïve T cells, Th1, Th2, and NK cells, where the Il4 and Il13 promoters associate with both the Th2 LCR (with the strongest interaction observed at RHS7), and HSs at the 3′ end of the Il4 gene. RHS7 also interacts with several HSs within and adjacent to Il4 and Il13, while the Il5 promoter interacts with these HSs as well, but does not directly interact with the Th2 LCR. A further layer of conformational structure is evident only in Th2 and NK cells, where the Il4 promoter and RHS3 (near the 5′ end of the Rad50 gene) interact (Spilianakis & Flavell, 2004; Fig. 2.5). These data indicate that robust, direct interactions between the Th2 LCR and the Il4 and Il13 genes, along with interactions between the Il5 promoter and sequences within Il4/Il13 that bring the Il5 promoter into close proximity to the Th2 LCR, result in a “poised” conformation at the Th2 locus in CD4+ T cells that enables optimal access for Th2-associated transcription factors as CD4+ T helper cell differentiation proceeds. In addition, because the Rad50 promoter fails to interact with any region of the Th2 locus, regardless of cell type, this suggests that regulation of this constitutively expressed gene involves mechanisms that are distinct from those at play at the Th2 locus (Spilianakis & Flavell, 2004).
The functional importance of RHS7 in the higher-order conformation of the Th2 locus has been clearly demonstrated by experiments with mice in which the site is deleted. In these animals, intrachromosomal interactions between the Il4 promoter and sites RHS4 and RHS6 in the Th2 LCR are abolished, and interaction between the Il4 promoter and the Il5 and Il13 promoters is reduced (Lee, Spilianakis, & Flavell, 2005). Notably, in mice lacking STAT6, interactions between RHS7 and other sites in the Th2 cytokine locus (and, reciprocally, between the Il4 promoter and sites in the Th2 LCR) are modestly impaired in naïve CD4+ cells, and more markedly impaired in Th1 and Th2 cells (Spilianakis & Flavell, 2004). GATA3 and NFAT proteins are sufficient to induce interactions between the Th2 LCR and the rest of the Th2 locus, as combined ionomycin treatment and ectopic expression of GATA3 in a murine fibroblast line induces interactions between the Th2 LCR and the rest of the locus (Spilianakis & Flavell, 2004). Thus, GATA3 and NFAT proteins participate in both the formation of the “poised” chromosomal conformation at the Th2 locus and transcriptional activation of the locus during Th2 differentiation. However, as the “poised” conformation of the Th2 locus is also present in Th1 cells, where GATA3 expression is very low, another factor, in combination with NFAT, is most likely capable of mediating this structural configuration.
The higher-order configuration of the murine Th2 cytokine locus is further organized into a dense series of chromatin loops mediated by the architectural protein SATB1. SATB1 expression is rapidly induced upon Th2 cell activation, and the protein binds to ATC-rich DNA sequences that readily separate into single strands upon superhelical strain, termed base-unpairing regions (BURs; Cai et al., 2006). SATB1 is recruited to nine sites within a ~200 kb region of chromosome 11 (consisting of the Th2 locus and flanking Kif3a and Sept8 genes) upon activation of a murine Th2 clone (Fig. 2.5). CNS−1 and CNS−2 were also identified as SATB1-binding regions, although interaction between SATB1 and these sites may be indirect given the lack of cognate SATB1-binding motifs in these sequences (Cai et al., 2006). By combining 3C and the ChIP-loop assay, which detects DNA loops in chromatin that are anchored by a specific protein (Horike, Cai, Miyano, Cheng, & Kohwi-Shigematsu, 2005), Cai et al. found several looped structures at the Th2 locus prior to activation: SBS-C1 (near the Il5 promoter) is spatially juxtaposed with CNS−2, SBS-C7 (which lies near CNS−2), and, weakly, SBS-C9 (downstream of Sept8), while SBS-C9 is spatially juxtaposed with the Il5 promoter, SBS-C2 (upstream of Rad50), and the 3′ end of the Th2 LCR near RHS7 (Cai et al., 2006). After activation of the Th2 clone, additional intrachromosomal loops rapidly form at the Th2 cytokine locus. Specifically, SBS-C1 forms additional contacts with SBS-C3 through C6 (in introns at the center of the Rad50 gene), the Il13 promoter, the Il4 promoter, and CNS−1, and its interaction with SBS-C9 is dramatically strengthened. In turn, SBS-C9 makes additional contacts with SBS-C3 through C6 and the Il13 and Il4 promoters. Direct analysis of promoter juxtapositions revealed weak interactions between the Il4 and Il13 promoters, and the Il4 and Il5 promoters, prior to stimulation; after cellular activation new interactions form between the Il5 and Il13 promoters, and the interaction between the Il4 and Il5 promoters is enhanced. The Rad50 promoter is excluded from this Il4/Il5/ Il13 promoter assembly. Ablation of SATB1 expression inhibits Il5, Il13, and, especially, Il4 expression in response to Th2 activation, and disrupts formation of the de novo SBS-C1 and SBS-C9 interactions that occur after cell stimulation (Cai et al., 2006). Expression of the transcription factor c-Maf, which is a critical mediator of Il4 transcriptional induction (Ho, Hodge, Rooney, & Glimcher, 1996), is also markedly diminished in stimulated SATB1-negative cells (Cai et al., 2006). Together, these findings suggest that activation of Th2 cells results in SATB1 binding to numerous sites throughout the Th2 locus, followed by condensation of the Th2 cytokine promoters at a core node formed by SATB1 association with the Rad50 intronic region and sites near the 5′ and 3′ ends of the locus. Intriguingly, SATB1 binding to the IL5 promoter during early human Th2 differentiation appears to suppress IL5 gene expression, in part via competition with GATA3 (Ahlfors et al., 2010). This suggests that the functions carried out by SATB1 may evolve over the course of full Th2 lineage commitment.
As discussed above, the murine Th2 cytokine locus on chromosome 11 engages in highly stable interchromosomal interactions with the Ifng locus on chromosome 10, and these loci colocalize to nonheterochromatic regions in naïve CD4+ T cells and NK cells, but not in B cells or fibroblasts (Spilianakis et al., 2005). In an interesting contrast to the intrachromosmal interactions at the Th2 locus, the Rad50 promoter participates in these inter-chromosomal interactions with the Ifng gene, along with the Il5 promoter and RHS6; while the crosslinking frequency of these interchromosomal interactions is reduced in both Th1 and Th2 cells relative to naïve CD4+ T cells, this reduction in overall cross-chromosomal interactions is most modest for the Rad50 promoter in Th2 cells (Spilianakis et al., 2005). In naive CD4+ T cells (as well as Th1 and Th2 cells) from the aforementioned RHS7-deficient mice (Lee, Spilianakis, et al., 2005), interchromosomal interactions are diminished based on both 3C and FISH analysis, and Ifng and Il5 gene transcription is delayed and attenuated (Spilianakis et al., 2005), indicating that these cross-chromosomal interactions influence stimulation-induced gene expression at loci associated with both helper cell types. In addition, in murine Th1 and Th2 cells, CTCF binds strongly to sites upstream of Il5, a site in the Il13/Il4 intergenic region, and a site within Kif3a, and knockout of CTCF markedly reduces IL-4, IL-5, and IL-13 production in Th2 cells, marginally reduces IFN-γ production in Th1 cells, and has no effect on IL-17 production in Th17 cells (Ribeiro de Almeida et al., 2009). Thus, CTCF also contributes to the formation and/or stability of long-range chromosomal interactions at the Th2 locus.
In summary, a series of interchromosomal and intrachromosomal interactions at the Th2 locus controls gene expression during Th2 lineage commitment. Based on these findings, a preliminary model of Th2 locus regulation can be proposed. In murine naïve CD4+ T cells, interchromsomal interactions place the Ifng and Th2 loci into close proximity, potentially placing the genes in a transcriptional hub sufficient to encourage their low level synthesis prior to cellular activation. A number of intrachromosomal interactions are also already present at the Th2 locus. Once TCR engagement occurs, Th2 polarizing conditions result in physical separation of the loci and, in turn, the establishment of GATA3/NFAT-mediated intrachromosomal interactions within the Th2 locus that place the Th2 LCR in close proximity to the Il4 and Il13 promoters. SATB1 is also recruited to the locus at this time, where it binds to several elements throughout the locus and condenses the locus into a node of transcriptionally competent loops. Finally, additional chromatin binding proteins and transcription factors, such as c-Maf, are recruited to the “primed” locus, and strong activation of Th2 cytokine synthesis proceeds (Amsen et al., 2009; Cai et al., 2006; Lee et al., 2006; Williams, Spilianakis, & Flavell, 2010).
2.2.3 The IL17A/IL17F locus
Due to its recent identification as a distinct CD4+ T helper lineage, fewer investigations have been performed of the epigenetic mechanisms involved in (i) the differentiation of Th17 cells from naïve precursors, and (ii) the regulation of the signature cytokines expressed by this helper subtype, including IL-17A, IL-17F, IL-21, IL-22 and, in humans, IL-26. A physiological role for pathogen-infected apoptotic APCs in directing Th17 differentiation has been identified (Torchinsky, Garaude, Martin, & Blander, 2009). However, as described in the introduction to this section, the precise set of factors required for Th17 lineage commitment, particularly in humans, remains a subject of debate.
In mammals the genes encoding IL-17A and IL-17F are oriented tail-to-tail and are separated by ~44 kb of sequence; the genes lie on chromosome 6 in mice and chromosome 1 in humans. In the murine Il17a/Il17f locus, a total of eight CNSs were initially characterized and designated CNS−1 through CNS−8; Th17-specific HSs have been found to correspond to several of these CNSs (Akimzhanov, Yang, & Dong, 2007; Mukasa et al., 2010; Fig. 2.6). Relative to naïve CD4+, Th1, and Th2 cells, hyper-acetylation of histone H3 is Th17-specific at all of these sites apart from CNS−5, and H3ac is also uniquely enriched at both the Il17a promoter and Il17f promoter in Th17 cells (Akimzhanov et al., 2007). In humans, anti-CD3/CD28 stimulation of naïve CD4+ T cells results in H3K18ac enrichment at CNS1, CNS2, CNS4, CNS5, CNS6 and the IL17A proximal promoter, and peripheral blood T cells isolated from patients with SLE, in whom circulating IL-17A levels are elevated, exhibit higher levels of H3K18ac at these sites than do CD4+ T cells from healthy controls (Hedrich, Rauen, Kis-Toth, Kyttaris, & Tsokos, 2012; Rauen, Hedrich, Juang, Tenbrock, & Tsokos, 2011).
Figure 2.6.
The IL17A/IL17F locus. Comparative histone posttranslational modifications, DNA methylation status, and intrachromosomal interactions are shown for the murine Il17a/Il17f locus in naïve CD4+ T, Th1, Th2, and Th17 cells. Position and transcriptional orientation of the Il17a, IL17f, Mcm3, and Phkd1 genes (gray boxes, arrows) and regulatory elements (black boxes) are shown, with sequence names indicated at the bottom. Red bars indicate the position of permissive histone modifications: H3 and H4 histone acetylation and trimethylation (3) at H3K4. Green bars indicate regions associated with the repressive histone mark H3K27me3. Regions of CpG hypomethylation and methylation are indicated by open and filled blue boxes, respectively. Intrachromosomal interactions that form in a Th17-specific fashion are shown by arrows at the bottom. ND, not determined in a given cell type.
With respect to histone methyl modifications at the Il17a/Il17f locus, one study reported that in murine Th17 cells, H3K4me3 is strongly enriched at the Il17a and Il17f promoters, present at low levels at the Ifng promoter, and absent from the Il4 promoter, while a second study confirmed H3K4me3 enrichment at the Il17a and Il17f promoters in murine Th17 cells (Akimzhanov et al., 2007; Mukasa et al., 2010). In addition, H3K4me3 is enriched at several other sites (excepting a site ~97 kb upstream of the Il17a TSS) throughout the Il17a/Il17f locus in Th17 cells as compared to murine naïve CD4+ T cells, while H3K4me3 is only elevated at a site ~10 kb downstream of the Il17a TSS in Th1 cells as compared to naïve cells (Mukasa et al., 2010). On the other hand, H3K27me3 is enriched at several sites throughout the Il17a/Il17f locus in murine Th1 cells as compared to naïve CD4+ T lymphocytes, but present at similar or lower levels at these sites in Th17 cells as compared to naïve CD4+T cells (Fig. 2.6). A recent study also reported that H3K27me3 levels are similarly enriched at seven distinct CNSs in the Il17a/Il17f locus in naïve CD4+ T cells and Th1 cells from mice, but strongly enhanced at these sites in Th2 cells; in Th17 cells H3K27me3 is lost, and H3K4me3 is deposited, at most of these sites (Thomas, Sai, & Wells, 2012; Fig. 2.6). Several Th17-associated cytokines have been found to promote changes in H3K4me3 levels at the Il17a and Il17f promoters in murine Th17 cells, with TGF-β driving increased H3K4me3 presence at both promoters, and IL-23 driving reduced H3K4me3 presence at the Il17a promoter; under the latter conditions Th17 cells primarily secrete IL17F (Mukasa et al., 2010; Thomas et al., 2012).
Th17 cells exhibit a surprising level of epigenetic plasticity. Treatment with IL-12 or, to a lesser extent, IL-23, in the absence of TGF-β drives murine Th17 cells to a Th1 phenotype characterized by loss of IL-17 expression and gain of IFN-γ expression (Lee, Turner, et al., 2009; Lexberg et al., 2008). IL-12 treat-ment of murine Th17 cells also induces H3K4me accumulationat the Ifng locus and concomitant H3K27me3 enrichment throughout the Il17a/IL17f locus, further emphasizing the in vitro epigenetic plasticity characteristic of murine Th17 cells (Mukasa et al., 2010). Several studies have found CD4+ T cells that express both IFN-γ and IL-17 at sites of inflammation in autoimmune disease (Aarvak, Chabaud, Miossec, & Natvig, 1999; Annunziato et al., 2007; Nistala et al., 2010). Comparative epigenetic profiling of these “Th1/Th17” cells, along with Th1 and Th17 cells isolated from peripheral human blood, revealed that H3K4me3 is enriched near the TSS of IFNG in both Th1 and Th1/Th17 cells, andnear theTSS ofIL17A in Th17cells, whileH3K27me3 is deposited at the IL17A TSS in Th1 and Th1/Th17 cells (Cohen et al., 2011). Furthermore, culturing human Th17 cells in Th1-promoting conditions or culturing human Th1 cells in Th17-promoting conditions (which induces IL-17A expression), leads to bivalent deposition of H3K4me3 and H3K27me3 at the IL17A promoter (Cohen et al., 2011). Intriguingly, the Rorc gene, which encodes the Th17 master regulator RORγt, is strongly marked in its 5′ region by H3K4me3 in murine Th17 cells and, conversely, by H3K27me3 throughout the coding region in Th1 cells; the Tbx21 gene, however, which encodes T-bet, is decorated at its 5′ end by high levels of H3K4me3 in Th1 cells, but by bivalent H3K4me3/H3K27me3 in murine Th17 cells, indicating that T-bet may be poised for induction under the right conditions (e.g. IL-12 exposure) in Th17 cells (Wei et al., 2009). The physiological importance of Th17 plasticity is underscored by findings that conversion of Th17 cells to Th1/Th17 and/or Th1 cells under certain stimulatory conditions in vivo is sufficient to drive inflammatory disease (Hirota et al., 2011; Lee, Turner, et al., 2009; Martin-Orozco, Chung, Chang, Wang, & Dong, 2009).
The roles of several histone-modifying proteins in the transcriptional regulation of the IL17A/IL17F locus have also been analyzed. At the murine locus, p300 associates with CNS−2 (~2 kb upstream of Il17a), the Il17a promoter, and the Il17f promoter in Th17 cells but not in Th1 cells; in reporter assays CNS−2 functions as a Th17-restricted enhancer not only for the Il17a and Il17f promoters, but also for the Ifng and Il4 promoters (Wang et al., 2012). The H3K27me3-specific histone demethylase JMJD3 is also recruited to CNS−2 in Th17 cells, consistent with earlier observations that this repressive histone mark is removed at the Il17a/Il17f locus during Th17 lineage commitment (Thomas et al., 2012). Deletion of CNS−2 in mice results in dramatically reduced secretion of IL-17A and IL-17F by in vitro-polarized Th17 cells, diminished recruitment of RNA Pol II and p300 to the Il17a and Il17f promoters, and increased H3K27 trimethylation at the two promoters; in all cases the observed effects were more modest at the Il17f promoter relative to the Il17a promoter (Wang et al., 2012). The CNS−2-deficient mice are also resistant to experimental autoimmune encephalomyelitis, a Th17 cell-dependent autoimmune disease model resembling human multiple sclerosis (Wang et al., 2012). The histone methyltransferase G9a has also been linked to regulation of the Il17a/Il17f locus, as CD4+ T cells isolated from mice deficient in T cell-specific expression of G9a produce elevated levels of IL-17A under neutral or Th2-polarizing conditions (Lehnertz et al., 2010). Increased IL-17A mRNA and/or protein expression in the mesenteric lymph nodes and intestines of these mice is found in response to infection with the helminth Trichuris muris, which primarily drives a Th2 response in wild-type animals (Lehnertz et al., 2010). Stimulation of CD4+ T cells under neutral and Th2-polarizing conditions in the presence of BIX-01294, a specific inhibitor of G9a methyl-transferase, also results in increased expression of IL-17A (Lehnertz et al., 2010). Consistent with this, when naïve CD4+ T cells from mice lacking hematopoietic-specific G9a expression are stimulated under neutral, Th1, and Th2 conditions, a strong reduction in H3K9me2 is found at several sites in the Il17a/Il17f locus, including the Il17a promoter, Il17a CNS−2, and Il17a CNS−3 (Lehnertz et al., 2010). Lysine-specific demethylase 1 (LSD1), which targets H3K4me1 and H3K4me2, has also been implicated in modulation of the Il17a/Il17f locus. In CD4+ T cells deficient in transcriptional repressor growth factor independent 1 (Gfi-1), which associates with LSD1 (Saleque, Kim, Rooke, & Orkin, 2007), Th2 polarization results in enrichment of H3K4me3 at the 5′ end of Rorc, while in wild-type Th2 cells this mark is almost completely absent at the Rorc locus. Il17a and Il17f gene expression can be inhibited by ectopic expression Gfi-1 in murine Th17 cells, while these cytokines are expressed by murine Th2 cells lacking Gfi-1 upon a shift to Th17-polarizing conditions, unlike wild-type Th2 cells (Zhu et al., 2009). Gfi-1 and LSD1 are also recruited to the Il17a/Il17f intergenic region, and association of LSD1 with the locus is erased in the absence of Gfi-1. As TGF-β treatment directly inhibits Gfi-1 synthesis, a mechanistic link between Th17 polarizing conditions and derepression of the Il17a/Il17f locus can be made (Zhu et al., 2009).
The relationship between gene transcription and DNA methylation at the CNSs of the murine Il17a/IL17f and human IL17A/IL17F loci has also been investigated. In the human locus, CNS1, CNS2 (proximal IL17A promoter), CNS3, CNS4, CNS5 (proximal IL17F promoter) and CNS6 display increased CpG methylation in non-IL-17A-secreting CD4+ T cells, and decreased CpG methylation in T cells from SLE patients (Hedrich et al., 2012; Rauen et al., 2011). Undifferentiated human naïve CD4+ cells exhibit high levels of CpG methylation in the IL17A proximal promoter, and culture of human CD4+ T cells in the presence of 5-aza-2′-deoxycytidine promotes IL17A expression (Janson et al., 2011). Furthermore, T cells from SLE patients show decreased recruitment of DNMT3A to a CRE site in the IL17A proximal promoter that is positioned at nucleotides −111 to −104 relative to the TSS and is recognized by cAMP-response element modulator α (CREMα), which interacts with DNMT3A (Rauen et al., 2011). Ectopic overexpression of DNMT3A in activated Jurkat T cells inhibits IL17A mRNA expression, and in these cells gene reporters driven by 195 bp of the IL17A proximal promoter are inhibited by increased methylation of the reporter plasmid (Rauen et al., 2011). CpG dinucleotides in the vicinity of the human IL17A TSS are strongly methylated in Th0 and Th1 cells, hypomethylated in Th17 cells, and partially demethylated (at sites downstream of the TSS) in Th1/Th17 cells; notably, culture of Th17 cells in Th1-polarizing conditions, or Th1 cells in Th17-polarizing conditions, has little effect on the methylation status of the TSS region (Cohen et al., 2011). In the case of the murine locus, the Il17a and Il17f promoters, as well as an enhancer 28 kb downstream of the Il17a TSS that promotes transcription of both genes, undergo Th17 lineage-specific DNA demethylation, which correlates with demethylation of H3K27 and increased H3K4 methylation in these regions. This CpG demethylation tends to coincide with STAT3 binding sites, and hypermethylation at one site in the Il17a proximal promoter blocks STAT3 binding and full promoter activity (Thomas et al., 2012). Furthermore, in Th17 cells cultured in the presence of IL-23, the Il17f promoter becomes preferentially demethylated, consistent with the aforementioned finding that exposure of murine Th17 cells to IL-23 shifts their cytokine secretion profile to one dominated by IL-17F instead of IL-17A (Thomas et al., 2012). Thus, as is found at Th1 and Th2 loci, CpG methylation at promoter and enhancer regions of IL17A/IL17F inversely correlates with activation of the locus.
Recently, 3C assays in murine Th17 and Th1 cells have provided a first glimpse into the higher-order conformation of the Il17a/Il17f locus (Wang et al., 2012). The CNS−2 enhancer, which interacts with p300 and JMJD3, makes Th17-specific intrachromosomal interactions with the Il17a and Il17f promoters (Wang et al., 2012; Fig. 2.6). As additional Th17-specific HS sites are present throughout the IL17a/IL17f locus, additional long-range chromosomal interactions may contribute to the epigenetic regulation of Th17-specific differentiation and cytokine synthesis.
2.3. Other loci
Examination of the TNF/LT, IFNG, Th2, and IL17A/IL17F loci has provided a wealth ofdata ontheepigeneticregulationofcritically importantfactors for both innateandadaptiveimmune responses. Inaddition, these locican serve as models for epigenetic modulation of other genes that are expressed in a cell-type and/or stimulus-specific manner in the immune system, as well as other tissues. For example, another key locus involved in CD4+ T helper cell differentiation is the locus that encodes the forkhead box p3 (Foxp3) master regulator, which is critical for regulatory CD4+ T cell (Treg) differentiation (Fontenot, Gavin, & Rudensky, 2003; Hori, Nomura, & Sakaguchi, 2003; Khattri, Cox, Yasayko, & Ramsdell, 2003). Several CNS elements in the locus, including the FOXP3 promoter, a TGF-β-sensitive element, and the Treg cell-specific demethylated region (TDSR), are regulated through histone modifications and changes in CpG methylation (Cavassani et al., 2010; Floess et al., 2007; Janson, Winerdal, et al., 2008; Kim & Leonard, 2007; Liu, Tahk, Yee, Fan, & Shuai, 2010; Mantel et al., 2006; Polansky et al., 2008).
Expression of the antiinflammatory cytokine IL-10 is also regulated epigenetically during CD4+ T helper cell differentiation. Unlike IFN-γ and IL-4, whose expression is tightly restricted to the Th1 and Th2 lineages, respectively, IL-10 is expressed by both subsets, albeit at a much higher level in Th2 cells than in Th1 cells (Fiorentino, Bond, & Mosmann, 1989; Jankovic et al., 2007). Relative to what is found in Th1 cells, high levels of histone H3 and H4 acetylation are observed at the Il10 locus in macrophages, which also produce high levels of IL-10 in response to activation, in naïve CD4+ T cells, and in Th2 cells (Chang, Helbig, et al., 2007; Lee, Sahoo, et al., 2009; Motomura et al., 2011; Saraiva et al., 2005; Shoemaker, Saraiva, & O’Garra, 2006; Villagra et al., 2009). H3K4 methylation is also higher at the Il10 locus in murine Th2 cells as compared to Th1 cells (Chang, Helbig, et al., 2007; Motomura et al., 2011), and phosphorylation at H3S10 is observed at the locus in murine macrophages stimulated by LPS treatment or FcγR ligation (Lucas, Zhang, Prasanna, & Mosser, 2005; Villagra et al., 2009; Zhang, Edwards, & Mosser, 2006). The SWI/SNF chromatin remodeling complex components Brg1 and Brm also associate with multiple HSs in the Il10 locus in murine Th1, Th2, and Th17 cells; futhermore, CBP and acetylated histone H3 are enriched at the locus in Th2 cells lacking the repressive SWI/SNF component BAF180 as compared to wild-type Th2 cells, and this correlates with an increase in Il10 gene expression (Wurster et al., 2012). Conversely, HDAC11 interacts with the distal region of the IL10 promoter, induces deacetylation of histones H3 and H4, and represses IL10 gene expression in human and murine APCs (Villagra et al., 2009). This was the first physiological role discovered for HDAC11, and revealed a mechanism that may be important for the establishment of immune tolerance. Repression of Il10 expression in Th1 cells has also been linked to Ets-1-dependent recruitment of HDAC1 (Lee et al., 2012). Finally, CpG dinucleotides in the human IL10 promoter are hypomethylated in PBMC, where the gene is active, but highly methylated in primary keratinoctyes and HeLa cells, where the gene is silent (Szalmás et al., 2008). While HSs and histone modifications have been characterized in the IL10 locus, the epigenetic mechanisms that regulate IL10 gene expression are still being elucidated (Lee, Sahoo, et al., 2009; Saraiva & O’Garra, 2010). In an intriguing recent study, histone marks associated with transcriptional competence, including H3K27ac, H3K4me3, and H3K4me1, were found to be enriched at the IL10 locus in human monocytes and mouse neu-trophils, which both express IL-10, but not in human neutrophils, which are unable to express IL-10 even after mitogenic stimulation (Tamassia et al., 2013). This provides the first evidence of a species-specific difference in epi-genetic regulation of the IL10 gene in a shared cell type. It is anticipated that characterization of intrachromosomal interactions at the IL10 locus will shed further light on the epigenetic regulation of this cytokine’s expression in an array of immune cells.
Macrophage differentiation is another process that is closely linked to differential cytokine expression, and for which there is strong evidence of epigenetic regulation. Macrophages can be polarized towards two major subtypes: M1 and M2. M1 macrophages develop in response to bacterial and viral infection and express high levels of TNF and other proinflammatory cytokines. M1 macrophages can be induced in vitro by treatment of primary monocytes with a combination of IFN-γ and TLR ligands or with granulocyte macrophage-colony stimulation factor (GM-CSF). By contrast, M2 macrophages are involved in the response to parasitic infection and other “alternative” activation signals. M2 macrophages can be induced in vitro by treatment of primary monocytes with macrophage-colony stimulation factor (M-CSF) and by IL-4 or IL-13 (Fleetwood, Lawrence, Hamilton, & Cook, 2007; Martinez, Gordon, Locati, & Mantovani, 2006; Verreck et al., 2004). The H3K27 demethylase JMJD3 is a critical player in M2 macrophage polarization, as JMJD3 is induced in a STAT6-dependent manner after IL-4 treatment of unpolarized murine macrophages, is recruited to the promoters of several M2 marker genes, and acts to demethylate H3K27 at these genes (Ishii et al., 2009). JMJD3 is also recruited at higher levels to M2 macrophage marker genes in peritoneal macrophages isolated from mice challenged with Schistosoma mansoni as compared to unchallenged mice (Ishii et al., 2009). A second study found that JMJD3 is also required for M2 macrophage polarization in mice in response to infection by the helminth Nippostrongylus brasiliensis or chitin administration, as JMJD3-deficient mice exhibit significantly reduced M2 macrophage activity when subjected to these conditions in comparison to wild-type mice (Satoh et al., 2010). In addition, JMJD3-deficient BMDMs demonstrate impaired M2 development in response to M-CSF, but not to IL-4. The expression of IRF-4, a key transcription factor in M2 macrophage polarization, is inhibited in macrophages lacking JMJD3, most likely due to loss of JMJD3-dependent H3K27 demethylation at the Irf4 promoter, which correlates with Irf4 transcriptional induction (Satoh et al., 2010).
3. PERSPECTIVES AND FUTURE DIRECTIONS
Here we have described the roles of a range of post-translational histone modifications, DNA methylation states, and higher-order chromatin interactions that control regulation of cytokine gene transcription. Epigenetic regulation strongly correlates with evolutionarily conserved regions of the genome where DNA is accessible to regulatory factors at DNase hypersensitive sites, often occurring in noncoding sequences separated by kilobases from the gene they regulate. These sites, in turn, associate with histones bearing specific, reversible covalent modifications and, in some cases, regions of hypo- or hypermethylation of cytosine at CpG dinucleotide motifs in DNA.
Histone modifications can promote gene transcription not only by weakening the nucleosome-DNA interaction and making DNA motifs more accessible to their cognate transcription factors and the general transcription machinery, but also by serving as docking sites for chromatin-modifying factors, which can exert permissive or repressive effects upon gene transcription. Furthermore, regulatory factors that interact with HSs can drive the formation of long-range intra- and interchromosomal interactions, which in turn place gene loci into regions of active transcription or into regions of transcriptional repression. In turn, these epigenetic regulatory mechanisms are profoundly influenced by cell type and stimulus, as well as developmental stage, which stimulate the epigenetic changes that control the transcriptional program.
The components of epigenetic cytokine gene regulation thus present potential targets for the manipulation of cytokine transcription in disease states that arise from, or are strongly influenced by, the dysregulation of cytokine gene expression occurring in specific cell or tissue types or stimulated by specific signaling pathways. Indeed, specific histone and DNA modifications have been associated with disease states, and in some cases compounds that reverse these epigenetic changes have been shown to be clinically effective. In particular, some HDAC inhibitors have been approved for use in cancer therapy. As more information becomes available about cell type- and stimulus-specific epigenetic modifications at key gene loci, it can be imagined that therapies can be designed to manipulate an individual gene’s expression in its native chromatin context and in tissues uniquely affected by its inappropriate expression by targeting these epigenetic control processes.
DNA methylation has been implicated in a number of autoimmune disease states, various cancers, chronic obstructive pulmonary disease, neurodegenerative diseases, and neurological disorders driven by chronic inflammation (Shanmugam & Sethi, 2012; Strickland & Richardson, 2008; Villagra et al., 2010). This is well illustrated in the case of SLE, in which strong phenotypic and functional similarities were observed between CD4+ cells isolated from patients with active SLE and experimentally manipulated mature human CD4+ T cells treated with agents that induce hypomethylation. Treatment of CD4+ T cells with 5-azacytidine results in an increase in transcription of ITGAL (integrin, alpha L), which codes for a subunit of the adhesion molecule lymphocyte function-associated antigen 1 (LFA-1), in expression of LFA-1, and in demethylation of alu elements 5′ of the ITGAL promoter. This treatment also results in autoreactive T cells, which respond to APCs without added antigen in a class II MHC-specific fashion (Lu et al., 2002; Richardson, 1986; Richardson et al., 1992). Coculture of 5-azacytidine-treated hypomethylated T cells with autologous B cells results in hypersecretion of IgG, partially mediated by IFN-γ, IL-4, and IL-6 (Quddus et al., 1993; Richardson, Liebling, & Hudson, 1990). Consistent with this observation, transcription of IL6, like that of IFNG and IL4, is repressed by DNA methylation (Reiner, 2005). Another intriguing example is provided by the lupus-like disease state that can result from drug therapy with the DNA methyltransferase inhibitors procainamide and hydralazine, an antiarrythmic and anti-hypertensive respectively (Deng et al., 2003; Lee, Yegnasubramanian, Lin, & Nelson, 2005; Mazari, Ouarzane, & Zouali, 2007; Yung & Richardson, 1994). This is of particular interest since T cells from patients with active SLE have decreased m5C content and DNMT1 mRNA relative to patients with inactive SLE and healthy controls (Richardson, Scheinbart, et al., 1990). Furthermore, T cells from SLE patients exhibit a range of similarities to experimentally demethylated T cells: for example, they induce hypersecretion of IgG in autologous B cells, and a subset of SLE T cells overexpress LFA-1, similar to 5-azacytidine-treated T cells (Oelke et al., 2004; Richardson et al., 1992). Moreover, demethylation of the alu elements upstream of the ITGAL promoter correlates with the activity of lupus disease (Lu et al., 2002). DNA hypomethylation has also been implicated in gene regulation underlying other autoimmune disease states. For example, studies with PBMCs from patients with rheumatoid arthritis revealed that hypomethylation at a single CpG site in the IL6 promoter correlates with both increased IL-6 expression and sustained inflammation (Nile, Read, Akil, Duff, & Wilson, 2008).
By contrast, a hallmark of cancer is selective hypermethylation and, as a result, persistent repression of the promoter regions of a wide range of genes, especially genes that encode for tumor suppressor proteins and proteins involved in DNA repair and the cell cycle (Heyn & Esteller, 2012; Shanmugam & Sethi, 2012). Although compounds that broadly inhibit DNA methylation can induce autoimmune disease-like states, they have proven to be clinically effective for patients in certain clinical entities: for example, 5-azacytidine (Vidaza) and 5-aza-2′-deoxycytidine (Dacogen) have been used as relatively low-toxicity therapies for myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML; Griffiths & Gore, 2013). Although our understanding of the role of DNA demethylation in these diseases is still emerging, it is anticipated that novel small molecules able to specifically modulate DNA methylation or demethylation at particular genomic regions may be of great benefit in treatment strategies for certain autoimmune and neoplastic disorders.
Considerable progress has been made in the design and the clinical application of compounds that interact specifically with histone modifying enzymes. As of the writing of this review, three second-generation HDAC inhibitors are in Phase III clinical trials or used in treatment (Arrowsmith et al., 2012). Panobinostat (LBH589), which targets HDAC1, HDAC2, HDAC3, and HDAC6, is in Phase III clinical trials for treatment of Hodgkin’s lymphoma and multiple myeloma (Arrowsmith et al., 2012; Zhou, Atadja, & Davidson, 2007). Suberoylanilide hydroxamic acid (SAHA), also called vorinostat (Zolinza), which targets HDAC1, HDAC2, HDAC3, and HDAC6, and romidepsin (Istodax), which targets HDAC1, HDAC2, HDAC3, and HDAC8 were approved for the treatment of cutaneous T-cell lymphoma (CTCL) in 2006 and 2009, respectively (Arrowsmith et al., 2012; Bertino & Otterson, 2011; Marks & Breslow, 2007; Prince, Bishton, & Harrison, 2009).
Notably, a novel indication for HDAC inhibition is treatment for HIV infection. A major obstacle to cure of HIV infection is the residual, latent viral reservoir that persists even after sustained and highly effective antire-troviral therapy (ART). Approaches have been attempted using HDACs to reactivate the viral reservoir, which then allows the combination of ART and the reconstituting T cell compartment to eliminate residual virus as it exits from its sequestered state. A number of HDAC inhibitors are capable of reactivating latent HIV that is integrated into the host genome (Hakre, Chavez, Shirakawa, & Verdin, 2011), and several have been tested in humans. For example, the HDAC1 inhibitor valproic acid (VPA) caused a decline in HIV-1 infection of resting CD4+ T cells in vivo in four patients when it was added to an intensified ART regimen (Lehrman et al., 2005). VPA is a weak HDAC inhibitor, however, and later clinical studies found no significant benefit for VPA in HIV treatment (Archin et al., 2008). A recent report found panobinostat to be superior to several other HDAC inhibitors, including VPA and SAHA, at inducing viral reactivation in both cell line and primary CD4+ T cell latency models (Rasmussen et al., 2013).
Other than HDAC inhibitors, no small-molecule inhibitors of other histone modifying enzymes are currently in clinical use; however, a number of the factors described in this review that are important in epigenetic cytokine gene regulation present attractive therapeutic targets. For example, aberrantly expressed mutant forms of the histone lysine methyltransferase EZH2 have been found in various types of leukemia and solid tumors (Simon & Lange, 2008), and certain cancers are marked by fusions of the bromodomain proteins BRD3 and BRD4 (Filippakopoulos et al., 2010) and the histone lysine methyltransferase MLL (Daigle et al., 2011; Okada et al., 2005). In some cases, in vitro studies have shown that the function of these histone modifying enzymes can be selectively inhibited by small-molecule drugs, including compounds with low nanomolar affinity for the bromodomains of members of the BET protein family (BRD2, BRD3, BRD4, and BRDT) (Chung et al., 2011; Dawson et al., 2011; Delmore et al., 2011; Filippakopoulos et al., 2010; Nicodeme et al., 2010). For example, the compound (+)-JQ1, which selectively interacts with BRD3 and BRD4, has been shown to promote terminal differentiation and inhibit proliferation of squamous carcinoma cells expressing the BRD4-NUT (nuclear protein in testis) fusion protein, displacing BRD4-NUT from acetylated chromatin through competitive binding (Filippakopoulos et al., 2010). Notably, BRD4 is a regulatory cofactor of the oncoprotein Myc, which has proven to be refractory to direct inhibition by therapeutic compounds (Arrowsmith et al., 2012). Inhibition of BRD4 activity, which has been shown to have an anti-proliferative effect in several models of AML, multiple myeloma, and mixed lineage leukemia, may thus allow for indirect inhibition of Myc (Dawson et al., 2011; Delmore et al., 2011; Zuber et al., 2011).
In addition to histone deacetylase “erasers” and bromodomain “readers,” histone acetyltransferase and histone methyltransferase “writers” and histone demethylase “erasers” present potential therapeutic targets. For example, specific histone acetyltransferases, deacetylases, and methyltransferases have all been linked to neuropsychiatric disorders: haploinsufficiency of CBP and HDAC4 lead to Rubinstein-Taybi syndrome and brachydactyly mental retardation syndrome, respectively (Petrij et al., 1995; Williams et al., 2010), and mutations in the histone lysine methyltransferase GLP1 result in a complex intellectual disability syndrome (Kleefstra et al., 2009; Kramer & van Bokhoven, 2009; Schaefer et al., 2009). Although appropriately selective HAT inhibitors have proven elusive, inhibitors have been developed for the histone lysine methyltransferases G9a and GLP-1. These include BIX-01294, which, as noted in the previous section, can increase Il17a transcription in cell-based assays (Kubicek et al., 2007; Lehnertz et al., 2010), and the more potent and selective second-generation inhibitor UNC638 (Vedadi et al., 2011). Moreover, the compound EPZ004777, a specific inhibitor of the histone methyltransferase DOT1-like (DOT1L), selectively induces apoptosis in cells containing MLL fusion proteins that directly or indirectly interact with DOT1L (Daigle et al., 2011). A selective inhibitor has also been successfully designed to block activity of the H3K27me3-specific demethylases JMJD3 and UTX; this compound, GSK-J4, was shown to inhibit LPS-induced expression of proinflammatory cytokines, including TNF, in human primary macrophages in vitro (Kruidenier et al., 2012).
Thus, new treatments for cancer, autoimmune diseases, neurodegenerative diseases, and chronic infections such as HIV will benefit from an improved understanding of the role of histone modifications, DNA methylation, and protein-DNA interactions involved in establishing functional intra- and interchromosomal interactions in gene expression. Moreover, such studies of epigenetic regulation will greatly enhance our general knowledge of how eukaryotic genes are regulated in a cell type- and inducer-specific manner.
Acknowledgments
The authors are indebted to current and former members of the Goldfeld lab whose discussions over the years have led to many insights contained in this review including Alla Tsytsykova, Nancy Chow, Shahin Ranjbar, Sebastian Biglione, Ricardo Rajsbaum and Robert Barthel. We thank David Tough (GlaxoSmithKline) for helpful discussions and Renate Hellmiss for graphic artwork. We gratefully acknowledge Karolin Luger (Colorado State University) for providing the three-dimensional nucleosome model used as a base for Figure 2.1. This work was supported by grants from the NIH (HL-059838 and GM076685) and a GlaxoSmithKline Alliance Research Project Grant to A. E. G. and a GlaxoSmithKline Alliance Postdoctoral Fellowship to L. D. J.
Footnotes
Gene names are capitalized when referring to loci in general and human loci specifically, and are in lowercase when referring to murine loci.
References
- Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. The Journal of Immunology. 1999;162:1246. [PubMed] [Google Scholar]
- Agalioti T, Chen G, Thanos D. Deciphering the transcriptional histone acetylation code for a human gene. Cell. 2002;111:381. doi: 10.1016/s0092-8674(02)01077-2. [DOI] [PubMed] [Google Scholar]
- Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell. 2000;103:667. doi: 10.1016/s0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Rao A. Long-range transcriptional regulation of cytokine gene expression. Current Opinion in Immunology. 1998a;10:345. doi: 10.1016/s0952-7915(98)80174-x. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998b;9:765. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- Ahlfors H, Limaye A, Elo LL, Tuomela S, Burute M, Gottimukkala KVP, et al. SATB1 dictates expression of multiple genes including IL-5 involved in human T helper cell differentiation. Blood. 2010;116:1443. doi: 10.1182/blood-2009-11-252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. The Journal of Biological Chemistry. 2007;282:5969. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Rhodes SJ, Bidwell JP. Context-dependent transcription: All politics is local. Gene. 2003;313:43. doi: 10.1016/s0378-1119(03)00627-9. [DOI] [PubMed] [Google Scholar]
- Amsen D, Spilianakis CG, Flavell RA. How are TH1 and TH2 effector cells made? Current Opinion in Immunology. 2009;21:153. doi: 10.1016/j.coi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression. Nature. 2003;423:659. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, et al. Phenotypic and functional features of human Th17 cells. The Journal of Experimental Medicine. 2007;204:1849. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A, Hampsey M. A role for the CPF 3′-end processing machinery in RNAP II-dependent gene looping. Genes & Development. 2005;19:2969. doi: 10.1101/gad.1362305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, Greenwald RJ, Agarwal S, Bassing CH, Monticelli S, Interlandi J, et al. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nature Immunology. 2004;5:1251. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, Martinson JA, Wiegand A, et al. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22:1131. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: A new frontier for drug discovery. Nature Reviews. Drug Discovery. 2012;11:384. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nature Immunology. 2002;3:643. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- Baena A, Mootnick AR, Falvo JV, Tsytskova AV, Ligeiro F, Diop OM, et al. Primate TNF promoters reveal markers of phylogeny and evolution of innate immunity. PLoS One. 2007;2:e621. doi: 10.1371/journal.pone.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet A, Bix M. Chromatin landscape dynamics of the Il4-Il13 locus during T helper 1 and 2 development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11410. doi: 10.1073/pnas.0403334101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani A, Mukasa R, Hatton RD, Weaver CT. Regulation of the Ifng locus in the context of T-lineage specification and plasticity. Immunological Reviews. 2010;238:216. doi: 10.1111/j.1600-065X.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani A, Shibata Y, Crawford GE, Baldwin AS, Hatton RD, Weaver CT. Modular utilization of distal cis-regulatory elements controls Ifng gene expression in T cells activated by distinct stimuli. Immunity. 2010;33:35. doi: 10.1016/j.immuni.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint BL, Gabor P, Nagy L. Genome-wide localization of histone 4 arginine 3 methylation in a differentiation primed myeloid leukemia cell line. Immunobiology. 2005;210:141. doi: 10.1016/j.imbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Balint BL, Szanto A, Madi A, Bauer UM, Gabor P, Benko S, et al. Arginine methylation provides epigenetic transcription memory for retinoid-induced differentiation in myeloid cells. Molecular and Cellular Biology. 2005;25:5648. doi: 10.1128/MCB.25.13.5648-5663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Research. 2011;21:381. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Pathophysiology of allergic inflammation. Immunological Reviews. 2011;242:31. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Barthel R, Goldfeld AE. T cell-specific expression of the human TNF-α gene involves a functional and highly conserved chromatin signature in intron 3. The Journal of Immunology. 2003;171:3612. doi: 10.4049/jimmunol.171.7.3612. [DOI] [PubMed] [Google Scholar]
- Barthel R, Tsytsykova AV, Barczak AK, Tsai EY, Dascher CC, Brenner MB, et al. Regulation of tumor necrosis factor alpha gene expression by mycobacteria involves the assembly of a unique enhanceosome dependent on the coactivator proteins CBP/p300. Molecular and Cellular Biology. 2003;23:526. doi: 10.1128/MCB.23.2.526-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baù D, Sanyal A, Lajoie BR, Capriotti E, Byron M, Lawrence JB, et al. The three-dimensional folding of the α-globin gene domain reveals formation of chromatin globules. Nature Structural & Molecular Biology. 2011;18:107. doi: 10.1038/nsmb.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bertino EM, Otterson GA. Romidepsin: A novel histone deacetylase inhibitor for cancer. Expert Opinion on Investigational Drugs. 2011;20:1151. doi: 10.1517/13543784.2011.594437. [DOI] [PubMed] [Google Scholar]
- Bestor T, Laudano A, Mattaliano R, Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. Journal of Molecular Biology. 1988;203:971. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- Bestor TH, Ingram VM. Two DNA methyltransferases from murine erythroleukemia cells: Purification, sequence specificity, and mode of interaction with DNA. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:5559. doi: 10.1073/pnas.80.18.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. Cachectin and tumor necrosis factor as two sides of the same biological coin. Nature. 1986;320:584. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Biglione S, Tsytsykova AV, Goldfeld AE. Monocyte-specific accessibility of a matrix attachment region in the tumor necrosis factor locus. The Journal of Biological Chemistry. 2011;286:44126. doi: 10.1074/jbc.M111.272476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes & Development. 2002;16:6. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bird A, Taggart M, Frommer M, Miller OJ, Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell. 1985;40:91. doi: 10.1016/0092-8674(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Botquin V, Hess H, Fuhrmann G, Anastassiadis C, Gross MK, Vriend G, et al. New POU dimer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by Oct-4 and Sox-2. Genes & Development. 1998;12:2073. doi: 10.1101/gad.12.13.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LYW, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates β-catenin signaling and NFκB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. The Journal of Biological Chemistry. 2009;284:2657. doi: 10.1074/jbc.M806708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis VA, Nadler LM, Strominger JL, Goldfeld AE. Tumor necrosis factor α is an autocrine growth factor for normal human B cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7007. doi: 10.1073/pnas.91.15.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AP, Davis S, Shulha HP, Meltzer P, Margulies EH, Weng Z, et al. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes & Development. 1993;7:592. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, et al. Lymphotoxin β, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- Brinkman BMN, Telliez JB, Schievella AR, Lin LL, Goldfeld AE. Engagement of tumor necrosis factor (TNF) receptor 1 leads to ATF-2- and p38 mitogen-activated protein kinase-dependent TNF-a gene expression. The Journal of Biological Chemistry. 1999;274:30882. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, tran-scriptionally active chromatin for coordinated expression of cytokine genes. Nature Genetics. 2006;38:1278. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- Cano E, Hazzalin CA, Kardalinou E, Buckle RS, Mahadevan LC. Neither ERK nor JNK/SAPK MAP kinase subtypes are essential for histone H3/ HMG-14 phosphorylation or c-fos and c-jun induction. Journal of Cell Science. 1995;108(Pt 11):3599. doi: 10.1242/jcs.108.11.3599. [DOI] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Molecular Cell. 2005;20:845. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Cavassani KA, Carson WF, 4th, Moreira AP, Wen H, Schaller MA, Ishii M, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115:4403. doi: 10.1182/blood-2009-09-241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Aune TM. Dynamic changes in histone-methylation ’marks’ across the locus encoding interferon-γ during the differentiation of T helper type 2 cells. Nature Immunology. 2007;8:723. doi: 10.1038/ni1473. [DOI] [PubMed] [Google Scholar]
- Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, et al. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. European Journal of Immunology. 2007;37:807. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Chen GY, Osada H, Santamaria-Babi LF, Kannagi R. Interaction of GATA-3/T-bet transcription factors regulates expression of sialyl Lewis X homing receptors on Th1/Th2 lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16894. doi: 10.1073/pnas.0607926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Molecular Cell. 2000;5:905. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Chung C-w, Coste H, White JH, Mirguet O, Wilde J, Gosmini RL, et al. Discovery and characterization of small molecule inhibitors of the BET family bromodomains. Journal of Medicinal Chemistry. 2011;54:3827. doi: 10.1021/jm200108t. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, Levings MK. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. The Journal of Immunology. 2011;187:5615. doi: 10.4049/jimmunol.1101058. [DOI] [PubMed] [Google Scholar]
- Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. The Journal of Experimental Medicine. 1995;182:1591. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PR. A model for all genomes: The role of transcription factories. Journal of Molecular Biology. 2010;395:1. doi: 10.1016/j.jmb.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3880. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21931. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross JGR, Harrison GA, Coggill P, Sims S, Beck S, Deakin JE, et al. Analysis of the genomic region containing the tammar wallaby (Macropus eugenii) orthologues of MHC class III genes. Cytogenetic and Genome Research. 2005;111:110. doi: 10.1159/000086379. [DOI] [PubMed] [Google Scholar]
- Cuturi MC, Murphy M, Costa-Giomi MP, Weinmann R, Perussia B, Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. The Journal of Experimental Medicine. 1987;165:1581. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JE, Papenfuss AT, Belov K, Cross JGR, Coggill P, Palmer S, et al. Evolution and comparative analysis of the MHC Class III inflammatory region. BMC Genomics. 2006;7:281. doi: 10.1186/1471-2164-7-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin D, Zaman Z, Liberatore RA, Ptashne M. Telomere looping permits gene activation by a downstream UAS in yeast. Nature. 2001;409:109. doi: 10.1038/35051119. [DOI] [PubMed] [Google Scholar]
- Dedon PC, Soults JA, Allis CD, Gorovsky MA. A simplified formaldehyde fixation and immunoprecipitation technique for studying protein-DNA interactions. Analytical Biochemistry. 1991;197:83. doi: 10.1016/0003-2697(91)90359-2. [DOI] [PubMed] [Google Scholar]
- Dekker J. A closer look at long-range chromosomal interactions. Trends in Biochemical Sciences. 2003;28:277. doi: 10.1016/S0968-0004(03)00089-6. [DOI] [PubMed] [Google Scholar]
- Dekker J. The three ’C’ s of chromosome conformation capture: Controls, controls, controls. Nature Methods. 2006;3:17. doi: 10.1038/nmeth823. [DOI] [PubMed] [Google Scholar]
- Dekker J. Gene regulation in the third dimension. Science. 2008;319:1793. doi: 10.1126/science.1152850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- de Laat W, Grosveld F. Spatial organization of gene expression: The active chromatin hub. Chromosome Research. 2003;11:447. doi: 10.1023/a:1024922626726. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, Rastegar M, Davie JR. Epigenetic control. Journal of Cellular Physiology. 2009;219:243. doi: 10.1002/jcp.21678. [DOI] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Lu Q, Zhang Z, Rao T, Attwood J, Yung R, et al. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis and Rheumatism. 2003;48:746. doi: 10.1002/art.10833. [DOI] [PubMed] [Google Scholar]
- Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8758. doi: 10.1073/pnas.1433065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- Dhar SS, Lee SH, Kan PY, Voigt P, Ma L, Shi X, et al. Trans-tail regulation of MLL4-catalyzed H3K4 methylation by H4R3 symmetric dimethylation is mediated by a tandem PHD of MLL4. Genes & Development. 2012;26:2749. doi: 10.1101/gad.203356.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Lopez-Berestein G. A distinct element involved in lipopolysaccharide activation of the tumor necrosis factor-α promoter in monocytes. Journal of Interferon & Cytokine Research. 2000;20:741. doi: 10.1089/10799900050116453. [DOI] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EA, Anest V, Cogswell P, Baldwin AS. The kinases MSK1 and MSK2 are required for epidermal growth factor-induced, but not tumor necrosis factor-induced, histone H3 Ser10 phosphorylation. The Journal of Biological Chemistry. 2006;281:12521. doi: 10.1074/jbc.M513333200. [DOI] [PubMed] [Google Scholar]
- Eivazova ER, Aune TM. Dynamic alterations in the conformation of the Ifng gene region during T helper cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:251. doi: 10.1073/pnas.0303919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eivazova ER, Gavrilov A, Pirozhkova I, Petrov A, Iarovaia OV, Razin SV, et al. Interaction in vivo between the two matrix attachment regions flanking a single chromatin loop. Journal of Molecular Biology. 2009;386:929. doi: 10.1016/j.jmb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Eivazova ER, Vassetzky YS, Aune TM. Selective matrix attachment regions in T helper cell subsets support loop conformation in the Ifng gene. Genes and Immunity. 2007;8:35. doi: 10.1038/sj.gene.6364349. [DOI] [PubMed] [Google Scholar]
- El Gazzar M, Yoza BK, Chen X, Hu J, Hawkins GA, McCall CE. G9a and HP1 couple histone and DNA methylation to TNFα transcription silencing during endotoxin tolerance. The Journal of Biological Chemistry. 2008;283:32198. doi: 10.1074/jbc.M803446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gazzar M, Yoza BK, Hu JYQ, Cousart SL, McCall CE. Epigenetic silencing of tumor necrosis factor α during endotoxin tolerance. The Journal of Biological Chemistry. 2007;282:26857. doi: 10.1074/jbc.M704584200. [DOI] [PubMed] [Google Scholar]
- Esensten JH, Tsytsykova AV, Lopez-Rodriguez C, Ligeiro FA, Rao A, Goldfeld AE. NFAT5 binds to the TNF promoter distinctly from NFATp, c, 3 and 4, and activates TNF transcription during hypertonic stress alone. Nucleic Acids Research. 2005;33:3845. doi: 10.1093/nar/gki701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euskirchen G, Auerbach RK, Snyder M. SWI/SNF chromatin-remodeling factors: Multiscale analyses and diverse functions. The Journal of Biological Chemistry. 2012;287:30897. doi: 10.1074/jbc.R111.309302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo JV, Brinkman BMN, Tsytsykova AV, Tsai EY, Yao TP, Kung AL, et al. A stimulus-specific role for CREB-binding protein (CBP) in T cell receptor-activated tumor necrosis factor α gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3925. doi: 10.1073/pnas.97.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo JV, Lin CH, Tsytsykova AV, Hwang PK, Thanos D, Goldfeld AE, et al. A dimer-specific function of the transcription factor NFATp. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19637. doi: 10.1073/pnas.0810648105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo JV, Parekh BS, Lin CH, Fraenkel E, Maniatis T. Assembly of a functional beta interferon enhanceosome is dependent on ATF-2—c-jun heterodimer orientation. Molecular and Cellular Biology. 2000;20:4814. doi: 10.1128/mcb.20.13.4814-4825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo JV, Tsytsykova AV, Goldfeld AE. Transcriptional control of the TNF gene. Current Directions in Autoimmunity. 2010;11:27. doi: 10.1159/000289196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo JV, Uglialoro AM, Brinkman BMN, Merika M, Parekh BS, Tsai EY, et al. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter. Molecular and Cellular Biology. 2000;20:2239. doi: 10.1128/mcb.20.6.2239-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar WL, Ruscetti FW, Young HA. 5-Azacytidine treatment of a murine cytotoxic T cell line alters interferon-γ gene induction by interleukin 2. The Journal of Immunology. 1985;135:1551. [PubMed] [Google Scholar]
- Feng Q, Zhang Y. The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes & Development. 2001;15:827. doi: 10.1101/gad.876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields PE, Kim ST, Flavell RA. Cutting edge: Changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. The Journal of Immunology. 2002;169:647. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Fierz B, Chatterjee C, McGinty RK, Bar-Dagan M, Raleigh DP, Muir TW. Histone H2B ubiquitylation disrupts local and higher-order chromatin compaction. Nature Chemical Biology. 2011;7:113. doi: 10.1038/nchembio.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Knapp S. The bromodomain interaction module. FEBS Letters. 2012;586:2692. doi: 10.1016/j.febslet.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of Experimental Medicine. 1989;170:2081. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. The Journal of Immunology. 2007;178:5245. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biology. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunology. 2003;4:330. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Ueda Y, Zhu Y, Gifford VR, Garofalo MR, Mohandas N, et al. Computational and biological analysis of 680 kb of DNA sequence from the human 5q31 cytokine gene cluster region. Genome Research. 1997;7:495. doi: 10.1101/gr.7.5.495. [DOI] [PubMed] [Google Scholar]
- Fujita N, Watanabe S, Ichimura T, Tsuruzoe S, Shinkai Y, Tachibana M, et al. Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression. The Journal of Biological Chemistry. 2003;278:24132. doi: 10.1074/jbc.M302283200. [DOI] [PubMed] [Google Scholar]
- Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. Journal of Molecular Biology. 1987;196:261. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Garrett S, Dietzmann-Maurer K, Song L, Sullivan KE. Polarization of primary human monocytes by IFN-γ induces chromatin changes and recruits RNA Pol II to the TNF-α promoter. The Journal of Immunology. 2008;180:5257. doi: 10.4049/jimmunol.180.8.5257. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature. 2010;467:967. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K, Kingsley C, Kirshner JR, Grosschedl R. Assembly and function of a TCRα enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes & Development. 1995;9:995. doi: 10.1101/gad.9.8.995. [DOI] [PubMed] [Google Scholar]
- Giese K, Pagel J, Grosschedl R. Functional analysis of DNA bending and unwinding by the high mobility group domain of LEF-1. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12845. doi: 10.1073/pnas.94.24.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, Doyle C, Maniatis T. Human tumor necrosis factor α gene regulation by virus and lipopolysaccharide. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9769. doi: 10.1073/pnas.87.24.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, Flemington EK, Boussiotis VA, Theodos CM, Titus RG, Strominger JL, et al. Transcription of the tumor necrosis factor α gene is rapidly induced by anti-immunoglobulin and blocked by cyclosporin A and FK506 in human B cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:12198. doi: 10.1073/pnas.89.24.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, Leung JY, Sawyer SA, Hartl DL. Post-genomicsand the neutral theory: Variation and conservation in the tumor necrosis factor-α promoter. Gene. 2000;261:19. doi: 10.1016/s0378-1119(00)00477-7. [DOI] [PubMed] [Google Scholar]
- Goldfeld AE, Maniatis T. Coordinate viral induction of tumor necrosis factor α and interferon β in human B cells and monocytes. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:1490. doi: 10.1073/pnas.86.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, McCaffrey PG, Strominger JL, Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. The Journal of Experimental Medicine. 1993;178:1365. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, Strominger JL, Doyle C. Human tumor necrosis factor α gene regulation in phorbol ester stimulated T and B cell lines. The Journal of Experimental Medicine. 1991;174:73. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfeld AE, Tsai E, Kincaid R, Belshaw PJ, Schrieber SL, Strominger JL, et al. Calcineurin mediates human tumor necrosis factor a gene induction in stimulated T and B cells. The Journal of Experimental Medicine. 1994;180:763. doi: 10.1084/jem.180.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göndör A, Ohlsson R. Chromosome crosstalk in three dimensions. Nature. 2009;461:212. doi: 10.1038/nature08453. [DOI] [PubMed] [Google Scholar]
- Gorham JD, Guler ML, Steen RG, Mackay AA, Daly MJ, Frederick K, et al. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12467. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grausenburger R, Bilic I, Boucheron N, Zupkovitz G, El-Housseiny L, Tschismarov R, et al. Conditional deletion of histone deacetylase 1 in T cells leads to enhanced airway inflammation and increased Th2 cytokine production. The Journal of Immunology. 2010;185:3489. doi: 10.4049/jimmunol.0903610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J, Hochschild A, Ptashne M. DNA loops induced by cooperative binding of γ repressor. Nature. 1986;322:750. doi: 10.1038/322750a0. [DOI] [PubMed] [Google Scholar]
- Griffiths EA, Gore SD. Epigenetic therapies in MDS and AML. Advances in Experimental Medicine and Biology. 2013;754:253. doi: 10.1007/978-1-4419-9967-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- Grogan JL, Wang ZE, Stanley S, Harmon B, Loots GG, Rubin EM, et al. Basal chromatin modification at the IL-4 gene in helper T cells. The Journal of Immunology. 2003;171:6672. doi: 10.4049/jimmunol.171.12.6672. [DOI] [PubMed] [Google Scholar]
- Guccione E, Bassi C, Casadio F, Martinato F, Cesaroni M, Schuchlautz H, et al. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- Guo L, Hu-Li J, Zhu J, Watson CJ, Difilippantonio MJ, Pannetier C, et al. In TH2 cells the Il4 gene has a series of accessibility states associated with distinctive probabilities of IL-4 production. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10623. doi: 10.1073/pnas.162360199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34:396. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakre S, Chavez L, Shirakawa K, Verdin E. Epigenetic regulation of HIV latency. Current Opinion in HIV and AIDS. 2011;6:19. doi: 10.1097/COH.0b013e3283412384. [DOI] [PubMed] [Google Scholar]
- Hamalainen-Laanaya HK, Kobie JJ, Chang C, Zeng WP. Temporal and spatial changes of histone 3 K4 dimethylation at the IFN-γ gene during Th1 and Th2 cell differentiation. The Journal of Immunology. 2007;179:6410. doi: 10.4049/jimmunol.179.10.6410. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6:1123. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hassan AH, Awad S, Al-Natour Z, Othman S, Mustafa F, Rizvi TA. Selective recognition of acetylated histones by bromodomains in transcriptional co-activators. Biochemistry Journal. 2007;402:125. doi: 10.1042/BJ20060907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Hatton RD, Harrington LE, Luther RJ, Wakefield T, Janowski KM, Oliver JR, et al. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hebbes TR, Thorne AW, Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. The EMBO Journal. 1988;7:1395. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- Hedrich CM, Rauen T, Kis-Toth K, Kyttaris VC, Tsokos GC. cAMP-responsive element modulator α (CREMα) suppresses IL-17F protein expression in T lymphocytes from patients with systemic lupus erythematosus (SLE) The Journal of Biological Chemistry. 2012;287:4715. doi: 10.1074/jbc.M111.323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nature Genetics. 2007;39:311. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- Heyn H, Esteller M. DNA methylation profiling in the clinic: Applications and challenges. Nature Reviews. Genetics. 2012;13:679. doi: 10.1038/nrg3270. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature Immunology. 2011;12:255. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Hochschild A, Ptashne M. Cooperative binding of γ repressors to sites separated by integral turns of the DNA helix. Cell. 1986;44:681. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- Hofmann SR, Morbach H, Schwarz T, Rosen-Wolff A, Girschick HJ, Hedrich CM. Attenuated TLR4/MAPK signaling in monocytes from patients with CRMO results in impaired IL-10 expression. Clinical Immunology. 2012;145:69. doi: 10.1016/j.clim.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226. [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Horike S, Cai S, Miyano M, Cheng JF, Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nature Genetics. 2005;37:31. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- Hsieh YJ, Kundu TK, Wang Z, Kovelman R, Roeder RG. The TFIIIC90 subunit of TFIIIC interacts with multiple components of the RNA polymerase III machinery and contains a histone-specific acetyltransferase activity. Molecular and Cellular Biology. 1999;19:7697. doi: 10.1128/mcb.19.11.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE. Solution structure and acetyl-lysine binding activity of the GCN5 bromodomain. Journal of Molecular Biology. 2000;304:355. doi: 10.1006/jmbi.2000.4207. [DOI] [PubMed] [Google Scholar]
- Hutchins AS, Mullen AC, Lee HW, Sykes KJ, High FA, Hendrich BD, et al. Gene silencing quantitatively controls the function of a developmental trans-activator. Molecular Cell. 2002;10:81. doi: 10.1016/s1097-2765(02)00564-6. [DOI] [PubMed] [Google Scholar]
- Iborra FJ, Pombo A, Jackson DA, Cook PR. Active RNA polymerases are localized within discrete transcription ‘factories’ in human nuclei. Journal of Cell Science. 1996;109(Pt 6):1427. doi: 10.1242/jcs.109.6.1427. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Watanabe S, Sakamoto Y, Aoto T, Fujita N, Nakao M. Transcriptional repression and heterochromatin formation by MBD1 and MCAF/AM family proteins. The Journal of Biological Chemistry. 2005;280:13928. doi: 10.1074/jbc.M413654200. [DOI] [PubMed] [Google Scholar]
- Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaldi MS, Karam CS, Corces VG. Phosphorylation of histone H3 at Ser10 facilitates RNA polymerase II release from promoter-proximal pausing in Drosophila. Genes & Development. 2007;21:2818. doi: 10.1101/gad.1604007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Hassan AB, Errington RJ, Cook PR. Visualization of focal sites of transcription within human nuclei. The EMBO Journal. 1993;12:1059. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Hod-Dvorai R, Ben-Mordechai OL, Boyko Y, Avni O. Dual function of polycomb group proteins in differentiated murine T helper (CD4+) cells. Journal of Molecular Signaling. 2011;6:5. doi: 10.1186/1750-2187-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E, Hod-Dvorai R, Schif-Zuck S, Avni O. Unconventional association of the polycomb group proteins with cytokine genes in differentiated T helper cells. The Journal of Biological Chemistry. 2008;283:13471. doi: 10.1074/jbc.M709886200. [DOI] [PubMed] [Google Scholar]
- Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. The Journal of Experimental Medicine. 2007;204:273. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson PCJ, Linton LB, Bergman EA, Marits P, Eberhardson M, Piehl F, et al. Profiling of CD4+ T cells with epigenetic immune lineage analysis. The Journal of Immunology. 2011;186:92. doi: 10.4049/jimmunol.1000960. [DOI] [PubMed] [Google Scholar]
- Janson PCJ, Marits P, Thörn M, Ohlsson R, Winqvist O. CpG methylation of the IFNG gene as a mechanism to induce immunosuppression in tumor-infiltrating lymphocytes. The Journal of Immunology. 2008;181:2878. doi: 10.4049/jimmunol.181.4.2878. [DOI] [PubMed] [Google Scholar]
- Janson PCJ, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS One. 2008;3:e1612. doi: 10.1371/journal.pone.0001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. The EMBO Journal. 2011;30:249. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Chen J. Inhibition of IFN-γ transcription by site-specific methylation during T helper cell development. The EMBO Journal. 2006;25:2443. doi: 10.1038/sj.emboj.7601148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJC, Wade PA, Vermaak D, Kass SU, Landsberger N, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genetics. 1998;19:187. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Joshi AA, Struhl K. Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Molecular Cell. 2005;20:971. doi: 10.1016/j.molcel.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Jüngel A, Distler JHW, Gay S, Distler O. Epigenetic modifications: Novel therapeutic strategies for systemic sclerosis? Expert Review of Clinical Immunology. 2011;7:475. doi: 10.1586/eci.11.37. [DOI] [PubMed] [Google Scholar]
- Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- Kalocsay M, Hiller NJ, Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Molecular Cell. 2009;33:335. doi: 10.1016/j.molcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Kanno Y, Vahedi G, Hirahara K, Singleton K, O’Shea JJ. Transcriptional and epigenetic control of T helper cell specification: Molecular mechanisms underlying commitment and plasticity. Annual Review of Immunology. 2012;30:707. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara TLA, Michishita E, Adler AS, Damian M, Berber E, Lin M, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136:62. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Schiltz L, Chiu R, Itakura K, Taira K, Nakatani Y, et al. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature. 2000;405:195. doi: 10.1038/35012097. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Kurdistani SK, Morris SA, Ahn SH, Podolny V, Collins SR, et al. Cotranscriptional Set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell. 2005;123:593. doi: 10.1016/j.cell.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4:337. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim T, Buratowski S. Dimethylation of H3K4 by Set1 recruits the Set3 histone deacetylase complex to 5′ transcribed regions. Cell. 2009;137:259. doi: 10.1016/j.cell.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Fields PE, Flavell RA. Demethylation of a specific hypersensitive site in the Th2 locus control region. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17052. doi: 10.1073/pnas.0708293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Guermah M, McGinty RK, Lee JS, Tang Z, Milne TA, et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: A role for DNA methylation. The Journal of Experimental Medicine. 2007;204:1543. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Koseki Y, Yamashita M, Watanabe N, Shimizu C, Katsumoto T, et al. Regulation of Th2 cell differentiation by mel-18, a mammalian Polycomb group gene. Immunity. 2001;15:275. doi: 10.1016/s1074-7613(01)00182-0. [DOI] [PubMed] [Google Scholar]
- Kirmizis A, Santos-Rosa H, Penkett CJ, Singer MA, Vermeulen M, Mann M, et al. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449:928. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishikawa H, Sun J, Choi A, Miaw SC, Ho IC. The cell type-specific expression of the murine IL-13 gene is regulated by GATA-3. The Journal of Immunology. 2001;167:4414. doi: 10.4049/jimmunol.167.8.4414. [DOI] [PubMed] [Google Scholar]
- Kizer KO, Phatnani HP, Shibata Y, Hall H, Greenleaf AL, Strahl BD. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Molecular and Cellular Biology. 2005;25:3305. doi: 10.1128/MCB.25.8.3305-3316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, van Zelst-Stams WA, Nillesen WM, Cormier-Daire V, Houge G, Foulds N, et al. Further clinical and molecular delineation of the 9q subtelomeric deletion syndrome supports a major contribution of EHMT1 haploinsufficiency to the core phenotype. Journal of Medical Genetics. 2009;46:598. doi: 10.1136/jmg.2008.062950. [DOI] [PubMed] [Google Scholar]
- Kleff S, Andrulis ED, Anderson CW, Sternglanz R. Identification of a gene encoding a yeast histone H4 acetyltransferase. The Journal of Biological Chemistry. 1995;270:24674. doi: 10.1074/jbc.270.42.24674. [DOI] [PubMed] [Google Scholar]
- Kochanek S, Radbruch A, Tesch H, Renz D, Doerfler W. DNA methylation profiles in the human genes for tumor necrosis factors α and β in subpopulations of leukocytes and in leukemias. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:5759. doi: 10.1073/pnas.88.13.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek S, Toth M, Dehmel A, Renz D, Doerfler W. Interindividual concordance of methylation profiles in human genes for tumor necrosis factors α and β. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8830. doi: 10.1073/pnas.87.22.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. The EMBO Journal. 1999;18:3090. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Issa JPJ. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Molecular and Cellular Biology. 2003;23:206. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Yan PS, Huang THM, Issa JPJ. Chromatin immunoprecipitation microarrays for identification of genes silenced by histone H3 lysine 9 methylation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7398. doi: 10.1073/pnas.0306641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T, Zou J, Bird S, Savan R, Sakai M, Secombes CJ. Identification and expression analysis of lymphotoxin-beta like homologues in rainbow trout Oncorhynchus mykiss. Molecular Immunology. 2006;43:1390. doi: 10.1016/j.molimm.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- Kouskouti A, Talianidis I. Histone modifications defining active genes persist after transcriptional and mitotic inactivation. The EMBO Journal. 2005;24:347. doi: 10.1038/sj.emboj.7600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Baguet A, Martens J, Margueron R, Jenuwein T, Bix M. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in TH1 cells. The Journal of Biological Chemistry. 2005;280:31470. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- Kramer JM, van Bokhoven H. Genetic and epigenetic defects in mental retardation. The International Journal of Biochemistry & Cell Biology. 2009;41:96. doi: 10.1016/j.biocel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Chung C-w, Cheng Z, Liddle J, Che K, Joberty G, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruys V, Thompson P, Beutler B. Extinction of the tumor necrosis factor locus, and of genes encoding the lipopolysaccharide signaling pathway. The Journal of Experimental Medicine. 1993;177:1383. doi: 10.1084/jem.177.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, et al. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Molecular Cell. 2007;25:473. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Kundu TK, Wang Z, Roeder RG. Human TFIIIC relieves chromatin-mediated repression of RNA polymerase III transcription and contains an intrinsic histone acetyltransferase activity. Molecular and Cellular Biology. 1999;19:1605. doi: 10.1128/mcb.19.2.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MH, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmondson DG, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383:269. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. The Journal of Immunology. 1999;162:4045. [PubMed] [Google Scholar]
- Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kurukuti S, Tiwari VK, Tavoosidana G, Pugacheva E, Murrell A, Zhao Z, et al. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10684. doi: 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell. 1997;89:349. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of Experimental Medicine. 2005;201:233. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PNI, Cheung P. Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes polycomb silencing. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2801. doi: 10.1073/pnas.1012798108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenu-Bombled C, Trainor CD, Makeh I, Romeo PH, Max-Audit I. Interleukin-13 gene expression is regulated by GATA-3 in T cells: Role of a critical association of a GATA and two GATG motifs. The Journal of Biological Chemistry. 2002;277:18313. doi: 10.1074/jbc.M110013200. [DOI] [PubMed] [Google Scholar]
- Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- Lee DU, Avni O, Chen L, Rao A. A distal enhancer in the interferon-γ (IFN-γ) locus revealed by genome sequence comparison. The Journal of Biological Chemistry. 2004;279:4802. doi: 10.1074/jbc.M307904200. [DOI] [PubMed] [Google Scholar]
- Lee GR, Fields PE, Flavell RA. Regulation of IL-4 gene expression by distal regulatory elements and GATA-3 at the chromatin level. Immunity. 2001;14:447. doi: 10.1016/s1074-7613(01)00125-x. [DOI] [PubMed] [Google Scholar]
- Lee GR, Fields PE, Griffin TJ, 4th, Flavell RA. Regulation of the Th2 cytokine locus by a locus control region. Immunity. 2003;19:145. doi: 10.1016/s1074-7613(03)00179-1. [DOI] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim NA, Sanford A, Sullivan KE. Histone acetylation and chromatin conformation are regulated separately at the TNF-α promoter in monocytes and macrophages. Journal of Leukocyte Biology. 2003;73:862. doi: 10.1189/jlb.1202618. [DOI] [PubMed] [Google Scholar]
- Lee GR, Kim ST, Spilianakis CG, Fields PE, Flavell RA. T helper cell differentiation: Regulation by cis elements and epigenetics. Immunity. 2006;24:369. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Lee CG, Kwon HK, Sahoo A, Hwang W, So JS, Hwang JS, et al. Interaction of Ets-1 with HDAC1 represses IL-10 expression in Th1 cells. The Journal of Immunology. 2012;188:2244. doi: 10.4049/jimmunol.1101614. [DOI] [PubMed] [Google Scholar]
- Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: A target for STAT6 but not GATA3. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16010. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Sahoo A, Im SH. Epigenetic regulation of cytokine gene expression in T lymphocytes. Yonsei Medical Journal. 2009;50:322. doi: 10.3349/ymj.2009.50.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Shukla A, Schneider J, Swanson SK, Washburn MP, Florens L, et al. Histone crosstalk between H2B monoubiquitination and H3 methylation mediated by COMPASS. Cell. 2007;131:1084. doi: 10.1016/j.cell.2007.09.046. [DOI] [PubMed] [Google Scholar]
- Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nature Immunology. 2005;6:42. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Yegnasubramanian S, Lin X, Nelson WG. Procainamide is a specific inhibitor of DNA methyltransferase 1. The Journal of Biological Chemistry. 2005;280:40749. doi: 10.1074/jbc.M505593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz B, Northrop JP, Antignano F, Burrows K, Hadidi S, Mullaly SC, et al. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. The Journal of Experimental Medicine. 2010;207:915. doi: 10.1084/jem.20100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, et al. Depletion of latent HIV-1 infection in vivo: A proof-of-concept study. The Lancet. 2005;366:549. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng J, Butcher BA, Egan CE, Abi Abdallah DS, Denkers EY. Toxo-plasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. The Journal of Immunology. 2009;182:489. doi: 10.4049/jimmunol.182.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, McKenzie FE, Uglialoro AM, Flores-Villanueva PO, Sorkin BC, Yunis EJ, et al. Identification of phylogenetic footprints in primate tumor necrosis factor-α promoters. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6614. doi: 10.1073/pnas.97.12.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexberg MH, Taubner A, Förster A, Albrecht I, Richter A, Kamradt T, et al. Th memory for interleukin-17 expression is stable in vivo. European Journal of Immunology. 2008;38:2654. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nature Reviews. Genetics. 2002;3:662. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- Li Q, Barkess G, Qian H. Chromatin looping and the probability of transcription. Trends in Genetics. 2006;22:197. doi: 10.1016/j.tig.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Li X, Hu X, Patel B, Zhou Z, Liang S, Ybarra R, et al. H4R3 methylation facilitates β-globin transcription by regulating histone acetyltransferase binding and H3 acetylation. Blood. 2010;115:2028. doi: 10.1182/blood-2009-07-236059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin Q, Yoon HG, Huang ZQ, Strahl BD, Allis CD, et al. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Molecular and Cellular Biology. 2002;22:5688. doi: 10.1128/MCB.22.16.5688-5697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-κB-dependent inflammatory genes. Relevance to diabetes and inflammation. The Journal of Biological Chemistry. 2008;283:26771. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nature Immunology. 2011;12:551. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, et al. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- Liu B, Tahk S, Yee KM, Fan G, Shuai K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science. 2010;330:521. doi: 10.1126/science.1193787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots GG, Locksley RM, Blankespoor CM, Wang ZE, Miller W, Rubin EM, et al. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science. 2000;288:136. doi: 10.1126/science.288.5463.136. [DOI] [PubMed] [Google Scholar]
- Love JJ, Li X, Case DA, Giese K, Grosschedl R, Wright PE. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature. 1995;376:791. doi: 10.1038/376791a0. [DOI] [PubMed] [Google Scholar]
- Lu Q, Kaplan M, Ray D, Zacharek S, Gutsch D, Richardson B. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis and Rheumatism. 2002;46:1282. doi: 10.1002/art.10234. [DOI] [PubMed] [Google Scholar]
- Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcγR ligation leads to chromatin modifications at the IL-10 locus. The Journal of Immunology. 2005;175:469. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- Luger K, Dechassa ML, Tremethick DJ. New insights into nucleosome and chromatin structure: An ordered state or a disordered affair? Nature Reviews. Molecular Cell Biology. 2012;13:436. doi: 10.1038/nrm3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Macdonald N, Welburn JPI, Noble MEM, Nguyen A, Yaffe MB, Clynes D, et al. Molecular basis for the recognition of phosphorylated and phosphoacetylated histone H3 by 14-3-3. Molecular Cell. 2005;20:199. doi: 10.1016/j.molcel.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. The American Journal of Pathology. 2012;181:8. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Makar KW, Pérez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nature Immunology. 2003;4:1183. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- Makar KW, Wilson CB. DNA methylation is a nonredundant repressor of the Th2 effector program. The Journal of Immunology. 2004;173:4402. doi: 10.4049/jimmunol.173.7.4402. [DOI] [PubMed] [Google Scholar]
- Mantel PY, Ouaked N, Rückert B, Karagiannidis C, Welz R, Blaser K, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. The Journal of Immunology. 2006;176:3593. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nature Biotechnology. 2007;25:84. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- Martin M, Cho J, Cesare AJ, Griffith JD, Attardi G. Termination factor-mediated DNA loop between termination and initiation sites drives mitochondrial rRNA synthesis. Cell. 2005;123:1227. doi: 10.1016/j.cell.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. The Journal of Immunology. 2006;177:7303. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. European Journal of Immunology. 2009;39:216. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazari L, Ouarzane M, Zouali M. Subversion of B lymphocyte tolerance by hydralazine, a potential mechanism for drug-induced lupus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6317. doi: 10.1073/pnas.0610434104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey PG, Goldfeld AE, Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-α gene transcription. The Journal of Biological Chemistry. 1994;269:30445. [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nature Immunology. 2009;10:314. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nature Reviews. Immunology. 2009;9:692. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human TH1 and TH2 lymphocytes. Nature Immunology. 2003;4:78. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. The Journal of Biological Chemistry. 2004;279:18091. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- Migliori V, Müller J, Phalke S, Low D, Bezzi M, Mok WC, et al. Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance. Nature Structural & Molecular Biology. 2012;19:136. doi: 10.1038/nsmb.2209. [DOI] [PubMed] [Google Scholar]
- Miller SA, Huang AC, Miazgowicz MM, Brassil MM, Weinmann AS. Coordinated but physically separable interaction with H3K27-demethylase and H3K4-methyltransferase activities are required for T-box protein-mediated activation of developmental gene expression. Genes & Development. 2008;22:2980. doi: 10.1101/gad.1689708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KHG. TLR-dependent T cell activation in autoimmunity. Nature Reviews. Immunology. 2011;11:807. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Miranda TB, Jones PA. DNA methylation: The nuts and bolts of repression. Journal of Cellular Physiology. 2007;213:384. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- Mirny LA. The fractal globule as a model of chromatin architecture in the cell. Chromosome Research. 2011;19:37. doi: 10.1007/s10577-010-9177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen CA, Yang XJ, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T, et al. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nature Immunology. 2011;12:450. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AC, Hutchins AS, High FA, Lee HW, Sykes KJ, Chodosh LA, et al. Hlx is induced by and genetically interacts with T-bet to promote heritable TH1 gene induction. Nature Immunology. 2002;3:652. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- Murrell A, Heeson S, Reik W. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nature Genetics. 2004;36:889. doi: 10.1038/ng1402. [DOI] [PubMed] [Google Scholar]
- Mutskov V, Felsenfeld G. Silencing of transgene transcription precedes methylation of promoter DNA and histone H3 lysine 9. The EMBO Journal. 2004;23:138. doi: 10.1038/sj.emboj.7600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, et al. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes & Development. 2006;20:966. doi: 10.1101/gad.1404206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedospasov SA, Hirt B, Shakhov AN, Dobrynin VN, Kawashima E, Accolla RS, et al. The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse. Nucleic Acids Research. 1986;14:7713. doi: 10.1093/nar/14.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell CL, Deisseroth AB, Lopez-Berestein G. Interaction of nuclear proteins with an AP-1/CRE-like promoter sequence in the human TNF-α gene. Journal of Leukocyte Biology. 1994;56:27. doi: 10.1002/jlb.56.1.27. [DOI] [PubMed] [Google Scholar]
- Ng HH, Robert F, Young RA, Struhl K. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Molecular Cell. 2003;11:709. doi: 10.1016/s1097-2765(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, et al. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nature Genetics. 1999;23:58. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung C-w, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu Y, Watanabe N, Neda H, Yamauchi N, Maeda M, Sone H, et al. Induction of synthesis of tumor necrosis factor in human and murine cell lines by exogenous recombinant human tumor necrosis factor. Cancer Research. 1988;48:5407. [PubMed] [Google Scholar]
- Nile CJ, Read RC, Akil M, Duff GW, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis and Rheumatism. 2008;58:2686. doi: 10.1002/art.23758. [DOI] [PubMed] [Google Scholar]
- Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14751. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, et al. Over-expression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis and Rheumatism. 2004;50:1850. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science. 2004;303:644. doi: 10.1126/science.1092727. [DOI] [PubMed] [Google Scholar]
- Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nature Genetics. 1998;19:219. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- Oliphant CJ, Barlow JL, McKenzie ANJ. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CT, Corces VG. Enhancers: Emerging roles in cell fate specification. EMBO Reports. 2012;13:423. doi: 10.1038/embor.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando V, Paro R. Mapping Polycomb-repressed domains in the bithorax complex using in vivo formaldehyde cross-linked chromatin. Cell. 1993;75:1187. doi: 10.1016/0092-8674(93)90328-n. [DOI] [PubMed] [Google Scholar]
- Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nature Genetics. 2004;36:1065. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- Osborne CS, Ewels PA, Young ANC. Meet the neighbours: Tools to dissect nuclear structure and function. Briefings in Functional Genomics. 2011;10:11. doi: 10.1093/bfgp/elq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan JM, Tan-Wong SM, Morillon A, Lee B, Coles J, Mellor J, et al. Gene loops juxtapose promoters and terminators in yeast. Nature Genetics. 2004;36:1014. doi: 10.1038/ng1411. [DOI] [PubMed] [Google Scholar]
- Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The b-globin nuclear compartment in development and erythroid differentiation. Nature Genetics. 2003;35:190. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- Pang Y, Norihisa Y, Benjamin D, Kantor RRS, Young HA. Interferon-γ gene expression in human B-cell lines: Induction by interleukin-2, protein kinase C activators, and possible effect of hypomethylation on gene regulation. Blood. 1992;80:724. [PubMed] [Google Scholar]
- Parekh BS, Maniatis T. Virus infection leads to localized hyperacetylation of histones H3 and H4 at the IFN-β promoter. Molecular Cell. 1999;3:125. doi: 10.1016/s1097-2765(00)80181-1. [DOI] [PubMed] [Google Scholar]
- Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell. 2008;132:422. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Parthun MR, Widom J, Gottschling DE. The major cytoplasmic histone acetyltransferase in yeast: Links to chromatin replication and histone metabolism. Cell. 1996;87:85. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- Patrinos GP, de Krom M, de Boer E, Langeveld A, Imam AMA, Strouboulis J, et al. Multiple interactions between regulatory regions are required to stabilize an active chromatin hub. Genes & Development. 2004;18:1495. doi: 10.1101/gad.289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Haykinson MJ, Johnson RC. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes & Development. 1993;7:1521. doi: 10.1101/gad.7.8.1521. [DOI] [PubMed] [Google Scholar]
- Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Pazin MJ, Kadonaga JT. What’s up and down with histone deacetylation and transcription? Cell. 1997;89:325. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- Peters AHFM, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AAHA, Perez-Burgos L, et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Molecular Cell. 2003;12:1577. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RCM, Masuno M, et al. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- Pil PM, Chow CS, Lippard SJ. High-mobility-group 1 protein mediates DNA bending as determined by ring closures. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9465. doi: 10.1073/pnas.90.20.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, et al. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. European Journal of Immunology. 2008;38:1654. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clinical Cancer Research. 2009;15:3958. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986;322:697. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Quddus J, Johnson KJ, Gavalchin J, Amento EP, Chrisp CE, Yung RL, et al. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. The Journal of Clinical Investigation. 1993;92:38. doi: 10.1172/JCI116576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran R, Garva R, Krstic-Demonacos M, Demonacos C. Sirtuins: Molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. Journal of Biomedicine & Biotechnology. 2011;2011:368276. doi: 10.1155/2011/368276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Braas D, Bhatt DM, Cheng CS, Hong C, Doty KR, et al. A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell. 2009;138:114. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, et al. Selective and antagonistic functions of SWI/SNF and Mi-2β nucleosome remodeling complexes during an inflammatory response. Genes & Development. 2006;20:282. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. Combinatorial complexity in chromatin structure and function: Revisiting the histone code. Current Opinion in Genetics & Development. 2012;22:148. doi: 10.1016/j.gde.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar S, Rajsbaum R, Goldfeld AE. Transactivator of transcription from HIV type 1 subtype E selectively inhibits TNF gene expression via interference with chromatin remodeling of the TNF locus. The Journal of Immunology. 2006;176:4182. doi: 10.4049/jimmunol.176.7.4182. [DOI] [PubMed] [Google Scholar]
- Rasmussen TA, Schmeltz Søgaard O, Brinkmann C, Wightman F, Lewin S, Melchjorsen J, et al. Comparison of HDAC inhibitors in clinical development: Effect on HIV production in latently infected cells and T-cell activation. Human vaccines & immunotherapeutics. 2013;9 doi: 10.4161/hv.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T, Hedrich CM, Juang YT, Tenbrock K, Tsokos GC. cAMP-responsive element modulator (CREM)α protein induces interleukin 17A expression and mediates epigenetic alterations at the interleukin-17A gene locus in patients with systemic lupus erythematosus. The Journal of Biological Chemistry. 2011;286:43437. doi: 10.1074/jbc.M111.299313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner SL. Epigenetic control in the immune response. Human Molecular Genetics. 2005;14(Spec No 1):R41. doi: 10.1093/hmg/ddi115. [DOI] [PubMed] [Google Scholar]
- Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG, Simon I, et al. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- Ribeiro de Almeida C, Heath H, Krpic S, Dingjan GM, van Hamburg JP, Bergen I, et al. Critical role for the transcription regulator CCCTC-binding factor in the control of Th2 cytokine expression. The Journal of Immunology. 2009;182:999. doi: 10.4049/jimmunol.182.2.999. [DOI] [PubMed] [Google Scholar]
- Richardson B. Effect of an inhibitor of DNA methylation on T cells. II. 5-Azacytidine induces self-reactivity in antigen-specific T4+ cells. Human Immunology. 1986;17:456. doi: 10.1016/0198-8859(86)90304-6. [DOI] [PubMed] [Google Scholar]
- Richardson BC, Liebling MR, Hudson JL. CD4+ cells treated with DNA methylation inhibitors induce autologous B cell differentiation. Clinical Immunology and Immunopathology. 1990;55:368. doi: 10.1016/0090-1229(90)90125-a. [DOI] [PubMed] [Google Scholar]
- Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis and Rheumatism. 1990;33:1665. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- Richardson BC, Strahler JR, Pivirotto TS, Quddus J, Bayliss GE, Gross LA, et al. Phenotypic and functional similarities between 5-azacytidine-treated T cells and a T cell subset in patients with active systemic lupus erythematosus. Arthritis and Rheumatism. 1992;35:647. doi: 10.1002/art.1780350608. [DOI] [PubMed] [Google Scholar]
- Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenetics and Cell Genetics. 1975;14:9. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Rippe K. Making contacts on a nucleic acid polymer. Trends in Biochemical Sciences. 2001;26:733. doi: 10.1016/s0968-0004(01)01978-8. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: A coactivator/ corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes & Development. 2006;20:1405. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Rougeulle C, Chaumeil J, Sarma K, Allis CD, Reinberg D, Avner P, et al. Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Molecular and Cellular Biology. 2004;24:5475. doi: 10.1128/MCB.24.12.5475-5484.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell E, Merkenschlager M, Wilson CB. Long-range regulation of cytokine gene expression. Current Opinion in Immunology. 2008;20:272. doi: 10.1016/j.coi.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BY, Anderson SL, Sullivan SA, Williamson BD, Carswell EA, Old LJ. Purification and characterization of a human tumor necrosis factor from the LuKII cell line. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6637. doi: 10.1073/pnas.82.19.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: Intricacy of writing and reading a single epigenetic mark. Molecular Cell. 2007;25:15. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-κB recruitment. Nature Immunology. 2002;3:69. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Saleque S, Kim J, Rooke HM, Orkin SH. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Molecular Cell. 2007;27:562. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K. SIRT1 longevity factor suppresses NF-kB -driven immune responses: Regulation of aging via NF-kB acetylation? BioEssays. 2008;30:939. doi: 10.1002/bies.20799. [DOI] [PubMed] [Google Scholar]
- Santangelo S, Cousins DJ, Winkelmann NEE, Staynov DZ. DNA methylation changes at human Th2 cytokine genes coincide with DNase I hypersensitive site formation during CD4+ T cell differentiation. The Journal of Immunology. 2002;169:1893. doi: 10.4049/jimmunol.169.4.1893. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NCT, et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Baù D, Martí-Renom MA, Dekker J. Chromatin globules: A common motif of higher order chromosome structure? Current Opinion in Cell Biology. 2011;23:325. doi: 10.1016/j.ceb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M, Christensen JR, Tsytsykova AV, Goldfeld AE, Ley SC, Kioussis D, et al. Identification of a macrophage-specific chromatin signature in the IL-10 locus. The Journal of Immunology. 2005;175:1041. doi: 10.4049/jimmunol.175.2.1041. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nature Reviews. Immunology. 2010;10:170. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nature Immunology. 2010;11:936. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- Savan R, Kono T, Igawa D, Sakai M. A novel tumor necrosis factor (TNF) gene present in tandem with theTNF-α gene on the same chromosome in teleosts. Immunogenetics. 2005;57:140. doi: 10.1007/s00251-005-0768-4. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, et al. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron. 2009;64:678. doi: 10.1016/j.neuron.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz RL, Mizzen CA, Vassilev A, Cook RG, Allis CD, Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. The Journal of Biological Chemistry. 1999;274:1189. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- Schneider G, Krämer OH, Schmid RM, Saur D. Acetylation as a transcriptional control mechanism—HDACs and HATs in pancreatic ductal adenocarcinoma. Journal of Gastrointestinal Cancer. 2011;42:85. doi: 10.1007/s12029-011-9257-1. [DOI] [PubMed] [Google Scholar]
- Schoenborn JR, Dorschner MO, Sekimata M, Santer DM, Shnyreva M, Fitzpatrick DR, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-γ. Nature Immunology. 2007;8:732. doi: 10.1038/ni1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz EG, Mariani L, Radbruch A, Höfer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-γ and interleukin-12. Immunity. 2009;30:673. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Selker EU. Trichostatin A causes selective loss of DNA methylation in Neurospora. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9430. doi: 10.1073/pnas.95.16.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimata M, Pérez-Melgosa M, Miller SA, Weinmann AS, Sabo PJ, Sandstrom R, et al. CCCTC-binding factor and the transcription factor T-bet orchestrate T helper 1 cell-specific structure and function at the interferon-γ locus. Immunity. 2009;31:551. doi: 10.1016/j.immuni.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvi BR, Batta K, Kishore AH, Mantelingu K, Varier RA, Balasubramanyam K, et al. Identification of a novel inhibitor of coactivator-associated arginine meth-yltransferase 1 (CARM1)-mediated methylation of histone H3 Arg-17. The Journal of Biological Chemistry. 2010;285:7143. doi: 10.1074/jbc.M109.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. κB-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor a gene in primary macrophages. The Journal of Experimental Medicine. 1990;171:35. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam MK, Sethi G. Role of epigenetics in inflammation-associated diseases. Subcellular Biochemistry. 2012;61:627. doi: 10.1007/978-94-007-4525-4_27. [DOI] [PubMed] [Google Scholar]
- Shebzukhov YV, Kuprash DV. Transcriptional regulation of TNF/LT locus in immune cells. Molecular Biology (Mosk) 2011;45:56. [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13225. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnyreva M, Weaver WM, Blanchette M, Taylor SL, Tompa M, Fitzpatrick DR, et al. Evolutionarily conserved sequence elements that positively regulate IFN-γ expression in T cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12622. doi: 10.1073/pnas.0400849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker J, Saraiva M, O’Garra A. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. The Journal of Immunology. 2006;176:3470. doi: 10.4049/jimmunol.176.6.3470. [DOI] [PubMed] [Google Scholar]
- Siegel MD, Zhang DH, Ray P, Ray A. Activation of the interleukin-5 promoter by cAMP in murine EL-4 cells requires the GATA-3 and CLE0 elements. The Journal of Biological Chemistry. 1995;270:24548. doi: 10.1074/jbc.270.41.24548. [DOI] [PubMed] [Google Scholar]
- Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutation Research. 2008;647:21. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Simonis M, Klous P, Splinter E, Moshkin Y, Willemsen R, de Wit E, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C) Nature Genetics. 2006;38:1348. doi: 10.1038/ng1896. [DOI] [PubMed] [Google Scholar]
- Sobel RE, Cook RG, Perry CA, Annunziato AT, Allis CD. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1237. doi: 10.1073/pnas.92.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. The EMBO Journal. 2003;22:2788. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MJ, Larsen PL, Varshavsky A. Mapping protein—DNA interactions in vivo with formaldehyde: Evidence that histone H4 is retained on a highly transcribed gene. Cell. 1988;53:937. doi: 10.1016/s0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- Solomon MJ, Varshavsky A. Formaldehyde-mediated DNA—protein crosslinking: A probe for in vivo chromatin structures. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:6470. doi: 10.1073/pnas.82.19.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutto M, Zhang F, Enerson B, Tong Y, Boothby M, Aune TM. A minimal IFN-γ promoter confers Th1 selective expression. The Journal of Immunology. 2002;169:4205. doi: 10.4049/jimmunol.169.8.4205. [DOI] [PubMed] [Google Scholar]
- Soutto M, Zhou W, Aune TM. Cutting edge: Distal regulatory elements are required to achieve selective expression of IFN-γ in Th1/Tc1 effector cells. The Journal of Immunology. 2002;169:6664. doi: 10.4049/jimmunol.169.12.6664. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nature Immunology. 2004;5:1017. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, et al. CTCF mediates long-range chromatin looping and local histone modification in the β-globin locus. Genes & Development. 2006;20:2349. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer JH, Kroeger KM, Abraham LJ, Joyce DA. Glucocorticoids suppress tumor necrosis factor-α expression by human monocytic THP-1 cells by suppressing transactivation through adjacent NF-κB and c-Jun-activating transcription factor-2 binding sites in the promoter. The Journal of Biological Chemistry. 2000;275:18432. doi: 10.1074/jbc.M906304199. [DOI] [PubMed] [Google Scholar]
- Steffen M, Ottmann OG, Moore MA. Simultaneous production of tumor necrosis factor-α and lymphotoxin by normal T cells after induction with IL-2 and anti-T3. The Journal of Immunology. 1988;140:2621. [PubMed] [Google Scholar]
- Stender JD, Pascual G, Liu W, Kaikkonen MU, Do K, Spann NJ, et al. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Molecular Cell. 2012;48:28. doi: 10.1016/j.molcel.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Strelkov IS, Davie JR. Ser-10 phosphorylation of histone H3 and immediate early gene expression in oncogene-transformed mouse fibroblasts. Cancer Research. 2002;62:75. [PubMed] [Google Scholar]
- Strickland FM, Richardson BC. Epigenetics in human autoimmunity. Epigenetics in autoimmunity—DNA methylation in systemic lupus erythematosus and beyond. Autoimmunity. 2008;41:278. doi: 10.1080/08916930802024616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikova M, Schagdarsurengin U, Kehlen A, Garbe JC, Stampfer MR, Dammann R. Chromatin inactivation precedes de novo DNA methylation during the progressive epigenetic silencing of the RASSF1A promoter. Molecular and Cellular Biology. 2005;25:3923. doi: 10.1128/MCB.25.10.3923-3933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, Reddy ABM, Dietzmann K, Suriano AR, Kocieda VP, Stewart M, et al. Epigenetic regulation of tumor necrosis factor alpha. Molecular and Cellular Biology. 2007;27:5147. doi: 10.1128/MCB.02429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, Suriano A, Dietzmann K, Lin J, Goldman D, Petri MA. The TNFa locus is altered in monocytes from patients with systemic lupus erythematosus. Clinical Immunology. 2007;123:74. doi: 10.1016/j.clim.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SSJ, Bjorndahl JM, Wang CY, Kao HT, Fu SM. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and anti-CD3 antibody. The Journal of Experimental Medicine. 1988;167:937. doi: 10.1084/jem.167.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SSJ, Jung LK, Walters JA, Chen W, Wang CY, Fu SM. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. The Journal of Experimental Medicine. 1988;168:1539. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Szalmás A, Bánáti F, Koroknai A, László B, Fehér E, Salamon D, et al. Lineage-specific silencing of human IL-10 gene expression by promoter methylation in cervical cancer cells. European Journal of Cancer. 2008;44:1030. doi: 10.1016/j.ejca.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Takebayashi S, Nakao M, Fujita N, Sado T, Tanaka M, Taguchi H, et al. 5-Aza-2′-deoxycytidine induces histone hyperacetylation of mouse centromeric heterochromatin by a mechanism independent of DNA demethylation. Biochemical and Biophysical Research Communications. 2001;288:921. doi: 10.1006/bbrc.2001.5863. [DOI] [PubMed] [Google Scholar]
- Takei S, Fernandez D, Redford A, Toyoda H. Methylation status of 5′-regulatory region of tumor necrosis factor α gene correlates with differentiation stages of monocytes. Biochemical and Biophysical Research Communications. 1996;220:606. doi: 10.1006/bbrc.1996.0450. [DOI] [PubMed] [Google Scholar]
- Takemoto N, Koyano-Nakagawa N, Yokota T, Arai N, Miyatake S, Arai K. Th2-specific DNase I-hypersensitive sites in the murine IL-13 and IL-4 intergenic region. International Immunology. 1998;10:1981. doi: 10.1093/intimm/10.12.1981. [DOI] [PubMed] [Google Scholar]
- Tamassia N, Zimmermann M, Castellucci M, Ostuni R, Bruderek K, Schilling B, et al. Cutting edge: An inactive chromatin configuration at the IL-10 locus in human neutrophils. The Journal of Immunology. 2013;190:1921. doi: 10.4049/jimmunol.1203022. [DOI] [PubMed] [Google Scholar]
- Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Tsukada J, Suzuki W, Hayashi K, Tanigaki K, Tsuji M, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. The Journal of Immunology. 1997;159:5956. [PubMed] [Google Scholar]
- Taylor JM, Wicks K, Vandiedonck C, Knight JC. Chromatin profiling across the human tumour necrosis factor gene locus reveals a complex, cell type-specific landscape with novel regulatory elements. Nucleic Acids Research. 2008;36:4845. doi: 10.1093/nar/gkn444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Sai H, Wells AD. Conserved intergenic elements and DNA methylation cooperate to regulate transcription at the il17 locus. The Journal of Biological Chemistry. 2012;287:25049. doi: 10.1074/jbc.M112.351916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/ HMG-14 kinase. The EMBO Journal. 1999;18:4779. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Mahadevan LC. Independent dynamic regulation of histone phosphorylation and acetylation during immediate-early gene induction. Molecular Cell. 2001;8:1231. doi: 10.1016/s1097-2765(01)00404-x. [DOI] [PubMed] [Google Scholar]
- Thorne JL, Ouboussad L, Lefevre PF. Heterochromatin protein 1 gamma and IκB kinase alpha interdependence during tumour necrosis factor gene transcription elongation in activated macrophages. Nucleic Acids Research. 2012;40:7676. doi: 10.1093/nar/gks509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. The EMBO Journal. 2009;28:1878. doi: 10.1038/emboj.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active b-globin locus. Molecular Cell. 2002;10:1453. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Tomotsune D, Shoji H, Wakamatsu Y, Kondoh H, Takahashi N. A mouse homologue of the Drosophila tumour-suppressor gene l(2)gl controlled by Hox-C8 in vivo. Nature. 1993;365:69. doi: 10.1038/365069a0. [DOI] [PubMed] [Google Scholar]
- Tong Y, Aune T, Boothby M. T-bet antagonizes mSin3a recruitment and transactivates a fully methylated IFN-γ promoter via a conserved T-box half-site. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2034. doi: 10.1073/pnas.0409510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature. 2009;458:78. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, et al. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Molecular and Cellular Biology. 2000;20:6084. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Molecular and Cellular Biology. 1996;16:459. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EY, Yie J, Thanos D, Goldfeld AE. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/ JUN. Molecular and Cellular Biology. 1996;16:5232. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- Tsytsykova AV, Falvo JV, Schmidt-Supprian M, Courtois G, Thanos D, Goldfeld AE. Post-induction, stimulus-specific regulation of tumor necrosis factor mRNA expression. The Journal of Biological Chemistry. 2007;282:11629. doi: 10.1074/jbc.M611418200. [DOI] [PubMed] [Google Scholar]
- Tsytsykova AV, Goldfeld AE. Nuclear factor of activated T cells transcription factor NFATp controls superantigen-induced lethal shock. The Journal of Experimental Medicine. 2000;192:581. doi: 10.1084/jem.192.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsytsykova AV, Goldfeld AE. Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes. Molecular and Cellular Biology. 2002;22:2620. doi: 10.1128/MCB.22.8.2620-2631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsytsykova AV, Rajsbaum R, Falvo JV, Ligeiro F, Neely SR, Goldfeld AE. Activation-dependent intrachromosomal interactions formed by the TNF gene promoter and two distal enhancers. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16850. doi: 10.1073/pnas.0708210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek-Plewa J, Jagodzinski PP. The role of mammalian DNA met-3 hyltransferases in the regulation of gene expression. Cellular & Molecular Biology Letters. 2005;10:631. [PubMed] [Google Scholar]
- Turner M, Londei M, Feldmann M. Human T cells from autoimmune and normal individuals can produce tumor necrosis factor. European Journal of Immunology. 1987;17:1807. doi: 10.1002/eji.1830171220. [DOI] [PubMed] [Google Scholar]
- Tykocinski LO, Hajkova P, Chang HD, Stamm T, Sözeri O, Löhning M, et al. A critical control element for interleukin-4 memory expression in T helper lymphocytes. The Journal of Biological Chemistry. 2005;280:28177. doi: 10.1074/jbc.M502038200. [DOI] [PubMed] [Google Scholar]
- Unoki M, Masuda A, Dohmae N, Arita K, Yoshimatsu M, Iwai Y, et al. Lysyl 5-hydroxylation, a novel histone modification, by Jumonji Domain Containing 6 (JMJD6) The Journal of Biological Chemistry. 2013;288:6053. doi: 10.1074/jbc.M112.433284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissière T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutation Research. 2008;659:40. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, et al. Proximity among distant regulatory elements at the β-globin locus requires GATA-1 and FOG-1. Molecular Cell. 2005;17:453. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1γ are associated with transcription elongation through mammalian chromatin. Molecular Cell. 2005;19:381. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Valapour M, Guo J, Schroeder JT, Keen J, Cianferoni A, Casolaro V, et al. Histone deacetylation inhibits IL4 gene expression in T cells. The Journal of Allergy and Clinical Immunology. 2002;109:238. doi: 10.1067/mai.2002.121145. [DOI] [PubMed] [Google Scholar]
- van den Berk LCJ, Jansen BJH, Siebers-Vermeulen KGC, Netea MG, Latuhihin T, Bergevoet S, et al. Toll-like receptor triggering in cord blood mesenchymal stem cells. Journal of Cellular and Molecular Medicine. 2009;13:3415. doi: 10.1111/j.1582-4934.2009.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berk LCJ, Jansen BJH, Siebers-Vermeulen KGC, Roelofs H, Figdor CG, Adema GJ, et al. Mesenchymal stem cells respond to TNF but do not produce TNF. Journal of Leukocyte Biology. 2010;87:283. doi: 10.1189/jlb.0709467. [DOI] [PubMed] [Google Scholar]
- van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12423. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Dekker J. Genomics tools for unraveling chromosome architecture. Nature Biotechnology. 2010;28:1089. doi: 10.1038/nbt.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, et al. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes & Development. 2006;20:1256. doi: 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedadi M, Barsyte-Lovejoy D, Liu F, Rival-Gervier S, Allali-Hassani A, Labrie V, et al. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nature Chemical Biology. 2011;7:566. doi: 10.1038/nchembio.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature Immunology. 2006;7:1151. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- Verreault A, Kaufman PD, Kobayashi R, Stillman B. Nucleosomal DNA regulates the core-histone-binding subunit of the human Hat1 acetyltransferase. Current Biology. 1998;8:96. doi: 10.1016/s0960-9822(98)70040-5. [DOI] [PubMed] [Google Scholar]
- Verreck FAW, de Boer T, Langenberg DML, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4560. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagra A, Cheng F, Wang HW, Suarez I, Glozak M, Maurin M, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nature Immunology. 2009;10:92. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagra A, Sotomayor EM, Seto E. Histone deacetylases and the immunological network: Implications in cancer and inflammation. Oncogene. 2010;29:157. doi: 10.1038/onc.2009.334. [DOI] [PubMed] [Google Scholar]
- Vogelauer M, Wu J, Suka N, Grunstein M. Global histone acetylation and deacetylation in yeast. Nature. 2000;408:495. doi: 10.1038/35044127. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nature Genetics. 2008;40:897. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Yang XO, Nurieva RI, Chang SH, Ojeda SS, et al. Transcription of Il17 and Il17f is controlled by conserved noncoding sequence 2. Immunity. 2012;36:23. doi: 10.1016/j.immuni.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Ishihara K, Hirosue A, Watanabe S, Hino S, Ojima H, et al. Higher-order chromatin regulation and differential gene expression in the human tumor necrosis factor/lymphotoxin locus in hepatocellular carcinoma cells. Molecular and Cellular Biology. 2012;32:1529. doi: 10.1128/MCB.06478-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby CJ, Wolf A, Gromak N, Dreger M, Kramer H, Kessler B, et al. Jmjd6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science. 2009;325:90. doi: 10.1126/science.1175865. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White GP, Hollams EM, Yerkovich ST, Bosco A, Holt BJ, Bassami MR, et al. CpG methylation patterns in the IFNγ promoter in naive T cells: Variations during Th1 and Th2 differentiation and between atopics and non-atopics. Pediatric Allergy and Immunology. 2006;17:557. doi: 10.1111/j.1399-3038.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Wicks K, Knight JC. Transcriptional repression and DNA looping associated with a novel regulatory element in the final exon of the lymphotoxin-β gene. Genes and Immunity. 2011;12:126. doi: 10.1038/gene.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Aldred MA, Der Kaloustian VM, Halal F, Gowans G, McLeod DR, et al. Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems. American Journal of Human Genetics. 2010;87:219. doi: 10.1016/j.ajhg.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Spilianakis CG, Flavell RA. Interchromosomal association and gene regulation in trans. Trends in Genetics. 2010;26:188. doi: 10.1016/j.tig.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunology. 2007;8:950. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nature Reviews. Immunology. 2009;9:91. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- Winter S, Simboeck E, Fischle W, Zupkovitz G, Dohnal I, Mechtler K, et al. 14-3-3 proteins recognize a histone code at histone H3 and are required for transcriptional activation. The EMBO Journal. 2008;27:88. doi: 10.1038/sj.emboj.7601954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Pazin MJ. BRG1-mediated chromatin remodeling regulates differentiation and gene expression of T helper cells. Molecular and Cellular Biology. 2008;28:7274. doi: 10.1128/MCB.00835-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Precht P, Becker KG, Wood WH, 3rd, Zhang Y, Wang Z, et al. IL-10 transcription is negatively regulated by BAF180, a component of the SWI/ SNF chromatin remodeling enzyme. BMC Immunology. 2012;13:9. doi: 10.1186/1471-2172-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würtele H, Chartrand P. Genome-wide scanning of HoxB1-associated loci in mouse ES cells using an open-ended Chromosome Conformation Capture methodology. Chromosome Research. 2006;14:477. doi: 10.1007/s10577-006-1075-0. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang K, Grunstein M. Acetylation in histone H3 globular domain regulates gene expression in yeast. Cell. 2005;121:375. doi: 10.1016/j.cell.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression. Nature. 2003;423:655. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24:611. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ukai-Tadenuma M, Kimura M, Omori M, Inami M, Taniguchi M, et al. Identification of a conserved GATA3 response element upstream proximal from the interleukin-13 gene locus. The Journal of Biological Chemistry. 2002;277:42399. doi: 10.1074/jbc.M205876200. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ukai-Tadenuma M, Miyamoto T, Sugaya K, Hosokawa H, Hasegawa A, et al. Essential role of GATA3 for the maintenance of type 2 helper T (Th2) cytokine production and chromatin remodeling at the Th2 cytokine gene loci. The Journal of Biological Chemistry. 2004;279:26983. doi: 10.1074/jbc.M403688200. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. The EMBO Journal. 2004;23:2369. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HA, Ghosh P, Ye J, Lederer J, Lichtman A, Gerard JR, et al. Differentiation of the T helper phenotypes by analysis of the methylation state of the IFN-γ gene. The Journal of Immunology. 1994;153:3603. [PubMed] [Google Scholar]
- Young HA, Komschlies KL, Ciccarone V, Beckwith M, Rosenberg M, Jenkins NA, et al. Expression of human IFN-γ genomic DNA in transgenic mice. The Journal of Immunology. 1989;143:2389. [PubMed] [Google Scholar]
- Yu Q, Thieu VT, Kaplan MH. Stat4 limits DNA methyltransferase recruitment and DNA methylation of the IL-18Rα gene during Th1 differentiation. The EMBO Journal. 2007;26:2052. doi: 10.1038/sj.emboj.7601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung RL, Richardson BC. Drug-induced lupus. Rheumatic Diseases Clinics of North America. 1994;20:61. [PubMed] [Google Scholar]
- Zeng L, Zhang Q, Gerona-Navarro G, Moshkina N, Zhou MM. Structural basis of site-specific histone recognition by the bromodomains of human coactivators PCAF and CBP/p300. Structure. 2008;16:643. doi: 10.1016/j.str.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Tesar PJ, Scacheri PC. Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Research. 2011;21:1273. doi: 10.1101/gr.122382.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Boothby M. T helper type 1-specific Brg1 recruitment and remodeling of nucleosomes positioned at the IFN-γ promoter are Stat4 dependent. The Journal of Experimental Medicine. 2006;203:1493. doi: 10.1084/jem.20060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Edwards JP, Mosser DM. Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription. The Journal of Immunology. 2006;177:1282. doi: 10.4049/jimmunol.177.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes & Development. 1999;13:1924. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Yang L, Ray A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. The Journal of Immunology. 1998;161:3817. [PubMed] [Google Scholar]
- Zhang F, Yu X. WAC, a functional partner of RNF20/40, regulates histone H2B ubiquitination and gene transcription. Molecular Cell. 2011;41:384. doi: 10.1016/j.molcel.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nature Structural & Molecular Biology. 2009;16:304. doi: 10.1038/nsmb.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Tavoosidana G, Sjölinder M, Göndör A, Mariano P, Wang S, et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epi-genetically regulated intra- and interchromosomal interactions. Nature Genetics. 2006;38:1341. doi: 10.1038/ng1891. [DOI] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor a (ER) gene expression without loss of DNA hypermethylation. Cancer Biology & Therapy. 2007;6:64. doi: 10.4161/cbt.6.1.3549. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunology. 2007;8:967. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhu P, Wang J, Pascual G, Ohgi KA, Lozach J, et al. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Molecular Cell. 2008;29:69. doi: 10.1016/j.molcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, et al. Down-regulation of Gfi-1 expression by TGF-β is important for differentiation of Th17 and CD103+ inducible regulatory T cells. The Journal of Experimental Medicine. 2009;206:329. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WG, Lakshmanan RR, Beal MD, Otterson GA. DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Research. 2001;61:1327. [PubMed] [Google Scholar]
- Zhu H, Yang J, Murphy TL, Ouyang W, Wagner F, Saparov A, et al. Unexpected characteristics of the IFN-γ reporters in nontransformed T cells. The Journal of Immunology. 2001;167:855. doi: 10.4049/jimmunol.167.2.855. [DOI] [PubMed] [Google Scholar]
- Zhu B, Zheng Y, Pham AD, Mandal SS, Erdjument-Bromage H, Tempst P, et al. Monoubiquitination of human histone H2B: The factors involved and their roles in HOX gene regulation. Molecular Cell. 2005;20:601. doi: 10.1016/j.molcel.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]