Abstract
Little is known about predictors of eczema severity in the US population. We sought to determine the distribution and associations of childhood eczema severity in the US. We analyzed data from the 2007 National Survey of Children's Health, a prospective questionnaire-based study of a nationally representative sample of 91,642 children (0-17yr). The prevalence of childhood eczema was 12.97% (95% confidence interval [95% CI]=12.42–13.53); 67.0% (95% CI: 64.8–69.2) had mild, 26.0% (95% CI: 23.9–28.1) moderate and 7.0% (95% CI: 5.8–8.3) severe disease. There was significant statewide-variation of the distribution of eczema severity (Rao-Scott chi square, P=0.004), with highest rates of severe disease in Northeastern and Midwestern states. In univariate models, eczema severity was increased with older age, African-American and Hispanic race/ethnicity, lower household income, oldest child in the family, home with a single mother, lower paternal/maternal education level, maternal general health, maternal/paternal emotional health, dilapidated housing and garbage on the streets. In multivariate survey logistic regression models using stepwise and backward selection, moderate–severe eczema was associated with older age, lower household income and fair or poor maternal health, but inversely associated with birthplace outside the US. These data indicate that environmental and/or lifestyle factors play an important role in eczema severity.
Keywords: eczema, atopic dermatitis, eczema severity, epidemiology, race, ethnicity, socioeconomic status
Introduction
Atopic dermatitis (AD) or eczema is a chronic inflammatory disorder of the skin that poses a significant public health burden. Several recent studies shed light on the epidemiology of eczema in the US. A study of 102,353 children from the 2003 National Survey of Children's Health (NSCH) found that the US prevalence of childhood eczema is 10.7% 1. A study of 27,157 adults from the 2010 National Health Interview Survey found a 10.2% prevalence of eczema 2. While these studies help to define the disease prevalence, little is known about the distribution of disease severity in this population.
While factors such as ethnicity, urban living 1, and climatic factors 3 are known to influence disease risk, it is unclear what role factors such as these may play in determining disease severity. Mutations in the skin barrier gene filaggrin, while playing an important role in disease risk, have inconsistently been linked to disease severity 4-10. Understanding the factors driving disease severity is important, as our previous work and the work of others revealed that disease severity directly correlates with the future risk for developing allergic comorbidities and asthma 11, 12. Additionally, children with severe disease are more likely to have a protracted disease course and a significantly worse quality of life compared to those with more mild disease 11. Understanding the distribution and risk factors for disease severity may help identify opportunities to intervene in the hopes of improving the overall disease course. We aimed to determine the distribution and associated factors of childhood eczema severity in the US using a large population-based cross-sectional database.
Methods
National Survey of children's Health (NSCH)
We used data from the 2007-2008 NSCH survey of 91,642 households, which was designed to estimate the prevalence of various child health issues. NSCH was sponsored by the Maternal and Child Health Bureau and the U.S. Department of Health and Human Services with a goal of >1,700 households per state. The National Center for Health Statistics conducted the study using the State and Local Area Integrated Telephone Survey program. The telephone numbers were chosen at random, followed by identification of the households with one or more children under the age of 18 and caregiver interview. Interviews were conducted in English, Spanish, and 4 Asian languages. Subsequently, caregivers were interviewed about a randomly selected child. The survey results were weighted by the NSCH to represent the population of non-institutionalized children nationally and in each state. Using the data from U.S. Bureau of the Census, sample weights were created that factored age, sex, race/ethnicity, household size, and educational attainment of the most educated household member using a multi-stage area probability sampling design by NSCH. These sample weights are needed to provide nationally representative prevalence estimates for each state's population of non-institutionalized children less than 18 years of age as previously described 13. All frequency data are presented as raw values, whereas prevalence estimates presented reflect this complex weighting. The National Center for Health Statistics of Center for Diseases Control and Prevention oversaw sampling and telephone interviews. Approval by the institutional review board was waived.
Eczema prevalence and severity
Eczema and skin allergy prevalence was determined using the NSCH question, “During the past 12 months, have you been told by a doctor or other health professional that (child) had eczema or any kind of skin allergy?” This is referred to as eczema throughout the manuscript. Since this question assessed healthcare diagnosed eczema, we excluded all subjects who responded “no” or “don’t know” to the question, “During the past 12 months, did (child) see a doctor, nurse, or other health care professional for any kind of medical care, including sick-child care, well-child check-ups, physical exams, and hospitalizations?” There were 91,642 children in the cohort; 79,667 of them had healthcare interaction in the previous year and were included in the final analysis. Severity of eczema was determined using the NSCH question, “Would you describe (child's) eczema or skin allergy as mild, moderate, or severe?” Responses were encoded as an ordinal variable, where 1=mild, 2=moderate and 3=severe.
Associations with eczema
A number of associations with eczema severity were examined, including age, sex, race/ethnicity, household income, family structure and size, highest level of maternal and paternal education, maternal and paternal birthplace, overall and mental health, primary language spoken in home, birthplace in the US, residence in a metropolitan area, smokers living in the home, smoking in the home, dilapidated housing, presence of garbage on the street, parks and sidewalks in the neighborhood. These covariates were selected for analysis based on a priori hypotheses and from the results of previous studies 1, 2, 14.
Data processing and statistical methods
All data processing and statistical analyses were performed in SAS version 9.2. Analyses of survey responses were performed using SURVEY procedures. Univariate associations were tested by Rao-Scott chi-square tests (SURVEYFREQ). Multivariate logistic regression models were constructed with eczema severity dichotomized into moderate-severe vs. mild (SURVEYLOGISTIC). Models included predictors from bivariate analysis with P-values <0.05. This approach was used over ordinal logistic regressions because the data did not meet the proportional odds assumption (Score test, P <0.01) and for parsimony. All variables were tested in the model using backward and stepwise selection with the same results. There were no differences between these approaches. Significant explanatory variables included in the final models are presented. Adjusted odds ratios and 95% confidence intervals (95% CI) were estimated for each covariate. Complete data analysis was performed, i.e. subjects with missing data were excluded.
Correction for multiple dependent tests (k = 27) was performed by minimizing the false discovery rate with the approach of Benjamini and Hochberg 15 and yielded a critical P-value of 0.026. Thus, uncorrected P-values are presented and P-value <0.026 was considered to be statistically significant.
Results
Eczema prevalence and severity
Eczema prevalence was 12.97% (95% CI = 12.42 – 13.53). Overall, sixty seven percent (95% CI: 64.8 – 69.2) of children with eczema reportedly had mild, 26.0% (95% CI: 23.9 – 28.1) moderate and 7.0% (95% CI: 5.8 – 8.3) severe disease. That is equivalent to 5.6, 2.2 and 0.6 million US children having mild, moderate and severe disease, respectively.
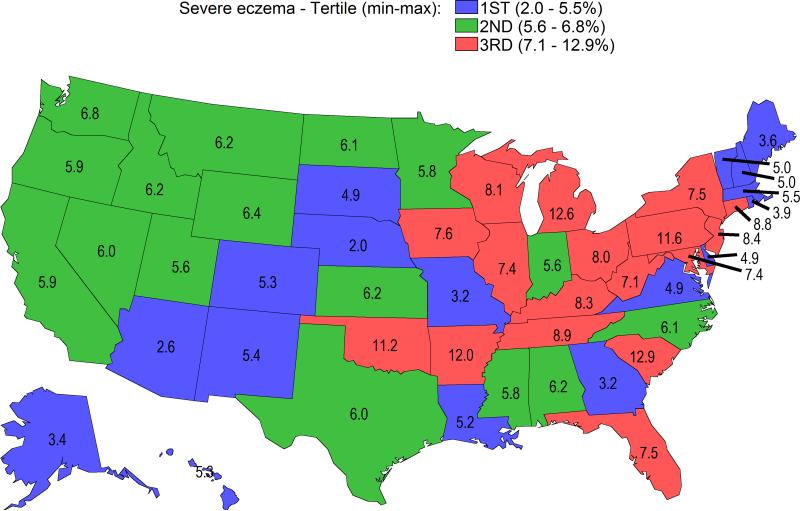
There was a significant variation of the distribution of eczema severity nationwide (Rao-Scott chi square, P = 0.004). Rates of severe eczema ranged from 2.0 – 12.9% across states and districts (Figure 1). The highest rates of severe eczema occurred predominantly located in Northeastern and Midwestern states.
Figure 1. Rates of severe eczema in the United States are highest in Northeastern and Midwestern states.
Data are presented as % per state. Severe eczema rates were divided into tertiles and color-coded: tertile-1 = blue, tertile-2 = green, tertile-3 = red.
Univariate analyses
Eczema severity was associated with older age (P = 0.0003), African-American and Hispanic race/ethnicity (P <0.0001) but not sex (P = 0.12) (Table 2). Several household factors were associated with increased eczema severity, including lower household income (P <0.0001), being the oldest child in the family (P = 0.02), home with a single mother and no father present (P <0.0001), but not number of children in the household (P = 0.03) or primary language spoken in home (P = 0.27).
Table 2.
Parental associations of childhood atopic dermatitis severity.
| Atopic dermatitis severity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mild (n = 7198) | Moderate (n = 2612) | Severe (n = 576) | ||||||
| Highest level of education | Freq | Percent (95% CI) | Freq | Percent (95% CI) | Freq | Percent (95% CI) | P-value* | |
| Father | < HS | 308 | 59.7 (51.1 – 68.3) | 151 | 32.1 (23.6 – 40.5) | 45 | 8.2 (4.6 – 11.9) | 0.001 |
| HS | 1100 | 64.9 (58.6 – 71.2) | 423 | 28.1 (22.0 – 34.2) | 89 | 7.0 (3.4 – 10.6) | ||
| > HS | 4153 | 73.1 (70.4 – 75.8) | 1373 | 23.5 (21.0 – 26.1) | 210 | 3.3 (2.2 – 4.4) | ||
| Mother | < HS | 381 | 62.8 (55.0 – 70.5) | 160 | 26.1 (19.2 – 33.1) | 64 | 11.1 (6.8 – 15.4) | <0.0001 |
| HS | 1158 | 59.7 (54.5 – 65.0) | 461 | 27.9 (23.2 – 32.5) | 135 | 12.4 (8.2 – 16.5) | ||
| > HS | 5238 | 69.9 (67.2 – 72.6) | 1836 | 25.5 (22.9 – 28.1) | 332 | 4.6 (3.5 – 5.6) | ||
| Birthplace in United States | ||||||||
| Father | No | 635 | 68.3 (61.6 – 74.9) | 219 | 26.1 (19.7 – 32.6) | 38 | 5.6 (2.9 – 8.3) | 0.72 |
| Yes | 4952 | 70.4 (67.7 – 73.1) | 1732 | 25.1 (22.6 – 27.7) | 310 | 4.5 (3.2 – 5.8) | ||
| Mother | No | 734 | 66.3 (59.5 – 73.1) | 280 | 27.1 (20.6 – 33.6) | 56 | 6.6 (3.3 – 9.9) | 0.94 |
| Yes | 6060 | 67.0 (64.5 – 69.4) | 2180 | 26.1 (23.8 – 28.4) | 477 | 7.0 (5.6 – 8.4) | ||
| Overall health | ||||||||
| Mother | Excellent | 2015 | 72.3 (67.8 – 76.9) | 567 | 22.7 (18.4 – 27.0) | 104 | 5.0 (2.6 – 7.4) | <0.0001 |
| Very good | 2724 | 70.7 (67.5 – 74.0) | 979 | 25.6 (22.4 – 28.7) | 159 | 3.7 (2.5 – 4.9) | ||
| Good | 1503 | 66.0 (61.1 – 71.0) | 575 | 25.9 (21.2 – 30.5) | 153 | 8.1 (5.5 – 10.7) | ||
| Fair | 459 | 46.6 (39.1 – 54.1) | 259 | 35.9 (28.0 – 32.8) | 84 | 17.5 (10.8 – 24.3) | ||
| Poor | 117 | 57.1 (43.9 – 70.3) | 88 | 30.6 (19.1 – 42.1) | 32 | 12.3 (5.0 – 19.7) | ||
| Father | Excellent | 1779 | 74.2 (70.1 – 78.3) | 537 | 20.6 (17.1 – 24.0) | 90 | 5.2 (2.5 – 8.0) | 0.15 |
| Very good | 2394 | 69.5 (65.4 – 73.5) | 849 | 26.2 (22.3 – 30.1) | 144 | 4.3 (2.6 – 6.1) | ||
| Good | 1131 | 67.6 (62.0 – 73.2) | 428 | 28.9 (23.3 – 34.4) | 68 | 3.5 (1.9 – 5.1) | ||
| Fair | 246 | 63.1 (52.0 – 74.2) | 110 | 30.9 (19.9 – 41.9) | 32 | 6.1 (2.3 – 9.8) | ||
| Poor | 56 | 69.9 (53.3 – 86.5) | 34 | 21.1 (7.8 – 34.3) | 15 | 9.0 (1.7 – 16.3) | ||
| Mental / emotional health | ||||||||
| Mother | Excellent | 2273 | 68.8 (64.7 – 72.9) | 682 | 26.4 (22.4 – 30.4) | 136 | 4.8 (2.9 – 6.6) | <0.0001 |
| Very good | 2844 | 71.8 (68.4 – 75.1) | 1009 | 24.5 (21.2 – 27.8) | 171 | 3.7 (2,6 0 4.9) | ||
| Good | 1262 | 64.5 (59.6 – 69.4) | 541 | 27.3 (22.7 – 31.8) | 139 | 8.2 (5.6 – 10.8) | ||
| Fair | 371 | 46.9 (37.5 – 56.4) | 192 | 31.5 (22.0 – 41.0) | 65 | 21.6 (12.6 – 30.5) | ||
| Poor | 63 | 47.5 (31.3 – 63.6) | 32 | 19.0 (8.6 – 29.3) | 23 | 33.6 (15.6 – 51.5) | ||
| Father | Excellent | 2189 | 71.5 (67.5 – 75.6) | 656 | 23.0 (19.4 – 26.7) | 117 | 5.5 (2.9 – 8.1) | 0.02 |
| Very good | 2371 | 71.7 (67.8 – 75.6) | 839 | 24.6 (20.8 0 28.5) | 133 | 3.7 (2.4 – 4.9) | ||
| Good | 827 | 67.5 (61.2 – 73.8) | 346 | 28.6 (22.4 – 34.8) | 67 | 3.9 (2.2 – 5.6) | ||
| Fair | 190 | 55.0 (42.1 – 67.8) | 97 | 37.6 (24.8 – 50.5) | 22 | 7.4 (1.2 – 13.7) | ||
| Poor | 21 | 42.9 (3.6 – 82.2) | 16 | 36.0 (5.1 – 67.0) | 10 | 21.1 (0.8 – 41.3) | ||
Rao-Scott chi-square tests comparing between mild, moderate and severe eczema.
Several parental factors were associated with more severe eczema, including paternal and maternal education level (P = 0.001 and <0.0001, respectively), maternal general health (P <0.0001) and maternal and paternal emotional health (P <0.0001 and 0.02, respectively). However, paternal, maternal and child birthplace inside the US were not associated with eczema severity (P ≥0.04).
Finally, several local environmental factors were associated with more severe eczema, including dilapidated housing (P = 0.0009) and garbage on the streets (P = 0.001). However, eczema severity was not associated with residence in a metropolitan area (P = 0.26), smokers living at home (P = 0.03), indoor smoking (P = 0.29) or the presence of a park (P = 0.53) or sidewalk (P = 0.94) in the neighborhood (Table 3).
Table 3.
Local environmental associations of childhood atopic dermatitis severity.
| Variable | Subgroup | Atopic dermatitis severity | ||||||
|---|---|---|---|---|---|---|---|---|
| Mild (n = 7198) | Moderate (n = 2612) | Severe (n = 576) | ||||||
| Freq | Percent (95% CI) | Freq | Percent (95% CI) | Freq | Percent (95% CI) | P-value* | ||
| Residence in metropolitan area | Yes | 4058 | 66.1 (63.4 – 68.8) | 1483 | 26.7 (24.1 – 29.3) | 342 | 7.2 (5.6 – 8.7) | 0.26 |
| No | 877 | 70.4 (66.2 – 74.6) | 327 | 22.8 (19.1 – 26.5) | 74 | 6.8 (4.2 – 9.4) | ||
| Smokers living at home | No | 5500 | 68.6 (66.0 – 71.2) | 1922 | 25.2 (22.7 – 27.6) | 358 | 6.2 (4.8 – 7.7) | 0.03 |
| Yes | 1651 | 62.6 (58.4 – 66.8) | 672 | 28.5 (24.4 – 32.6) | 213 | 8.9 (6.7 – 11.1) | ||
| Smoking in home | No | 1205 | 61.8 (56.7 – 66.9) | 495 | 29.9 (24.9 – 24.9) | 148 | 8.3 (5.7 – 10.9) | 0.29 |
| Yes | 445 | 64.8 (57.9 – 71.6) | 177 | 24.4 (18.2 – 30.5) | 65 | 10.9 (6.5 – 15.2) | ||
| Dilapidated housing | No | 6054 | 68.7 (66.3 – 71.1) | 2110 | 25.3 (23.1 – 27.6) | 434 | 6.0 (4.8 – 7.2) | 0.0009 |
| Yes | 1073 | 59.0 (52.9 – 65.0) | 472 | 29.4 (23.7 – 35.2) | 136 | 11.6 (7.6 – 15.6) | ||
| Garbage on street | No | 5839 | 69.0 (66.6 – 71.4) | 2051 | 25.0 (22.7 – 27.3) | 425 | 6.0 (4.8 – 7.2) | 0.001 |
| Yes | 1296 | 59.6 (54.4 – 64.8) | 535 | 29.9 (24.8 – 34.9) | 144 | 10.6 (6.9 – 14.3) | ||
| Park in neighborhood | No | 1265 | 64.7 (59.8 – 69.7) | 525 | 27.1 (22.8 – 31.4) | 117 | 8.2 (4.9 – 11.5) | 0.53 |
| Yes | 5870 | 67.5 (65.0 – 70.0) | 2064 | 25.8 (23.4 – 28.2) | 452 | 6.7 (5.4 – 8.0) | ||
| Sidewalk in neighborhood | No | 1771 | 67.4 (63.3 – 71.5) | 669 | 26.0 (22.0 – 29.9) | 166 | 6.6 (4.9 – 8.4) | 0.94 |
| Yes | 5366 | 66.9 (64.2 – 69.5) | 1920 | 26.1 (23.6 – 28.5) | 403 | 7.1 (5.5 – 8.6) | ||
Rao-Scott chi-square tests comparing between mild, moderate and severe eczema.
Multivariate analysis
Multivariate survey logistic regression models were constructed using backward and stepwise selection from all significant variables from univariate analyses. Moderate – severe eczema was associated with older age (adjusted odds ratio [95% confidence interval], P-value; 1.05 [1.03 – 1.07], P < 0.0001), lower household income (0-99% FPL: 1.91 [1.39 – 2.63], P < 0.0001; 100-199% FPL: 1.60 [1.20 – 2.12], P = 0.001; 200-399% FPL: 1.39 [1.06 – 1.83], P = 0.01) and fair or poor maternal health (2.16 [1.47 – 3.16], P < 0.0001), but inversely associated with birthplace outside the US (0.44 [0.22 – 0.89], P = 0.02).
Discussion
Using a population-based sample, we present the distribution and associations of eczema severity in the US. The findings of this study suggest a multifactorial role of genetic, environmental and lifestyle factors on eczema severity. First, we found significant statewide variation of eczema severity, with severe eczema occurring predominantly in Northeastern and Midwest states. Further, eczema severity was associated with household income, family structure, parental education and health, dilapidated housing and the presence of garbage on local streets and inversely associated with birthplace in the US. Previous international studies found only weak associations 5-7, 9, 10 or no associations 4, 8 between filaggrin mutations and AD severity. Together, these suggest that environmental and/or lifestyle factors drive disease severity in the US and play as much, or even more important role than genetics.
The present study assessed self-reported eczema severity over a 1 year period. This measure provides an overall assessment of eczema severity over a relatively broad time period and is distinct from disease prevalence or incidence. This approach is quite useful because assessment of eczema severity at a single time point may not capture the true disease severity given the waxing and waning nature of the disease. We found that the distribution of eczema severity was 67% mild, 26% moderate and 7% severe. This is a slightly more severe disease distribution than the distribution of 82% mild, 12% moderate and 6% severe found in a previous study in the United Kingdom using a novel eczema severity score 16. Another UK study using clinician assessment of severity found 84% mild, 14% moderate and 2% severe 17. The different distributions may reflect population-based differences of eczema severity between the US and UK. Alternatively, caregiver-report of AD severity may differ from that of the Nottingham Eczema Severity Score or clinician assessment used in those studies 16, 17. Nevertheless, we previously demonstrated that caregiver-report of eczema severity strongly correlated with quality-of-life reporting and healthcare utilization in the US 12. Thus, the distribution of eczema severity found in this study appears to be a good indicator of the public health burden of eczema in the US.
The distribution of eczema severity found in this study is also distinct from that of the distribution of US prevalence of eczema previously demonstrated, where peak prevalences occurred on the East coast and Pacific Northwest 1. Many of the states with higher rates of severe eczema have large population-densities with different racial/ethnic distributions 18, more air pollution 19 and colder climates. It is unknown what role, if any, these factors have on eczema severity. We previously found that climate conditions influence the prevalence of pediatric eczema in the US, with disease risk increasing in areas of lower relative humidity, lower UV index, lower temperatures and more precipitation and increased days needing indoor heating 3. The Northeastern states generally have lower UV index and relative humidity, which suggests that climate may influence disease severity as well. It may be that low indoor humidity during the winter months in Northeastern and Midwest states causes xerotic or irritant contact dermatitis in at risk individuals, thereby predisposing to the development of ESA. Further studies are needed to determine whether climatic or other factors further drive eczema severity in the US.
We found that severe eczema was associated with Black race and Hispanic ethnicity. No studies have examined the role of racial/ethnic disparities in determining eczema severity. Previous studies found that eczema prevalence was higher in children of African descent compared with Caucasians 1, 20-22. Studies of racial/ethnic disparities in asthma found that African-Americans have significantly worse asthma outcomes, while Hispanics have lower quality of life secondary to asthma 23. African American children are less likely to use preventive medications for asthma than White or Hispanic children 24 and to have lower health literacy 23. Together, there appear to be significant racial/ethnic disparities for AD and other allergic disease.
Severe eczema was also associated with lower socioeconomic status, including lower household income, single-parent households, lower parental education, dilapidated housing and garbage on the street. Hanifin and Reed found that the US prevalence of AD and eczema decreased significantly with higher household income, but did not compare AD severity by household income 25. However, Shaw et al. found that eczema prevalence in the US increased with higher household incomes 1. Several other studies in the US and internationally found that markers of atopy such as skin-prick test positivity and immunoglobulin E levels were increased with higher socioeconomic status 26-28. Future studies are needed to elucidate these points and to determine the cause(s) of racial/ethnic and perhaps socioeconomic disparities.
Eczema severity was significantly associated with being the oldest child in the household. This may be due to parental inexperience dealing with eczema skin care in their children, which would improve with subsequent children. This emphasizes the importance of education of parents about proper use of emollients and medications by dermatologists and other providers. Alternatively, there may be different exposures in children with older siblings living in the home, such as earlier introduction of certain foods that might result in tolerance or greater exposure to allergens in the physical environment. Flohr et al. recently demonstrated increased sensitization to a variety of food allergens in children that were exclusively breastfed, suggesting that sensitization occurs via transcutaneous sensitization 29. Family size and structure, household dietary and cultural practices may contribute to such transcutaneous sensitization. Future studies are needed to better understand the role of these factors on eczema.
Moderate - severe eczema was also associated with older age, which remained significant in multivariate models. Previous studies found lower prevalence of childhood eczema and eczema with increasing age 1, 12, 25. Together, these findings suggest that mild to moderate eczema generally becomes more quiescent with age and that children whose eczema persists are likely to have more severe disease. It is therefore important to study this group in the future to better understand the determinants of severe eczema in the population.
We found that birthplace outside the US was associated with decreased eczema severity. This is consistent with previous studies of Silverberg et al. that showed children born outside the US had significantly lower odds of atopic disease compared with those born in the US, including asthma, eczema, hay fever and food allergies 14. Interestingly, children born outside the US who lived in the US for >10 years when compared with only 0-2 years had significantly higher odds of developing eczema and other atopic disorders. Shaw et al. found that the prevalence of childhood eczema was lower in children who were born outside the US or whose parents were born outside the US 1. Similarly, Silverberg and Hanifin found that the prevalence of eczema in US adults was lower in those born outside the US compared with those born in the US 2. Several other studies found that birthplace outside the US was associated with lower prevalence of asthma 30-33. The results of this study suggest that birthplace outside the US may also have a protective effect for eczema severity.
The strengths of this study include being large-scale, US population-based and using backward and stepwise selection approaches in multivariate survey logistic regression models. However, the study also has some limitations. The NSCH question encompassed both eczema and skin allergy. We were therefore unable to distinguish between these two entities. However, the preponderant etiology of eczema in children is related to AD. Is it possible that responses are skewed by inclusion of allergic or irritant contact dermatitis, which may increase with older age. However, this is less likely because the population we have categorized as eczema demonstrate a disease prevalence, comorbidity profile, and a natural history course consistent with AD 12. This group of children has a prevalence of eczema similar to two other studies of the prevalence of AD in the US using more strict criteria 34. A lower prevalence of affirmative responses to this question with age is consistent with the natural course of the disease. Lastly, children with a “yes” response to this question have a higher risk of asthma, food allergy and hay fever with odds ratios very similar to previously published numbers for AD populations. History and severity of eczema were defined by caregiver report. Previous studies using parental recall of history of physician-diagnosed eczema 34, 35 and severity of disease 36 have been validated and found to correlate well with physician exam. Moreover, the perception of severe eczema by parents and children is likely a better determinant of quality of life impairment and healthcare utilization than physician assessment. We reported that caregiver-report of severe eczema was associated with poorer overall health and greater healthcare utilization 12. Caregiver-report of severe eczema was associated with increased mental health comorbidity, including attention deficit hyperactivity disorder, depression, anxiety, conduct disorder and autism 37. Thus, the results of this survey based approach using a patient / caregiver centered outcome are likely meaningful and accurate. The study is cross-sectional and therefore provides a snapshot view of eczema severity nationwide. Of course, eczema has a waxing and waning course, which may not be accurately represented in a cross-sectional study. However, the measure used in the study was report of overall severity of eczema over a 1 year period, which better describes the variable disease course than current severity. Nevertheless, future longitudinal studies are needed to identify risk factors for disease severity and flares.
In conclusion, childhood eczema severity varies widely nationwide and is associated with older age, African and Hispanic descent and lower socioeconomic status. Future studies are needed to verify these associations and to further identify the environmental factors that drive eczema severity in the US.
Table 1.
Personal and household associations of childhood atopic dermatitis severity (n=79,667).
| Variable | Subgroup | Atopic dermatitis severity | ||||||
|---|---|---|---|---|---|---|---|---|
| Mild (n = 7198) | Moderate (n = 2612) | Severe (n = 576) | ||||||
| Freq | Percent (95% CI) | Freq | Percent (95% CI) | Freq | Percent (95% CI) | P-value* | ||
| Age (yr) | <1 | 392 | 76.9 (69.9 – 84.0) | 93 | 17.7 (11.2 – 24.2) | 25 | 5.4 (2.0 – 8.7) | 0.0003 |
| 1-3 | 1182 | 76.2 (72.2 – 80.2) | 352 | 18.6 (15.2 – 22.0) | 61 | 5.2 (2.8 – 7.6) | ||
| 3-7 | 1890 | 67.7 (63.3 – 72.2) | 648 | 27.1 (22.9 – 31.3) | 123 | 5.2 (2.9 – 7.4) | ||
| 7-13 | 2003 | 63.1 (59.0 – 67.3) | 784 | 27.8 (24.0 – 31.5) | 196 | 9.1 (6.4 – 11.9) | ||
| 13-17 | 1731 | 62.6 (57.4 – 67.7) | 735 | 29.5 (24.3 – 34.6) | 171 | 8.0 (5.7 – 10.3) | ||
| Sex | Male | 3725 | 65.0 (61.9 – 68.1) | 1354 | 26.9 (24.1 – 29.8) | 295 | 8.1 (6.0 – 10.1) | 0.12 |
| Female | 3466 | 68.9 (65.8 – 72.1) | 1258 | 25.1 (22.0 – 28.2) | 281 | 6.0 (4.7 – 7.3) | ||
| Race/ethnicity | Hispanic | 801 | 61.8 (54.2 – 69.4) | 324 | 30.2 (22.7 – 37.7) | 85 | 8.0 (3.9 – 12.2) | <0.00001 |
| White, non-Hispanic | 4500 | 71.8 (69.2 – 74.3) | 1530 | 23.1 (20.8 – 25.4) | 285 | 5.1 (3.8 – 6.5) | ||
| African American, non-Hispanic | 1045 | 60.5 (55.5 – 65.4) | 435 | 28.4 (23.6 – 33.2) | 135 | 11.2 (8.1 – 14.3) | ||
| Multi/Other, non-Hispanic | 741 | 66.5 (60.3 – 72.8) | 282 | 29.8 (23.6 – 36.0) | 54 | 3.6 (2.0 – 5.3) | ||
| Child's birthplace in the US | No | 123 | 78.4 (68.6 – 88.3) | 40 | 13.4 (6.5 – 20.2) | 11 | 8.2 (1.6 – 14.9) | 0.04 |
| Yes | 7004 | 66.7 (64.5 – 69.0) | 2548 | 26.3 (24.2 – 28.4) | 570 | 7.0 (5.7 – 8.2) | ||
| Household income | 0-99% FPL | 779 | 58.3 (52.8 – 63.8) | 339 | 27.5 (22.7 – 32.3) | 128 | 14.2 (10.1 – 18.3) | <0.0001 |
| 100-199% FPL | 1137 | 64.2 (59.2 – 69.1) | 470 | 27.4 (22.6 – 32.2) | 146 | 8.4 (6.0 – 10.9) | ||
| 200-399% FPL | 2395 | 66.9 (62.8 – 71.0) | 897 | 27.8 (24.0 – 31.7) | 131 | 5.3 (3.0 – 7.5) | ||
| ≥400% FPL | 7198 | 74.0 (70.3 – 77.8) | 2612 | 22.2 (18.6 – 25.8) | 576 | 3.7 (2.2 – 5.3) | ||
| Primary language spoken in home | English | 6843 | 66.7 (64.4 – 69.0) | 2478 | 26.1 (24.0 – 28.3) | 550 | 7.2 (5.9 – 8.5) | 0.27 |
| Not English | 352 | 71.3 (63.4 – 79.2) | 133 | 24.4 (16.9 – 31.9) | 25 | 4.3 (2.1 – 6.6) | ||
| Number of children in household | 1 | 2950 | 67.5 (64.1 – 70.8) | 1041 | 24.8 (22.0 – 27.6) | 229 | 7.8 (5.2 – 10.3) | 0.03 |
| 2 | 2783 | 67.4 (63.9 – 80.8) | 1008 | 26.7 (23.4 – 30.0) | 206 | 5.9 (4.2 – 7.7) | ||
| 3 | 1027 | 64.2 (59.0 – 69.4) | 415 | 29.4 (24.2 – 34.6) | 95 | 6.4 (4.2 – 8.6) | ||
| ≥4 | 438 | 71.2 (64.4 – 78.0) | 148 | 17.7 (12.9 – 22.4) | 46 | 11.1 (5.8 – 16.4) | ||
| Birth order in families with 2 or more children | Oldest child | 2950 | 67.5 (64.1 – 70.8) | 1041 | 24.8 (22.0 – 27.6) | 229 | 7.8 (5.2 – 10.3) | 0.02 |
| 2nd oldest child | 1644 | 63.5 (58.6 – 68.4) | 606 | 29.8 (25.0 – 34.6) | 134 | 6.7 (4.1 – 9.3) | ||
| 3rd oldest child | 1935 | 65.2 (61.3 – 69.2) | 730 | 27.8 (24.0 – 31.7) | 157 | 6.9 (4.9 – 9.0) | ||
| ≥4th oldest child | 502 | 75.8 (70.2 – 81.5) | 185 | 17.5 (12.7 – 22.3) | 41 | 6.6 (3.4 – 9.8) | ||
| Family structure | Two parent biological/adopted | 5084 | 70.5 (67.8 – 73.1) | 1724 | 25.3 (22.8 – 27.8) | 287 | 4.2 (3.0 – 5.5) | <0.0001 |
| Two parent stepfamily | 415 | 64.7 (56.0 – 73.4) | 194 | 26.4 (17.9 – 34.9) | 58 | 8.9 (5.0 – 12.9) | ||
| Single mother/no father present | 1295 | 57.4 (52.3 – 62.5) | 535 | 28.5 (23.7 – 33.2) | 189 | 14.1 (10.5 – 17.7) | ||
| Other | 367 | 69.5 (62.9 – 76.2) | 144 | 23.9 (17.9 – 30.0) | 38 | 6.5 (3.2 – 9.9) | ||
Rao-Scott chi-square tests comparing between mild, moderate and severe eczema.
Acknowledgments
Funding/Support: This project was supported in part by a Mentored Patient-oriented Research Career Development Award (K23)–award number K23AR057486 for Eric Simpson from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Abbreviations used
- CI
confidence interval
- OR
odds ratio
- aOR
adjusted odds ratio
- AD
atopic dermatitis
- NSCH
National Survey of Children's Health
Footnotes
Statistical analysis: JI Silverberg
Conflicts of Interest: None reported.
Financial Disclosure: None reported.
Bibliography
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg JI, Hanifin J. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population-based study. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.08.031. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752–9. doi: 10.1038/jid.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballardini N, Kull I, Soderhall C, Lilja G, Wickman M, Wahlgren CF. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588–94. doi: 10.1111/bjd.12196. [DOI] [PubMed] [Google Scholar]
- 5.Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132:751–62. doi: 10.1038/jid.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekelund E, Lieden A, Link J, Lee SP, D'Amato M, Palmer CN, et al. Loss-of-function variants of the filaggrin gene are associated with atopic eczema and associated phenotypes in Swedish families. Acta Derm Venereol. 2008;88:15–9. doi: 10.2340/00015555-0383. [DOI] [PubMed] [Google Scholar]
- 7.Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. 2010;163:1333–6. doi: 10.1111/j.1365-2133.2010.10068.x. [DOI] [PubMed] [Google Scholar]
- 8.Hubiche T, Ged C, Benard A, Leaute-Labreze C, McElreavey K, de Verneuil H, et al. Analysis of SPINK 5, KLK 7 and FLG genotypes in a French atopic dermatitis cohort. Acta Derm Venereol. 2007;87:499–505. doi: 10.2340/00015555-0329. [DOI] [PubMed] [Google Scholar]
- 9.Morar N, Cookson WO, Harper JI, Moffatt MF. Filaggrin mutations in children with severe atopic dermatitis. J Invest Dermatol. 2007;127:1667–72. doi: 10.1038/sj.jid.5700739. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361–70. e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long-term follow-up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol. 2006;55:765–71. doi: 10.1016/j.jaad.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476–86. doi: 10.1111/pai.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumberg SJ, Welch EM, Chowdhury SR, Upchurch HL, Parker EK, Skalland BJ. Design and operation of the National Survey of Children with Special Health Care Needs, 2005-2006. Vital Health Stat 1. 2008:1–188. [PubMed] [Google Scholar]
- 14.Silverberg JI, Simpson EL, Durkin HG, Joks R. Prevalence of allergic disease in foreign-born American children. JAMA Pediatr. 2013;167:554–60. doi: 10.1001/jamapediatrics.2013.1319. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 16.Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol. 2000;142:288–97. doi: 10.1046/j.1365-2133.2000.03300.x. [DOI] [PubMed] [Google Scholar]
- 17.Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol. 1998;139:73–6. doi: 10.1046/j.1365-2133.1998.02316.x. [DOI] [PubMed] [Google Scholar]
- 18.Interactive Population Map. 2010 Available from http://www.census.gov/2010census/popmap/
- 19.America's Health Ranking: Air Pollution 2012 Available from http://www.americashealthrankings.org/ALL/air/2012.
- 20.Davis LR, Marten RH, Sarkany I. Atopic eczema in European and Negro West Indian infants in London. Br J Dermatol. 1961;73:410–4. doi: 10.1111/j.1365-2133.1961.tb14988.x. [DOI] [PubMed] [Google Scholar]
- 21.Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157–64. doi: 10.1111/j.1525-1470.1983.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams HC, Pembroke AC, Forsdyke H, Boodoo G, Hay RJ, Burney PG. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212–7. doi: 10.1016/0190-9622(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 23.Curtis LM, Wolf MS, Weiss KB, Grammer LC. The impact of health literacy and socioeconomic status on asthma disparities. J Asthma. 2012;49:178–83. doi: 10.3109/02770903.2011.648297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel MK, Waldfogel J. Racial and ethnic differences in the management of childhood asthma in the United States. J Asthma. 2012;49:785–91. doi: 10.3109/02770903.2012.702840. [DOI] [PubMed] [Google Scholar]
- 25.Hanifin JM, Reed ML, Eczema P, Impact Working G A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SA, Weiss ST, Platts-Mills TA, Syring M, Gold DR. Association of specific allergen sensitization with socioeconomic factors and allergic disease in a population of Boston women. J Allergy Clin Immunol. 2001;107:615–22. doi: 10.1067/mai.2001.113523. [DOI] [PubMed] [Google Scholar]
- 27.Ring J, Kramer U, Schafer T, Abeck D, Vieluf D, Behrendt H. Environmental risk factors for respiratory and skin atopy: results from epidemiological studies in former East and West Germany. Int Arch Allergy Immunol. 1999;118:403–7. doi: 10.1159/000024148. [DOI] [PubMed] [Google Scholar]
- 28.Strachan DP, Harkins LS, Johnston ID, Anderson HR. Childhood antecedents of allergic sensitization in young British adults. J Allergy Clin Immunol. 1997;99:6–12. doi: 10.1016/s0091-6749(97)70294-x. [DOI] [PubMed] [Google Scholar]
- 29.Flohr C, Perkin M, Logan K, Marrs T, Radulovic S, Campbell LE, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345–50. doi: 10.1038/jid.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brugge D, Lee AC, Woodin M, Rioux C. Native and foreign born as predictors of pediatric asthma in an Asian immigrant population: a cross sectional survey. Environ Health. 2007;6:13. doi: 10.1186/1476-069X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eldeirawi K, McConnell R, Freels S, Persky VW. Associations of place of birth with asthma and wheezing in Mexican American children. J Allergy Clin Immunol. 2005;116:42–8. doi: 10.1016/j.jaci.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Holguin F, Mannino DM, Anto J, Mott J, Ford ES, Teague WG, et al. Country of birth as a risk factor for asthma among Mexican Americans. Am J Respir Crit Care Med. 2005;171:103–8. doi: 10.1164/rccm.200402-143OC. [DOI] [PubMed] [Google Scholar]
- 33.Lee T, Brugge D, Francis C, Fisher O. Asthma prevalence among inner-city Asian American schoolchildren. Public Health Rep. 2003;118:215–20. doi: 10.1016/S0033-3549(04)50242-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–55. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 35.Flohr C, Weinmayr G, Weiland SK, Addo-Yobo E, Annesi-Maesano I, Bjorksten B, et al. How well do questionnaires perform compared with physical examination in detecting flexural eczema? Findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2009;161:846–53. doi: 10.1111/j.1365-2133.2009.09261.x. [DOI] [PubMed] [Google Scholar]
- 36.Magin PJ, Pond CD, Smith WT, Watson AB, Goode SM. Correlation and agreement of self-assessed and objective skin disease severity in a cross-sectional study of patients with acne, psoriasis, and atopic eczema. Int J Dermatol. 2011;50:1486–90. doi: 10.1111/j.1365-4632.2011.04883.x. [DOI] [PubMed] [Google Scholar]
- 37.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:428–33. doi: 10.1016/j.jaci.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]