Abstract
Saturated fatty acids like palmitate contribute to muscle atrophy in a number of conditions (e.g., Type II diabetes) by altering insulin signaling. Akt is a key modulator of protein balance that inhibits the FoxO transcription factors (e.g., FoxO3) which selectively induce the expression of atrophy-inducing genes (atrogenes) in the ubiquitin-proteasome and autophagy-lysosome systems. Conversely, omega-3 polyunsaturated fatty acids have beneficial effects on insulin signaling and may preserve muscle mass. In an earlier report, the omega-3 fatty acid docosahexaenoic acid (DHA) protected myotubes from palmitate-induced atrophy; the mechanisms underlying the alterations in protein metabolism were not identified. This study investigated whether DHA prevents a palmitate-induced increase in proteolysis by restoring Akt/FoxO signaling. Palmitate increased the rate of protein degradation, while co-treatment with DHA prevented the response. Palmitate reduced the activation state of Akt and increased nuclear FoxO3 protein while decreasing its cytosolic level. Palmitate also increased the mRNAs of two FoxO3 atrogene targets, the E3 ubiquitin ligase atrogin-1/MAFbx and the autophagy mediator Bnip3. DHA attenuated the effects of palmitate on Akt activation, FoxO3 localization, and atrogene mRNAs. DHA, alone or in combination with palmitate, decreased the ratio of LC3B-II:LC3B-I protein as well as the rate of autophagosome formation, as indicated by reduced LC3B-II protein in the presence of 10 mmol/L methylamine, suggesting an independent effect of DHA on the macroautophagy pathway. These data indicate that palmitate induces myotube atrophy, at least in part, by activating multiple proteolytic systems and that DHA counters the catabolic effects of palmitate by restoring Akt/FoxO signaling.
Keywords: palmitate, docosahexaenoic acid, muscle atrophy, proteolysis, macroautophagy, FoxO3
Introduction
Skeletal muscle atrophy contributes to a debilitating loss of functional independence and increases the rate of mortality in individuals with numerous chronic illnesses, including diabetes, renal and heart failure, and cancer [1]. Currently there are few effective treatments that counteract muscle atrophy [2]. Lipotoxicity contributes to muscle atrophy in a number of conditions [3]. In obesity and Type II diabetes, there is an increase in lipid flux and accumulation in skeletal muscle [4]. Recent evidence indicates that both the quantity and saturation of intramyocellular and extramyocellular lipid depots are elevated in skeletal muscle from diabetic patients compared to healthy controls [5]. Rodents that are fed a high-fat diet develop obesity and insulin resistance which have been linked to muscle atrophy [6]. Direct lipotoxicity is among the proposed mechanisms for obesity-related insulin resistance. In support of this hypothesis, treatment of cultured myotubes with the saturated fatty acid palmitate causes similar defects in insulin signaling and reduces myotube diameter [7].
Reduced insulin signaling through the protein kinase Akt can result in the loss of muscle protein through up-regulation of multiple proteolytic systems, including the ubiquitin-proteasome (UbP) and autophagy-lysosome pathways [8, 9]. Akt inactivates the Forkhead box (FoxO3) transcription factors, including FoxO3, which induce the expression of several key atrophy-inducing genes, termed “atrogenes,” including the muscle-specific E3 ubiquitin ligases MuRF1 and atrogin-1/MAFbx [10, 11] and the autophagy mediator Bnip3 [12]. Enhanced expression of MuRF-1 and atrogin-1/MAFbx is linked to muscle atrophy in a variety of disease models and targeted inactivation of either of them reduces muscle loss [13–15]. Bnip3 is important for macroautophagic remodeling of the mitochondrial network [16]. Increasing expression of Bnip3 by constitutively active FoxO3 enhances macroautophagy and decreases muscle mass, while knockdown of Bnip3 reduces autophagosome formation induced by either constitutively active FoxO3 or fasting conditions [12]. LC3 is a small ubiquitin-like molecule required for membrane commitment and growth into autophagosomes [16]. It is enzymatically cleaved to form LC3-II, which allows the protein to localize to autophagosomes and bind phosphatidylethanolamine (i.e. undergo lipidation) [17]. Lipidated LC3-II binds to the cargo adaptor protein p62 which tags cellular targets for macroautophagy [18]. Thus, LC3-II is indicative of autophagosome formation [12].
Unlike saturated fatty acids, plasma levels of the polyunsaturated omega-3 fatty acids (ω-3 FA) are positively correlated with insulin sensitivity in individuals with type 2 diabetes [19], and they prevent insulin resistance induced by saturated fatty acids in myotubes [20] and by high-fat feeding in rodents [21]. Apart from the effects on insulin sensitivity, there is other inferential evidence that ω-3 FA may affect muscle mass. Transgenic fat-1 mice have elevated endogenous ω-3 FA levels, and their muscle fibers retain their cross-sectional areas following sciatic nerve injury compared to wild-type mice [22]. Supplementing the diets of rodents with fish oil, but not corn oil, prevents the increase in MuRF1 and atrogin-1 mRNA levels induced by hindlimb mobilization [23]. Fish oil supplementation also has beneficial effects on muscle mass in cancer patients undergoing chemotherapy [24]. Interpretation of the biological effects of ω-3 FA on muscle mass in these studies is complicated because commercial fish oil is a highly enriched mixture of ω-3 FA, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which are reported to exert different physiologic and cellular effects [25, 26]. DHA is the terminal fatty acid in the omega-3 conversion pathway. Studies show that consumption of preformed DHA is the best way to increase its level in the blood while EPA is more readily formed from alpha-linolenic acid which is highly enriched in plant-based foods [25]. It was previously reported that co-treating C2C12 myotubes with DHA and palmitate protects them from atrophy induced by the saturated fatty acid [7]; however, the cellular processes underlying the fatty acid-induced alterations in protein metabolism remain unclear. Since it is well-established that FoxO3 mediates the loss of muscle protein in chronic illness by increasing protein degradation [16, 27], the purpose of this study was two-fold: to investigate whether palmitate increases protein degradation in muscle cells and whether DHA counters the atrophy-inducing effects of palmitate by restoring Akt/FoxO signaling. By using the C2C12 myotube model, we ensure that the observed responses to palmitate and DHA are due to their direct effects on muscle cells.
Methods and Materials
Cultured myotube model
Mouse C2C12 myoblasts (American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5 g/L glucose and supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA) plus antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin; Invitrogen, Carlsbad, CA, USA). At 90–95% confluence, cells were induced to differentiate into myotubes in DMEM containing 4.5 g/L glucose plus 2% horse serum (Invitrogen) and antibiotics for 3–4 days before treatment with fatty acids.
Experimental treatments
Palmitic acid and cis-4, 7, 10, 13, 16, 19-docosahexaenoic acid (DHA) (Sigma Aldrich, St. Louis, MO, USA) were dissolved in ethanol and diluted to 500 µmol/L and 100 µmol/L, respectively, in DMEM containing 2% Fraction V protease-free bovine serum albumin (BSA; Product number 03117332001, Roche, Indianapolis, IN, USA), 2% FBS (Atlanta Biologicals, Inc., Flowery Branch, GA), 2 mmol/L L-carnitine (Sigma Aldrich), and 1% antibiotics (“treatment media”). Control cells were incubated in treatment media with an equal amount of ethanol substituted for palmitate and DHA. It is reported that 2% BSA and 500 µmol/L free fatty acids result in a final molar ratio that closely resembles that of human plasma [28] and that fasting plasma nonesterified fatty acids are around 600 µmol/L in obese adults with normal or impaired glucose tolerance or diabetes [29]. Additionally, the circulating level of palmitate is between 3–11 times higher than that of DHA in humans after 6 weeks of DHA supplementation [30]. Lastly, previous dose-response experiments in C2C12 myotubes showed that 100 µmol/L was the lowest tested concentration of DHA that could prevent palmitate-induced defects in energy-sensing pathways [7]. Myotubes were incubated in treatment media for 2–24 h, as indicated in the figure legends. In some experiments to measure converted LC3-II, some myotubes were treated with 10 mmol/L methylamine (Tokyo Chemical Industry Co, Ltd, Tokyo, Japan) to inhibit lysosomal activity for 3 h prior to harvest.
Protein degradation assay
Protein degradation was measured in differentiated myotubes as previously described [31]. Briefly, cellular proteins were radiolabeled with 14C-phenylalanine (Phe) for 48 h. After a 2 h chase period to remove labeled Phe released from short-lived proteins, treatment media was applied and the amount of 14C-Phe released into the media was measured in serial aliquots at intervals up to 28 h. The rate of protein degradation was calculated as described by Gulve and Dice [32].
RNA isolation and qPCR analysis
RNA was isolated using TRIzol (Invitrogen) and reverse transcribed using the Superscript III First-Strand Synthesis kit (Invitrogen) according to the manufacturer’s instructions. mRNA was measured using quantitative real time PCR with the BioRad iCycler and the iQ SYBR Green reagent (BioRad Laboratories, Hercules, CA, USA). Previously published primer sets for MuRF1, atrogin-1, and Bnip3 were used to perform PCR reactions [12, 33]; β-actin was used as the normalization control. The data were analyzed for fold change (ΔΔCt) using the iCycler software, as previously described [10]. Melting curve analyses were performed to analyze and verify the specificity of the reaction.
Isolation of cytosolic and nuclear cell fractions
Cells were rinsed 3 times and scraped from culture dishes in ice-cold phosphate-buffered saline (PBS), followed by centrifugation at 1500 × g for 5 min at 4°C. The pellet was re-suspended in a solution containing 0.01 mol/L HEPES (pH 7.6–7.8), 1.5 mmol/L MgCl2, 2 mmol/L KCl, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 10 µg/mL aprotinin, 5 µg/mL leupeptin, 5 µg/mL pepstatin A, and 1 mmol/L sodium orthovanadate and incubated on ice for 15 min. After addition of Nonidet P-40 (final concentration 0.5%), samples were mixed vigorously for 10 seconds and centrifuged at 1500 × g for 30–60 seconds at 4°C. The supernatant (cytosolic fraction) was transferred to a new tube and stored at −80°C until further analysis. The pellet was re-suspended in a solution containing 0.02 mmol/L HEPES (pH 7.6–7.8), 1.5 mmol/L MgCl2, 2 mmol/L KCl, 0.4 mol/L NaCl, 0.2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF, 10 µg/mL aprotinin, 5 µg/mL leupeptin, 5 µg/mL pepstatin A, 1 mmol/L sodium orthovanadate, and 25% glycerol, and samples were incubated on a shaking platform for 15–60 min at 4°C. Samples were centrifuged at 21000 × g for 20 min at 4°C, and the supernatant (nuclear fraction) was transferred to a new tube and stored at −80°C until further analysis.
Western blot analysis
Whole cell lysates were prepared using the buffers specified by the antibody vendors. Protein concentrations of cleared lysates, including the cytosolic and nuclear fractions described above, were measured using a BioRad DC protein assay kit (BioRad Laboratories). Western analyses were performed as described [34] using commercial antibodies to phospho-Akt (S473), total Akt, FoxO3a, LC3B, β-actin (Cell Signaling Technology, Beverly, MA, USA), and p62 (Sigma Aldrich) [10]. Antibodies to GAPDH, Histone H1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and Na+-K+ ATPase (Cell Signaling Technology) were used to assess the purity of the nuclear and cytosolic samples. Equal loading of total protein in the sample lanes was verified by Ponceau S Red staining [10].
Statistical analyses
Data are presented as mean percentage of control ± SE. Differences between 2 treatment groups are compared by two-tailed Student’s t test. Differences between ≥3 treatments are compared by one-way ANOVA with post-hoc analysis by Tukey’s test for multiple comparisons. Results are considered statistically significant at P < 0.05.
Results
DHA attenuates the effects of palmitate on protein degradation
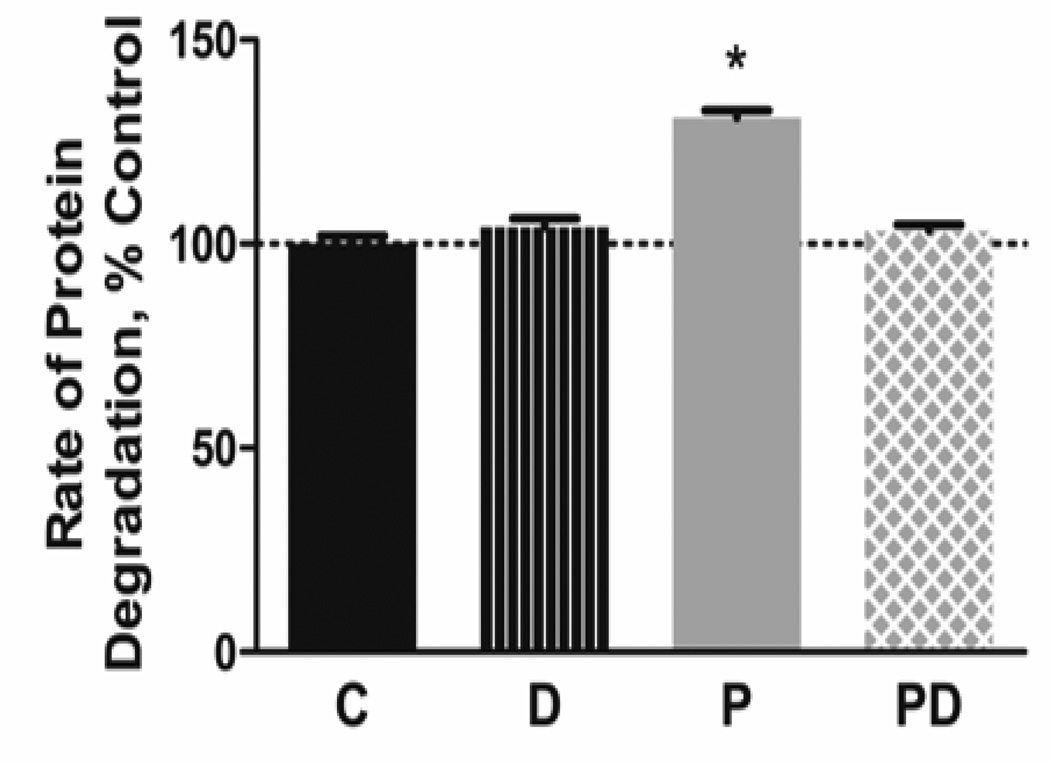
Previous experiments in C2C12 myotubes indicated that addition of DHA reverses the reduction in myotube size induced by palmitate [7]. To determine if changes in protein degradation contribute to these responses, we measured the rate of protein degradation in myotubes treated with each lipid alone and in combination. Palmitate alone increased proteolysis by 31% whereas DHA alone had no effect on the rate of proteolysis (Figure 1). Co-treatment of DHA with palmitate prevented the proteolytic response to the saturated fatty acid.
Figure 1.
Docosahexaenoic acid prevents palmitate-induced protein degradation. C2C12 myotubes were pre-labeled with 14C-phenylalanine then treated with 500 µmol/L palmitate (PA) and/or 100 µmol/L docosahexaenoic acid (DHA) for 28 h. The rate of protein degradation was calculated by measuring the release of 14C-phenylalanine into the culture media. PA increased the rate of protein degradation, while co-treatment with DHA prevented the response. Representative results from 1 of 3 independent experiments are shown (*p<0.01 versus other groups, n=6 per group per experiment)
DHA attenuates palmitate-induced inhibition of Akt-FoxO3 signaling
Akt is a key regulator of protein degradation in muscle because it phosphorylates FoxO3, thereby inhibiting its function. We therefore tested whether palmitate and DHA alter the Akt/FoxO3 pathway. Treating myotubes with palmitate consistently suppressed the phospho:total Akt ratio over treatment times between 2–24 h (Figure 2A). DHA alone had no effect on Akt phosphorylation whereas co-treatment of myotubes with DHA and palmitate for 4 h (Figure 2B) and 24 h (Figure 2C) restored Akt phosphorylation to near control levels. The lack of reduction in Akt activation by DHA was not simply due to a lower molarity of fatty acid compared to palmitate because we tested 500 µmol/L DHA and it improved Akt phosphorylation compared to control samples (data not shown). This indicates that the type of fatty acid, rather than concentration, is primarily responsible for their disparate effects on Akt signaling in this model.
Figure 2.
DHA prevents palmitate-induced suppression of Akt activation. C2C12 myotubes were treated with 500 µmol/L PA (P) for 2–24 h and the protein levels of phospho(S473)-Akt and total Akt were evaluated by western blot analysis. A) PA reduced the ratio of phospho(S473):total Akt at all timepoints, indicating a continuous suppression of Akt activation by the saturated fatty acid (*p<0.05 versus control, n=3–5/timepoints; #p<0.01 versus control, n=5/timepoint). B/C) Co-treatment with 100 µmol/L DHA (PD) attenuates the effects of PA on the Akt ratio after B) 4 h (*p<0.01 versus all other groups, n=3–4/group) and C) 24 h (*p<0.001 versus all other groups, n=5–6/group).
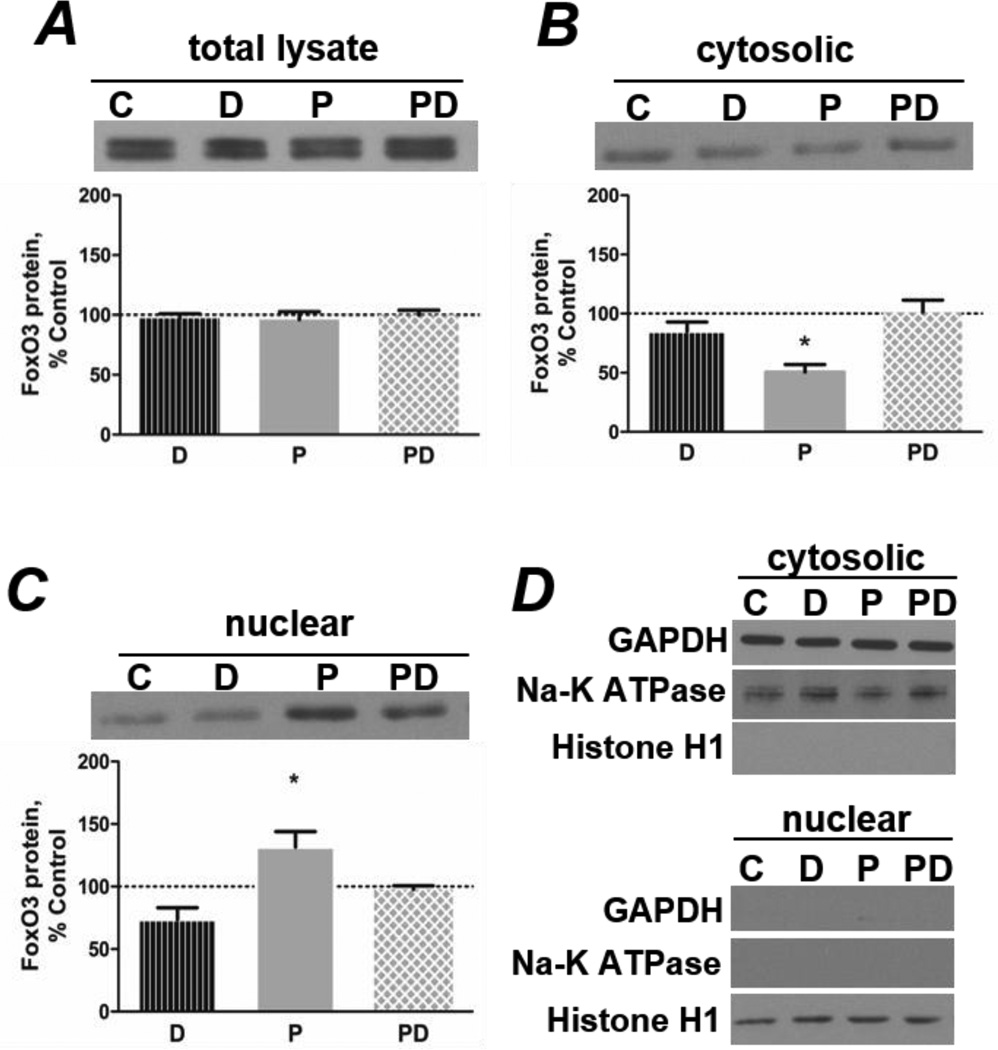
The level of FoxO3 protein in total cell lysates was unchanged by the fatty acid treatments (Figure 3A). Examination of cytosolic and nuclear cell fractions revealed that palmitate decreased FoxO3 protein in the cytosol (Figure 3B) while simultaneously increasing its level in the nucleus (Figure 3C). Co-treatment with DHA restored the levels of FoxO3 in the cytosol and nucleus to control levels (Figures 3B and 3C). The purity of the cytosolic and nuclear fractions was evaluated by testing for the presence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, cytosolic), Na+-K+ ATPase (cell membrane) and histone H1 (nuclear) proteins in each of the samples. No obvious contamination between fractions was detected (Figure 3D).
Figure 3.
Co-treatment with DHA prevents palmitate-induced alterations in FoxO3 localization. A) Treatment (4 h) with 500 µmol/L PA (P) and/or 100 µmol/L DHA (D) does not alter FoxO3 protein in total cell lysates (n=3/group). PA decreases cytosolic (B) and increases nuclear FoxO3 protein (C), while co-treatment with DHA (PD) prevents the responses (*p<0.01 versus all other groups, n=4/group). D) Representative western blots show markers of cytosolic (GAPDH), nuclear (histone H1), and cell membrane (Na+-K+ ATPase) proteins to confirm the purity of cell fractions.
DHA attenuates the effects of palmitate on proteolytic systems
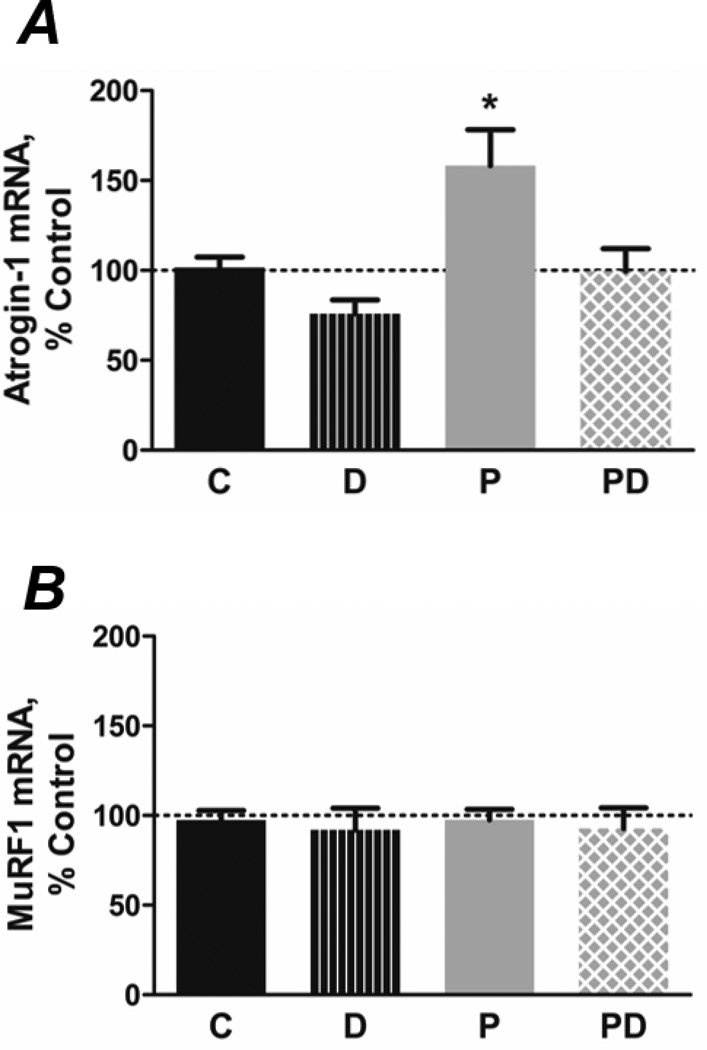
FoxO3 promotes protein degradation by enhancing expression of key components of multiple proteolytic systems. Therefore, we evaluated whether the fatty acid treatments altered markers of the UbP and autophagy pathways. Palmitate increased the level of E3 ubiquitin ligase atrogin-1/MAFbx mRNA after 4 h compared to control cells, while co-treatment with DHA prevented the response (Figure 4A). Surprisingly, the level of MuRF1 mRNA was unchanged by treatment with the fatty acids (Figure 4B).
Figure 4.
Co-treatment with DHA prevents the PA-induced increase in atrogin-1 mRNA levels. A) PA (P; 500 µmol/L, 4 h) increases mRNA encoding the muscle-specific E3 ubiquitin ligase atrogin-1 (*p<0.01 versus all other groups, n=8–12/group), while co-treatment with 100 µmol/L DHA (PD) prevents these responses. B) mRNA encoding the MuRF-1 E3 ligase is unchanged by fatty acids (4 h, n=7/group).
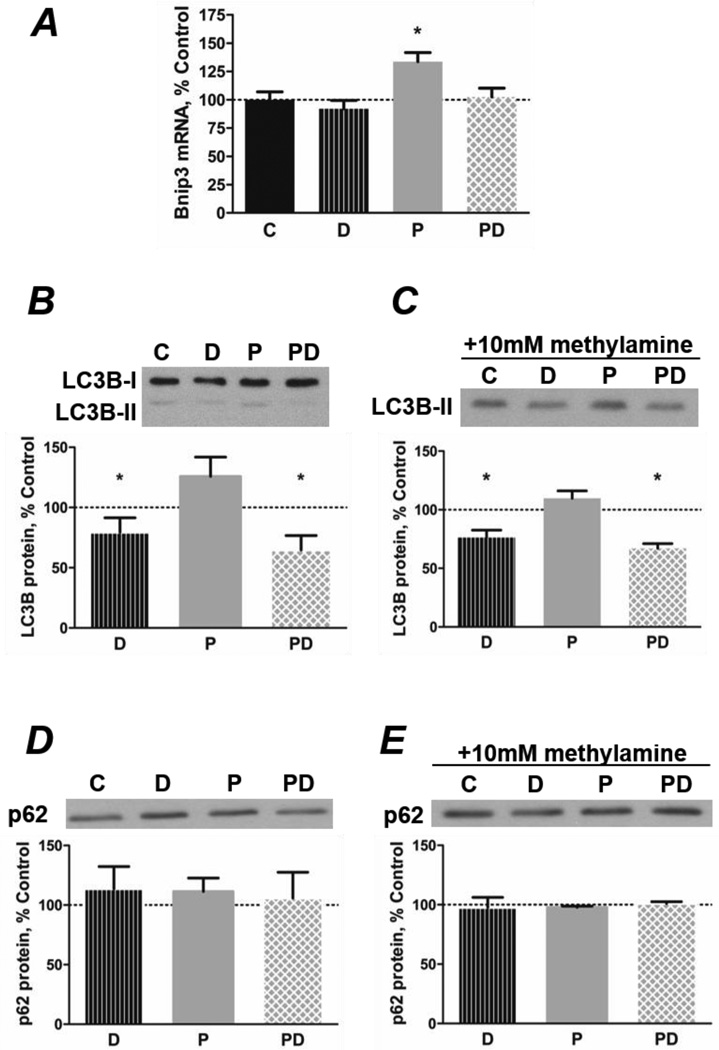
Regarding macroautophagy, palmitate increased the level of Bnip3 mRNA whereas co-treatment with DHA reduced the level to that in control cells (Figure 5A). LC3-II protein was unchanged by palmitate but was reduced by DHA, regardless of whether palmitate was present (Figure 5B). To test whether DHA regulates the rate of autophagosome formation, degradation of LC3-II-containing autophagosomes was inhibited by adding methylamine to the media. The level of LC3-II was still reduced in DHA-treated cells after inhibition of lysosomal activity, indicating that DHA significantly reduces autophagic flux while palmitate has no effect (Figure 5C). The levels of p62 were not altered by either fatty acid, regardless of whether lysosomes were active or not (Figures 5D and 5E).
Figure 5.
Co-treatment with DHA prevents the PA-induced increase in macroautophagy. A) PA (P; 500 µmol/L, 24 h) increases mRNA levels of the macroautophagy mediator Bnip3, while cotreatment with 100 µmol/L DHA (PD) prevents the response (*p<0.01 versus all other groups, n=5–6). B/C) DHA inhibits autophagosome formation. B) DHA decreases the ratio of LC3BII: LC3B-I protein in the absence (D) or presence (PD) of PA (24 h, *p<0.05 versus PA, n=4). C) Myotubes were treated with 10 mmol/L methylamine to inhibit lysosomal degradation of autophagosome contents for 3 h before lysis. The level of LC3B-II protein remained decreased, indicating an independent effect of the omega-3 fatty acid to inhibit macroautophagy (24 h, *p<0.001 versus control and PA, n=3/group). D/E) p62 protein levels remain unchanged by treatment with PA or DHA for 24 h in the D) absence (n=4/group) or E) presence (n=3/group) of 10 mmol/L methylamine.
Discussion
The objective of this study was to determine whether palmitate enhances protein degradation in myotubes and to investigate how DHA protects against palmitate-induced muscle atrophy. We hypothesized that DHA antagonizes the effects of palmitate on the Akt/FoxO3 axis because it is a key determinant of the activity of various proteolytic pathways in muscle cells. Our findings indicate that DHA counteracts the palmitate-induced increase in protein degradation by preventing the upregulation of the UbP and autophagy systems. DHA’s effects are mediated, at least in part, through restoration of Akt activation and FoxO3 inhibition (Figure 6). Although several in vivo studies have documented that ω-3 fatty acids, primarily in the form of fish oil, exert beneficial effects on protein metabolism in muscle [22–24], it remained unclear whether these fatty acids directly target muscle signaling pathways that control protein balance or exert indirect effects on muscle via systemic metabolic alterations. The current experiments establish that ω-3 fatty acids, specifically DHA, act directly on myotubes to regulate proteolytic pathways in a high fat environment.
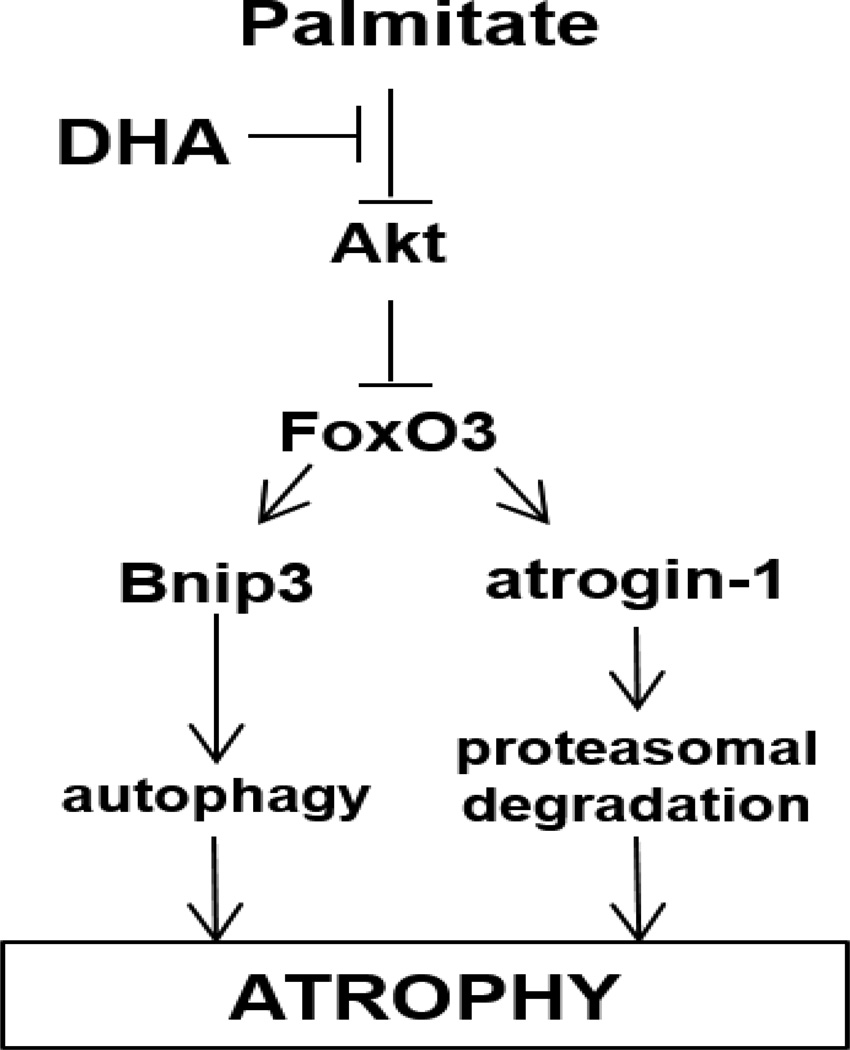
Figure 6.
The Akt/FoxO3 axis is a key regulator of the activity of various proteolytic pathways in muscle cells, including the ubiquitin-proteasome and macroautophagy systems. Palmitate induces the activity of multiple proteolytic systems, resulting in myotube atrophy. DHA counteracts the effects of palmitate by restoring Akt activation and FoxO3 inhibition, thus preventing the upregulation of protein degradation.
Previous experiments demonstrated that co-treatment with DHA prevents the palmitate-induced decrease in the diameter of C2C12 myotubes [7]. To elucidate the mechanisms underlying the anti-atrophy effect of DHA, we first determined whether the fatty acids altered the rate of protein degradation in myotubes. As hypothesized, palmitate increased proteolysis while co-treatment with DHA inhibited the effect of palmitate. Consistent with an earlier study [35], DHA alone did not alter the rate of protein degradation. Although the present work does not address the possibility that DHA alters protein synthesis, the earlier report indicated that 50 µmol/L DHA had no effect on the process in C2C12 myotubes [35]. This finding, however, does not preclude the possibility that DHA prevents a negative effect of palmitate on protein synthesis. Regardless, our results indicate that the beneficial effects of DHA are more apparent in the presence of an atrophy-inducing signal such as palmitate.
Akt is a key regulator of protein degradation in skeletal muscle. Under normal healthy conditions, it inactivates FoxO3 through phosphorylation, thereby inhibiting its nuclear localization and suppressing the expression of key components of the muscle cell’s proteolytic systems [36]. Reduced signaling through Akt is a common feature of most cell and animal models of atrophy [8]. In agreement with other studies [37–39], palmitate caused a prolonged suppression of Akt activation, as indicated by decreased phospho:total Akt ratio compared to control cells. Coincident with the change in Akt phosphorylation, the level of nuclear Foxo3 also increased. Importantly, co-treatment with DHA preserved Akt activation as well as restored cytosolic and nuclear FoxO3 protein to control levels. This suggests that the protective effect of DHA against palmitate-induced protein degradation is mediated, at least in part, by reestablishing a normal Akt/FoxO3 axis.
Activation of FoxO3 stimulates multiple proteolytic systems, including the UbP and autophagy pathways. The muscle-specific E3 ubiquitin ligases MuRF1 and atrogin-1/MAFbx are key components of the atrophy program that selectively target specific muscle proteins for ubiquitination and degradation by the 26S proteasome [13, 15]. Their mRNAs are typically elevated in models of atrophy [13, 14]. Overexpression of MAFbx is sufficient to induce atrophy in myotubes, while MAFbx/atrogin-1 or MuRF1 deficiency attenuates muscle loss due to denervation in mice [15]. We hypothesized that the palmitate-induced enhancement in nuclear localization of FoxO3 would result in increased expression of atrophy-related FoxO3 targets. Consistent with this prediction, increased atrogin-1 mRNA expression indicates that the UbP plays a role in palmitate-induced protein degradation. The effects of DHA on atrogene expression have not been investigated previously in a high fat model. Our finding that co-treatment with DHA prevented the palmitate-induced increase in atrogin-1 mRNA further supports the assertion that DHA plays a protective role against the atrophy-inducing effects of palmitate by restoring inhibition of FoxO3 and reducing proteolysis.
Unexpectedly, palmitate increased the level of mRNA encoding atrogin-1 but not MuRF1. Although this is an unusual result, it is not without precedent and may be attributable to the atrophy stimulus in our high fat model. Peterson and colleagues [40] reported that incubating C2C12 myotubes with 200 µmol/L palmitate increased the mRNA for atrogin-1, but not MuRF1; in these experiments, myotubes were co-cultured with either fibroblasts or macrophages. They also noted that 200 µmol/L DHA did not alter the expression of either atrogene (in the absence of palmitate) [40]. Again, this is consistent with our data and further suggests that the disparate effects of palmitate and DHA on atrogene expression are not due to differences in molarity of the fatty acids. We also examined this possibility by incubating myotubes with 500 µmol/L DHA and/or 500 µmol/L palmitate and found no reduction in the phospho-Akt:total Akt ratio due to the higher concentration of DHA (data not shown), confirming that a higher concentration of DHA does not have detrimental effects akin to those of palmitate.
Until recently, the importance of the autophagy pathway in skeletal muscle atrophy has been underappreciated. Several studies have identified autophagy-related atrogene mRNAs, including Bnip3 and LC3, which are increased in some models of atrophy [16, 41]. Mammucari et al. [12] demonstrated that a FoxO3-mediated increase in Bnip3, but not LC3, is necessary and sufficient to induce autophagy and muscle atrophy. In the current study, co-treatment with DHA counteracted the induction of Bnip3 mRNA by palmitate. Surprisingly, palmitate did not significantly increase LC3-II or alter p62 protein; however, DHA did decrease autophagosome formation regardless of whether palmitate was present or not. Therefore, it appears that DHA has an independent effect to inhibit autophagy in muscle cells. This effect alone is not sufficient to significantly reduce the overall rate of protein degradation (Fig. 1), likely because macroautophagy quantitatively represents only a small fraction of total cellular proteolysis in skeletal muscle [42]. Others have suggested that mitochondria may be a critical target for macroautophagy in muscle wasting [16]. This is an interesting corollary to the present work and could be the basis of future experiments to delineate the effects of DHA and palmitate on mitochondrial number and function.
In summary, our data demonstrate that a palmitate-induced increase in protein degradation via suppression of Akt/FoxO3 signaling and induction of the UbP and autophagy pathways contributes to the muscle cell atrophy seen with lipotoxicity. DHA, a major ω-3 fatty acid constituent of fish oil, protects against the detrimental effects of palmitate. It acts by reestablishing the Akt-mediated inhibition of FoxO3, thereby suppressing atrogene expression and limiting induction of autophagy. These results underscore the potential for future therapeutic application of the omega-3 fatty acids in counteracting muscle loss due to chronic illness.
Acknowledgements
The authors would like to thank Sara Zoromsky for her assistance with the protein degradation assay.
This work was supported by NIH RO1DK95610, AHA GRNT7660020 and VA MERIT X01BX001456 awarded to S. Russ Price.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Little JP, Phillips SM. Resistance exercise and nutrition to counteract muscle wasting. Applied Physiology, Nutrition, and Metabolism. 2009;34:817–828. doi: 10.1139/H09-093. [DOI] [PubMed] [Google Scholar]
- 2.Eddins MJ, Marblestone JG, Suresh Kumar KG, Leach CA, Sterner DE, Mattern MR, et al. Targeting the ubiquitin E3 ligase MuRF1 to inhibit muscle atrophy. Cell Biochemistry and Biophysics. 2011;60:113–118. doi: 10.1007/s12013-011-9175-7. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, Liu L, Liu N, Liu Y. Muscular response and adaptation to diabetes mellitus. Frontiers in Bioscience. 2008;13:4765–4794. doi: 10.2741/3038. [DOI] [PubMed] [Google Scholar]
- 4.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. The FASEB Journal. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 5.Srikanthan P, Singhal A, Lee CC, Nagarajan R, Wilson N, Roberts CK, et al. Characterization of Intra-myocellular Lipids using 2D Localized Correlated Spectroscopy and Abdominal Fat using MRI in Type 2 Diabetes. Magnetic Resonance Insights. 2012;5:29–36. doi: 10.4137/MRI.S10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Experimental Physiology. 2011;96:179–193. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 7.Bryner RW, Woodworth-Hobbs ME, Williamson DW, Alway SE. Docosahexaenoic Acid Protects Muscle Cells from Palmitate-Induced Atrophy. International Scholarly Research Network Obesity. 2012 doi: 10.5402/2012/647348. Article ID 647348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. Journal of the American Society of Nephrology. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 9.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. Journal of the American Society of Nephrology. 2006;17:1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- 10.Zheng B, Ohkawa S, Li H, Roberts-Wilson TK, Price SR. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. The FASEB Journal. 2010;24:2660–2669. doi: 10.1096/fj.09-151480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metabolism. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. The FASEB Journal. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 14.Franch HA, Price SR. Molecular signaling pathways regulating muscle proteolysis during atrophy. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8:271–275. doi: 10.1097/01.mco.0000165005.01331.45. [DOI] [PubMed] [Google Scholar]
- 15.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 16.Sandri M. New findings of lysosomal proteolysis in skeletal muscle. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14:223–229. doi: 10.1097/MCO.0b013e3283457a75. [DOI] [PubMed] [Google Scholar]
- 17.Tanida I, Ueno T, Kominami E. Human light chain 3/MAP1LC3B is cleaved at its carboxyl-terminal Met121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. The Journal of Biological Chemistry. 2004;279:47704–47710. doi: 10.1074/jbc.M407016200. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura Y, Komatsu M. Pathophysiological role of autophagy: lesson from autophagy-deficient mouse models. Experimental Animals / Japanese Association for Laboratory Animal Science. 2011;60:329–345. doi: 10.1538/expanim.60.329. [DOI] [PubMed] [Google Scholar]
- 19.Huang T, Wahlqvist ML, Xu T, Xu A, Zhang A, Li D. Increased plasma n-3 polyunsaturated fatty acid is associated with improved insulin sensitivity in type 2 diabetes in China. Molecular Nutrition & Food Research. 2010;54(Suppl 1):S112–S119. doi: 10.1002/mnfr.200900189. [DOI] [PubMed] [Google Scholar]
- 20.Lam YY, Hatzinikolas G, Weir JM, Janovska A, McAinch AJ, Game P, et al. Insulin-stimulated glucose uptake and pathways regulating energy metabolism in skeletal muscle cells: the effects of subcutaneous and visceral fat, and long-chain saturated, n-3 and n-6 polyunsaturated fatty acids. Biochimica et Biophysica Acta. 2011;1811:468–475. doi: 10.1016/j.bbalip.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. American Journal of Physiology Endocrinology and Metabolism. 2002;282:E664–E671. doi: 10.1152/ajpendo.00320.2001. [DOI] [PubMed] [Google Scholar]
- 22.Smith BK, Holloway GP, Reza-Lopez S, Jeram SM, Kang JX, Ma DW. A decreased n-6/n-3 ratio in the fat-1 mouse is associated with improved glucose tolerance. Applied Physiology, Nutrition, and Metabolism. 2010;35:699–706. doi: 10.1139/H10-066. [DOI] [PubMed] [Google Scholar]
- 23.You JS, Park MN, Song W, Lee YS. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Applied Physiology, Nutrition, and Metabolism. 2010;35:310–318. doi: 10.1139/H10-022. [DOI] [PubMed] [Google Scholar]
- 24.Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011;117:1775–1782. doi: 10.1002/cncr.25709. [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? The Journal of Nutrition. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khairallah RJ, O'Shea KM, Brown BH, Khanna N, Des Rosiers C, Stanley WC. Treatment with docosahexaenoic acid, but not eicosapentaenoic acid, delays Ca2+-induced mitochondria permeability transition in normal and hypertrophied myocardium. The Journal of Pharmacology and Experimental Therapeutics. 2010;335:155–162. doi: 10.1124/jpet.110.170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Current Opinion in Clinical Nutrition and Metabolic Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. American Journal of Physiology Endocrinology and Metababolism. 2010;299:E1096–E1105. doi: 10.1152/ajpendo.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 30.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. Journal of Lipid Research. 1998;39:286–292. [PubMed] [Google Scholar]
- 31.Franch HA, Raissi S, Wang X, Zheng B, Bailey JL, Price SR. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. American Journal of Physiology Renal Physiology. 2004;287:F700–F706. doi: 10.1152/ajprenal.00440.2003. [DOI] [PubMed] [Google Scholar]
- 32.Gulve EA, Dice JF. Regulation of protein synthesis and degradation in L8 myotubes. Effects of serum, insulin and insulin-like growth factors. The Biochemical Journal. 1989;260:377–387. doi: 10.1042/bj2600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. American Journal of Physiology Endocrinology and Metabolism. 2004;287:E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Ordas R, Klein JD, Price SR. Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3-kinase and MEK-1/2. Journal of Applied Physiology. 2008;105:1772–1778. doi: 10.1152/japplphysiol.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamolrat T, Gray SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochemical and Biophysical Research Communications. 2013;432:593–598. doi: 10.1016/j.bbrc.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 36.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Molecular Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Du J, Hu Z, Walsh K, Wang XH. Evidence for adipose-muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and Fatty acids. Endocrinology. 2007;148:5696–5705. doi: 10.1210/en.2007-0183. [DOI] [PubMed] [Google Scholar]
- 38.Peterson JM, Wang Y, Bryner RW, Williamson DL, Alway SE. Bax signaling regulates palmitate-mediated apoptosis in C(2)C(12) myotubes. American Journal of Physiology Endocrinology and Metabolism. 2008;295:E1307–E1314. doi: 10.1152/ajpendo.00738.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. Journal of Cellular Physiology. 2010;222:187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- 40.Finlin BS, Varma V, Nolen GT, Dube J, Starnes CP, Rasouli N, et al. DHA reduces the atrophy-associated Fn14 protein in differentiated myotubes during coculture with macrophages. The Journal of Nutritional Biochemistry. 2012 Aug;23:885–891. doi: 10.1016/j.jnutbio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. American Journal of Respiratory and Critical Care Medicine. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 42.Price SR, Bailey JL, Wang X, Jurkovitz C, England BK, Ding X, et al. Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin-proteasome proteolytic pathway by a mechanism including gene transcription. The Journal of Clinical Investigation. 1996;98:1703–1708. doi: 10.1172/JCI118968. [DOI] [PMC free article] [PubMed] [Google Scholar]