Abstract
Alcohol abuse is a widespread problem among those at risk for and living with HIV and can impact transmission and disease progression. In this study we sought to use the simian immunodeficiency virus (SIV)-macaque model to evaluate the immunological and virological changes in the genital microenvironment of females exposed to chronic alcohol. Female rhesus macaques were treated with alcohol (n=6) or isocaloric sucrose (n=6) for 3 months and then inoculated with SIVmac251. To assess the effects of chronic alcohol on SIV disease and the genital microenvironment, we quantified plasma and genital SIV levels, measured inflammatory cells in genital fluids, and characterized microbial flora by gram stains over 10 weeks post-SIV infection. Following 3 months of alcohol/sucrose treatment, significant differences were observed in the vaginal microenvironment of alcohol-treated animals as compared to controls. Microbial flora of alcohol-treated animals had decreased levels of lactobacillus morphotypes and increased levels of gram-positive cocci relative to sucrose controls. Alcohol-treated animals were also more likely to have white blood cells in vaginal fluids prior to SIV inoculation, which persisted through viral set point. Similar levels of cell-free SIV were observed in plasma and vaginal fluids of both groups, but alcohol-treated animals had a higher incidence and levels of cell-associated SIV shed in vaginal secretions. Chronic alcohol treatment negatively impacts the genital microenvironment prior to and over the course of SIV infection and may increase the risk of genital virus shedding and transmission.
Introduction
Approximately 35 million people are infected with human immunodeficiency virus (HIV) worldwide, and women comprise 50% of the cases.1 Among the HIV+ population, alcohol consumption is more common than that observed in the general population, with some studies reporting that more than 50% of HIV+ individuals chronically abuse alcohol.2–5 Additionally, one study found 40% of young women (ages 15–24) at risk for sexually transmitted infections (STIs) participate in alcohol binge drinking.6 Currently, the majority of HIV infections occur through sexual transmission, and alcohol use is associated with high-risk sexual behavior, particularly among HIV+ women.7–14
High plasma viral load (PVL) and genital HIV expression in women are both independently associated with an increased likelihood of sexual and mother-to-infant HIV transmission.15–17 The presence of STIs and ulcerative genital lesions has been associated with higher levels of HIV in genital secretions and, therefore, an increased risk of HIV transmission.18–20 Similarly, bacterial vaginosis (BV), consisting of low levels of lactobacilli, increased quantities of anaerobic bacteria, and high vaginal pH (pH>4.5), has been associated with increased genital HIV levels and higher transmission rates in comparison to women with normal flora.21–25 Increased PVL has been correlated with higher levels of genital HIV; however genital HIV shedding also occurs among women with undetectable PVL, suggesting local HIV reservoirs.26,27 HIV in the genital tract can be detected as free virions or provirally infected cells within cervical or vaginal fluids.28 Other factors have also been associated with increased genital virus levels, including local inflammation, decreased penetration of antiretroviral therapy (ART) in the genital tract, and alcohol use.29–32
Many studies have associated chronic alcohol abuse with increased transmission, higher plasma viral load, and disease acceleration; however, the mechanisms by which this occurs are poorly defined.33–36 Studies have shown chronic alcohol use impairs host defenses against HIV and stimulates proinflammatory cytokines.37–40 Additionally, alcohol may alter the genital microbial flora, as one study correlated alcohol abuse with decreased levels of lactobacilli in the vaginal tract.41 Increased HIV shedding in vaginal secretions of women who abuse alcohol has also been observed, which has serious implications for viral transmission.7,32
The widespread use of alcohol and the potential impact on HIV transmission are important public health concerns. Deciphering mechanisms by which alcohol affects HIV transmission among women is difficult due to the variation in alcohol consumption and behaviors of individuals. The simian immunodeficiency virus (SIV)-infected rhesus macaque exposed to chronic binge alcohol (CBA) is an ideal model to decipher these potential mechanisms because controlled amounts of alcohol are delivered and changes in the genital microenvironment can be evaluated in the absence of additional confounding behavioral factors.35,36 Previous studies with male macaques have associated CBA with increased plasma viral loads and more rapid disease progression.35,36 The goal of this study was to evaluate how CBA affects HIV levels and the genital microenvironment over the initial disease course in females, using the SIV-macaque model.
For this study, we utilized 12 female rhesus macaques treated with alcohol or isocaloric sucrose and inoculated with SIVmac251 and assessed the effects of CBA on the female genital tract. Plasma and genital viral levels, genital inflammatory cells, and microbial flora were evaluated through 10 weeks postinoculation (p.i.). Although significant differences were not observed in cell-free plasma and genital viral loads, higher levels of cell-associated SIV were found in vaginal secretions from alcohol-treated macaques as compared to controls. The most significant differences in the genital microenvironment were observed after 3 months of alcohol and prior to SIV infection. These included decreased levels of lactobacilli morphotypes, increased levels of gram-positive cocci, and increased vaginal white blood cells (WBCs) in alcohol-treated animals, which persisted through 10 weeks p.i. This study identifies potential mechanisms by which alcohol may change the genital microenvironment and increase HIV shedding in genital secretions. Additionally, it establishes the utility of this model to evaluate how CBA affects susceptibility to HIV, as well as HIV prevention and therapeutic strategies.
Materials and Methods
Animals
For these studies, 12 female rhesus macaques (Macaca mulatta) of Indian origin were obtained from the breeding colony at the Tulane National Primate Research Center (TNPRC) in Covington, Louisiana. The Institutional Animal Care and Use Committees at TNPRC and Louisiana State University Health Sciences Center, New Orleans (LSUHSC-NO) approved all procedures, and TNPRC is an AAALAC International accredited institution. Healthy, age-matched female macaques (4–11 years) were selected for the study and housed at TNPRC as previously detailed.36 Intragastric catheters were surgically implanted for alcohol (ALC) or sucrose (SUC) administration as described.36 Macaques received daily intoxicating doses of ethanol (to achieve 50–60 mM blood alcohol concentration) or isocaloric sucrose via gastric catheter beginning 3 months prior to SIV inoculation and continued throughout the duration of the study.36 Animals received twice-daily checks and weekly physical examinations, including assessments of cervical friability, vaginal discharge, and vaginal bleeding. Menstrual cycling was evaluated based on daily observations of vaginal bleeding.42
SIV inoculation and sample collection
After 3 months of daily alcohol or sucrose delivery, females were intrarectally inoculated with 250 TCID50 of SIVmac251. Four sucrose-treated animals were not infected with the initial inoculation and were reinoculated intrarectally with the same dose. Two of these animals failed to become infected after the second intrarectal challenge, and they were successfully infected by intravenous inoculation with the same dose. Samples of peripheral blood were collected and plasma aliquots were preserved.43 Vaginal specimens were collected using polyester-tipped swabs following the insertion of a speculum. Specimens collected during menses were not utilized. One vaginal specimen was smeared onto a glass slide, heat-fixed, and used for microscopic evaluations. A second vaginal swab specimen was eluted into RNAlater solution (Life Technologies, Carlsbad, CA) for virion and cell preservation and subsequent viral load quantification. Genital fluids eluted into RNAlater solution were preserved overnight at 4°C, and then separated into cellular and supernatant fractions before storage at −70° C. Vaginal fluids eluted into RNAlater solution were diluted 1:1 with phosphate-buffered saline (PBS) prior to freezing. Samples of endocervical cells were obtained by insertion and rotation of a cytobrush into the cervical os. Cells were eluted into RPMI medium for immediate analysis of cell types.
Viral load and T cell quantification
Plasma, vaginal, and cell-associated SIV levels were quantified using a sensitive real-time polymerase chain reaction (PCR) assay that targets the gag gene of SIV as described.44 Briefly, viral RNA was isolated from 1 ml of plasma or 0.5 ml vaginal fluids by high-speed centrifugation, reverse transcribed; viral sequences were then amplified by PCR with a TaqMan assay (Life Technologies) and quantified using SIV gag RNA standards. The limit of quantification is 50 copies/ml. DNA was isolated from the cellular fraction of vaginal specimens and quantified similarly using DNA standards. The cellular fraction contained both epithelial and immune cells. SIV DNA copies were normalized to total vaginal cell numbers determined by amplification of a cellular target. Samples with undetectable virus levels were assigned a value of 25 copies/ml for viral RNA measures and 1 copy/100,000 cells for viral DNA. Peripheral blood T-lymphocytes were quantified from whole blood samples by using fluorochrome-conjugated monoclonal antibodies against human phenotypic cell surface antigens followed by flow cytometric analysis as previously described.45
Gram stain
Slides containing vaginal secretions were gram stained (BD Diagnostics, Sparks, MD), then analyzed and scored using the Nugent Scoring System.46 Other bacterial morphotypes that were frequently identified in rhesus macaques were also quantified in four different fields (1,000×objective).47,48 Morphotypes assessed included gram-positive cocci (GPC), gram-positive diplococci (GPDC), gram-positive cocci in chains (GPC chains) and gram-positive cocci in clusters (GPC clusters), small gram-variable rods (SGVR), curved gram-negative rods (CGNR), and large gram-positive rods (LGPR). Morphotypes were quantified as absent, <1, 1–4, 5–30, and >30. These categories were ranked and relative abundance was calculated by the rank of a particular morphotype in comparison to the total rank of all morphotypes present in the specimen.
Genital cell counts
WBCs found in vaginal secretions were quantified from the gram-stained vaginal smears. Slides with blood contamination [>20 red blood cells (RBC)/field] were not used for white blood cell (WBC) quantification. The average number of WBCs found in four fields at 400×was recorded. Cells contained in endocervical fluid samples were stained with an Endtz staining method (myeloperoxidase assay) to differentiate the inflammatory cells present.49 An aliquot of the sample was mixed with benzidine (Sigma, St. Louis, MO) solution and allowed to incubate briefly at room temperature, followed by microscopic evaluation using a hemocytometer to enumerate neutrophils (peroxidase-positive cells) and mononuclear cells (based on size and morphology). Total cell counts per sample were reported.
Statistical analysis
Viral load data were log10 transformed for analyses. Longitudinal response variables were analyzed using a repeated measures two-way model with time (week p.i.) being a repeated factor and treatment (ALC vs. SUC) a fixed effect. The dependences between observations on the same animal were modeled by an autoregressive type I model. The denominator degrees of freedom of the approximate F- tests and t-tests were determined by applying the Kenward–Roger type method. Unless otherwise stated, the multiple comparisons significance level was adjusted using the Tukey–Kramer type inequality. Cellular infiltrate and Nugent score classification were analyzed with respect to the two treatment groups using a chi-square test at baseline. The difference in mean bacterial morphotypes between treatment groups was analyzed prior to inoculation by t-test. The nominal significance level was set to 0.05 for all tests. The statistical analysis was carried out using the SAS Statistical software package Version 9.4
Results
Clinical disease parameters
To evaluate SIV disease progression, levels of virus and T-lymphocytes in peripheral blood were monitored over a 10-week period p.i. Mean plasma viral loads did not differ between the two treatment groups at peak or set point time periods (Fig. 1A). After 3 months of alcohol treatment, no differences were detected in the mean levels of both CD4+ and CD8+ T cells in alcohol-treated and sucrose-treated animals. Following SIV infection, the mean CD4+ T cell numbers progressively declined in both groups (Fig. 1B). The mean levels of CD8+ T cells were unchanged at 2 weeks p.i. in both treatment groups; however, a significant increase was observed in sucrose-treated animals at 10 weeks p.i. (p<0.05) (Fig. 1C).
FIG. 1.
(A) Plasma viral load in six sucrose and six alcohol-treated macaques were quantified by RT-qPCR and log10 transformed. Shown are the peak (2 or 3 weeks p.i.) and set point (10 weeks p.i.) viral load. Symbols represent individual viral levels and lines represent mean group levels. (B) Levels of CD4+ and (C) CD8+ T-lymphocytes determined by flow cytometry. Shown are mean cell counts±SEM from six sucrose and six alcohol-treated macaques at time points “0” (pre-SIV inoculation at 12 weeks post-treatment) and 2 and 10 weeks post-SIV infection.
Vaginal SIV levels
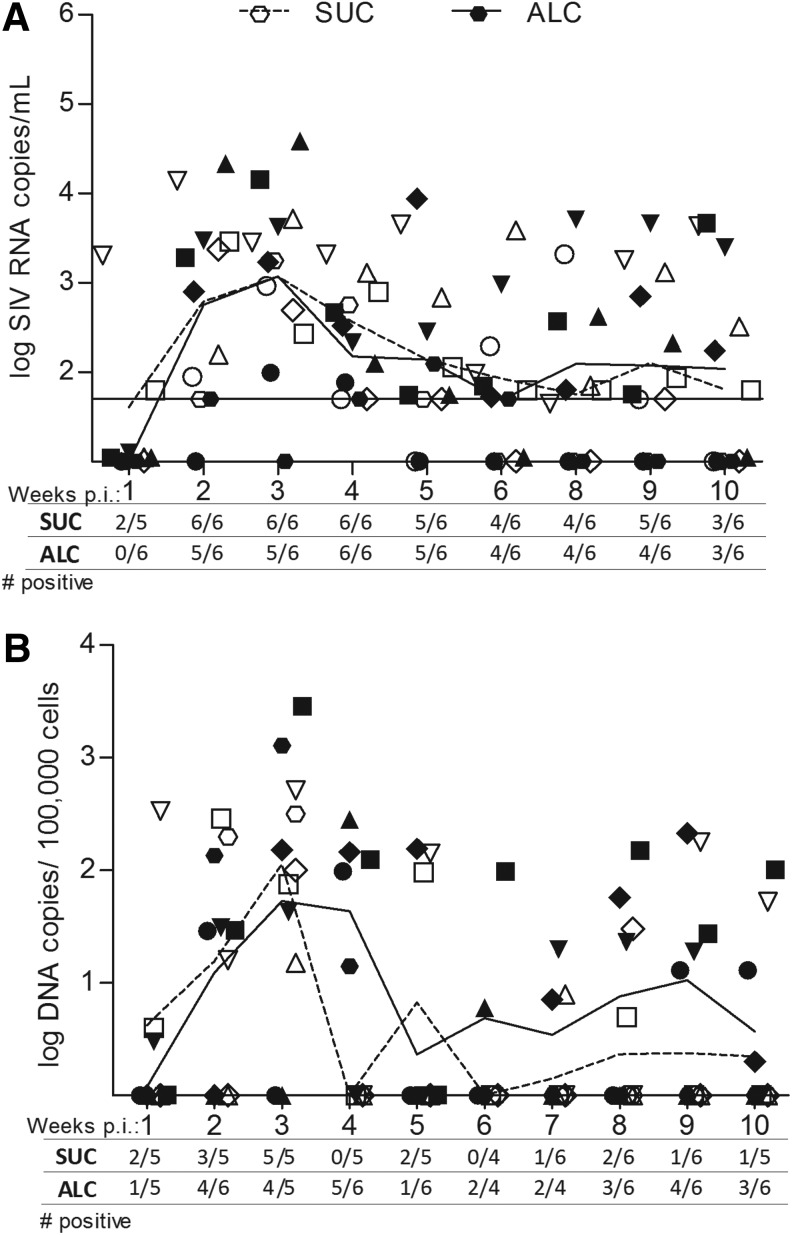
To evaluate the risk of sexual transmission due to CBA, we measured cell-free and cell-associated SIV levels in genital secretions (Fig. 2). In both groups of females, vaginal virus levels from individual animals fluctuated over time and were approximately 3.5 logs lower than plasma levels. The mean levels of cell-free virus shed in vaginal fluids did not differ between ALC and SUC macaques over the disease course, with mean SIV RNA levels over 10 weeks of 1,945 and 1,109 copies/ml vaginal fluid, respectively. Cell-associated viral DNA levels in vaginal fluids also varied in both ALC and SUC groups, but overall, ALC animals had higher levels of cell-associated virus as compared to SUC animals. Mean cell-associated SIV levels over the 10-week period were 103 and 48 SIV DNA copies/100,000 vaginal cells, respectively. The levels and incidence of infected cells shed in genital fluids varied between 4 and 6 weeks of SIV in both groups, but at 4 weeks p.i., ALC animals had higher mean levels of cell-associated virus p.i. as compared to sucrose-treated animals (p<0.006). Over 7 to 10 weeks p.i., vaginal proviral levels became more stable among individual animals and remained higher in alcohol-treated animals. Also over this time frame, the incidence of proviral DNA was higher among alcohol-treated animals, with 55% (12/22) of samples positive for SIV as compared to only 22% (5/23) from sucrose controls. Furthermore, four of six ALC/SIV+ females had persistent shedding evident by SIV detection in at least 75% of the samples. In comparison, shedding rates were lower among SUC/SIV+ females; one animal had provirus detection rates of 50% while all others were 25% or lower.
FIG. 2.
SIV levels in genital fluids quantified in weekly vaginal fluid samples from six alcohol-treated and six sucrose-treated animals. Genital samples at the time of menses were not included. The number of animals with detectable virus out of the total analyzed at that time point is shown on the x-axis. Symbols represent viral levels from individual animals and lines represent mean group levels. Open symbols and dashed line represent SUC animals, and closed symbols and solid line represent ALC animals. (A) Levels of virions shed in the vaginal fluid were quantified with RT-qPCR on cell-free secretions and expressed as log10 RNA copies/ml of vaginal fluid supernatant. The limit of quantification (50 copies or 1.7 log) is delineated on the figure. (B) Levels of cell-associated SIV DNA in the cellular fraction of vaginal secretions were quantified by qPCR and expressed as DNA copies/100,000 cells. The limit of quantification was 1 copy.
Cervicovaginal cellular infiltrates
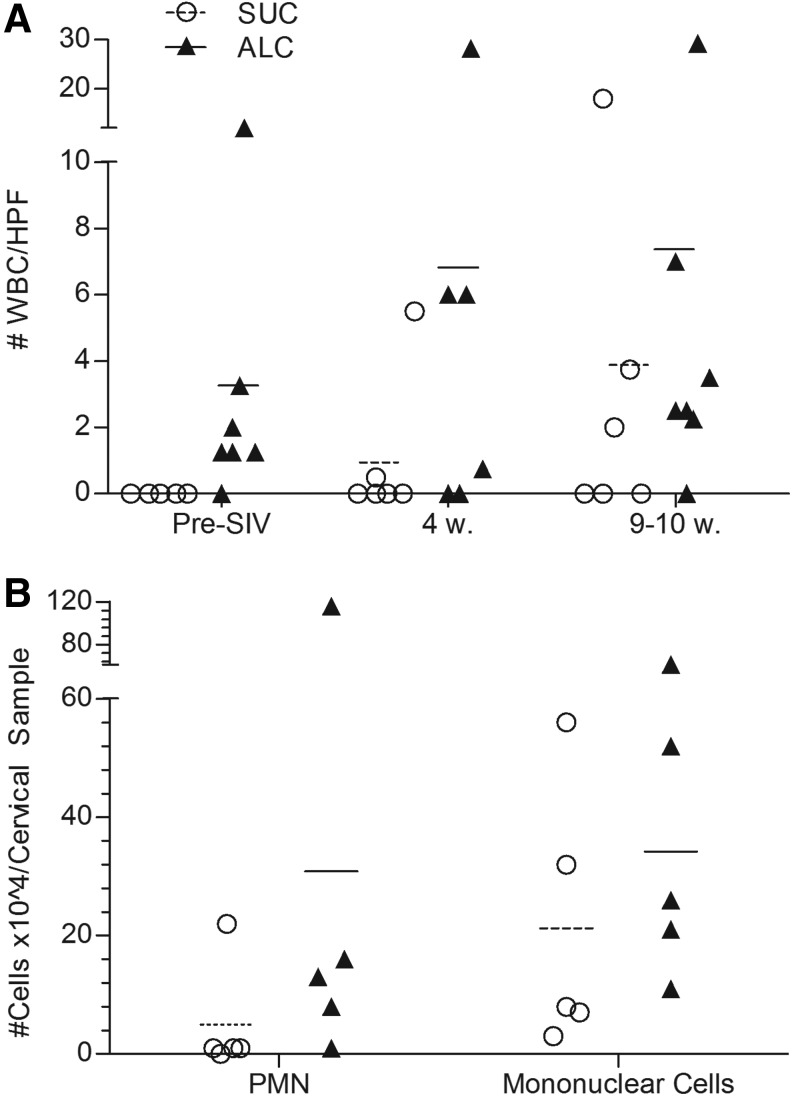
Previous studies have associated a proinflammatory state with CBA,50–52 therefore we sought to evaluate inflammatory cell changes in the female genital tract (Fig. 3A). We quantified the numbers of WBCs contained in smears of vaginal secretions. Prior to SIV infection, vaginal WBCs were not identified in samples from each of the six sucrose-treated macaques, but were observed in five of the six alcohol-treated macaques (p=0.006). After SIV infection, WBCs were persistently found in vaginal fluids from ALC/SIV+ females and levels increased approximately 2-fold by 10 weeks p.i. WBCs also increased in SUC/SIV+ females as the disease course progressed; however, alcohol-treated macaques consistently had more vaginal WBCs/high power field (HPF) in comparison to sucrose-treated controls. Overall, vaginal WBCs were present at relatively low levels (<10 WBC/HPF) in all animals.
FIG. 3.
Evaluation of immune cells in vaginal fluids in alcohol and sucrose treated macaques. Symbols represent individual levels in individual animals and lines represent mean group levels. (A) Number of WBCs/high power field (HPF) quantified in vaginal smears (n=36). One sample/animal was analyzed at: pre-SIV (9 to 12 weeks post-alcohol treatment), 4 weeks and 9–10 weeks post SIV infection. (B) Levels of cervical neutrophils and mononuclear cells contained in cervical samples obtained from brush samplings of the endocervix in five alcohol and five sucrose treated animals at 9 or 10 weeks post SIV infection.
As an additional measure of genital inflammation, we sampled and quantified the cellular populations of the endocervix from females at 9–10 weeks p.i. (Fig. 3B). Using Endtz stain, we determined that the mean number of neutrophils in cervical samples was higher in ALC/SIV+ animals in comparison to controls (SUC: 5×104 cells vs. ALC: 31×104 cells). However, the mean number of mononuclear cells was similar between both treatments. Throughout the study, none of the females demonstrated signs of genital tract infections, cervical friability, malodorous vaginal discharge, or abnormal vaginal bleeding.
Vaginal flora
The general composition and fluctuations of the genital microbial flora were evaluated over time to assess the impact of CBA. Bacterial morphotypes were identified in vaginal smears using gram stains and the Nugent scoring system, which is used clinically to diagnose bacterial vaginosis (BV) in women. The system grades flora into three different classifications: normal, intermediate, or positive for bacterial vaginosis.46 Prior to SIV infection, the majority of vaginal samples from sucrose and alcohol-treated macaques were classified as intermediate; but 27% of vaginal samples (3/11) from sucrose-treated macaques were classified as normal in comparison to none of the alcohol-treated macaque samples (Table 1). Following SIV infection, the Nugent score classification in both treatment groups did not significantly fluctuate over time; however, flora of alcohol-treated macaques was more frequently classified as BV+. A significant correlation was not detected between Nugent score and vaginal viral load.
Table 1.
Nugent Score Classification of 46 Vaginal Samples from 12 Macaques
| Preinoculation | ||||
|---|---|---|---|---|
| Macaque # | Sample 1 | Sample 2 | 4 Weeks SIV | Set point SIV |
| Sucrose treated | ||||
| A | 3 | N/A | 4 | 3 |
| B | 3 | 3 | 5 | 4 |
| C | 4 | 4 | 4 | 4 |
| Da | 4 | 5 | 6 | 6 |
| Ea | 5 | 6 | 5 | 5 |
| F | 6 | 6 | 5 | 10 |
| Alcohol treated | ||||
| G | 4 | 4 | 4 | 6 |
| H | 4 | 4 | 6 | 6 |
| I | 4 | 4 | 7 | 5 |
| J | 4 | 6 | 0 | 4 |
| K | 4 | 9 | 7 | 9 |
| L | 7 | N/A | 8 | 9 |
Delineates animals that were intravenously inoculated.
Preinoculation samples were obtained between 0 and 3 weeks prior to rectal simian immunodeficiency virus (SIV) inoculation (9–12 weeks after initiation of daily alcohol/sucrose). The remaining samples were obtained at time points relative to SIV inoculation that resulted in infection. Samples for set point SIV were obtained at either 9 or 10 weeks p.i. The Nugent scoring system classifies a score of 0 to 3 as normal (not bold, not italics), 4 to 6 as intermediate (italics), and 7 to 10 as bacterial vaginosis (BV)+ (bold).
N/A signifies that samples were unavailable to be analyzed.
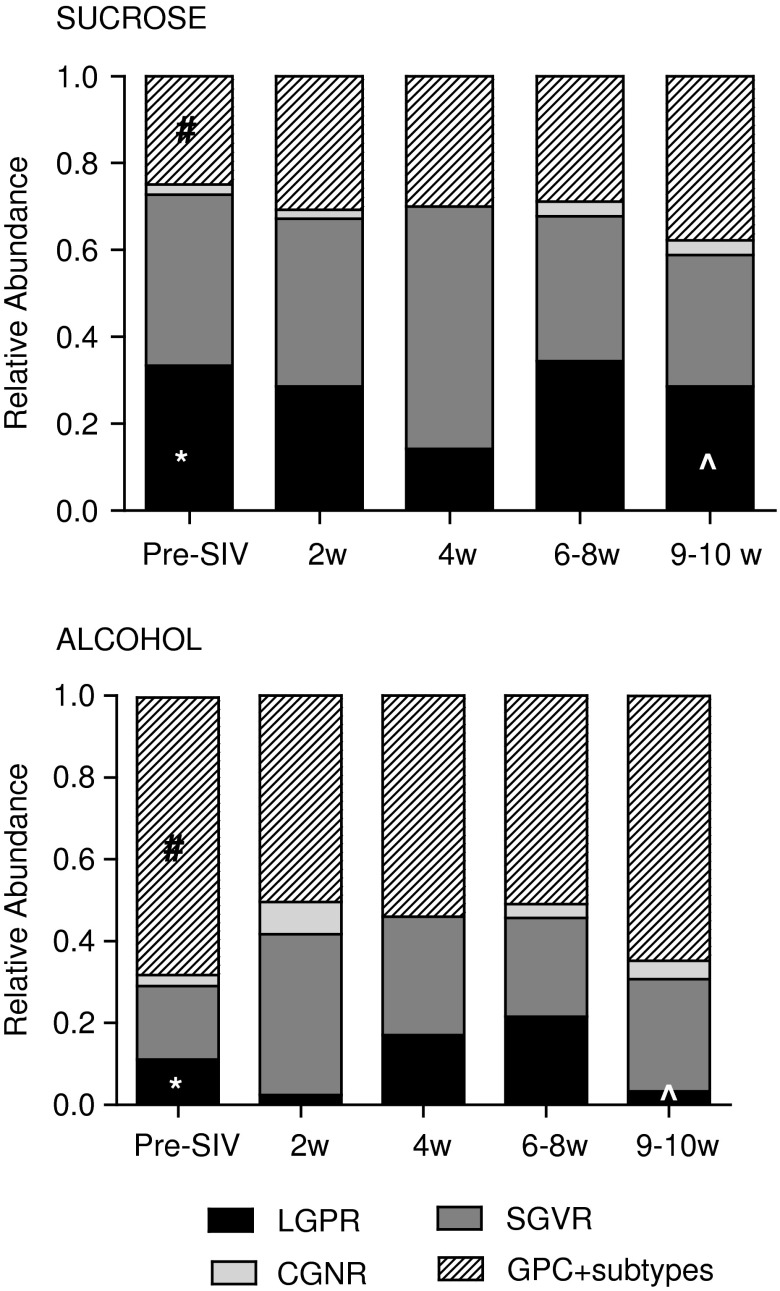
While the Nugent scoring system served as a way to initially characterize the flora of the macaques, we and others commonly observed GPC in the macaque vaginal flora; these were identified in chains, clusters, as diplococci, or individual cells.47,48 GPC are not commonly identified in gram stains of human vaginal secretions designated as normal or BV+, although they have been noted in women.53 Therefore, we included levels of GPC morphotypes in our analyses. Using this modified Nugent scoring system, we quantified the relative abundance of various morphotypes. Following 3 months of treatment, ALC animals had significantly lower mean levels of LGPR, which are suggestive of Lactobacillus spp. relative to sucrose (p<0.02). We also detected significantly higher levels of GPC morphotypes in ALC animals (p<0.01) (Fig. 4).
FIG. 4.
Relative abundance of bacterial morphotypes observed in 70 vaginal samples collected from six sucrose and six alcohol treated macaques over time. Two pre-SIV samples were collected from each animal when available (n=22, detailed in Table 1). At the remaining indicated time points, one sample/animal was analyzed. Morphotypes evaluated included large gram positive rods (LGPR), small gram variable rods (SGVR), curved gram negative rods (GNR); gram-positive cocci (GPC) and GPC subtypes, which include GPC found in chains, clusters, as diplococci, or individual cells. Levels of morphotypes found in alcohol and time-matched sucrose controls were compared at baseline (*LGPR p<0.02, #GPC p<0.01); at 10 weeks p.i., comparisons were adjusted for pre-SIV baseline levels (^LGPR, LSmeans=0.341, stderr=0.149, p<0.05).
Further characterization of this morphotype into subcategories did not reveal significant differences in the composition of GPC among treatment groups (data not shown). Following SIV infection, there were minor fluctuations in the relative abundance of bacterial morphotype among the groups, but significant changes were not observed over time. At 9–10 weeks p.i., significantly lower mean levels of LGPR were still observed in ALC/SIV+ animals as compared to SUC/SIV+ controls after adjusting for pre-SIV baseline levels (p<0.05).
Discussion
The SIV-infected rhesus macaque model has been instrumental in understanding HIV pathogenesis and evaluating mucosal viral infection.50,54–56 We have extended this model to evaluate how CBA impacts the genital microenvironment and SIV disease in females. Following 3 months of chronic alcohol administration, we were able to longitudinally monitor viral, cellular, and microbial changes for 10 weeks following SIV infection. We observed significant alterations in the cellular infiltrate and microbial flora in the genital tract of the alcohol-treated animals as compared to controls, which may have important implications for genital virus shedding and sexual transmission of HIV.
Following 3 months of alcohol delivery, but prior to SIV inoculation, significantly more alcohol-treated macaques had WBCs in vaginal fluids. After SIV infection, genital cell infiltrates increased in both groups of animals, but the alcohol group consistently had higher vaginal WBCs and cervical neutrophils. Although the absolute levels of genital cell infiltrates in the macaques were low, and never reached levels that would be clinically significant in women, environment and behavior were controlled, and the risk of vaginitis due to infectious agents was minimized. Additional studies using flow cytometry and immunohistochemistry are needed to more fully characterize the cellular populations in this compartment; however, these studies will require tissue biopsy in order to obtain sufficient cells for analyses.
It is plausible that CBA would exacerbate inflammation and increase the levels of virus shed into the genital tract in the presence of a concurrent STD infection. A recent study conducted with SIV-infected male macaques found that animals exposed to CBA and inoculated with a common respiratory pathogen, Streptococcus pneumoniae, had enhanced and prolonged SIV expression in the lung compartment relative to controls.43 Coinfections with STDs are commonly observed in women at risk for HIV and abuse alcohol, therefore future studies should be designed to evaluate the effect of alcohol on inflammation and compartmentalized shedding of SIV in the presence of genital coinfections.
SIV-infected cells in vaginal fluids were detected more frequently and at higher levels in the alcohol-treated animals as compared to the sucrose controls. The incidence and levels of SIV-infected cells were highly variable, and often did not correlate with cell-free levels in this compartment. The observed differences in proviral DNA levels did not reach statistical significance over 7–10 weeks p.i., but our sample size was relatively small and animals were monitored only through 10 weeks. The increased incidence of vaginal SIV detection in alcohol-treated macaques may have important implications for sexual transmission of HIV, since alcohol abusers also have an increased risk of unsafe sexual practices.7–13 It is unclear whether free virions or cell-associated virus play a greater role in initiating mucosal infection.57–60 A recent study using a colon explant model showed that cell-associated virus (infected lymphocytes) transmits the virus across the rectal mucosa more efficiently than cell-free virus.61 Additionally, the levels of HIV-infected cells in breast milk have been associated with postpartum HIV transmission via breastfeeding.62–64
Previous clinical studies have associated chronic alcohol abuse with increased plasma HIV levels,33,65–67 as have studies with the SIV-infected male rhesus macaque exposed to chronic alcohol.36 This study in females did not find a significant effect of CBA on plasma viral load. The male studies, however, used larger groups of animals (24 to 32), which were monitored until end-stage SIV. Others have identified sex differences in which females had lower plasma HIV levels relative to males during primary HIV infection (controlled for CD4+ count), although survival and disease progression rates were similar.34,68,69 Additional long-term studies are needed to evaluate how CBA influences chronic disease progression in females and address potential sex differences.
The vaginal flora of rhesus macaques has been characterized by 16S deep sequencing, and similarities to the flora present in BV+ women were observed.47 Our study also found that the majority of samples from both treatment groups were classified as intermediate to BV+, although alcohol-treated macaques had more BV+ samples than controls. Several studies have associated BV with an increased risk of acquiring HIV as well as higher genital HIV levels in comparison to women with normal flora.23–25 To more thoroughly characterize the bacterial flora from the gram stain, we expanded the Nugent scoring criteria to quantify the relative abundance of bacterial morphotypes commonly found in rhesus macaques.47,48 Alcohol-treated macaques had significantly greater levels of GPC morphotypes and significantly decreased levels of LGPR (suggestive of Lactobacillus spp.) prior to SIV inoculation.
Numerous studies have touted the protective effect of lactobacilli morphotypes and its prevention against HIV infection; additional studies in the macaque model are needed to evaluate protective factors within the genital environment, such as lactic acid.21,24,41,70,71 The adapted Nugent scoring system provided a novel and efficient tool to longitudinally compare changes in the macaque vaginal microenvironment, but it has its limitations. Analyses by 16S deep sequencing are warranted, as they will allow for quantification and identification of specific bacterial species, including those that have been implicated in increased rates of HIV and SIV replication.21,22,72,73 Moreover, longitudinal evaluation of the microbial flora prior to and over the course of alcohol treatment will allow for a more thorough characterization of alcohol's impact amid the diversity present in individual macaques.
We identified significant differences in the genital microenvironment of CBA females as compared to controls, which may influence the susceptibility to HIV/SIV acquisition via vaginal exposure. The females in this study were inoculated with SIV via a rectal route as part of our overall study design for comparison with male macaques similarly exposed to daily alcohol. Therefore genital inflammation and microflora composition at the time of SIV exposure could not be directly related to SIV acquisition. Future studies with CBA females and low-dose vaginal SIV challenge are needed to directly address the effects of CBA on HIV/SIV acquisition.
This study provided a unique opportunity to evaluate the longitudinal effects of alcohol on primary SIV infection in the periphery and genital compartment under highly controlled conditions using the well-established SIV-infected rhesus macaque model. Our overall findings suggest that chronic alcohol consumption negatively impacts the genital microenvironment by increasing vaginal inflammation, decreasing beneficial bacterial species, and increasing adverse bacterial morphotypes. Further studies are warranted to examine the mechanisms by which these changes influence cell-associated virus in the genital compartment.
This model provides an invaluable tool to decipher the manner in which alcohol alters the genital microenvironment and impacts SIV transmission. These studies also have important implications for HIV prevention and the efficacy of microbicides in reducing viral transmission among alcohol abusers.
Acknowledgments
The authors would like to acknowledge the contributions of Nedra Lacour, Judy Burnett, Jane A. Schexnayder, Jean W. Carnal, Constance Porretta, Spencer Robichaux, Meredith Booth, Amy B. Weinberg, Rhonda R. Martinez, and Joseph Soblosky for technical and analytical assistance. From TNRPC we thank Dr. Preston Marx for providing the SIVmac251 inoculum and Larissa Devlin, Wayne A. Cyprian, and Nancy Dillman for animal assistance. This work was supported by NIH grants P60-AA09803, T32-AA07577, and P51-OD011104.
Parts of the data were presented at the 2013 Research Society for Alcoholism Meeting, Orlando, Florida.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS: 2013 Global Fact Sheet. 2013
- 2.Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, and Justice AC: How harmful is hazardous alcohol use and abuse in HIV infection: Do health care providers know who is at risk? J Acquir Immune Defic Syndr 2003;33(4):521–525 [DOI] [PubMed] [Google Scholar]
- 3.Chander G, Josephs J, Fleishman JA, et al. : Alcohol use among HIV-infected persons in care: Results of a multi-site survey. HIV Med 2008;9(4):196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook RL. and Clark DB: Is there an association between alcohol consumption and sexually transmitted diseases? A systematic review. Sex Transmit Dis 2005;32(3):156–164 [DOI] [PubMed] [Google Scholar]
- 5.Stinson FS. and DeBakey SF: Prevalence of alcohol problems among selected AIDS risk groups: United States, 1988. Addiction 1993;88(8):1139–1147 [DOI] [PubMed] [Google Scholar]
- 6.Samet JH, Phillips SJ, Horton NJ, Traphagen ET, and Freedberg KA: Detecting alcohol problems in HIV-infected patients: Use of the CAGE questionnaire. AIDS Res Hum Retroviruses 2004;20(2):151–155 [DOI] [PubMed] [Google Scholar]
- 7.Theall K, Clark R, Powell A, Smith H, and Kissinger P: Alcohol consumption, art usage and high-risk sex among women infected with HIV. AIDS Behav 2007;11(2):205–215 [DOI] [PubMed] [Google Scholar]
- 8.Weiser SD, Leiter K, Heisler M, et al. : A population-based study on alcohol and high-risk sexual behaviors in Botswana. PLoS Med 2006;3(10):e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shuper PA, Joharchi N, Irving H, and Rehm J: Alcohol as a correlate of unprotected sexual behavior among people living with HIV/AIDS: Review and meta-analysis. AIDS Behav 2009;13(6):1021–1036 [DOI] [PubMed] [Google Scholar]
- 10.Kiene SM, Simbayi LC, Abrams A, Cloete A, Tennen H, and Fisher JD: High rates of unprotected sex occurring among HIV-positive individuals in a daily diary study in South Africa: The role of alcohol use. J Acquir Immune Defic Syndr 2008;49(2):219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenstein V, Horton NJ, and Samet JH: Inconsistent condom use among HIV-infected patients with alcohol problems. Drug Alcohol Depend 2004;73(2):159–166 [DOI] [PubMed] [Google Scholar]
- 12.Karim QA, Sibeko S, and Baxter C: Preventing HIV infection in women: A global health imperative. Clin Infect Dis 2010;50(Suppl 3):S122–S129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutton HE, McCaul ME, Santora PB, and Erbelding EJ: The relationship between recent alcohol use and sexual behaviors: Gender differences among sexually transmitted disease clinic patients. Alcoholism Clin Exp Res 2008;32(11):2008–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schellenberg JJ. and Plummer FA: The microbiological context of HIV resistance: Vaginal microbiota and mucosal inflammation at the viral point of entry. Int J Inflam 2012;2012:131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten JM, Kahle E, Lingappa JR, et al. : Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Translation Med 2011;3(77):77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John GC, Nduati RW, Mbori-Ngacha DA, et al. : Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: Association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis 2001;183(2):206–212 [DOI] [PubMed] [Google Scholar]
- 17.Quinn TC, Wawer MJ, Sewankambo N, et al. : Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000;342(13):921–929 [DOI] [PubMed] [Google Scholar]
- 18.Galvin SR. and Cohen MS: The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2(1):33–42 [DOI] [PubMed] [Google Scholar]
- 19.Ghys PD, Fransen K, Diallo MO, et al. : The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Côte d'Ivoire. AIDS 1997;11(12):F85–F93 [DOI] [PubMed] [Google Scholar]
- 20.McClelland RS, Wang CC, Overbaugh J, et al. : Association between cervical shedding of herpes simplex virus and HIV-1. AIDS 2002;16(18):2425–2430 [DOI] [PubMed] [Google Scholar]
- 21.Sha BE, Zariffard MR, Wang QJ, et al. : Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis 2005;191(1):25–32 [DOI] [PubMed] [Google Scholar]
- 22.Al-Harthi L, Roebuck KA, Olinger GG, et al. : Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. J Acquir Immune Defic Syndr 1999;21(3):194–202 [DOI] [PubMed] [Google Scholar]
- 23.Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, and Spear GT: A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: Implications for HIV and SIV susceptibility. AIDS Res Hum Retroviruses 2012;28(1):76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin HL, Richardson BA, Nyange PM, et al. : Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180(6):1863–1868 [DOI] [PubMed] [Google Scholar]
- 25.Coleman JS, Hitti J, Bukusi EA, et al. : Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS 2007;21(6):755–759 [DOI] [PubMed] [Google Scholar]
- 26.Hart CE, Lennox JL, Pratt-Palmore M, et al. : Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis 1999;179(4):871–882 [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Bujalance S, Ruiz G, De Guevara CL, et al. : Quantitation of human immunodeficiency virus type 1 RNA loads in cervicovaginal secretions in pregnant women and relationship between viral loads in the genital tract and blood. Eur J Clin Microbiol Infect Dis 2004;23(2):111–115 [DOI] [PubMed] [Google Scholar]
- 28.Ellerbrock TV, Lennox JL, Clancy KA, et al. : Cellular replication of human immunodeficiency virus type 1 occurs in vaginal secretions. J Infect Dis 2001;184(1):28–36 [DOI] [PubMed] [Google Scholar]
- 29.Kwara A, Delong A, Rezk N, et al. : Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis 2008;46(5):719–725 [DOI] [PubMed] [Google Scholar]
- 30.Cu-Uvin S, DeLong AK, Venkatesh KK, et al. : Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS 2010;24(16):2489–2497 [DOI] [PubMed] [Google Scholar]
- 31.Theall KP, Amedee A, Clark RA, Dumestre J, and Kissinger P: Alcohol consumption and HIV-1 vaginal RNA shedding among women. J Studies Alcohol Drugs 2008;69(3):454–458 [DOI] [PubMed] [Google Scholar]
- 32.Homans J, Christensen S, Stiller T, et al. : Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acquir Immune Defic Syndr 2012;60(1):99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baum MK, Rafie C, Lai S, Sales S, Page JB, and Campa A: Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses 2010;26(5):511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rompalo AM, Astemborski J, Schoenbaum E, et al. : Comparison of clinical manifestations of HIV infection among women by risk group, CD4+ cell count, and HIV-1 plasma viral load. J Acquir Immune Defic Syndr 1999;20(5):448–454 [DOI] [PubMed] [Google Scholar]
- 35.Bagby GJ, Stoltz DA, Zhang P, et al. : The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcoholism Clin Exp Res 2003;27(3):495–502 [DOI] [PubMed] [Google Scholar]
- 36.Bagby GJ, Zhang P, Purcell JE, Didier PJ, and Nelson S: Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcoholism Clin Exp Res 2006;30(10):1781–1790 [DOI] [PubMed] [Google Scholar]
- 37.MacGregor RR: Alcohol and immune defense. JAMA 1986;256(11):1474–1479 [PubMed] [Google Scholar]
- 38.Barve SS, Kelkar SV, Gobejishvilli L, Joshi-Barve S, and McClain CJ: Mechanisms of alcohol-mediated CD4+ T lymphocyte death: Relevance to HIV and HCV pathogenesis. Front Biosci 2002;7:1689–1696 [DOI] [PubMed] [Google Scholar]
- 39.Szabo G: Consequences of alcohol consumption on host defence. Alcohol 1999;34(6):830–841 [DOI] [PubMed] [Google Scholar]
- 40.Nair MP, Kumar NM, Kronfol ZA, et al. : Selective effect of alcohol on cellular immune responses of lymphocytes from AIDS patients. Alcohol 1994;11(2):85–90 [DOI] [PubMed] [Google Scholar]
- 41.Baeten JM, Hassan WM, Chohan V, et al. : Prospective study of correlates of vaginal Lactobacillus colonisation among high-risk HIV-1 seronegative women. Sex Transmit Infect 2009;85(5):348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowell TE: Behaviour and female reproductive cycles of rhesus macaques. J Reprod Fertil 1963;6:193–203 [DOI] [PubMed] [Google Scholar]
- 43.Nelson S, Happel KI, Zhang P, Myers L, Dufour JP, and Bagby GJ: Effect of bacterial pneumonia on lung simian immunodeficiency virus (SIV) replication in alcohol consuming SIV-infected rhesus macaques. Alcoholism Clin Exp Res 2013;37(6):969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina PE, Amedee AM, Lecapitaine NJ, et al. : Modulation of gut-specific mechanisms by chronic delta9-THC administration in male rhesus macaques infected with simian immunodeficiency virus: A systems biology analysis. AIDS Res Hum Retroviruses 2014;30(6):567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin LN, Murphey-Corb M, Soike KF, Davison-Fairburn B, Baskin GB: Effects of initiation of 3'-azido,3'-deoxythymidine (zidovudine) treatment at different times after infection of rhesus monkeys with simian immunodeficiency virus. J Infect Dis 1993;168(4):825–835 [DOI] [PubMed] [Google Scholar]
- 46.Nugent RP, Krohn MA, and Hillier S: Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29(2):297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spear GT, Gilbert D, Sikaroodi M, et al. : Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: Implications for use as an animal model for HIV vaginal infection. AIDS Res Hum Retroviruses 2010;26(2):193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doyle L, Young CL, Jang SS, and Hillier SL: Normal vaginal aerobic and anaerobic bacterial flora of the rhesus macaque (Macaca mulatta). J Med Primatol 1991;20(8):409–413 [PubMed] [Google Scholar]
- 49.Endtz AW: A rapid staining method for differentiating granulocytes from “germinal cells” in Papanicolaou-stained semen. Acta Cytol 1974;18(1):2–7 [PubMed] [Google Scholar]
- 50.Poonia B, Nelson S, Bagby GJ, and Veazey RS: Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: Implications for SIV/HIV infection. J Acquir Immune Defic Syndr 2006;41(5):537–547 [DOI] [PubMed] [Google Scholar]
- 51.McClain C, Hill D, Schmidt J, and Diehl AM: Cytokines and alcoholic liver disease. Semin Liver Dis 1993;13(2):170–182 [DOI] [PubMed] [Google Scholar]
- 52.Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, and Veazey RS: Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses 2005;21(10):863–868 [DOI] [PubMed] [Google Scholar]
- 53.Ison CA. and Hay PE: Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect 2002;78(6):413–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spira AI, Marx PA, Patterson BK, et al. : Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 1996;183(1):215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keele BF, Li H, Learn GH, et al. : Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 2009;206(5):1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng B, Voltan R, Lim L, et al. : Rhesus macaque resistance to mucosal simian immunodeficiency virus infection is associated with a postentry block in viral replication. J Virol 2002;76(12):6016–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson DJ. and Yunis EJ: “Trojan Horse” leukocytes in AIDS. N Engl J Med 1983;309(16):984–985 [PubMed] [Google Scholar]
- 58.Kashuba AD, Dyer JR, Kramer LM, Raasch RH, Eron JJ, and Cohen MS: Antiretroviral-drug concentrations in semen: Implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother 1999;43(8):1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virgin HW. and Walker BD: Immunology and the elusive AIDS vaccine. Nature 2010;464(7286):224–231 [DOI] [PubMed] [Google Scholar]
- 60.Vernazza PL: HIV in semen: Still more to be learned. AIDS Res Ther 2005;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolodkin-Gal D, Hulot SL, Korioth-Schmitz B, et al. : Efficiency of cell-free and cell-associated virus in mucosal transmission of human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 2013;87(24):13589–13597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ndirangu J, Viljoen J, Bland RM, et al. : Cell-free (RNA) and cell-associated (DNA) HIV-1 and postnatal transmission through breastfeeding. PloS One 2012;7(12):e51493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slyker JA, Chung MH, Lehman DA, et al. : Incidence and correlates of HIV-1 RNA detection in the breast milk of women receiving HAART for the prevention of HIV-1 transmission. PloS One 2012;7(1):e29777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valea D, Tuaillon E, Al Tabaa Y, et al. : CD4+ T cells spontaneously producing human immunodeficiency virus type I in breast milk from women with or without antiretroviral drugs. Retrovirology 2011;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samet JH, Horton NJ, Traphagen ET, Lyon SM, and Freedberg KA: Alcohol consumption and HIV disease progression: Are they related? Alcoholism Clin Exp Res 2003;27(5):862–867 [DOI] [PubMed] [Google Scholar]
- 66.Cook RT, Stapleton JT, Ballas ZK, and Klinzman D: Effect of a single ethanol exposure on HIV replication in human lymphocytes. J Invest Med 1997;45(5):265–271 [PubMed] [Google Scholar]
- 67.Ickovics JR, Hamburger ME, Vlahov D, et al. : Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV epidemiology research study. JAMA 2001;285(11):1466–1474 [DOI] [PubMed] [Google Scholar]
- 68.Farzadegan H, Hoover DR, Astemborski J, et al. : Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998;352(9139):1510–1514 [DOI] [PubMed] [Google Scholar]
- 69.Junghans C, Ledergerber B, Chan P, Weber R, and Egger M: Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1999;353(9152):589. [DOI] [PubMed] [Google Scholar]
- 70.Gustafsson R, Ahrne S, Jeppsson B, et al. : The Lactobacillus flora in vagina and rectum of fertile and postmenopausal healthy Swedish women. BMC Women's Health 2011;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eschenbach DA, Davick PR, Williams BL, et al. : Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 1989;27(2):251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simoes JA, Hashemi FB, Aroutcheva AA, et al. : Human immunodeficiency virus type 1 stimulatory activity by Gardnerella vaginalis: Relationship to biotypes and other pathogenic characteristics. J Infect Dis 2001;184(1):22–27 [DOI] [PubMed] [Google Scholar]
- 73.Hashemi FB, Ghassemi M, Roebuck KA, and Spear GT: Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis 1999;179(4):924–930 [DOI] [PubMed] [Google Scholar]