Abstract
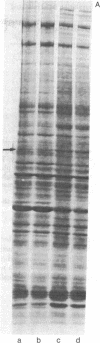
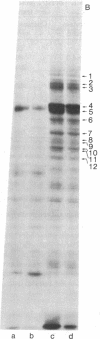
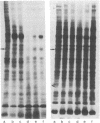
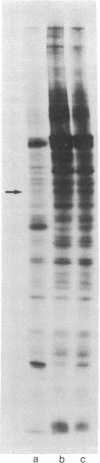
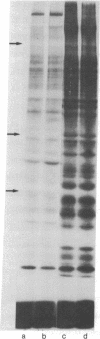
Protein phosphorylation in normal and in simian virus 40-transformed human skin fibroblasts was assessed by two different methods: incubation of whole-cell homogenates with [γ-32P]ATP or labeling of intact cells with Na2H32PO4. Phosphorylated proteins were detected by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and autoradiography. With both methods, the Coomassie-blue-stained protein patterns of the three transformed cell lines studied were similar to the patterns of the nontransformed normal human cells. However, although the phosphoprotein autoradiograms of the three transformed cell lines were nearly identical, their patterns were strikingly different from those of the nontransformed cells. Each of the three transformed lines tested showed approximately 25-30 phosphoprotein bands that were significantly enhanced when compared to the patterns of the nontransformed cells. Quantitation of 12 of the enhanced phosphoprotein bands in one of the transformed cell lines showed an average of 4.4 times as much phosphorylation as in the normal cells. The enhanced phosphorylation observed in the transformed cell lines was not dependent on the growth rate of the cells or on cyclic AMP. Furthermore, when homogenates of transformed and nontransformed cells were mixed prior to incubation with [γ-32P]ATP, the resultant phosphoprotein patterns resembed those obtained with transformed cells alone. In addition, an evaluation of the time course of protein phosphorylation revealed that the initial reaction rate was greater in the transformed than in the normal cells, although in both cell types the reaction was complete after 1 min. The results suggest that the simian virus 40-transformed human fibroblasts possess an increased ability to phosphorylate proteins rather than that the normal cells possess a diffusible inhibitor. There appear to be many endogenous cellular substrates for this increased activity.
Keywords: transformation, protein kinase, cancer
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byus C. V., Klimpel G. R., Lucas D. O., Russell D. H. Type I and type II cyclic AMP-dependent protein kinase as opposite effectors of lymphocyte mitogenesis. Nature. 1977 Jul 7;268(5615):63–64. doi: 10.1038/268063a0. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Zubialde J. P. Increase of cyclic AMP-dependent protein kinase type II as an early event in hormone-dependent mammary tumor regression. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1150–1155. doi: 10.1016/0006-291x(78)90662-9. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cell cycle-specific activity of type I and type II cyclic adenosine 3':5'-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J Biol Chem. 1976 Jun 10;251(11):3313–3319. [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. G1 specific increases in cyclic AMP levels and protein kinase activity in Chinese hamster ovary cells. Biochim Biophys Acta. 1976 Mar 4;425(2):246–255. doi: 10.1016/0005-2787(76)90031-9. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Walton K. G., Curran P. F., Greengard P. Regulation of phosphorylation of a specific protein in toad-bladder membrane by antidiuretic hormone and cyclic AMP, and its possible relationship to membrane permeability changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):880–884. doi: 10.1073/pnas.70.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J., Breslow J. L. Increased resistance of cystic fibrosis fibroblasts to ouabain toxicity. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1676–1679. doi: 10.1073/pnas.74.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Griffin B. E. Organization of the genomes of polyoma virus and SV40. Adv Cancer Res. 1977;24:67–113. doi: 10.1016/s0065-230x(08)61013-1. [DOI] [PubMed] [Google Scholar]
- Gharrett A. J., Malkinson A. M., Sheppard J. R. Cyclic AMP-dependent protein kinases from normal and SV40-transformed 3T3 cells. Nature. 1976 Dec 16;264(5587):673–675. doi: 10.1038/264673a0. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann N. S., Rae P. A., Schimmer B. P. Altered cyclic AMP-dependent protein kinase activity in a mutant adrenocortical tumor cell line. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):451–460. doi: 10.1002/jcp.1040970320. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975 Nov 28;190(4217):896–898. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Miller M. R., Pardee A. B. Unique cytoplasmic phosphoproteins are associated with cell growth arrest. Nature. 1977 Nov 3;270(5632):57–59. doi: 10.1038/270057a0. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L., Branton P. E. Immunoprecipitation of protein kinase activity from adenovirus 5-infected cells using antiserum directed against tumour antigens. Nature. 1979 Jan 18;277(5693):241–243. doi: 10.1038/277241a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Pumo D. E., Stein G. S., Kleinsmith L. J. Stimulated phosphorylation of non-histone phosphoproteins in SV-40 transformed WI-38 human diploid fibroblasts. Biochim Biophys Acta. 1975 Aug 6;402(1):125–130. doi: 10.1016/0005-2787(75)90376-7. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Segawa K., Oda K., Yuasa Y., Shiroki K., Shimojo H. Phosphorylation of chromatin- and ribosome-associated proteins in cells transformed by adenovirus, murine sarcoma virus, and methylcholanthrene. J Virol. 1978 Sep;27(3):800–808. doi: 10.1128/jvi.27.3.800-808.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K., Yamaguchi N., Oda K. Simian virus 40 gene A regulates the association between a highly phosphorylated protein and chromatin and ribosomes in simian virus 40-transformed cells. J Virol. 1977 Jun;22(3):679–693. doi: 10.1128/jvi.22.3.679-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]