Abstract
A molecular analysis of HIV-1 subtypes and recombinants circulating in cities in the Russian Far East was performed. The study included samples from 201 outpatients from Vladivostok, Khabarovsk, and Blagoveshchensk. In most parts of Russia, patients are infected with HIV-1 subtype A, known as the IDU-A variant. Subtype B, including the IDU-B variant, is rare in Russia but widespread in the Ukraine, and the CRF02_AG is prevalent in Central Asian countries and Siberia, Russia. One of the challenges of this study in the Far East was to determine whether the molecular landscape of HIV infection in this region is influenced by the bordering countries, including China and Japan, where a distinct set of HIV subtypes is circulating, such as B′, C, and CRF01_AE. The distribution of HIV-1 genetic variants in the cities studied was as follows: subtype A (IDU-A), 55.7%; subtype B, 25.3% (IDU-B variant—24.3%); subtype C, 10.0%; CRF02_AG, 1.5%; and CRF63_02A1, 7.5%. A phylogenetic analysis confirmed the relationship of subtype A viruses with the IDU-A variant predominating in Ukraine, Russia and other former Soviet Union (FSU) countries, of subtype B viruses with IDU-B in the Ukraine and of CRF02_AG variants with variants in Uzbekistan, Russia, and other former USSR countries. Subtype C sequences were not uniform, and most clustered between each other and HIV-1 sequences originating from Africa; there was only one sample possibly related to Chinese variants. Thus, despite close cultural and commercial relationships among Russia, China, and Japan, the distribution of HIV-1 subtypes in the Russian Far East is still primarily influenced by contacts with the countries of the former USSR.
Introduction
The large-scale HIV epidemic in Russia started approximately 20 years ago, and the incidence rate of HIV infection in Russia is still among the highest in the world. According to the Russian Federal AIDS Center (www.hivrussia.org), the cumulative number of HIV cases in Russia as of December 2012 was 720,000, with approximately 50,000 new cases reported each year. The prevalence of HIV was 428.8 per 100,000 people, and the average incidence in 2012 was 37.7 per 100,000 people in the overall population.
When the epidemic started in mid-1990s, it primarily affected injecting drug users (IDUs) and their immediate sexual partners. The first outbreaks of HIV infection were registered in Central Russia1,2 and St. Petersburg,3 with subsequent distribution in all Eastern European and certain Siberian regions. At this stage, the epidemic was nearly fully homogenic and was caused by the subtype A virus, which was widespread among IDUs in the Ukraine and was designated as IDU-A.4 More recent studies showed that the massive Ukrainian epidemic resulted from a single event introducing the A1 genetic variant, which most likely originated from the Republic of Guinea5 or the Democratic Republic of Congo.3 The IDU-A variant proved to be very tenacious and, during subsequent years, caused the epidemics in Belarus,6 Kazakhstan,7 Kyrgyzstan,8 and other FSU countries.
Another HIV-1 strain was found in the IDU population in southern Ukraine in 19949,10; this strain belonged to subtype B and was given the name IDU-B. This genetic variant is still relatively widespread in the Ukraine11 but does not have a pronounced effect in Russian HIV epidemics, as it is rarely found in IDUs and heterosexuals.
According to early work,9 both of these strains gave rise to the recombinant form CRF03_AB, which caused a sizable HIV outbreak in the Kaliningrad region (an enclave in Western Russia) in 1998–1999. More recent work10 found the origin of subtype B to be unclear. This HIV genetic form has been found only occasionally in different Russian cities over many years. A marked explosion of HIV infection caused by CRF03_AB was registered in the city of Cherepovets, Vologda region, Northern Russia, in 2004–2005.12 CRF03_AB is also highly prevalent in Ekaterinburg, Central Russia, where it is responsible for 23% of infections.13
Finally, HIV subtype B, which is different from IDU-B and is called the “Western B variant,” was detected in men who have sex with men (MSM), but only with low prevalence relative to the overall epidemiology.
All of these observations were mostly made in the European parts of Russia and Siberia14 due to research laboratories concentrating on these areas. The molecular epidemiological studies were conducted by different groups of specialists during the first decade of the HIV epidemic in Russia. As for the current situation, studies have confirmed the predominance of the IDU-A variant in all of the territories explored, with subtype B being much less common and with other subtypes and recombinants being detected occasionally. However, certain tendencies are gradually changing the molecular landscape of HIV infection in Russia.15 Among them are the stable increase in heterosexuals among newly HIV-infected people; the emergence and increasing distribution of HIV recombinant forms, such as CRF02_AG and its derivatives16 and new recombinants between the IDU-A and the IDU-B subtypes17; and the mutual penetration of genetic variants between risk groups.
To date, certain regions distant from the main territories have never been involved in regular HIV molecular monitoring studies. From this perspective, one of the most interesting areas is the Far Eastern region (or the so-called Russian Far East), where no work has been performed on the surveillance of HIV subtypes in the general population. This area is located close to Japan and China, where HIV is highly prevalent (Fig. 1).
FIG. 1.
Map of Russia showing the geographic localization of the cities studied.
Historically, the Far East served as a Russian military and commercial outpost, participating in not only local but also worldwide communications. The region continues to be industrially and commercially useful, although it has an acute shortage of labor forces, which was aggravated during the past decades due to political changes. The main cities of interest are the largest Russian seaport, Vladivostok, on the Japanese seashore, which works on a global scale, and two cities, Khabarovsk and Blagoveshchensk on the Amur River, separating the Russian and Chinese territories. These three cities have been attracting considerable interest from merchants, tourists, and labor migrants from both remote countries and FSU countries, including Russia and the Ukraine. Regarding the bordering countries, the region has very intensive trade connections with both Japan and China, although few Japanese people live in the Russian Far East. The situation of Chinese people is relatively different; according to the last official data (Russian Census, 2002) the number of residents originating from China was 9,677, but unofficial opinion estimates that the number of Chinese ranges from several hundred thousand to two million people, with most of them being illegal labor migrants or tradesmen. Trade and social relations with North Korea are limited, with little known about HIV infection in this country.
The HIV epidemics in China and Japan differ not only from each other but also from Russia. In the first stage of the epidemic, HIV transmission in China mainly occurred among IDUs and plasma donors, but now heterosexual sex has become the dominant transmission route.18,19 Sex between men accounts for 1.3–17.7% of new infections. In contrast, male-to-male sexual contacts cause the majority of new HIV cases in Japan (61.8% of all new cases), whereas very few HIV cases are the result of intravenous drug usage. Additionally, approximately one-third of HIV-positive people are infected through heterosexual contacts.20 There is a significant distinction between the prevalence of HIV subtypes in China and Japan as well. The HIV infection epidemic in China is driven by four major strains, including the prevailing strain, subtype B′ (founded by a single lineage of pandemic subtype B); CRF07_BC; CRF08_BC; and CRF01_AE.21,22 The pandemic variant of subtype B is predominant in Japan, with a frequency of 88.7%, followed by CRF01_AE and C.23 Hence, the geographic localization of and growing economic and social relations between Russia, China, and Japan are the basis for possible HIV transmission due to residents' and visitors' risky behavior. Therefore, the penetration of unusual HIV subtypes into the local population may occur, and the regional molecular landscape may predictably differ from that of the main Russian territory.
To test this hypothesis, HIV samples collected from outpatients visiting clinics at AIDS centers in Vladivostok, Khabarovsk, and Blagoveshchensk and their surroundings were studied (Fig. 1). These cities are the administrative centers of Primorsky, Khabarovsky, and Amursky Krays, respectively. In December 2012 (www.hivrussia.org), 10,376, 1,996, and 410 individuals, respectively, were registered as HIV positive in these territories. The main routes of HIV transmission are intravenous drug usage and heterosexual contacts, with the latter becoming dominant during the past few years. The large-scale program of antiretroviral treatment in this region started in the early 2000s, concurrently with programs in other Russian regions.
Materials and Methods
Blood samples from both naive (152) and treated (56) patients were collected in 2011–2012 when they came to AIDS centers in Vladivostok, Khabarovsk, and Blagoveshchensk for regular examination, without any additional criteria. All individuals were diagnosed as HIV positive in the period between 2002 and 2012. A questionnaire, collecting information including age, sex, transmission group, the date of diagnosis, and the place of infection, among other information, was answered by each of the patients; certain epidemiological characteristics are presented in Table 1. Each participant provided informed consent following approval from the ethical review committee (SRC VB VECTOR, IRB00001360, FWA00000621).
Table 1.
Epidemiological Data and HIV-1 Subtypes in Individuals Studied
| Transmission route | Gender | HIV-1 subtype (pol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Residence | Number of patients | IDU | Het | Other | Male | Female | A | B | C | CRF02_AG | CRF63_02A1 |
| Blagoveshchensk | 40 | 14 | 25 | 1 | 28 | 12 | 37 | 1 | — | — | 2 |
| Khabarovsk | 88 | 59 | 29 | — | 27 | 61 | 58 | 11 | 4 | 2 | 13 |
| Vladivostok | 73 | 42 | 30 | 1 | 33 | 40 | 17 | 39 | 16 | 1 | — |
| 201 | 115 (57.2%) | 84 (41.8%) | 2 (1.0%) | 88 (43.8%) | 113 (56.2%) | 112 (55.7%) | 51 (25.3%) | 20 (10.0%) | 3 (1.5%) | 15 (7.5%) | |
HIV-1 subtypes according to REGA/Comet proved by phylogenetic analysis.
IDU, intravenous drug use; HET, heterosexual.
Peripheral blood mononuclear cells (PBMCs) were washed and pelleted, and both the PBMCs and plasma were then frozen and kept in the laboratories until transportation to Moscow. Total RNA (viral load ranging from 40 to 1,490,000 RNA copies/ml) and proviral DNA were extracted using the DNA/RNA extractor QIAcube (QIAGEN, Hilden, Germany) following the manufacturer's instructions. HIV-1 sequences [pol gene; full protease (PR) and partial reverse transcriptase (RT) regions], with 1,302 or 914 nucleotides in total, depending of the method, were obtained in two different ways. A standard method, using the ViroSeq HIV-1 Genotyping System v. 2.0 (Celera Diagnostics, Alameda, CA), was applied to the samples with a viral load >500 copies/ml, and an in-house method of amplification of proviral DNA8 was used when the viral load was <500 copies/ml or undetectable. The genetic analyzer ABI Prism 3130 (Applied Biosystems, Foster City, CA) was used for automated DNA sequencing. Sequences from individual primers were assembled using the program BioEdit version 7.0.5.3 (www.mbio.ncsu.edu/bioedit/bioedit.html), with the HXB2 strain as the reference.
The assembled sequences and HIV-1 reference strains, taken from the Los Alamos HIV database (www.hiv.lanl.gov), were aligned using MEGA5.0.24 The search of the best nucleotide substitution model for phylogenetic analysis was performed using jModelTest v.0.1.1 (http://darwin.uvigo.es/). Phylogenetic trees were constructed using the PhyML tool by the maximum likelihood method, the bootstrap method of branch support (100 replicates), and the GTR+gamma nucleotide substitution model (www.hiv.lanl.gov). Optimization of phylogenetic trees was carried out by FigTree v.1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/). To find the HIV sequences that were most similar to the samples studied, the program HIV BLAST (www.hiv.lanl.gov/content/sequence/BASIC_BLAST) was used.
For HIV-1 subtype determination, before the phylogenetic analysis, online reference programs were used, including the REGA HIV-1 Subtyping Tool (version 2) (http://hivdb.stanford.edu) and COMET HIV-1 (http://comet.retrovirology.lu/) (“REGA/Comet analysis”). To analyze possible recombination, a Recombinant Identification Program (RIP) was applied (www.hiv.lanl.gov/content/sequence/RIP/RIP.html). For drug resistance analysis, an HIVdb Program: Sequence Analysis (http://sierra2.stanford.edu/sierra/servlet/JSierra?action=sequenceInput) was employed.
Results
The REGA/Comet subtype analysis of the pol regions [protease (PR) and reverse transcriptase (RT)] showed that the cities studied essentially differ from each other (Tables 1 and 2). In Blagoveshchensk, the overwhelming majority of patients were infected with HIV-1 subtype A1 (92.5%; 37/40), followed by CRF02_AG and subtype B, which were found in two patients and one patient of 40, respectively. Most infections resulted from heterosexual contacts (62.5%; 25/40), and only subtype A1 was detected in IDUs (35.0%; 14/40).
Table 2.
The Distribution of HIV-1 Subtypes by Routes of Transmission
| Subtype A1 | Subtype B | Subtype C | CRF02_AG | |||||
|---|---|---|---|---|---|---|---|---|
| IDUs | Het | IDUs | Het | IDUs | Het | IDUs | Het | |
| Blagoveshchensk | 14 | 22 | — | 1 | — | — | — | 2 |
| Khabarovsk | 31 | 27 | 10 | 1 | 3 | 1 | 15 | — |
| Vladivostok | 9 | 8 | 26 | 13 | 7 | 8 | — | 1 |
| 54 | 57 | 36 | 15 | 10 | 9 | 15 | 3 | |
HIV-1 subtypes according to REGA/Comet analysis.
In Khabarovsk, the virus seemed to be much more diverse, with the greatest variety found in the IDU group. Subtype A1 was the most common in this risk group (52.5%; 31/59), and subtype B (16.9%; 10/59), CRF02_AG (25.4%; 15/59) and subtype C (5.1%; 3/59) were additionally found. The majority of heterosexually infected individuals displayed subtype A1 (93.1%; 27/29), and subtypes B and C were found in two cases.
The most striking results were revealed in Vladivostok, where subtype B prevailed in IDUs (61.9%; 26/42) and was widespread in heterosexuals (43.3%; 13/30). Subtype A1 was relatively abundant in the population studied as well (17/73; 23.3%). A significant proportion of viruses was represented by subtype C (16/73; 21.9%), and CRF02_AG was found in one patient.
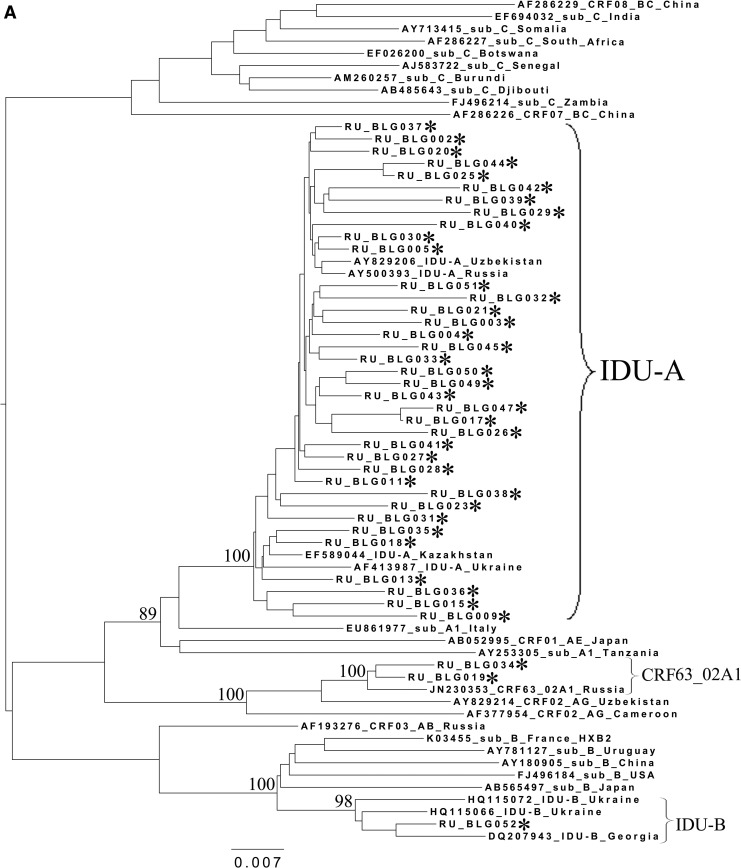
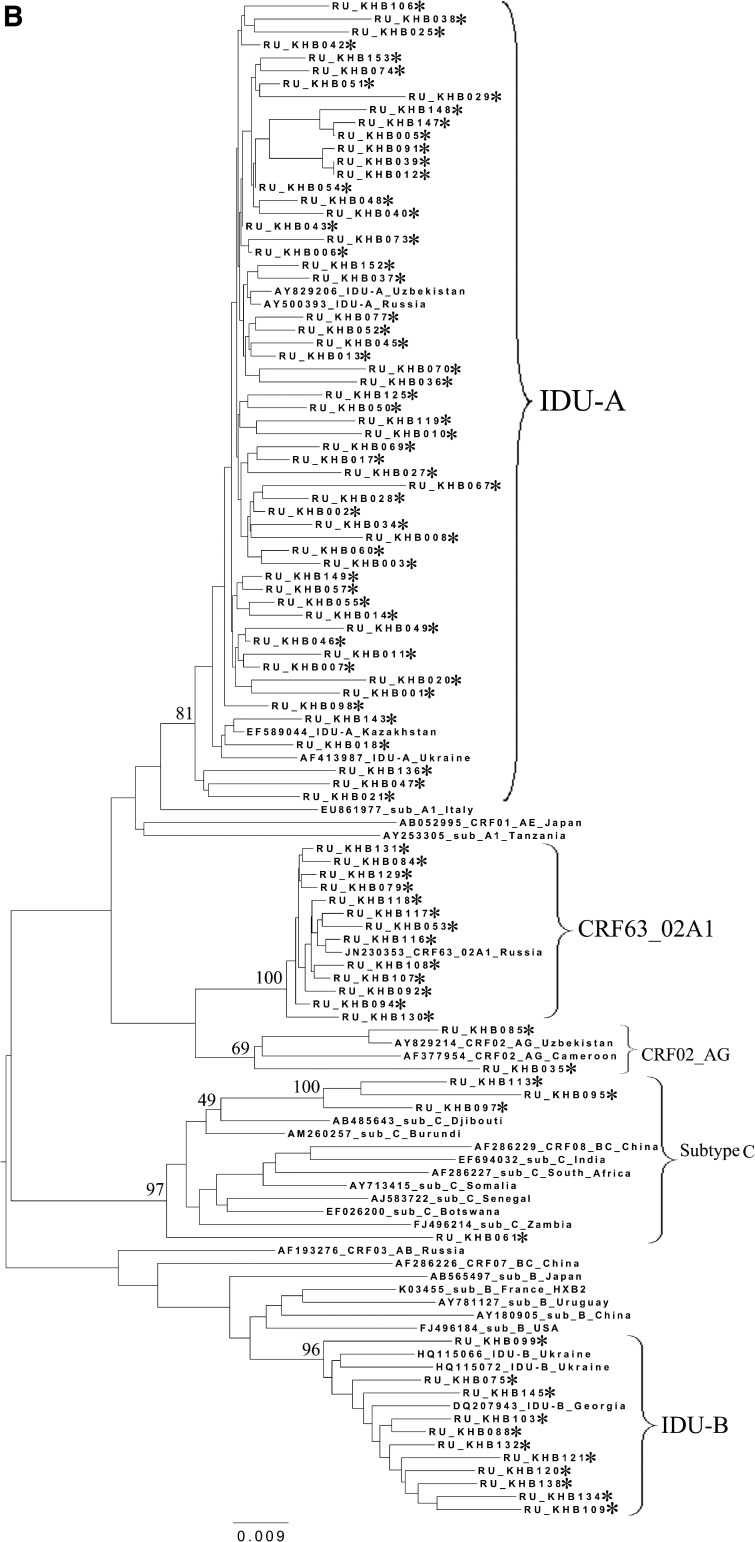
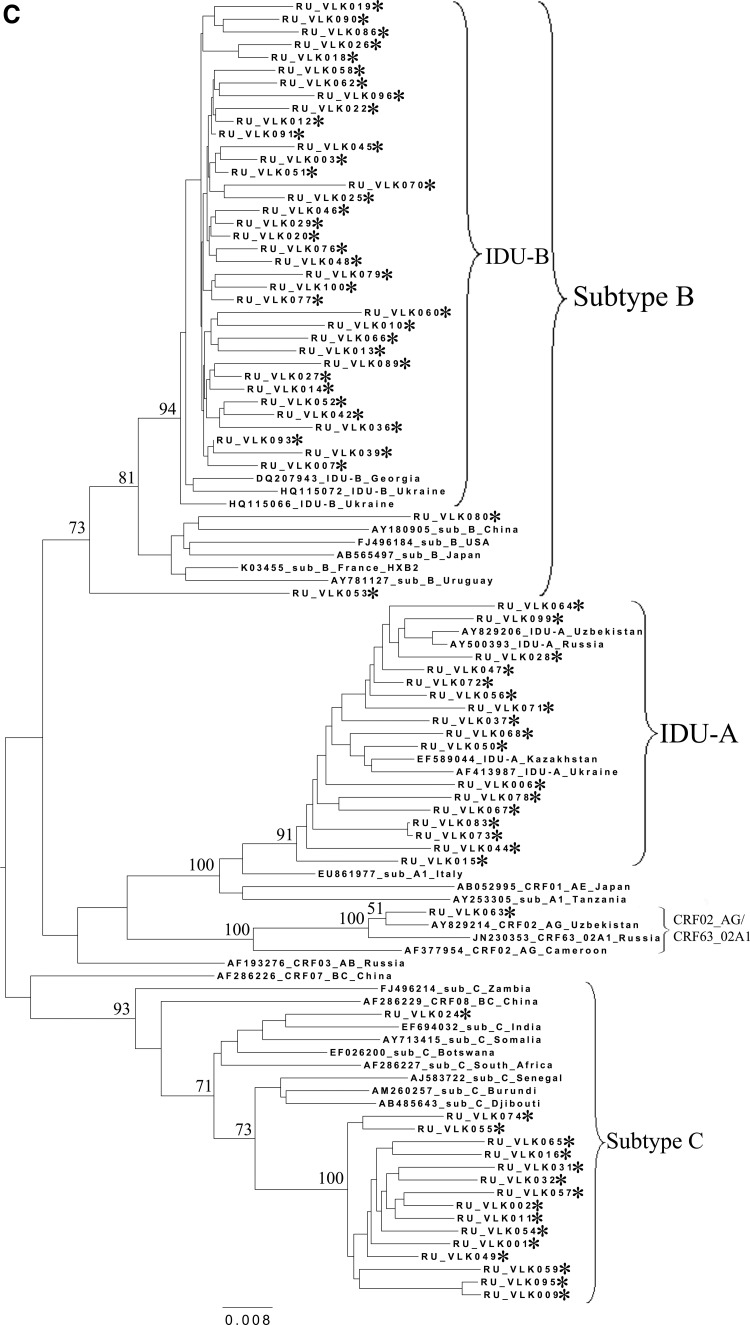
To analyze the possible origin of the HIV-1 variants circulating in the cities studied, a phylogenetic analysis of the pol sequences coding for the PR and part of the RT was performed (Fig. 2). A set of subtype reference sequences included the sequences originating from China and Japan. The IDU-B sequences were obtained from the patients in the Ukraine (HQ115066 and HQ115072) and Georgia (DQ207943) and proved to belong to the IDU-B variant by comparison with IDU-B samples kindly provided by A. Masharsky10 (not included in the phylogenetic trees due to the shorter length); all the patients were infected in Nikolaev, the Ukraine (personal communication).
FIG. 2.
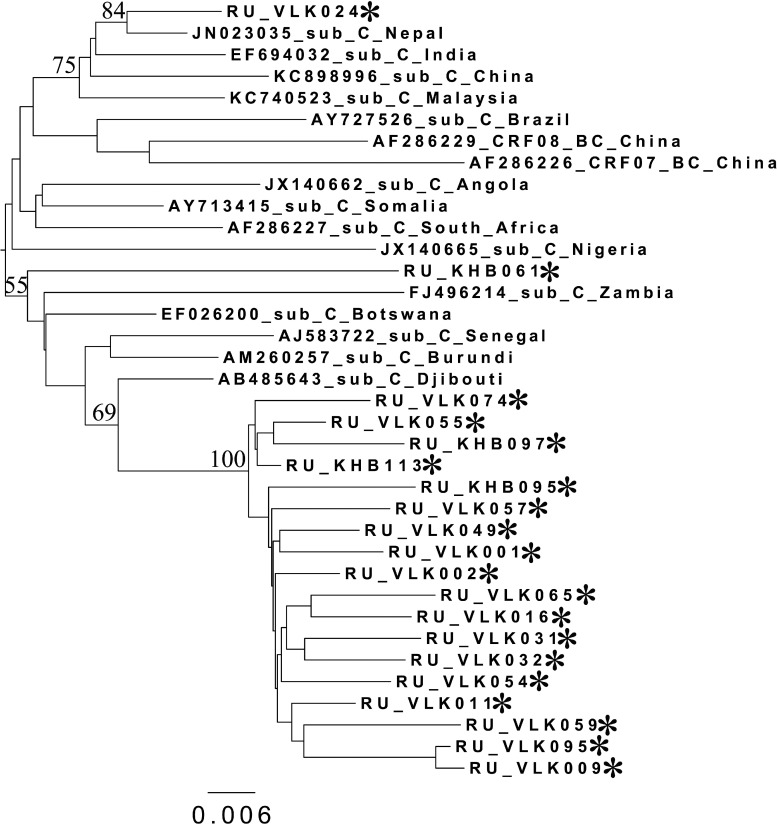
Phylogenetic analysis of HIV-1 pol sequences coding the Pro and reverse transcriptase (RT) regions from samples collected in Blagoveshchensk (2253–3554 bp) (A), Khabarovsk (2253–3554 bp) (B), and Vladivostok (2253–3167 bp) (C). A phylogenetic tree was constructed using the PhyML tool by the maximum likelihood method of phylogeny, the bootstrap method of branch support (100 replicates), and the GTR+gamma nucleotide substitution model. Optimization of the phylogenetic tree was carried out by FigTree v.1.3.1. Nucleotide positions are shown with respect to the HXB2 genome (GenBank number K03455). The sequences obtained in this study are marked with snowflakes. Reference isolates are represented by their subtypes and sampling countries. Node support values as percentages are indicated at the nodes.
Figure 2A–C shows the phylogenetic trees for Blagoveshchensk, Khabarovsk, and Vladivostok, respectively. As may be observed in the figures, in 100% of cases, the subtype A1 sequences clustered close together with the sequences typical of the FSU countries' subtype A1 strain, IDU-A.4
All CRF02_AG variants clustered with each other and with recombinants from other FSU countries, such as Uzbekistan25,26 and Russia, including the newly registered CRF63_02A1 variant.16,17 Recently, similar HIV-1 variants were found in Kazakhstan27 and Kyrgyzstan.8 For example, the Khabarovsk sample RU_KHB035 (Fig. 2B) formed a common branch with an Uzbekistan strain, which may be indicative of the possible penetration of HIV immediately from the territory of this bordering country. Furthermore, most of the Khabarovsk sequences (13/15) shared a common node branch with the CRF63_02A1 variant from Russia, suggesting common ancestry. The CRF02_AG samples from Blagoveshchensk and Vladivostok clustered close to CRF63_02A1 and the Uzbekistan sequences, respectively.
Regarding the HIV-1 subtype C variants from Khabarovsk and Vladivostok, most of them (18/20) clustered together on the phylogenetic tree (Figs. 2B, 2C, and 3), suggesting the epidemiological links between them. Interestingly, most of these samples belonged to either IDU men or heterosexual women. There were two outlier samples from Vladivostok (RU_VLK024) and Khabarovsk (RU_KHB061), which were subjected to further analysis by RIP and BLAST programs. As was shown, both of them belonged to subtype C and showed maximum similarity with Indian/Nepal/China and African (Burundi) HIV-1 sequences, correspondingly.
FIG. 3.
Phylogenetic analysis of HIV-1 pol sequences coding the Pro and RT regions (2253–3179 bp) from subtype C samples analyzed in this study and reference sequences of subtype C and BC recombinants. The phylogenetic tree was constructed using the PhyML tool by the maximum likelihood method of phylogeny, the bootstrap method of branch support (100 replicates), and the GTR+gamma nucleotide substitution model. The optimization of the phylogenetic tree was carried out by FigTree v.1.3.1. Nucleotide positions are shown with respect to the HXB2 genome (GenBank number K03455). Samples from Khabarovsk and Vladivostok are marked as RU_KHB*** and RU_VLK***, respectively. Reference isolates are represented by their subtypes and sampling countries. Node support values as percentages are indicated at the nodes.
Nearly all HIV-1 subtype B variants from the Far East were very close to each other and differed from Chinese and Japanese strains and the subtype B variants found in other countries. Of prime importance was that subtype B's origin was in the Ukraine, where this variant predominated. As can be clearly observed, the majority of the sequences studied clustered with the Eastern European variant of subtype B (IDU-B9). There were only two sequences (RU_VLK053 and RU_VLK080) outside the common branch and close to the pandemic (“Western B”) variant of HIV-1 subtype B. This variant was previously found in Russia in MSM in contact with developed countries in Europe and North America. The epidemiological data confirmed that RU_VLK080 belonged to an MSM patient. As to the other one (RU_VLK053), it was obtained from a woman infected through heterosexual contact. The detailed analysis using RIP classified it as the unique recombinant formed by subtype C (protease) and subtype B (reverse transcriptase) and having no relationship with CRF07_BC and CRF08_BC. All of the data described above were confirmed when studied using the HIV BLAST program.
The presence of mutations that conferred resistance to available first-line regimens was evaluated in all patients. Fourteen sequences were found to bear drug resistance mutations, among which seven samples belonged to antiretroviral treatment (ART) recipients and seven belonged to naive patients (4.6%; 7/152) (Table 3). Among the naive patients, the mutations K103N, M184V, T215Y, and Y181C were detected, all from the WHO 2009 List of Mutations for Surveillance of Transmitted Drug Resistant HIV Strains.28 The lack of epidemiological data made it difficult to conclude with certainty whether the mutations observed among the drug-naive patients were transmitted.
Table 3.
The Results of Resistance Mutations Analysis (WHO List, 2009)
| Number of naïve patients | Number of mutation bearing sequences in naïve patients (%) | Number of patients on ART | Number of mutation bearing sequences in patients on ART | |
|---|---|---|---|---|
| Blagoveshchensk | 39 | 3 (7.7) | 1 | 0 |
| Khabarovsk | 77 | 3 (3.9) | 11 | 2 |
| Vladivostok | 36 | 1 (2.8) | 37 | 5 |
ART, antiretroviral treatment.
Discussion
This study was the first to examine HIV-1 subtypes and recombinants circulating in the Russian Far East. The study is limited by the small number of patients available and can provide only a rough idea about the prevalence of HIV-1 variants in this geographic area. Even so, these first observations were interesting and relatively unexpected.
At the beginning of this study, no data on the prevalence of HIV subtypes in these regions were available. The only exception was our own study, conducted in 2000 and based on heteroduplex mobility assay results, which revealed the uniform HIV-1 subtype A variant in 28 Khabarovsky and Amursky Krays residents (unpublished data).
The massive propagation of HIV-1 by drug usage in the Far East started in 1999, when the main territory of Russia was already seized by an epidemic of HIV infection. The IDU-A variant was first introduced into Russia after 1995 and very rapidly spread among IDUs, thus becoming the founder strain for the entire epidemic. Not surprisingly, the overwhelming distribution of IDU-A in the European and Siberian parts of Russia influenced the Far East region, and the 2000 study mentioned above confirmed this assumption. Thus, our most predictable finding in the Far East was the wide prevalence of the IDU-A variant, which is typical of all of the Russian territories (55.7% in total). As Table 2 shows, subtype A1 was equally represented by IDUs and heterosexuals, which corresponded to the growth of the sexual route of HIV transmission in the epidemiological structure as a whole.
The main hypothesis of the present study was that HIV-1 variants that were different from the strains circulating in the main Russian territory and that were typical of the bordering countries, consisting of China and Japan, would be found in the Russian Far East. In practice, this speculation was nearly completely disproved. The molecular landscape of the Far East region was in fact relatively diverse and different from that of Central Russia. In the entire period of the HIV epidemic molecular monitoring in Russia, this study was the first to reveal that subtypes B and C caused infection in large groups of patients. Nevertheless, there was little evidence that B and C virus infections are significantly influenced by the close relationship between Russian citizens and travelers from China and Japan.
All but two sequences belonging to subtype B clustered together with the IDU-B variant; this variant is now widespread in the Ukraine and caused the explosion of HIV infection among IDUs in the 1990s. The migration of the Ukrainian labor forces to the Far East region started in the mid-1990s and is currently very intensive. The origin of subtype B in the cities studied was apparently associated with this social phenomenon. In view of this observation, the distribution of subtype B cases between IDUs and heterosexuals (36:15) is not surprising and can be explained by subtype B's heterosexual transmission from IDUs to their partners.
The cases of HIV-1 subtype C infection were extraordinarily frequent and, in fact, represented the first detection of an outbreak of the epidemic caused by this genetic variant in Russia.
The phylogenetic analysis showed the close cases' relationships between each other, except for two cases, and to the African subtype C variants. The majority of subtype C-infected patients were found in Vladivostok, a city known as a large seaport where contact with many countries, including African countries, occurs. Thus, this subtype C explosion was possibly caused by heterosexual transmission from travelers abroad. The subtype C samples were almost equally distributed between IDUs and heterosexuals (7:8) (in one case the route of transmission was unknown). So, the transmission from heterosexuals to IDUs could occur, which opens new fields of work on HIV prevention in the region. Moreover, the influence of China on the Far East epidemic cannot be excluded because one sample (RU_VLK024) demonstrated a close relationship with Chinese and Indian HIV-1 sequences. It is well known that Chinese migrants in this region try to maintain a traditional lifestyle and do not have much contact with the residents. The abovementioned findings suggest that this situation may gradually change.
Regarding the cases of HIV infection caused by CRF02_AG and CRF63_02A1, most (83.3%; 15/18) were found in Khabarovsk and in the IDU group only. The corresponding sequences formed common branches with HIV-1 samples from FSU countries in Central Asia, such as Uzbekistan25,26 and Russia.16 It is worth noting, that the epidemiological data did not find any links between the patients with dates of infection ranging from 2001 to 2011. Moreover, 10 of 15 patients reported an infection incidence far outside Khabarovsky Kray. The influx of Central Asian and Siberian labor forces to the Far East region has been increasing annually, so the penetration of HIV-1 genetic forms typical of Uzbekistan, Russia, and other countries was relatively predictable.
In summary, this study demonstrates that despite the great variety of HIV-1 subtypes in the bordering countries, the molecular epidemiological situation in the Russian Far East is still determined by links with FSU countries, and primarily the Ukraine, Uzbekistan, and the major territory of Russia. The subtype A variant, which predominated in Russia and other FSU countries in previous years, is still the most prevalent, accounting for 55.7% of all subtypes in the region. The subtype B variant, normally found in the Ukraine, was the second most prevalent (24.3%) in this study. Similarly, the CRF02_AG and CRF63_02A1 viruses (9.0% in total), derived from Uzbekistan and Russian regions, came to the Far East with great expectancy. The only HIV-1 genetic variant clearly originating from abroad was subtype C (10.0%), found mainly where sailors arrived and likely originating from the countries that trade with Russia. The possible influx of Chinese subtype C variants was noted for the first time. All of these epidemiological hypotheses should receive further support through the extension and prolongation of studies in this in many ways interesting region.
Sequence Data
The GenBank accession numbers for the sequences described in this article are KC665916–KC665944, KC509837–509885, KC156532, KC156533, KC208003–208005, KC254581–254615, KF177159–KF177168, KC312893–KC312930, KC701380–KC701384, KF205368–KF205391, KF220493, KF290961–KF290993, KF971919–KF971945, KF768002, KF768004–KF768008, and KJ466102.
Acknowledgments
We thank our colleagues from the Vladivostok, Khabarovsk, and Blagoveshchensk AIDS centers, namely, Lydia Sklyar, Svetlana Kruglyak, Galina Voronsova, Anna Kuznetsova, Ekaterina Loyfman, Irina Staruchina, Olga Kustova, Natalya Lipskaya, and Natalya Polovica for collecting the blood samples and epidemiological data. The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under the project “Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN)”—grant agreement N 223131. The study was supported in part by the Ministry of Education and Science of the Russian Federation, agreements 8148, 8154, and 8479.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bobkov AF, Pokrovskii VV, Selimova LM, et al. : [Genotyping and phylogenetic analysis of HIV-1 isolates circulating in Russia]. Vopr Virusol 1997;42(1):13–16 [PubMed] [Google Scholar]
- 2.Bobkov AF, Pokrovskii VV, Selimova LM, et al. : [Genetic characteristics of variants of human immunodeficiency virus type 1, causing an epidemic among substance abusers in Commonwealth of Independent States countries]. Vopr Virusol 1998;43(6):253–256 [PubMed] [Google Scholar]
- 3.Thomson MM, de Parga EV, Vinogradova A, et al. : New insights into the origin of the HIV type 1 subtype A epidemic in former Soviet Union's countries derived from sequence analyses of preepidemically transmitted viruses. AIDS Res Hum Retroviruses 2007;23(12):1599–1604 [DOI] [PubMed] [Google Scholar]
- 4.Bobkov A, Cheingsong-Popov R, Selimova L, et al. : An HIV type 1 epidemic among injecting drug users in the former Soviet Union caused by a homogeneous subtype A strain. AIDS Res Hum Retroviruses 1997;13(14):1195–1201 [DOI] [PubMed] [Google Scholar]
- 5.Riva C, Romano L, Saladini F, et al. : Identification of a possible ancestor of the subtype A1 HIV Type 1 variant circulating in the former Soviet Union. AIDS Res Hum Retroviruses 2008;24(10):1319–1325 [DOI] [PubMed] [Google Scholar]
- 6.Lazouskaya NV, Eremin VF, Adema KW, et al. : The HIV type 1 epidemic in Belarus: Predominance of Eastern European subtype A strains and circulation of subtype B viruses. AIDS Res Hum Retroviruses 2005;21(9):830–833 [DOI] [PubMed] [Google Scholar]
- 7.Bobkov AF, Kazennova EV, Sukhanova AL, et al. : An HIV type 1 subtype A outbreak among injecting drug users in Kazakhstan. AIDS Res Hum Retroviruses 2004;20(10):1134–1136 [DOI] [PubMed] [Google Scholar]
- 8.Laga V, Kazennova EV, Vasil'ev AV, et al. : [Molecular-genetic characterization of the HIV-1 variants abundant in Kirghizia]. Vopr Virusol 2012;57(5):26–32 [PubMed] [Google Scholar]
- 9.Bobkov A, Kazennova E, Selimova L, et al. : A sudden epidemic of HIV type 1 among injecting drug users in the former Soviet Union: Identification of subtype A, subtype B, and novel gagA/envB recombinants. AIDS Res Hum Retroviruses 1998;14(8):669–676 [DOI] [PubMed] [Google Scholar]
- 10.Masharsky AE, Klimov NA, and Kozlov AP: Molecular cloning and analysis of full-length genome of HIV type 1 strains prevalent in countries of the former Soviet Union. AIDS Res Hum Retroviruses 2003;19(10):933–939 [DOI] [PubMed] [Google Scholar]
- 11.Saad MD, Shcherbinskaya AM, Nadai Y, et al. : Molecular epidemiology of HIV Type 1 in Ukraine: Birthplace of an epidemic. AIDS Res Hum Retroviruses 2006;22(8):709–714 [DOI] [PubMed] [Google Scholar]
- 12.Smolskaya T, Liitsola K, Zetterberg V, et al. : HIV epidemiology in the Northwestern Federal District of Russia: Dominance of HIV type 1 subtype A. AIDS Res Hum Retroviruses 2006;22(11):1074–1080 [DOI] [PubMed] [Google Scholar]
- 13.Dement'eva N, Sizova N, Lisitsyna Z, et al. : Analysis of HIV subtypes and drug-resistant variants circulating in Saint-Petersburg. HIV Infect Immune Disorders 2011;3(4):34–43 [Google Scholar]
- 14.Kazennova E, Vasilyev A, Lapovok I, et al. : HIV-1 genetic variants in Asian part of Russia: Study 2005–2010. Vopr Virusol 2013;58(4):28–35 [PubMed] [Google Scholar]
- 15.Bobkova M: Current status of HIV-1 diversity and drug resistance monitoring in the former USSR. AIDS Rev 2013;15(4):204–212 [PubMed] [Google Scholar]
- 16.Baryshev PB, Bogachev VV, and Gashnikova NM: Genetic characterization of an isolate of HIV type 1 AG recombinant form circulating in Siberia, Russia. Arch Virol 2012;157(12):2335–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gashnikova NM, Totmenin AV, Bocharov EF, et al. : [New recombinant variant of human immunodeficiency virus of type 1, subtype envB/envA, isolated in Novosibirsk]. Zh Mikrobiol Epidemiol Immunobiol 2004;(5):53–58 [PubMed] [Google Scholar]
- 18.UNAIDS: Report on the global AIDS epidemic, 2008
- 19.HIV & AIDS in China, 2013; Available from www.avert.org/aidschina.htm
- 20.Masaki I. The current situation of HIV/AIDS epidemic in Japan, 2012. Available from www.ajf.gr.jp/aidsinjapan/stats.htm
- 21.Li Z, He X, Wang Z, et al. : Tracing the origin and history of the HIV-1 subtype B′ epidemic by near full-length genome analyses. AIDS 2012;26(7):877–884 [DOI] [PubMed] [Google Scholar]
- 22.Liao L, Xing H, Shang H, et al. : The prevalence of transmitted antiretroviral drug resistance in treatment-naive HIV-infected individuals in China. J Acquir Immune Defic Syndr 2010;53(Suppl 1):S10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima Y, Kawahata T, Mori H, et al. : Prevalence and epidemiological traits of HIV infections in populations with high-risk behaviours as revealed by genetic analysis of HBV. Epidemiol Infect 2013;141(11):2410–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Peterson D, Peterson N, et al. : MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28(10):2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carr JK, Nadai Y, Eyzaguirre L, et al. : Outbreak of a West African recombinant of HIV-1 in Tashkent, Uzbekistan. J Acquir Immune Defic Syndr 2005;39(5):570–575 [PubMed] [Google Scholar]
- 26.Kurbanov F, Kondo M, Tanaka Y, et al. : Human immunodeficiency virus in Uzbekistan: Epidemiological and genetic analyses. AIDS Res Hum Retroviruses 2003;19(9):731–738 [DOI] [PubMed] [Google Scholar]
- 27.Laga V, Lapovok I, and Vasilyev A: CRF02_AG recombinants in Russia and Central Asia countries. 14th European AIDS Conference, October16–19, 2013, Brussels, Belgium [Google Scholar]
- 28.Bennett DE, Camacho RJ, Otelea D, et al. : Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009;4(3):e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]