Abstract
Multiple sclerosis (MS) and its animal model of experimental autoimmune encephalomyelitis (EAE) are characterized by focal inflammatory infiltrates into the central nervous system, demyelinating lesions, axonal damage, and abundant production of cytokines that activate immune cells and damage neurons and oligodendrocytes, including interleukin-12 (IL-12), IL-6, IL-17, IL-21, IL-23, granulocyte macrophage-colony stimulating factor, and interferon-gamma. The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signaling pathway mediates the biological activities of these cytokines and is essential for the development and regulation of immune responses. Dysregulation of the JAK/STAT pathway contributes to numerous autoimmune diseases, including MS/EAE. The JAK/STAT pathway is aberrantly activated in MS/EAE because of excessive production of cytokines, loss of expression of negative regulators such as suppressors of cytokine signaling proteins, and significant enrichment of genes encoding components of the JAK/STAT pathway, including STAT3. Specific JAK/STAT inhibitors have been used in numerous preclinical models of MS and demonstrate beneficial effects on the clinical course of disease and attenuation of innate and adaptive immune responses. In addition, other drugs such as statins, glatiramer acetate, laquinimod, and fumarates have beneficial effects that involve inhibition of the JAK/STAT pathway. We conclude by discussing the feasibility of the JAK/STAT pathway as a target for neuroinflammatory diseases.
Introduction
Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory demyelinating immune-mediated disease of the central nervous system (CNS; brain, spinal cord, optic nerves) of unknown etiology and heterogeneous clinical symptoms and course (Mayo and others 2012). A combination of immunologic, environmental, and genetic factors is thought to cause and/or contribute to MS. Symptoms are varied, ranging from numbness in limbs to severe disease, including paralysis or loss of vision. Further, cognitive impairment can occur. In approximately 85% of MS patients, disease is characterized by a relapsing-remitting (RR) stage, followed by a secondary progressive (SP) phase (Lopez-Diego and Weiner 2008). The RR stage involves activities of Th1 and Th17 cells that infiltrate the CNS, and the SP phase is triggered by inflammation caused by activation of the innate immune system (Weiner 2008). Hallmarks of MS are demyelination; inflammatory lesions; axonal damage; inappropriate activation of interferon-gamma (IFN-γ)-producing Th1 cells, and interleukin-17 (IL-17)-producing Th17 cells, as well as CD8+T-cells and B-cells; hyperactivation of innate immune cells such as macrophages/microglia, neutrophils, and dendritic cells (DCs); astrocyte activation; and exuberant production of cytokines/chemokines (Bhat and Steinman 2009; Ransohoff 2009; Disanto and others 2012). Existing FDA-approved drugs for MS patients such as IFN-β, Glatiramer Acetate (GA), Mitroxantrone, Natalizumab, and, most recently, Fingolimod, Tecfidera, and Aubagio are only partially effective (Lopez-Diego and Weiner 2008; Axtell and others 2010; Lalive and others 2011; Hauser and others 2013), indicating a clear need for new therapies.
Experimental autoimmune encephalomyelitis
Experimental autoimmune encephalomyelitis (EAE), which has been widely used as a model of MS, is induced by active immunization with CNS antigens or by adoptive transfer of CNS-reactive T-cells (Ponomarev and others 2007; Bailey-Bucktrout and others 2008; Nair and others 2008; Linker and Lee 2009; Barr and others 2012). The pathogenesis of EAE is complex, with both IFN-γ-producing Th1 cells and IL-17-producing Th17 cells having pivotal roles in the development of neuroinflammation (Goverman 2009; Axtell and others 2010; Domingues and others 2010; Becher and Segal 2011). Th1 and Th17 cells also produce granulocyte macrophage-colony stimulating factor (GM-CSF), which is essential to induce EAE, and sustains neuroinflammation by recruitment of myeloid cells to the CNS (Kroenke and others 2010; Codarri and others 2011; El-Behi and others 2011; McGeachy 2011). In both EAE and MS, it is particularly important to limit the entry of Th1 and Th17 cells into the CNS and/or limit expansion of these cells once they have breached the blood–brain barrier. Th2 cells, which produce high levels of IL-4 and IL-10, are correlated with resolution of EAE, and CD25+Foxp3+T regulatory cells (Tregs) function as inhibitors of CNS inflammation (Goverman 2009; Kuchroo and others 2012). In addition, innate immune cells such as DCs, neutrophils, macrophages, and microglia have critical roles in EAE development (Bhat and Steinman 2009; Goverman 2009; Steinman 2010; Ajami and others 2011; Kuchroo and others 2012; Mayo and others 2012; Starossom and others 2012). Similar to MS, EAE is characterized by the heighted production of many proinflammatory cytokines and chemokines, including IL-12, IL-6, IL-17A, IL-17F, IL-21, IL-23, GM-CSF, IL-1, TNF, IFN-γ, CCL2, and CXCL10.
Janus kinase/signal transducers and activators of transcription pathway
The Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signaling pathway is the predominant signal transduction cascade utilized by numerous cytokines and is critical for initiating innate immunity, orchestrating adaptive immune systems, and ultimately constraining inflammatory and immune responses (O'Shea and Plenge 2012). Cytokines activate receptor-associated JAKs, which phosphorylate the receptor cytoplasmic domain on tyrosine residues, leading to recruitment of STATs. The JAKs then tyrosine phosphorylate STATs, promoting their activation. Once activated, STATs dimerize, translocate to the nucleus, and bind to regulatory elements to induce transcription of target genes (Fig. 1A,B). Over 60 cytokines and growth factors use the JAK/STAT pathway (O'Shea and Plenge 2012). There are 4 JAKs (JAK1, JAK2, JAK3, and TYK2) and a total of 7 STATs (STAT 1, 2, 3, 4, 5a, 5b, and 6). Various combinations of JAK/STAT usage result in differential gene expression, particularly depending on the STAT transcription factor(s) that is activated. Cytokines, through activation of the JAK/STAT pathway, are of paramount importance in regulating the development, differentiation, and function of T-cells and myeloid cells (Weaver and others 2007; Geissmann and others 2010b). Specifically, Th1 cell differentiation is induced by IL-12 through activation of JAK2/TYK2 and STAT4, Th2 differentiation is induced by IL-4 activation of JAK1/3 and STAT6, while Th17 cell differentiation requires IL-6 and IL-23, which signal through JAK1/2 and STAT3 (Fig. 2) (Harris and others 2007; Weaver and others 2007). STATs also regulate innate immune cells (Geissmann and others 2010a; Galli and others 2011). JAK1/2 and STAT1 activation mediate the effects of IFN-γ on macrophage function, JAK1/2 and STAT3 are involved in IL-6 family signaling, and GM-CSF signals through JAK2 and STAT5 to affect myeloid development. JAKs and STATs are essential mediators of almost all biological signaling events initiated by cytokines. As such, unrestrained activation of the JAK/STAT pathway is detrimental and has been associated with numerous immune-mediated and autoimmune diseases, including MS (O'Shea and Plenge 2012). Indeed, a number of STAT target genes, including IL-23R, IL-17A, IL-17F, IL-21, IL-22, IL-6, IFN-γ, RORγt, T-bet, CXCR3, and HLA-DR, are overexpressed in both MS and EAE, and have been implicated in contributing to disease pathogenesis. Activating mutations in STAT proteins are rare; thus, STAT hyperactivation is usually caused by an overabundance of cytokines, and/or dysregulation of endogenous negative regulators of JAKs, most notably, suppressors of cytokine signaling (SOCS) proteins. The SOCS family is composed of 8 members, CIS and SOCS1–7, and serve to restrict the duration of activation of cytokine-induced signaling by inhibiting JAK kinase activity after it has been turned on (Fig. 3A) (Yoshimura and others 2012). SOCS proteins contain an N-terminal variable region, a classical SH2 domain, and a C-terminal SOCS box (Fig. 3B). SOCS proteins are not constitutively expressed, but rather induced by cytokines, creating a negative feedback loop to prevent excessive activation of cytokine-induced JAK/STAT signaling. SOCS proteins bind to activated JAKs and to certain cytokine receptors via their SH2 domains, thereby suppressing further signaling events. In addition, the SOCS box interacts with components of the ubiquitin ligase machinery and mediates proteosomal degradation of associated proteins, most commonly, JAKs. SOCS1 and SOCS3 are unique among the SOCS proteins in terms of containing a 12 amino acid kinase-inhibitory region (KIR) (Fig. 3B), which acts as a pseudosubstrate for JAKs, conferring inhibition of JAK kinase activity. SOCS1 and SOCS3 in particular have critical functions in repressing innate and adaptive immunity, in part by inhibiting STAT activation induced by IFN-γ, IL-6, IL-12, IL-23, and GM-CSF, which are all implicated in MS and EAE pathogenesis (Yoshimura and others 2012; Kershaw and others 2013).
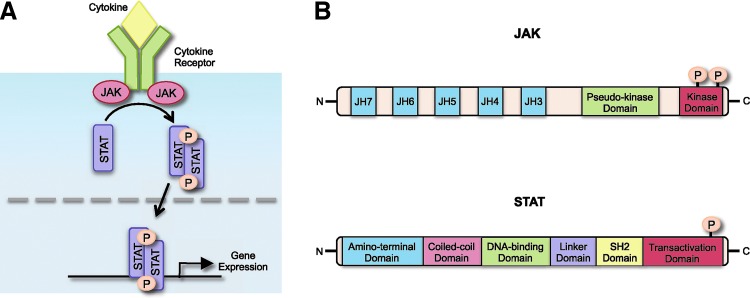
FIG. 1.
The JAK/STAT signaling pathway and domain structure of JAK and STAT proteins. (A) Various cytokines bind to their corresponding cytokine receptor and activate receptor-associated JAKs, leading to recruitment of STATs. JAKs then tyrosine phosphorylate STATs, promoting their activation. STATs dimerize, translocate to the nucleus, and bind to regulatory elements to induce transcription of target genes. (B) JAK and STAT protein domain structure. JAK proteins contain 7 JH domains including the pseudo-kinase domain (JH2) and the kinase domain (JH1). Trans- and autophosphorylation of tyrosine residues in the C-terminal kinase domain lead to the recruitment and activation of STATs. STAT protein structure consists of an amino-terminal domain, a coiled-coil domain, a DNA-binding domain, a linker domain, an SH2 domain, and a transactivation domain. Phosphorylation in the C-terminal transactivation domain by JAKs leads to STAT activation and dimerization. JAK/STAT, Janus kinase/signal transducer and activator of transcription; JH, JAK homology.
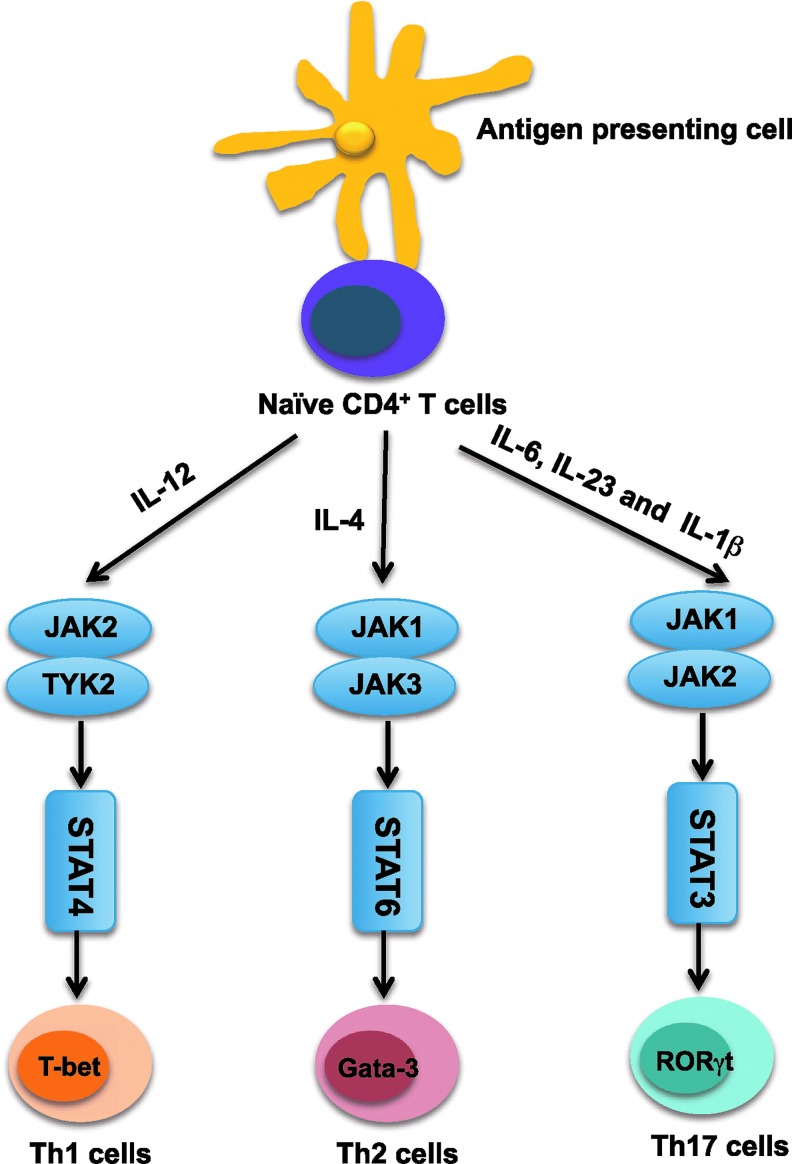
FIG. 2.
JAK/STAT signaling is critical for the differentiation of CD4+T-cells. Naive CD4+Th cells differentiate into various distinct functional subsets depending on the cytokines they encounter. Specifically, Th1 cell differentiation is induced by IL-12 through activation of JAK2/TYK2 and STAT4, Th2 cell differentiation is induced by IL-4 activation of JAK1/3 and STAT6, while Th17 cell differentiation requires IL-6 and IL-23, which signal through JAK1/2 and STAT3, as well as IL-1β. IL, interleukin; Th, T helper.
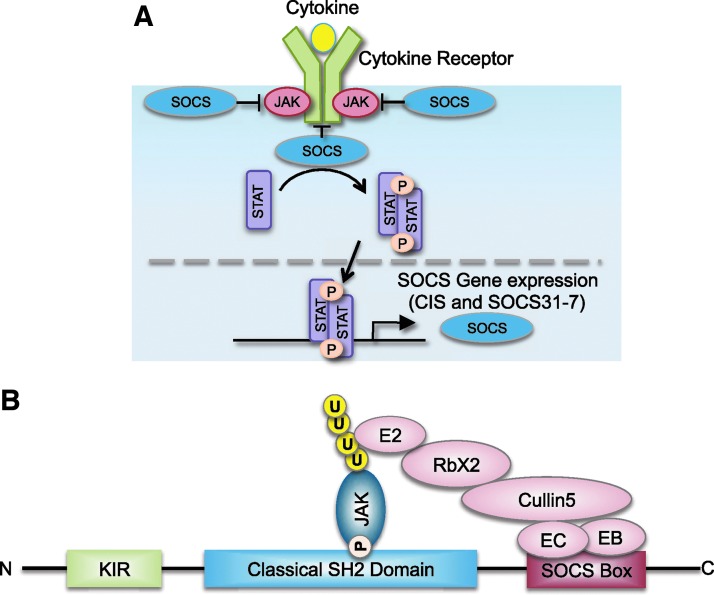
FIG. 3.
Induction of SOCS expression, and domain structure of SOCS3. (A) SOCS proteins (CIS and SOCS1–7) are not constitutively expressed; rather, they are induced by cytokines, creating a negative feedback loop to prevent excessive activation of cytokine-induced JAK/STAT signaling. (B) SOCS proteins contain an N-terminal variable region, a classical SH2 domain, and a C-terminal SOCS box. The SOCS box interacts with components of the ubiquitin ligase machinery and mediates proteosomal degradation of associated proteins, most commonly, JAKs. SOCS1 and SOCS3 are unique among the SOCS proteins as they contain a KIR, which acts as a pseudosubstrate for JAKs, conferring inhibition of JAK kinase activity. KIR, kinase-inhibitory region; SOCS, suppressors of cytokine signaling.
Dysregulation of the JAK/STAT Pathway in MS
Genome-wide association studies have shown that cytokines, their receptors, JAKs, STATs, and SOCS proteins are associated with human autoimmune diseases, especially pathways leading to aberrant STAT3 and STAT4 activation (Oksenberg and Baranzini 2010; Sawcer and others 2011; Vandenbroeck 2012). In MS, there is significant overexpression of immunologically relevant genes involved in Th cell differentiation and antigen presentation. These include cytokine and cytokine receptor genes such as IL-7, IL-12, IL-2RA, IL-7R, IL-28RA, OSMR, and IL-22R (Bronson and others 2010; Oksenberg and Baranzini 2010; Couturier and others 2011; Oksenberg and Hauser 2011; Zuvich and others 2011; Vandenbroeck and others 2012; Beecham and others 2013; IMSGC 2013). A TYK2 variant in MS patients is a protective allele that results in diminished TYK2 kinase activity, leading to a decrease in STAT1 activation (Couturier and others 2011). This promotes a deviation of Th cell differentiation to the Th2 phenotype, and is associated with a decreased risk of MS. STAT3 has been identified as an MS susceptibility gene (Baranzini and others 2009; Jakkula and others 2010; Oksenberg and Baranzini 2010), and independent replication supports the association between STAT3 and an increase in MS risk (Lill and others 2012). In addition, genes encoding components of the JAK/STAT pathway were recently demonstrated to be significantly enriched in MS patients (IMSGC 2013). T-cells and monocytes from MS patients during relapse have elevated levels of activated STAT3 compared with cells from patients in remission, which is correlated with low levels of SOCS3 (Frisullo and others 2006). This suggests an association of decreased SOCS3 expression, increased STAT3 activation, and MS relapse. Furthermore, high levels of activated STAT3 in T-cells from patients with clinically isolated syndrome predict conversion to clinically defined MS (Frisullo and others 2008). A SOCS1 variant was recently validated as a novel risk factor for MS (Vandenbroeck and others 2012), although the functional significance of this single-nucleotide polymorphism is not known. Interestingly, single-nucleotide polymorphisms related to various components of the NF-κB pathway have been identified in MS patients (Beecham and others 2013), suggesting overactivation of this pathway. This is relevant to the JAK/STAT pathway as there is considerable crosstalk between these two signaling cascades, and they function in a feed-forward loop to ensure continuous activation (McFarland and others 2013).
Statins have been intensely studied for immunomodulatory and anti-inflammatory properties (Zamvil and Steinman 2002; Weber and others 2007b). Simvastatin has been shown to induce SOCS3 expression in monocytes from MS patients with RR disease, which was associated with diminished STAT1 and STAT3 activation (Zhang and others 2008). This also decreased the production of IL-6 and IL-23 by monocytes, leading to diminished IL-17 production by T-cells. Furthermore, simvastatin directly inhibits Th17 cell differentiation in RR MS patients (Zhang and others 2011). This is accomplished by inhibition of IRF-4 expression, which is a key transcription factor for human Th17 cell differentiation, leading to decreased IL-17A, IL-17F, IL-21, and IL-22 production (Zhang and others 2011). Lastly, simvastatin directly targets DCs from MS patients, which causes an induction of SOCS1 and SOCS3 expression, and decreased STAT1 and STAT3 activation (Zhang and others 2013). This in turn decreased expression of IL-1, IL-23, TGF-β, IL-21, and IL-12 from DCs, providing an inhibitory cytokine environment for Th1 and Th17 cell differentiation (Zhang and others 2013). These studies collectively suggest that SOCS proteins may represent therapeutic targets in MS (Ramgolam and Markovic-Plese 2011). Indeed, a SOCS1 mimetic has efficacy in various EAE models, which is described in more detail below.
Dysregulation of the JAK/STAT Pathway in EAE
Activation of the JAK/STAT pathway, particularly STAT1, STAT3, and STAT4 activation, is observed in various models of EAE, including active induction of classical and atypical EAE, as well as Th1 and Th17 cell-adoptive transfer models of EAE (Zaheer and others 2007; Chen and others 2009; Jia and others 2011; Qin and others 2012b; Egwuagu and Larkin 2013; Liu and others 2014). Mice with targeted deletion of STAT proteins have been used to determine the functional role of different STAT members in EAE. Given the importance of STAT3 in differentiation of Th17 cells, several groups have examined mice with targeted deletion of STAT3 in T-cells for susceptibility to EAE. Loss of STAT3 in T-cells renders mice resistant to EAE disease (Harris and others 2007; Liu and others 2008). These studies demonstrate that STAT3, by regulating the expression of the transcription factor RORγt and the IL-23R, is essential for the development of Th17 cells. As such, STAT3 knock-out mice cannot generate Th17 cells and thus are protected from developing EAE (Harris and others 2007; Liu and others 2008). In addition, T-cells from these mice are defective in expression and activation of the integrins α4β1 and α4β7 and cannot traffic into the CNS (Liu and others 2008). STAT4-deficient mice are defective in the differentiation of Th1 cells and are resistant to EAE induction (Chitnis and others 2001). STAT4 knockout mice have been shown to have a predominantly Th2 phenotype, which is associated with high levels of IL-4 and IL-5. As such, the resistance to EAE may be because of the protective effects of Th2-derived cytokines. Interestingly, mice lacking STAT1 are highly susceptible to EAE (Bettelli and others 2004). It is possible that in the absence of STAT1, STAT3 and/or STAT4 signaling may compensate to drive Th1 cell responses as these mice are characterized by IFN-γ–producing Th1 cells. It has been suggested that mice lacking STAT1 cannot benefit from the protective effect of IFN-γ and thus have severe disease. IL-6 has a deleterious role in EAE by activation of STAT3, which is pivotal for induction of pathogenic Th17 cells, and trafficking of Th1 and Th17 cells into the CNS (Yang and others 2007; Jin and others 2009; Quintana and others 2009; Scheller and others 2011). Thus, IL-6-deficient mice have been shown to be resistant to EAE induction because of a lack of development of Th17 cells (Quintana and others 2009).
The JAK/STAT pathway also regulates the innate immune response in EAE. Macrophages, microglia, and DCs can promote both injury and repair, and have detrimental and protective roles in diseases such as EAE and MS (Block and others 2007; Gensel and others 2009; Mildner and others 2009; Rivest 2009; Shechter and others 2009; Zhu and others 2011; Yogev and others 2012). These divergent functions are dictated by their microenvironment, which can promote a spectrum of macrophage/microglia phenotypes (Fig. 4). Polarized macrophages are termed proinflammatory, classically activated M1, and anti-inflammatory, alternatively activated M2, which represent 2 extremes of the macrophage continuum (Gordon 2003; Mantovani and others 2005; Cassol and others 2010). Macrophages are polarized to the M1 phenotype by LPS, IFN-γ, and GM-CSF (Mantovani and others 2007; van der Does and others 2010; Krausgruber and others 2011); produce high levels of IL-12, IL-6, IL-1β, IL-23, CXCL10, and CCL2, increased levels of reactive oxygen species, and low levels of IL-10; and participate in the induction of Th1 and Th17 responses (Mantovani and others 2005; Cassol and others 2010; Krausgruber and others 2011). We have recently shown that the absence of SOCS3 in macrophages leads to a “heightened” M1 phenotype associated with excessive STAT activation (Qin and others 2012a,b). Furthermore, mice with targeted deletion of SOCS3 in myeloid cells develop a severe, nonresolving atypical form of EAE, which is associated with lesions in the cerebellum, rather than the spinal cord (Qin and others 2012b). These mice have elevated levels of M1 macrophages; exhibit hyperactivation of STAT1, STAT3, and STAT4; and have elevated numbers of inflammatory cells in the cerebellum, including macrophages and neutrophils, and a prominent Th1 and Th17 cell infiltrate (Qin and others 2012b). IL-4, IL-13, and M-CSF induce M2 macrophages, which upregulate scavenger and mannose receptors, the IL-1 receptor antagonist, and express high levels of IL-10, CCL17, CCL18, and CCL22 (Gordon 2003). M2 macrophages resolve inflammation and promote Th2 responses (Wang and others 2010). M2 macrophages are protective in multiple models of EAE. Weber and others (2007a) demonstrated that M2 macrophages are induced by GA treatment, characterized by increased secretion of IL-10 and TGF-β and diminished STAT1 activation, and inhibit EAE disease upon adoptive transfer by suppressing Th17 development and promoting Th2 and Tregs. Laquinimod, an agent under evaluation for RR MS, is protective in EAE models by induction of M2 macrophages, also characterized by low STAT1 activation (Schulze-Topphoff and others 2012; Thone and others 2012). Fumurates, which were approved by the FDA in 2013 for treatment of RR MS patients, protect mice from EAE by the generation of anti-inflammatory type II DCs, which induce Th2 cells (Ghoreschi and others 2011a). The type II DCs have impaired STAT1 phosphorylation. Thus, agents that are protective in EAE models and in MS (GA, laquinimod, fumarates) promote protective M2 macrophage and type II DC phenotypes, which are associated with diminished STAT1/3 activation, decreased production of IL-6, IL-12, and IL-23, and elevated secretion of IL-10. We have also shown that M2 macrophages are protective in the atypical model of EAE associated with SOCS3 deficiency in myeloid cells (Qin and others 2012b). Adoptive transfer of M2 macrophages diminished the inflammatory infiltrate observed in the cerebellum, reduced Th1 and Th17 cells while enhancing expression of Th2 and Tregs, and inhibited STAT activation in the cerebellum (Qin and others 2012b).
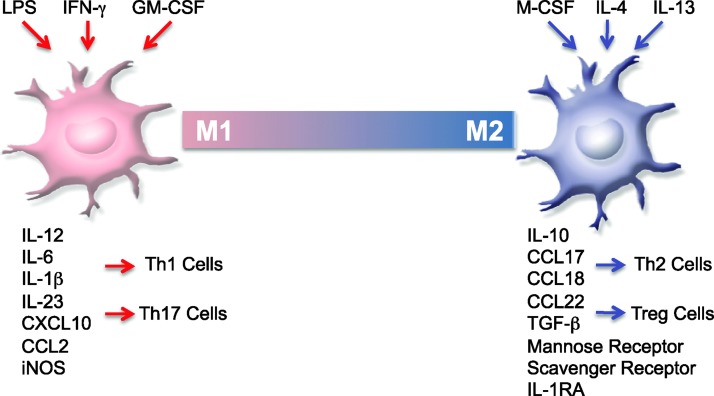
FIG. 4.
M1 and M2 macrophage polarization. Polarized macrophages are termed proinflammatory, classically activated M1, and anti-inflammatory, alternatively activated M2. Macrophages are polarized to the M1 phenotype by LPS, IFN-γ, and GM-CSF, produce high levels of IL-12, IL-6, IL-1β, IL-23, CXCL10, and CCL2, increase levels of reactive oxygen species, and participate in the induction of Th1 and Th17 responses. M-CSF, IL-13, and IL-4 induce M2 macrophages, which upregulate scavenger and mannose receptors, the IL-1 receptor antagonist, and express high levels of IL-10, CCL17, CCL18, CCL22, and TGF-β. M2 macrophages can also be induced by GA and Laquinimod treatment. GM-CSF, granulocyte macrophage-colony stimulating factor; IFN, interferon.
As mentioned previously, SOCS1 and SOCS3 are critically involved in regulating innate and adaptive immune responses (Yoshimura and others 2012). In EAE models, expression of both SOCS1 and SOCS3 is detected predominantly in macrophages during the early stages of disease, as well as in active disease (Stark and Cross 2006; Berard and others 2010). In a study comparing RR EAE to chronic (CH) EAE, it was noted that the number of SOCS1-expressing macrophages at the peak of RR disease was significantly higher than in CH-EAE (Berard and others 2010). This correlated with diminished expression of iNOS, whose expression is regulated by SOCS1, in RR-EAE mice compared with CH-EAE mice. The authors speculate that the inhibition of iNOS may promote remission in the RR-EAE model, and that SOCS1 expression by macrophages may contribute to induction of remissions. SOCS3 expression in DCs is also protective in EAE (Li and others 2006). Adoptive transfer of SOCS3-expressing DCs at EAE induction or at disease onset reduces the clinical severity of EAE compared with control DCs. This was associated with a limited differentiation of Th1 and Th17 cells and a robust induction of Th2 cells, which provide protection in EAE. As discussed previously, IL-6 and IL-23 activation of STAT3 is critical for Th17 cell differentiation (Basu and others 2013). Deficiency of SOCS3 in T-cells leads to preferential polarization to the Th17 phenotype, because of heightened STAT3 phosphorylation and subsequent IL-17A and IL-17F expression (Chen and others 2006). TGF-β is also required for Th17 cell differentiation (Basu and others 2013). We have shown that TGF-β inhibits SOCS3 expression, leading to enhancement of STAT3 activation and Th17 cell polarization (Qin and others 2009). These results indicate that SOCS3 expression is critical to constrain the differentiation of Th17 cells.
Therapeutic intervention of the JAK/STAT pathway in EAE
The JAK/STAT pathway has received significant attention as a therapeutic target in inflammation, autoimmune diseases, solid and liquid tumors, and transplant rejection (Opar 2010; O'Shea and Plenge 2012; Seavey and Dobrzanski 2012). A variety of JAK inhibitors have been developed with varying degrees of specificity for JAK1, JAK2, JAK3, and TYK2, and have demonstrated clinical efficacy in rheumatoid arthritis and other inflammatory disorders (Fridman and others 2010; Opar 2010; Ghoreschi and others 2011b; Stump and others 2011; O'Shea and Plenge 2012; O'Shea and others 2013a). Two JAK inhibitors have been approved by the FDA: Ruxolitinib, a JAK1/JAK2 inhibitor, was approved in 2011 for patients with myelofibrosis and polycythaemia vera, and Tofacitinab, a JAK3/JAK1 inhibitor, was approved in 2012 for treatment of patients with rheumatoid arthritis (O'Shea and others 2013a,b). JAK inhibitors interrupt signaling downstream of multiple cytokines, representing a useful approach for MS, which is characterized by a “cytokine storm” in the periphery and CNS. Many of the key immunoregulatory cytokines involved in EAE and MS, including IL-6, IL-12, IL-23, IFN-γ, and GM-CSF, require activation of JAK1, JAK2, or both for subsequent activation of STATs, and ultimate biological responses (O'Shea and Plenge 2012). As such, a number of studies have examined direct inhibition of the JAK/STAT pathway in EAE. Bright and others (1999) previously demonstrated that tyrphostin B42, a JAK2 inhibitor, reduced severity of EAE. This was accomplished by inhibiting IL-12-induced activation of JAK2 in T-cells, leading to a decrease in Th1 cell polarization. In vivo, tyrphostin B42 decreased Th1 cell development, thereby reducing the incidence and severity of EAE. We have recently demonstrated that AZD1480, a JAK1/JAK2 inhibitor, has striking clinical efficacy in multiple models of EAE (Liu and others 2014). In vitro, AZD1480 inhibits the differentiation of both human and murine Th1 cells by inhibiting STAT1 and STAT4 activation, and decreasing levels of IFN-γ and T-bet, the transcription factor critical for Th1 cell polarization. AZD1480 also inhibited the differentiation of human and murine Th17 cells by inhibiting STAT3 activation, and downstream STAT3 target genes such as IL-17A, RORγt, IL-22, and IL-23R. AZD1480 also influenced macrophages and DCs by inhibiting STAT1, STAT3, and STAT5 activation, and expression of genes such as iNOS, Class II MHC, and CD40 (Liu and others 2014). Our results indicate that AZD1480 does not promote the M2 macrophage phenotype but exerts an inhibitory effect on M1 polarization. In vivo, AZD1480 had a significant protective effect in 5 EAE models: classical EAE, atypical EAE, RR EAE, Th1 cell-mediated EAE, and Th17 cell-mediated EAE. The beneficial immunomodulatory effects of AZD1480 were associated with deactivation of myeloid cells, diminished polarization of Th1 and Th17 cells, decreased expression of proinflammatory cytokines/chemokines, and reduced infiltration of immune cells (Liu and others 2014). Importantly, AZD1480 treatment was administered at the onset of disease and in a therapeutic manner after the appearance of clinical symptoms, with potent clinical efficacy.
Studies on other pharmacologic inhibitors have implicated the JAK/STAT axis in regulating clinical manifestations of EAE. Peroxisome proliferator activated receptor-γ (PPARγ) and COX2 inhibitors suppress EAE severity, in part, by inhibiting IL-12-induced activation of the JAK/STAT pathway, and subsequent suppression of Th1 cell differentiation (Natarajan and Bright 2002; Muthian and others 2006). This was accomplished by inhibiting STAT3 and STAT4 activation in T-cells. The protective effect of GA in EAE is in part caused by inhibition of STAT3 phosphorylation in T-cells, leading to inhibition of RORγt expression, and suppression of Th17 cell differentiation (Chen and others 2009). Several herbal compounds, including plumbagin (PL), berberine, and quercetin, exert protective effects in EAE models by inhibiting STAT activation and Th1 and Th17 cell differentiation (Muthian and Bright 2004; Qin and others 2010; Jia and others 2011). Furthermore, both PL and berberine inhibited NF-κB activation in antigen-presenting cells, which diminished expression of IL-6 and iNOS by these cells. The lack of IL-6 likely contributes to the decrease in Th17 cell differentiation, while reductions in iNOS may promote the development of Tregs (Lee and others 2011), which aid in resolution of disease.
Lastly, there is much interest in the use of SOCS mimetics in neuroinflammatory diseases. SOCS1 and SOCS3 contain a KIR domain that binds to tyrosine-phosphorylated JAKs and inhibits their kinase activity (Fig. 3B). SOCS1 mimetics have been made that bind to the JAK2 autophosphorylation site, preventing activation of STAT1 and STAT3. The SOCS1 mimetic (Tkip) inhibits IFN-γ and IL-6 activation of STAT proteins in vitro, thereby suppressing downstream gene expression (Flowers and others 2004). The SOCS1 mimetic has been tested in several EAE models. Administration of Tkip before EAE induction in NZW mice prevents acute EAE, while in SJL/J mice, administration blocks the acute and relapse phases of EAE, even when given after establishment of disease (Mujtaba and others 2005). In C57BL/6 mice with CH EAE, Tkip reduced disease severity (Berard and others 2010). A more detailed analysis demonstrated that Tkip inhibits expansion of Th17 cells in EAE by blocking IL-23 activation of STAT3 (Jager and others 2011). The therapeutic efficacy of Tkip in EAE was associated with a reduction in cellular infiltration into the CNS. These findings collectively indicate that the SOCS1 mimetic can attenuate neuroinflammatory responses in the CNS and may have therapeutic value in MS patients.
STAT inhibitors
There is also interest in the development of STAT inhibitors, particularly inhibitors of STAT3 and STAT5, as their aberrant activation is associated with a wide range of cancers (Brantley and Benveniste 2008; Yu and others 2009; Walker and others 2013). In addition, STAT3 mediates many of the inflammatory responses associated with signaling by the IL-6 family of cytokines and has relevance as a target in numerous autoimmune diseases, including MS. Thus, STAT transcription factors appear to be targets with a high therapeutic index. However, the ability to target STATs has lagged far behind the great progress in targeting JAKs. Although STATs lack enzymatic activity, they do contain clearly defined functional domains that can serve as targets (Fig. 1B). A number of strategies have been employed, including (1) creating phosphopeptide mimetic prodrugs that target the SH2 domain, thereby preventing dimerization of STATs (McMurray and others 2012); (2) targeting of the N-terminal domain of STATs to modulate JAK/STAT signaling (Timofeeva and Tarasova 2012); (3) development of decoy oligonucleotides that bind activated STATs, thereby interfering with DNA binding and transcriptional activity (Sen and Grandis 2012); and (4) screening chemical libraries for STAT modulators (Nelson and others 2011; Walker and Frank 2012). A number of STAT3 inhibitors have been described and tested successfully in preclinical models (Nelson and others 2011; Zhang and others 2012; Miklossy and others 2013). More importantly, a STAT3 decoy oligonucleotide has been tested in a phase 0 clinical trial in patients with head and neck cancers. The results demonstrate evidence of inhibition of STAT3 target genes in the tumor and are the first to document the efficacy of STAT3 inhibitors in humans (Sen and others 2012). In addition, a small molecule inhibitor of STAT3 is being tested in a clinical trial in patients with chronic lymphocytic leukemia (Frank 2012). Of relevance to neuroinflammation, a STAT3 inhibitor (ORLL-NIH001) has been tested in experimental autoimmune uveitis, a model of human posterior uveitis (Yu and others 2012). Treatment with ORLL-NIH001 attenuated disease severity by inhibiting the inflammatory properties of T-cells, inhibiting entry of T-cells into the retina, and reducing expression of CCR6 and CXCR3 (Yu and others 2012). In addition, ORLL-NIH001 inhibited the expansion of human Th17 cells in vitro. These encouraging findings bode well for the future development and use of STAT3 inhibitors in MS patients.
Conclusions
The JAK/STAT pathway is one of the most critical signal transduction systems utilized by cells of the innate and adaptive immune systems to initiate and regulate immune responses. Aberrant activation of this pathway promotes dysregulation of innate and adaptive immunity, including activation of pathogenic Th1 and Th17 cells, activation of macrophages, neutrophils, and DCs, and excessive production of proinflammatory cytokines, all of which contribute to the pathogenesis of MS. There have been remarkable advances in the development of specific JAK inhibitors that show great promise in the treatment of autoimmune diseases, as well as a variety of cancers (O'Shea and others 2013a,b). Common adverse effects associated with the JAK inhibitors include bacterial, fungal, and viral infections, but opportunistic infections are uncommon. Presumably because of interference with EPO signaling and signaling by colony stimulating factors via JAK2 inhibition, anemia and neutropenia can also occur. Hypercholesterolemia is observed with the use of JAK inhibitors, which may be due to blockade of IL-6 signaling. Because of the relatively short half-life of the JAK inhibitors, the drug can be stopped if adverse effects such as infections become severe. Another possibility may be to administer JAK inhibitors in a pulsatile manner.
Given the profound role of cytokines in autoimmunity, JAK inhibitors have great potential utility. As JAK inhibitors can interrupt the signaling of numerous cytokines, we believe that they may be useful for the treatment of MS as simultaneous inhibition of cytokine signaling involved in activation of innate and adaptive immune responses may break the cycle of inflammation characteristic of MS. Other diseases such as Parkinson's disease, spinal cord injury, and Alzheimer's disease, which have a prominent neuroinflammatory component, may also benefit from JAK inhibitors. In this regard, preliminary findings from our laboratory using a JAK1/2 inhibitor in a rat model of Parkinson's disease demonstrate a reduction in macrophage/microglial activation, and sparing of dopaminergic neurons (unpublished data). These findings collectively suggest that the JAK/STAT axis may serve as a therapeutic target for neuroinflammatory and neurodegenerative diseases.
Acknowledgments
This work was supported in part by National Institutes of Health Grants NS45290 and NS57563 (to E.N.B. and H.Q.), National Multiple Sclerosis Society Grant CA-1059-A-13 (to E.N.B.), a grant from the Michael J. Fox Foundation (to E.N.B. and H.Q.), and a grant from the American Brain Tumor Foundation (to B.C.M.). The authors thank Cheryl Lyles and Kim Sanders for assistance.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. 2011. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14(9):1142–1149 [DOI] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. 2010. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med 16(4):406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. 2008. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol 180(10):6457–6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranzini SE, Galwey NW, Wang J, Khankhanian P, Lindberg R, Pelletier D, Wu W, Uitdehaag BM, Kappos L, Polman CH, Matthews PM, Hauser SL, Gibson RA, Oksenberg JR, Barnes MR. 2009. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum Mol Genet 18(11):2078–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B.O'Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209(5):1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Hatton RD, Weaver CT. 2013. The Th17 family: flexibility follows function. Immunol Rev 252(1):89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Segal BM. 2011. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol 23(6):707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A, Oturai A, Saarela J, Fontaine B, Hemmer B, Martin C, Zipp F, D'Alfonso S, Martinelli-Boneschi F, Taylor B, Harbo HF, Kockum I, Hillert J, Olsson T, Ban M, Oksenberg JR, Hintzen R, Barcellos LF, Agliardi C, Alfredsson L, Alizadeh M, Anderson C, Andrews R, Sondergaard HB, Baker A, Band G, Baranzini SE, Barizzone N, Barrett J, Bellenguez C, Bergamaschi L, Bernardinelli L, Berthele A, Biberacher V, Binder TM, Blackburn H, Bomfim IL, Brambilla P, Broadley S, Brochet B, Brundin L, Buck D, Butzkueven H, Caillier SJ, Camu W, Carpentier W, Cavalla P, Celius EG, Coman I, Comi G, Corrado L, Cosemans L, Cournu-Rebeix I, Cree BA, Cusi D, Damotte V, Defer G, Delgado SR, Deloukas P, di Sapio A, Dilthey AT, Donnelly P, Dubois B, Duddy M, Edkins S, Elovaara I, Esposito F, Evangelou N, Fiddes B, Field J, Franke A, Freeman C, Frohlich IY, Galimberti D, Gieger C, Gourraud PA, Graetz C, Graham A, Grummel V, Guaschino C, Hadjixenofontos A, Hakonarson H, Halfpenny C, Hall G, Hall P, Hamsten A, Harley J, Harrower T, Hawkins C, Hellenthal G, Hillier C, Hobart J, Hoshi M, Hunt SE, Jagodic M, Jelcic I, Jochim A, Kendall B, Kermode A, Kilpatrick T, Koivisto K, Konidari I, Korn T, Kronsbein H, Langford C, Larsson M, Lathrop M, Lebrun-Frenay C, Lechner-Scott J, Lee MH, Leone MA, Leppa V, Liberatore G, Lie BA, Lill CM, Linden M, Link J, Luessi F, Lycke J, Macciardi F, Mannisto S, Manrique CP, Martin R, Martinelli V, Mason D, Mazibrada G, McCabe C, Mero IL, Mescheriakova J, Moutsianas L, Myhr KM, Nagels G, Nicholas R, Nilsson P, Piehl F, Pirinen M, Price SE, Quach H, Reunanen M, Robberecht W, Robertson NP, Rodegher M, Rog D, Salvetti M, Schnetz-Boutaud NC, Sellebjerg F, Selter RC, Schaefer C, Shaunak S, Shen L, Shields S, Siffrin V, Slee M, Sorensen PS, Sorosina M, Sospedra M, Spurkland A, Strange A, Sundqvist E, Thijs V, Thorpe J, Ticca A, Tienari P, van Duijn C, Visser EM, Vucic S, Westerlind H, Wiley JS, Wilkins A, Wilson JF, Winkelmann J, Zajicek J, Zindler E, Haines JL, Pericak-Vance MA, Ivinson AJ, Stewart G, Hafler D, Hauser SL, Compston A, McVean G, De Jager P, Sawcer SJ, McCauley JL. 2013. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45(11):1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard JL, Kerr BJ, Johnson HM, David S. 2010. Differential expression of SOCS1 in macrophages in relapsing-remitting and chronic EAE and its role in disease severity. Glia 58(15):1816. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. 2004. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 200(1):79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Steinman L. 2009. Innate and adaptive autoimmunity directed to the central nervous system. Neuron 64(1):123–132 [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69 [DOI] [PubMed] [Google Scholar]
- Brantley E, Benveniste EN. 2008. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res 6(5):675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Du C, Sriram S. 1999. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus Kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol 162(10):6255–6262 [PubMed] [Google Scholar]
- Bronson PG, Caillier S, Ramsay PP, McCauley JL, Zuvich RL, De Jager PL, Rioux JD, Ivinson AJ, Compston A, Hafler DA, Sawcer SJ, Pericak-Vance MA, Haines JL, Hauser SL, Oksenberg JR, Barcellos LF. 2010. CIITA variation in the presence of HLA-DRB1*1501 increases risk for multiple sclerosis. Hum Mol Gen 19(11):2331–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol E, Cassetta L, Alfano M, Poli G. 2010. Macrophage polarization and HIV-1 infection. J Leukoc Biol 87(4):599–608 [DOI] [PubMed] [Google Scholar]
- Chen C, Liu X, Wan B, Zhang JZ. 2009. Regulatory properties of copolymer I in Th17 differentiation by altering STAT3 phosphorylation. J Immunol 183(1):246–253 [DOI] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. 2006. Selective regulatory function of SOCS3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA 103:8137–8142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. 2001. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest 108(5):739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. 2011. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12(6):560–567 [DOI] [PubMed] [Google Scholar]
- Couturier N, Bucciarelli F, Nurtdinov RN, Debouverie M, Lebrun-Frenay C, Defer G, Moreau T, Confavreux C, Vukusic S, Cournu-Rebeix I, Goertsches RH, Zettl UK, Comabella M, Montalban X, Rieckmann P, Weber F, Muller-Myhsok B, Edan G, Fontaine B, Mars LT, Saoudi A, Oksenberg JR, Clanet M, Liblau RS, Brassat D. 2011. Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility. Brain 134(Pt 3):693–703 [DOI] [PubMed] [Google Scholar]
- Disanto G, Morahan JM, Barnett MH, Giovannoni G, Ramagopalan SV. 2012. The evidence for a role of B cells in multiple sclerosis. Neurology 78(11):823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. 2010. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One 5(11):e15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egwuagu CE, Larkin J. 2013. Therapeutic targeting of STAT pathways in CNS autoimmune diseases. JAK-STAT 2(1):e24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 12(6):568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers LO, Johnson HM, Mujtaba MG, Ellis MR, Haider SM, Subramaniam PS. 2004. Characterization of a peptide inhibitor of Janus Kinase 2 that mimics Suppressor Of Cytokine Signaling 1 function. J Immunol 172(12):7510–7518 [DOI] [PubMed] [Google Scholar]
- Frank DA. 2012. Targeting STATS for cancer therapy: “undruggable” no more. JAK-STAT 1(4):261–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K. 2010. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 184(9):5298–5307 [DOI] [PubMed] [Google Scholar]
- Frisullo G, Angelucci F, Caggiula M, Nociti V, Iorio R, Patanella AK, Sancricca C, Mirabella M, Tonali PA, Batocchi AP. 2006. P-STAT1, P-STAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J Neurosci Res 84(5):1027–1036 [DOI] [PubMed] [Google Scholar]
- Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, Mirabella M, Tonali PA, Batocchi AP. 2008. The persistency of high levels of P-STAT3 expression in circulating CD4+T cells from CIS patients favors the early conversion to clinically defined multiple sclerosis. J Neuroimmunol 205(1–2):126–134 [DOI] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA. 2011. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12(11):1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. 2010a. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10(6):453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. 2010b. Development of monocytes, macrophages, and dendritic cells. Science 327(5966):656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. 2009. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci 29(12):3956–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Bruck J, Kellerer C, Deng C, Peng H, Rothfuss O, Hussain RZ, Gocke AR, Respa A, Glocova I, Valtcheva N, Alexander E, Feil S, Feil R, Schulze-Osthoff K, Rupec RA, Lovett-Racke AE, Dringen R, Racke MK, Rocken M. 2011a. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 208(11):2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, Warner JD, Tanaka M, Steward-Tharp SM, Gadina M, Thomas CJ, Minnerly JC, Storer CE, LaBranche TP, Radi ZA, Dowty ME, Head RD, Meyer DM, Kishore N, O'Shea JJ. 2011b. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 186(7):4234–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. 2003. Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35 [DOI] [PubMed] [Google Scholar]
- Goverman J. 2009. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 9(6):393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV, Maris CH, Housseau F, Yu H, Pardoll DM, Drake CG. 2007. Cutting edge: an in vivo requirement for STAT3 signaling in Th17 development and Th17-dependent autoimmunity. J Immunol 179(7):4313–4317 [DOI] [PubMed] [Google Scholar]
- Hauser SL, Chan JR, Oksenberg JR. 2013. Multiple sclerosis: prospects and promise. Ann Neurol 74(3):317–327 [DOI] [PubMed] [Google Scholar]
- IMSGC. 2013. Network-Based multiple sclerosis pathway analysis with GWAS data from 15,000 cases and 30,000 controls. Am J Hum Genet 92(6):854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager LD, Dabelic R, Waiboci LW, Lau K, Haider MS, Ahmed CM, Larkin J 3rd, David S, Johnson HM. 2011. The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis. J Neuroimmunol 232(1–2):108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkula E, Leppa V, Sulonen AM, Varilo T, Kallio S, Kemppinen A, Purcell S, Koivisto K, Tienari P, Sumelahti ML, Elovaara I, Pirttila T, Reunanen M, Aromaa A, Oturai AB, Sondergaard HB, Harbo HF, Mero IL, Gabriel SB, Mirel DB, Hauser SL, Kappos L, Polman C, De Jager PL, Hafler DA, Daly MJ, Palotie A, Saarela J, Peltonen L. 2010. Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Gen 86(2):285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Jing J, Bai Y, Li Z, Liu L, Luo J, Liu M, Chen H. 2011. Amelioration of experimental autoimmune encephalomyelitis by plumbagin through down-regulation of JAK-STAT and NF-kappaB signaling pathways. PLoS One 6(10):e27006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Zhou XF, Yu J, Cheng X, Sun SC. 2009. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood 113(26):6603–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw NJ, Murphy JM, Lucet IS, Nicola NA, Babon JJ. 2013. Regulation of Janus Kinases by SOCS proteins. Biochem Soc Trans 41(4):1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. 2011. IRF5 promotes inflammatory macrophage polarization and Th1-Th17 responses. Nat Immunol 12(3):231–238 [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Chensue SW, Segal BM. 2010. EAE mediated by a non-IFN-gamma/non-IL-17 pathway. Eur J Immunol 40(8):2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Ohashi PS, Sartor RB, Vinuesa CG. 2012. Dysregulation of immune homeostasis in autoimmune diseases. Nat Med 18(1):42–47 [DOI] [PubMed] [Google Scholar]
- Lalive PH, Neuhaus O, Benkhoucha M, Burger D, Hohlfeld R, Zamvil SS, Weber MS. 2011. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 25(5):401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Choi H, Eun SY, Fukuyama S, Croft M. 2011. Nitric oxide modulates TGF-β-directive signals to suppress Foxp3+regulatory T cell differentiation and potentiate Th1 development. J Immunol 186(12):6972–6980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chu N, Rostami A, Zhang GX. 2006. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs Type 2 Th cell differentiation in vitro and in vivo. J Immunol 177(3):1679–1688 [DOI] [PubMed] [Google Scholar]
- Lill CM, Schjeide BM, Akkad DA, Blaschke P, Winkelmann A, Gerdes LA, Hoffjan S, Luessi F, Dorner T, Li SC, Steinhagen-Thiessen E, Lindenberger U, Chan A, Hartung HP, Aktas O, Lohse P, Kumpfel T, Kubisch C, Epplen JT, Zettl UK, Bertram L, Zipp F. 2012. Independent replication of STAT3 association with multiple sclerosis risk in a large German case-control sample. Neurogenetics 13(1):83–86 [DOI] [PubMed] [Google Scholar]
- Linker RA, Lee DH. 2009. Models of autoimmune demyelination in the central nervous system: on the way to translational medicine. Exp Transl Stroke Med 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lee YS, Yu CR, Egwuagu CE. 2008. Loss of STAT3 in CD4+T cells prevents development of experimental autoimmune diseases. J Immunol 180(9):6070–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Holdbrooks AT, De Sarno P, Rowse AL, Yanagisawa LL, McFarland BC, Harrington LE, Raman C, Sabbaj S, Benveniste EN, Qin H. 2014. Therapeutic efficacy of suppressing the JAK/STAT pathway in multiple models of experimental autoimmune encephalomyelitis. J Immunol 192:59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Diego RS, Weiner HL. 2008. Novel therapeutic strategies for multiple sclerosis—a multifaceted adversary. Nat Rev Drug Discov 7(11):909–925 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. 2005. Macrophage polarization comes of age. Immunity 23(4):344–346 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. 2007. New vistas on macrophage differentiation and activation. Eur J Immunol 37(1):14–16 [DOI] [PubMed] [Google Scholar]
- Mayo L, Quintana FJ, Weiner HL. 2012. The innate immune system in demyelinating disease. Immunol Rev 248(1):170–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BC, Hong SW, Rajbhandari R, Twitty GB, Jr., Gray GK, Yu H, Benveniste EN, Nozell SE. 2013. NF-kappaB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS One 8(11):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ. 2011. GM-CSF: the secret weapon in the Th17 arsenal. Nat Immunol 12(6):521–522 [DOI] [PubMed] [Google Scholar]
- McMurray JS, Mandal PK, Liao WS, Klostergaard J, Robertson FM. 2012. The consequences of selective inhibition of signal transducer and activator of transcription 3 (STAT3) tyrosine705 phosphorylation by phosphopeptide mimetic prodrugs targeting the Src homology 2 (SH2) domain. JAK-STAT 1(4):263–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy G, Hilliard TS, Turkson J. 2013. Therapeutic modulators of STAT signalling for human diseases. Nat Rev Drug Discov 12(8):611–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, Hille A, Priller J, Prinz M. 2009. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132(Pt 9):2487–2500 [DOI] [PubMed] [Google Scholar]
- Mujtaba MG, Flowers LO, Patel CB, Patel RA, Haider MI, Johnson HM. 2005. Treatment of mice with the suppressor of cytokine signaling-1 mimetic peptide, tyrosine kinase inhibitor peptide, prevents development of the acute form of experimental allergic encephalomyelitis and induces stable remission in the chronic relapsing/remitting form. J Immunol 175:5077–5086 [DOI] [PubMed] [Google Scholar]
- Muthian G, Bright JJ. 2004. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol 24(5):542–552 [DOI] [PubMed] [Google Scholar]
- Muthian G, Raikwar HP, Johnson C, Rajasingh J, Kalgutkar A, Marnett LJ, Bright JJ. 2006. COX-2 inhibitors modulate IL-12 signaling through JAK-STAT pathway leading to Th1 response in experimental allergic encephalomyelitis. J Clin Immunol 26(1):73–85 [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. 2008. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65(17):2702–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Bright JJ. 2002. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun 3(2):59–70 [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sharma SV, Settleman J, Frank DA. 2011. A chemical biology approach to developing STAT inhibitors: molecular strategies for accelerating clinical translation. Oncotarget 2(6):518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg JR, Baranzini SE. 2010. Multiple sclerosis genetics—is the glass half full, or half empty? Nat Rev Neurol 6(8):429–437 [DOI] [PubMed] [Google Scholar]
- Oksenberg JR, Hauser SL. 2011. Decoding multiple sclerosis. Ann Neurol 70(6):A5–A7 [DOI] [PubMed] [Google Scholar]
- Opar A. 2010. Kinase inhibitors attract attention as oral rheumatoid arthritis drugs. Nat Rev Drug Discov 9(4):257–258 [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. 2013a. Janus Kinase inhibitors in autoimmune diseases. Ann Rheum Dis 72Suppl 2:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Laurence A, McInnes IB. 2013b. Back to the future: oral targeted therapy for RA and other autoimmune diseases. Nat Rev Rheumatol 9(3):173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Plenge R. 2012. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36(4):542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. 2007. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol 178(1):39–48 [DOI] [PubMed] [Google Scholar]
- Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. 2012a. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol 189(7):3439–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wang L, Feng T, Elson CO, Niyongere SA, Lee SJ, Reynolds SL, Weaver CT, Roarty K, Serra R, Benveniste EN, Cong Y. 2009. TGF-β promotes Th17 cell development through inhibition of SOCS3. J Immunol 183(1):97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Yeh W-I, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox TH III, Park K, Harrington LE, Raman C, Benveniste EN. 2012b. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci USA 109(13):5004–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Guo BT, Wan B, Fang L, Lu L, Wu L, Zang YQ, Zhang JZ. 2010. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol 185(3):1855–1863 [DOI] [PubMed] [Google Scholar]
- Quintana A, Muller M, Frausto RF, Ramos R, Getts DR, Sanz E, Hofer MJ, Krauthausen M, King NJ, Hidalgo J, Campbell IL. 2009. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol 183(3):2079–2088 [DOI] [PubMed] [Google Scholar]
- Ramgolam VS, Markovic-Plese S. 2011. Regulation of suppressors of cytokine signaling as a therapeutic approach in autoimmune diseases, with an emphasis on multiple sclerosis. J Signal Transduct 2011:635721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. 2009. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31(5):711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. 2009. Regulation of innate immune responses in the brain. Nat Rev Immunol 9(6):429–439 [DOI] [PubMed] [Google Scholar]
- Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D'alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D'hooghe MB, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppä V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Rückert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sørensen PS, Søndergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvänen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A. 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359):214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813(5):878–888 [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U, Shetty A, Varrin-Doyer M, Molnarfi N, Sagan SA, Sobel RA, Nelson PA, Zamvil SS. 2012. Laquinimod, a quinoline-3-carboxamide, induces Type II myeloid cells that modulate central nervous system autoimmunity. PLoS One 7(3):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seavey MM, Dobrzanski P. 2012. The many faces of Janus Kinase. Biochem Pharmacol 83(9):1136–1145 [DOI] [PubMed] [Google Scholar]
- Sen M, Grandis JR. 2012. Nucleic acid-based approaches to STAT inhibition. JAK-STAT 1(4):285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S, Freilino ML, Shi H, Li C, Ly D, Rapireddy S, Etter JP, Li PK, Wang L, Chiosea S, Seethala RR, Gooding WE, Chen X, Kaminski N, Pandit K, Johnson DE, Grandis JR. 2012. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: Implications for cancer therapy. Cancer Discov 2(8):694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. 2009. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6(7):e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Cross AH. 2006. Differential expression of suppressors of cytokine signaling-1 and -3 and related cytokines in central nervous system during remitting versus non-remitting forms of experimental autoimmune encephalomyelitis. Int Immunol 18(2):347–353 [DOI] [PubMed] [Google Scholar]
- Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez SF, Bassil R, Croci DO, Cerliani JP, Delacour D, Wang Y, Elyaman W, Khoury SJ, Rabinovich GA. 2012. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37(2):249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. 2010. Mixed results with modulation of Th-17 cells in human autoimmune diseases. Nat Immunol 11(1):41–44 [DOI] [PubMed] [Google Scholar]
- Stump KL, Lu LD, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Ator MA, Dorsey BD, Ruggeri BA, Seavey MM. 2011. A highly selective, orally active inhibitor of Janus Kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthr Res Ther 13(2):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thone J, Ellrichmann G, Seubert S, Peruga I, Lee DH, Conrad R, Hayardeny L, Comi G, Wiese S, Linker RA, Gold R. 2012. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol 180(1):267–274 [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Tarasova NI. 2012. Alternative ways of modulating JAK-STAT pathway: looking beyond phosphorylation. JAK-STAT 1(4):274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroeck K. 2012. Cytokine gene polymorphisms and human autoimmune disease in the era of genome-wide association studies. J Interferon Cytokine Res 32(4):139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroeck K, Alvarez J, Swaminathan B, Alloza I, Matesanz F, Urcelay E, Comabella M, Alcina A, Fedetz M, Ortiz MA, Izquierdo G, Fernandez O, Rodriguez-Ezpeleta N, Matute C, Caillier S, Arroyo R, Montalban X, Oksenberg JR, Antiguedad A, Aransay A. 2012. A cytokine gene screen uncovers SOCS1 as genetic risk factor for multiple sclerosis. Genes Immun 13(1):21–28 [DOI] [PubMed] [Google Scholar]
- van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff TH, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH. 2010. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol 185(3):1442–1449 [DOI] [PubMed] [Google Scholar]
- Walker SR, Frank DA. 2012. Screening approaches to generating STAT inhibitors: allowing the hits to identify the targets. JAK-STAT 1(4):292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SR, Xiang M, Frank DA. 2013. Distinct roles of STAT3 and STAT5 in the pathogenesis and targeted therapy of breast cancer. Mol Cell Endocrinol 382(1):616–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. 2010. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 70(12):4840–4049 [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. 2007. IL-17 family cytokines and the expanding diversity of effector T-cell lineages. Ann Rev Immunol 25: 821–852 [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. 2007a. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med 13(8):935–943 [DOI] [PubMed] [Google Scholar]
- Weber MS, Steinman L, Zamvil SS. 2007b. Statins—treatment option for central nervous system autoimmune disease? Neurotherapeutics 4(4):693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL. 2008. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J Neurol 255Suppl 1:3–11 [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282(13):9358–9363 [DOI] [PubMed] [Google Scholar]
- Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E H.-P.robst C, van den Broek M, Riethmacher D, Birnberg T, Blank T, Reizis B, Korn T, Wiendl H, Jung S, Prinz M, Kurschus FC, Waisman A. 2012. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor+regulatory T cells. Immunity 37(2):264–275 [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. 2012. SOCS, inflammation, and autoimmunity. Front Immunol 3(20):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Lee YS, Mahdi RM, Surendran N, Egwuagu CE. 2012. Therapeutic targeting of STAT3 (signal transducers and activators of transcription 3) pathway inhibits experimental autoimmune uveitis. PLoS One 7(1):e29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer S, Wu Y, Bassett J, Yang B, Zaheer A. 2007. Glia maturation factor regulation of STAT expression: a novel mechanism in experimental autoimmune encephalomyelitis. Neurochem Res 32(12):2123–2131 [DOI] [PubMed] [Google Scholar]
- Zamvil SS, Steinman L. 2002. Cholesterol-lowering statins possess anti-inflammatory activity that might be useful for treatment of MS. Neurology 59(7):970–971 [DOI] [PubMed] [Google Scholar]
- Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. 2008. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+lymphocytes. J Immunol 180(10):6988–6996 [DOI] [PubMed] [Google Scholar]
- Zhang X, Tao Y, Troiani L, Markovic-Plese S. 2011. Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+T cells derived from patients with multiple sclerosis. J Immunol 187(6):3431–3437 [DOI] [PubMed] [Google Scholar]
- Zhang X, Tao Y, Wang J, Garcia-Mata R, Markovic-Plese S. 2013. Simvastatin inhibits secretion of Th17-polarizing cytokines and antigen presentation by DCs in patients with relapsing remitting multiple sclerosis. Eur J Immunol 43(1):281–289 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. 2012. Orally bioavailable small-molecule inhibitor of transcription factor STAT3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA 109(24):9623–9628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Kennedy JK, Wang Y, Sandoval-Garcia C, Cao L, Xiao S, Wu C, Elyaman W, Khoury SJ. 2011. Plasticity of Ly-6C(hi) myeloid cells in T cell regulation. J Immunol 187(5):2418–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuvich RL, Bush WS, McCauley JL, Beecham AH, De Jager PL, Ivinson AJ, Compston A, Hafler DA, Hauser SL, Sawcer SJ, Pericak-Vance MA, Barcellos LF, Mortlock DP, Haines JL. 2011. Interrogating the complex role of chromosome 16p13.13 in multiple sclerosis susceptibility: independent genetic signals in the CIITA-CLEC16A-SOCS1 gene complex. Hum Mol Gen 20(17):3517–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]