Abstract
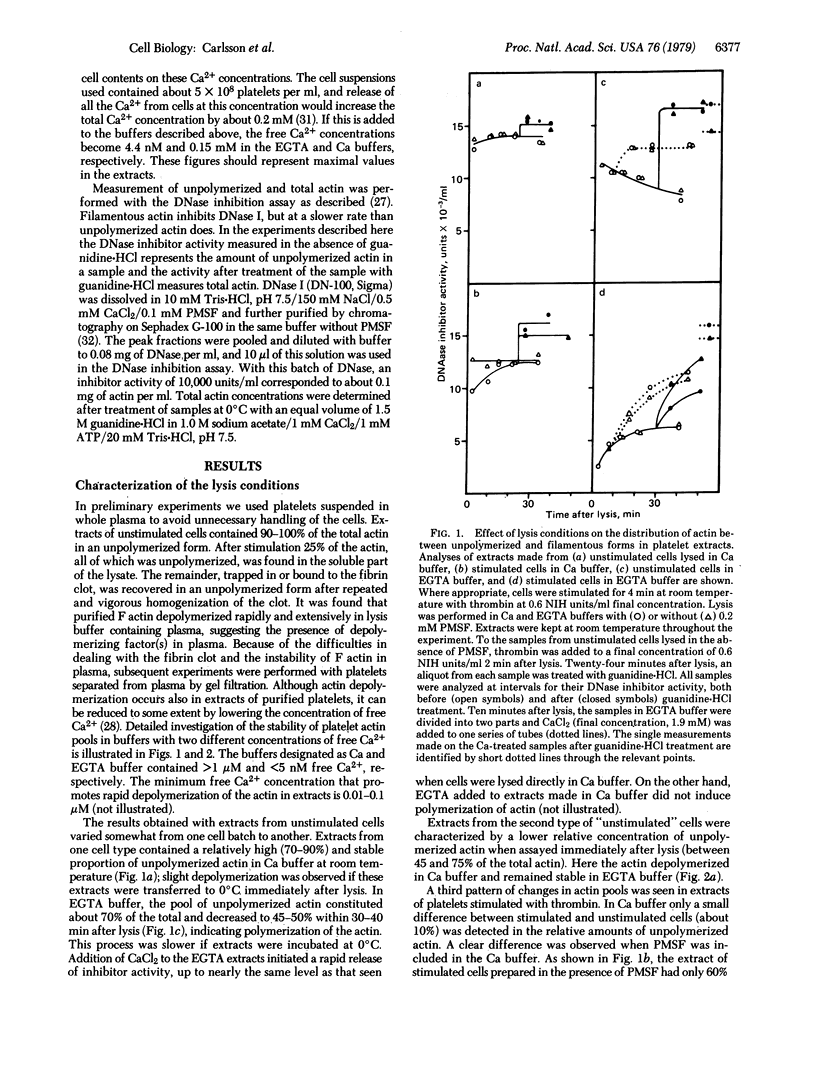
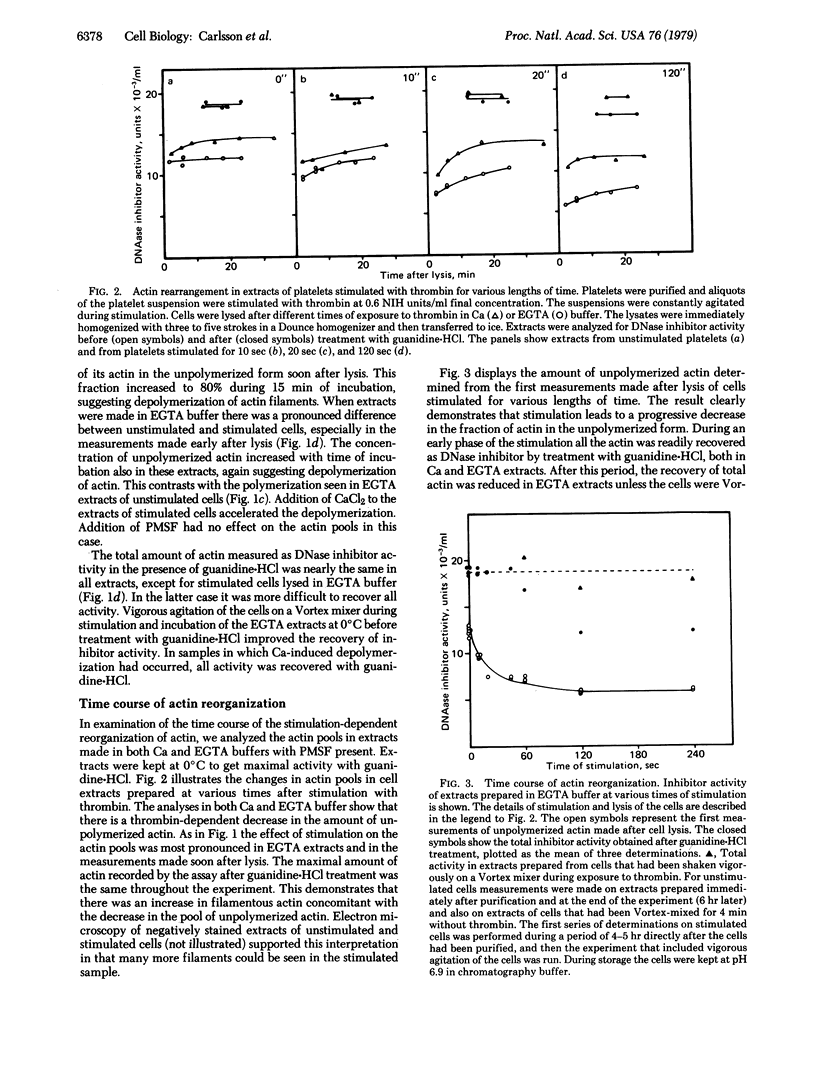
The effect of thrombin stimulation on actin organization in human platelets has been analyzed by using the DNase I inhibition assay, which is selective for unpolymerized and filamentous actin. The results provide biochemical evidence for the suggestion that stimulation leads to rapid polymerization of actin. The measurements also reveal changes in the polymerization state of actin occurring after cell lysis. These changes are influenced by the concentration of free calcium in the extracts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R. Physarum action. Observations on its presence, stability, and assembly in plasmodial extracts and development of an improved purification procedure. Biochemistry. 1977 Nov 1;16(22):4862–4871. doi: 10.1021/bi00641a018. [DOI] [PubMed] [Google Scholar]
- Behnke O., Kristensen B. I., Nielsen L. E. Electron microscopical observations on actinoid and myosinoid filaments in blood platelets. J Ultrastruct Res. 1971 Nov;37(3):351–369. doi: 10.1016/s0022-5320(71)80129-6. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977 Sep 25;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Chao F. C., Shepro D., Tullis J. L., Belamarich F. A., Curby W. A. Similarities between platelet contraction and cellular motility during mitosis: role of platelet microtubules in clot retraction. J Cell Sci. 1976 May;20(3):569–588. doi: 10.1242/jcs.20.3.569. [DOI] [PubMed] [Google Scholar]
- Cohen I., Gabbay J., Glaser T., Oplatka A. Fibrin-blood platelet interaction in a contracting clot. Br J Haematol. 1975 Sep;31(1):45–50. doi: 10.1111/j.1365-2141.1975.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Cooke R. The role of the bound nucleotide in the polymerization of actin. Biochemistry. 1975 Jul 15;14(14):3250–3256. doi: 10.1021/bi00685a035. [DOI] [PubMed] [Google Scholar]
- Geisow M. J. Calcium control of enzyme reactions. Nature. 1978 Nov 16;276(5685):211–212. doi: 10.1038/276211a0. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Boyer J. L., Korn E. D. Comparative biochemistry of non-muscle actins. J Biol Chem. 1977 Nov 25;252(22):8300–8309. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Harris H. E., Weeds A. G. Platelet actin: sub-cellular distribution and association with profilin. FEBS Lett. 1978 Jun 1;90(1):84–88. doi: 10.1016/0014-5793(78)80303-2. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of motility in nonmuscle cells. J Cell Biol. 1977 Jul;74(1):1–15. doi: 10.1083/jcb.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Setkowsky C. A. Secretory mechanisms. Behaviour of adenine nucleotides during the platelet release reaction induced by adenosine diphosphate and adrenaline. Biochem J. 1972 Aug;129(1):67–82. doi: 10.1042/bj1290067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Stormorken H. The blood platelet release reaction. Scand J Haematol Suppl. 1969;8:3–26. [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. ATP binding to a protease-resistant core of actin. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2742–2746. doi: 10.1073/pnas.73.8.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U. Molecular weight and amino acid composition of deoxyribonuclease I. Biochemistry. 1967 Jan;6(1):335–342. doi: 10.1021/bi00853a050. [DOI] [PubMed] [Google Scholar]
- Markey F., Lindberg U., Eriksson L. Human platelets contain profilin, a potential regulator of actin polymerisability. FEBS Lett. 1978 Apr 1;88(1):75–79. doi: 10.1016/0014-5793(78)80610-3. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Nachmias V., Sullender J., Asch A. Shape and cytoplasmic filaments in control and lidocaine-treated human platelets. Blood. 1977 Jul;50(1):39–53. [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Probst E., Lüscher F. Studies on thrombosthenin a, the actin-like moiety of the contractile protein from blood platelets. I. Isolation, characterization and evidence for two forms of thrombosthenin A. Biochim Biophys Acta. 1972 Oct 31;278(3):577–584. doi: 10.1016/0005-2795(72)90017-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Skaer R. J. Elemental composition of platelet dense bodies. Ciba Found Symp. 1975;35:239–259. doi: 10.1002/9780470720172.ch12. [DOI] [PubMed] [Google Scholar]
- Tangen O., Andrae M. L., Nilsson B. E. Nucleotide leakage from platelets in artificial media: prevention by albumin and other macromolecules and relation to ADP-induced platelet aggregation. Scand J Haematol. 1973;11(3):241–248. doi: 10.1111/j.1600-0609.1973.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Hatano S., Ishikawa H., Mooseker M. S. The polymerization of actin: its role in the generation of the acrosomal process of certain echinoderm sperm. J Cell Biol. 1973 Oct;59(1):109–126. doi: 10.1083/jcb.59.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter F., Engel J. Association kinetics and binding constants of nucleoside triphosphates with G-actin. Eur J Biochem. 1977 Apr 1;74(2):227–232. doi: 10.1111/j.1432-1033.1977.tb11385.x. [DOI] [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- Wohlfarth-Bottlermann K. E., Fleischer M. Cycling aggregation patterns of cytoplasmic F-actin coordinated with oscillating tension force generation. Cell Tissue Res. 1976 Jan 27;165(3):327–344. doi: 10.1007/BF00222437. [DOI] [PubMed] [Google Scholar]


