Abstract
There is increasing support for the importance of risk factors such as genetic makeup, obesity, smoking, vitamin D insufficiency, and antibiotic exposure contributing to the development of autoimmune diseases, including human multiple sclerosis (MS). Perhaps the greatest environmental risk factor associated with the development of immune-mediated conditions is the gut microbiome. Microbial and helminthic agents are active participants in shaping the immune systems of their hosts. This concept is continually reinforced by studies in the burgeoning area of commensal-mediated immunomodulation. The clinical importance of these findings for MS is suggested by both their participation in disease and, perhaps of greater clinical importance, attenuation of disease severity. Observations made in murine models of central nervous system demyelinating disease and a limited number of small studies in human MS suggest that immune homeostasis within the gut microbiome may be of paramount importance in maintaining a disease-free state. This review describes three immunological factors associated with the gut microbiome that are central to cytokine network activities in MS pathogenesis: T helper cell polarization, T regulatory cell function, and B cell activity. Comparisons are drawn between the regulatory mechanisms attributed to first-line therapies and those described in commensal-mediated amelioration of central nervous system demyelination.
Introduction
Bacterial agents colonize the human host from birth and work in tandem with the cells of the young growing organism to establish immune and physiologic equilibrium. The result of this intricately orchestrated set of processes is appropriate development of host tissues and an environment within which commensals may thrive. Cell-for-cell the multicellular host is outnumbered 100 to 1 by the large number (1014) and species variety (500–1,000) of commensal bacteria existing within the gastrointestinal tract (Huttenhower 2012). The cast of characters typically includes hundreds of bacterial species, viruses, and, in some instances, helminths. Microbial commensals, collectively referred to as the microbiota, impact a surprising range of developmental and homeostatic processes in their hosts such as metabolism, vessel formation, and bone reabsorption, highlighting the importance of maintained symbiosis (Sommer and Bäckhed 2013). Roles for the microbiota in development and function of host immune responses are also well established. Commensals may influence both innate and adaptive arms of host immune systems, including mucosal barrier formation, production of antimicrobial peptides, as well as humoral and cellular immune responses (Kamada and others 2013). Of clinical relevance, both the microbiota and helminths have been shown to reduce severity of experimentally induced organ-specific inflammation in animals. Notably, therapeutic relevance of these findings has been extended to human disease as reported by observational studies and clinical trials for inflammatory bowel disease and multiple sclerosis (MS).
MS is a disease characterized by formation of plaque-like lesions on the brain or spinal cord as a result of aberrant inflammatory activity localized within these central nervous system (CNS) sites. The majority of patients diagnosed with MS present with a relapsing-remitting form of the disease (RRMS) where a transient period of CNS-localized inflammation and demyelination results in sensory and/or motor dysfunction. MS lesions are often associated with an array of leukocytes from both the myeloid and lymphoid lineage, suggesting that the interface between the CNS and the immune system is key to the pathogenesis of RRMS (Noseworthy and others 2000; Sospedra and Martin 2005). Recent studies suggest that the immune dysfunction associated with MS continues as patients move into a secondary, more progressive phase of the disease process. Furthermore, current disease-modifying therapies target various immune system compartments. While current understanding of MS has revealed a plethora of mechanisms underlying disease, this review will focus on the impact of commensal-mediated effector and regulatory cytokine expression by three centrally implicated cell populations: T helper (Th) cells, T regulatory cells (Tregs), and B cells.
Commensal Agents May Facilitate Induction of Pathogenic Th1 and Th17 Cells
The archetypical immune response is characterized by a number of interactions between T cells of the adaptive immune system and antigen presenting cells (APCs) of the innate immune system. Recognition and presentation of antigen by APCs such as dendritic cells (DCs), monocytes, or B cells lead to increased expression of costimulatory molecules on the surface of APCs, including CD80 and CD86, resulting in the survival and full activation of cognate T cells. Reciprocally, engagement of CD40 ligand (expressed by activated T cells) with CD40 constitutively present on the surface of APCs promotes APC maturation, which may include enhanced phagocytosis, trafficking to draining lymph nodes, or antibody isotype switching. In addition to these interactions, cytokines secreted by both APCs and T cells play pivotal roles in directing pathogen-appropriate immune responses that are capable of promoting or dampening inflammatory environments. Production of either IL-12, or IL-4 or a combination of IL-1 β, IL-6, and IL-23 by APCs causes increased expression of the transcription factors T-bet, GATA-3, or RoRγt in naïve Th cells, resulting in polarization of these cells toward Th1, Th2, or Th17 subtypes, respectively. Polarized Th cells are distinguished by production of hallmark cytokines: Th1 cells are primary producers of the cytokine IFNγ; Th2 cells are associated with IL-4, IL-13, IL-10, and TGF-β; finally, Th17 cells are primary sources of IL-17 and IL-22 (Zhu and others 2010).
Use of germ-free (GF) mice as well as antibiotic treatment of conventionally colonized mice have nuanced our understanding of how the microbiota can affect aspects of host immune responses such as Th polarization. Bred and raised in sterile conditions, GF mice lack the influence of commensals on their biological systems, resulting in profound developmental defects among gut-associated lymphoid tissue (GALT) such as peyer's patches, cryptopatches, and innate lymphoid follicles (Hooper 2004; Sommer and Bäckhed 2013). However, immunological discrepancies are not limited to the GALT. Extraintestinal secondary lymphoid structures, including lymph nodes, and spleen are also underdeveloped and relatively disorganized compared with same strain mice housed under specific-pathogen-free (SPF) conditions (Macpherson and Harris 2004). Correspondingly, GF mice exhibit altered Th polarization, reflected in their Th2-dominated immune system characterized by substantially reduced frequencies of Th1 and Th17 cells. The introduction of commensal agents is sufficient to resolve structural and organizational deficiencies (Macpherson and Harris 2004). Similarly, colonization of GF mice with particular species of the microbiota such as Bacteroides fragilis and segmented filamentous bacteria (SFB), a murine-specific commensal, can regulate the frequency of Th cell populations. Colonization of the GF mice with B. fragilis, a human commensal, corrects the Th2 imbalance characteristic of these mice, promoting Th1 polarization evidenced by reduced serum IL-4 and enhanced IFNγ-producing CD4+ T cells (Mazmanian and others 2005). Likewise, while GF mice possess no inherent capacity to produce IL-17 or IL-22, colonization of GF mice with SFB resulted in potent induction of CD4+ T cells producing these cytokines (Ivanov and others 2009).
Based on numerous studies in experimental allergic (or autoimmune) encephalomyelitis (EAE), the most commonly used animal model of human CNS demyelinating disease, MS has been considered a Th1-dominated condition along a Th1/Th2 axis of polarization. The presence of T cells in CNS tissue and cerebrospinal fluid (CSF) (Zhang and others 1994) as well as increased levels of Th1 cytokines in MS patient peripheral circulation provided the precedent for this belief. Additionally, treatment of a cohort of patients with the Th1-polarized cytokine, IFNγ, exacerbated disease in a number of patients (Panitch and others 1987). However, opposing the role of Th1-mediated inflammation in MS immunopathogenesis was the clinical trial failure of anti-IL-12p40 in RRMS (Segal and others 2008). Recently, a more complex immunopathology underlying EAE has been suggested, particularly since the condition could still be induced, and in fact exacerbated, in mice lacking factors necessary for Th1 cell polarization (Gran and others 2002). It is now understood that Th17 cells are key in clinical outcomes of EAE. Importantly, the central role of Th17 cells in CNS autoimmunity in EAE has been upheld in human MS. Enhanced frequencies of T cells expressing IL-17 are observed in patient blood and found postmortem in MS patient CNS tissue (Lock and others 2002; Durelli and others 2009).
Given the centrality of Th1 and Th17 cells to MS and murine EAE, promotion or suppression of these cells is of critical relevance to MS pathogenesis. Accordingly, the ability for the microbiota to dramatically influence susceptibility to CNS demyelinating disease may be a function of their capacity to polarize Th responses. GF mice afflicted with EAE exhibit a less severe disease marked by reduced myelin oligodendrocyte glycoprotein (MOG)-specific IFNγ, and IL-17 production compared with SPF mice (Lee and others 2011), perhaps reflecting their Th2-skewed T cell responses. In contrast, colonization of GF mice with Th17-promoting SFB restores susceptibility to EAE. A similar trend is observed in anti-MOG TCR transgenic SJL/J mice that are prone to developing spontaneous EAE. Whereas 80% of SPF SJL/J mice experience spontaneous relapsing-remitting EAE within the first 3–8 months of life, GF mice of this genetic background do not develop disease throughout the course of their lives (Berer and others 2012). Whether gut-derived Th17 cells traverse the blood–brain barrier and contribute to CNS demyelination remains to be formally demonstrated. However, in support of this notion Berer and colleagues (2012) observe a drastic reduction of Th17 cells in the GALT but not in extraintestinal secondary lymphoid sites of their GF mice. Thus, microbiota-induced Th17 cells seemingly contribute to CNS demyelination in this model. Further evidence for the encephalitogenic potential of gut-derived Th17 cells is provided by Ito and others (2011), who report expression of both CNS and gut-homing receptors by CD4 T cell infiltrates in their spontaneous EAE model.
Th2 Polarization Is Characteristic of Commensal-Mediated Protection from EAE
Th1 and Th17 cells are prime therapeutic targets for a number of MS treatments. Glatiramer acetate (GA) is a frequently utilized first-line therapy comprised of a synthetic polymer made up of 4 amino acids (glutamic acid, lysine, alanine, and tyrosine) in a specific molar concentration. Administration of GA in EAE-afflicted mice results in reduced Th1 and Th17 activity along with upregulated Th2 cytokines (Begum-Haque and others 2008). A separate study showed that murine GA-specific T cells lose their ability to produce IFNγ, and instead produce Th2 cytokines (Aharoni and others 1997). This was paralleled in patients treated with GA, who exhibited elevated frequencies of Th2 cytokine-producing GA-specific cells (Neuhaus and others 2000). Thus, GA may facilitate polarization away from potentially pathogenic inflammatory Th1 and Th17 cells and instead promote of a protective Th2 program. Interferon-β (IFB-β) therapy represents a class of first-line treatments based on injection of the recombinant cytokine IFB-β, which, like GA, promotes production of Th2-associated cytokine IL-10. Support for this stems from observations that IFB-β reduced IL-12p40 and IL-23 levels while promoting IL-10 in MS patients (Alexander and others 2010).
We have shown that altering the microbial load of the gut by treating conventionally colonized mice with an oral antibiotic cocktail (ampicillin, vancomycin, neomycin sulfate, and metronidazole) parallels these therapeutic trends significantly dampening IFNγ, IL-6, and IL-17 within GALT and extraintestinal secondary lymphoid sites. Concomitantly, this commensal shift enhances IL-13 and IL-10 levels while rendering disease-susceptible mice resistant to EAE (Ochoa-Repáraz and others 2009). In contrast, intraperitoneal delivery with the same antibiotic cocktail fails to affect both qualitative and quantitative bacterial load and, moreover, has no impact on disease susceptibility. Furthermore, we demonstrated a broad therapeutic relevance of these trends by reducing disease severity in two genetically different strains of mice upon administration of this antibiotic cocktail (Ochoa-Repáraz and others 2009). In a related study, transfer of IL-10-producing B cells from antibiotic-treated mice into EAE-afflicted recipient mice reverses disease susceptibility and is associated with enhanced MOG-specific production of IL-10 and IL-13 along with reduction of IFNγ and IL-17 (Ochoa-Repáraz and others 2010b). Work by Lavasani and others (2010) in which several lactobacillus strains were orally administered to EAE-afflicted mice demonstrated similarly enhanced Th2 cytokines (IL-4, IL-10, and TGF-β) and reduced TNF-α, IFNγ, and IL-17. Importantly, this Th2 induction correlated with protection from disease both prophylactically and therapeutically after disease onset (Lavasani and others 2010).
Helminth Administration Yields Protective Th2 Responses in Mice
Helminths, like the microbiota, are capable of altering disease course for EAE and other autoimmune disease models (Cooke and others 1999; Araujo and others 2000; Elliott and others 2003; Kitagaki and others 2006). Injection of Schistosoma mansoni ova or oral administration of Schistosoma japonicum soluble egg antigen (SEA) before EAE induction results in delayed onset of symptoms and attenuated disease severity (Sewell and others 2003; Zheng and others 2008). Similar to microbiota-induced therapy, helminth-mediated attenuation of EAE is often associated with enhanced Th2 cytokines along with reduction of Th1 as well as Th17 cytokines. Protection from EAE by oral administration of S. mansoni ova correlates with enhanced IL-4, and reduced IFNγ production in splenocytes isolated from infected SJL/J mice. Th2-based protection from CNS inflammation is also reported when administering adult S. mansoni, as protection is ablated in STAT-6 KO mice (Sewell and others 2003; Zheng and others 2008). Likewise, administration of S. japonicum SEA attenuates disease severity while yielding enhanced IL-4 and reduced IFNγ (Zheng and others 2008). Similar to our observations in which enhanced CNS antigen-specific Th2 cytokine production results from transfer of B cells taken from antibiotic-treated mice (Ochoa-Repáraz and others 2010b), S. mansoni ova administration increases numbers of IL-4-producing, but not IFNγ-producing, T cells in the CNS of infected mice (Sewell and others 2003). These therapeutic CNS-specific Th2 responses are reminiscent of findings by Aharoni and others (1997, 2003), which demonstrated GA-specific Th2 cell activity in the CNS of GA-treated mice.
Helminth-Mediated Attenuation of Human MS Is Associated with Th2 Polarization
Efficacy of helminth infection in dampening human MS has been recently demonstrated in a fascinating longitudinal observational study and a small pilot trial. Although appropriate blinding and placebo controls were missing from these studies, the suggested clinical benefit and positive treatment safety profile has prompted several larger studies (HINT-2, WIRMS, TRIOMS), which are currently underway. Correale and Farez (2011) followed the progress of 12 individuals with RRMS whom had contracted helminth infections through natural means. Nine patients presented with infections of Hymenolepis nana, Trichuris trichiura, or Ascaris lumbricoides. Two additional patients presented with Strongyloides stercolaris and one with Enterobius vermicularis. Clinical, radiological, and cytokine readouts made every three or six months were compared with 12 geographically matched healthy individuals as well as 12 age-, sex-, and disease duration-matched but uninfected RRMS patients who were in clinical remission. Individuals were observed for a total period of approximately 90 months. During this time the infected group of patients experienced 3 clinical relapses compared with 56 that occurred in the uninfected group. Uninfected RRMS patients also presented with greater gadolinium-enhancing lesion load and higher expanded disability status scale scores compared to infected counterparts. Immunologic readouts reflected clinical and neurological activity. When assayed for cytokine production, peripheral blood mononuclear cells (PBMCs) from infected RRMS patients produced significantly more IL-10 and TGF-β and less IL-12 and IFNγ compared with uninfected patients. Infected patients also yielded greater numbers of IL-10- and TGF-β-secreting T cell clones that were specific to MBP83–102 (Correale and Farez 2007). Conversely, infected patients produced fewer cells that secreted IFNγ.
In the HINT-1 pilot trial, five individuals newly diagnosed with RRMS who were naïve to treatment were given 2,500 Tricuris suis ova (TSO) orally every two weeks. After three months of treatment, MRI and serum readouts were compared with baseline (before treatment). The mean number of gadolinium-enhancing lesions fell from 6.6 at baseline to 2.0 after treatment. Two months after cessation of treatment, this number rose to 5.8. Cytokine serum levels suggest a systemic Th2 response to TSO treatment evidenced by average relative increases of IL-4, IL-5, IL-13, and IL-10. Four of the infected patients were given anti-helminth therapy after presenting with gastrointestinal disturbances. Remarkably, soon after treatment the average clinical and immunologic readouts of these patients resembled uninfected individuals complete with neurologic disease activity and corresponding cytokine profiles (Fleming and others 2011). As noted by study investigators, the readouts and assessment of this study were unblinded. Nonetheless, their findings suggest a proof of concept that helminths exhibit a potent capacity to effectively regulate MS through mechanisms, which include enhancement of Th2 cytokines. The results of this pilot trial suggest clinically favorable trends; however, its short duration and the limited numbers of individuals enrolled preclude definitive interpretation regarding the efficacy of TSO treatment.
MS Pathogenesis Features Dampened T-Regulatory Cell Function
Tolerogenic mechanisms are critical factors that work in concert with Th polarization facilitating careful application of immune responses. Central tolerance in the thymus minimizes the circulating pool of autoreactive T cells. This is accomplished in the thymus via positive and negative selection of bone marrow-derived Th cell progenitors. A majority of T cells, including those that react strongly to self-antigens, fail this selection process and are deleted. However, this selection process is imperfect, resulting in a number of autoreactive T cells among the peripheral repertoire (Nepom 2005). Responsibility to curtail pathological inflammation caused by self-antigen-specific autoimmune responses therefore falls to peripheral tolerance mechanisms. These mechanisms may act via localized cell-mediated anti-inflammatory responses in the form of Treg activity, or cytokine-mediated bystander suppression from other sources.
Current definitions of human Tregs include both cells that are enriched for and those that lack the forkhead box protein 3 (Foxp3) transcription factor (Sakaguchi and others 2010). Tr1 cells are a class of suppressive T cells in humans lacking Foxp3 expression that predominantly mediate their suppressive effects by production of IL-10. Foxp3+ Tregs are generally defined as CD4+ T cells expressing moderate to high levels of CD25 and diminished levels of CD127. Within this definition, expression of CD45RA and CD45RO distinguishes naïve and memory Tregs, respectively; furthermore, naïve Tregs may convert into memory Tregs upon TCR stimulation (Booth and others 2010). These Treg subsets demonstrate several important functional distinctions in regard to rate of proliferation, resistance to CD95L-mediated cell death, and secretion of cytokines (Fritzsching and others 2006). Overall naïve Tregs seemingly represent a basal population from which shorter-lived, cytokine-secreting memory Tregs may generate (Miyara and others 2009). Memory Tregs, particularly those expressing ICOS, are copious producers of IL-10, while ICOS− Tregs predominantly secrete TGF-β (Ito and others 2008). CD161-expressing memory Tregs have been demonstrated to secrete inflammatory cytokines such as IL-17, further emphasizing the importance of phenotypic classification in disease-relevant activities of these cells (Afzali and others 2013; Pesenacker and others 2013).
Absence or dysfunction of Tregs results in autoimmune pathology highlighting the critical function of these suppressive cells (Asano and others 1996). Indeed, while self-reactive T cells against several CNS epitopes such as MBP and MOG circulate in the periphery of healthy individuals, T cell reactivity in MS patients toward these antigens is potentiated (Zhang and others 1994; Traggiai and others 2001; Nepom 2005). Treg dysfunction in MS is supported by both in vitro experimental data and genome-wide association studies linking MS pathogenesis to several molecules associated with Treg survival and function, including CD127 and CD58 (Hafler and others 2007; Rubio and others 2008). Work by Hafler and others (2007) demonstrate that while MS patients possessed a comparable frequency of Tregs to healthy donors, they demonstrate a functional disturbance marked by a reduction of in vitro suppressive activity (Viglietta 2004). Analysis of various Treg subsets supports and nuances this observation. Tr1 cells derived from MS patients secrete less IL-10 compared with healthy donors when stimulated via CD46 (Astier and others 2006). A subset of the naïve Treg compartment known as recent thymic emigrant (RTE)-Tregs, which express CD31, is deficient in limiting effector cell proliferation in vitro (Haas and others 2007). Furthermore, as individuals with RRMS possess RTEs containing consistently lower levels of short joining chain T-cell receptor excision circles compared with healthy donors, this dysfunction may reflect premature thymic involution (Hug and others 2003; Venken and others 2010). Suppressive deficiency has also been observed in a Foxp3+ subset expressing CD39, an ectonuclease enzyme found predominantly on memory Tregs. CD39 cleaves extracellular ATP into ADP, reducing the potentially inflammatory contribution of this molecule. Fletcher and colleagues (2009) demonstrated that CD39+ Tregs were exclusively capable of suppressing IL-17 production in vitro. Unlike age-matched healthy donors, CD39+ Tregs taken from MS patients were unable to suppress production of IL-17 by autologous stimulated effectors in vitro, implying a potentially critical role for CD39+ Tregs in MS.
Current Therapies and Commensal Modification Facilitate Enhanced Treg Frequency and Function
Enhanced function and frequency of Foxp3+ Tregs after treatment with GA or IFNβ-1 further supports the importance of Treg function in MS. When treated with GA, EAE-afflicted mice exhibit enhanced frequencies of Tregs that express greater levels of Foxp3. Accordingly, these Tregs are more suppressive (Jee and others 2007). Similarly, while individuals with RRMS typically express lower levels of Foxp3 (Venken and others 2008b) GA-treated MS patients exhibit enhanced Foxp3 expression among CD4+ T cells (Hong and others 2005). Frequencies of both naïve and memory Tregs are enhanced in MS patients after GA and IFN-β treatment, and the increased presence of RTE-Tregs correlate with restored suppressive function in these patients (Venken and others 2008a).
Paralleling these observations, similar trends may be observed upon altering the microbiota with oral administration of antibiotics, commensal species, or certain commensal antigens. EAE-afflicted mice subjected to significant changes in the heterogeneity of the gut microbiota by treatment with antibiotics incur a greater frequency of Foxp3+ Tregs in GALT, including mesenteric lymph nodes, as well as extraintestinal sites such as spleen, and cervical lymph nodes, suggesting a systemic effect. These antibiotic-induced Tregs also possess greater suppressive function, capable of more potently attenuating EAE compared with the same phenotype of Tregs derived from mice that did not receive antibiotics treatment (Ochoa-Repáraz and others 2009). Oral delivery of lactobacillus strains enhances the frequency of Tregs in the CNS of EAE-afflicted mice, which dampens disease in an IL-10-dependent manner (Lavasani and others 2010). Induction of Treg cells has also been associated with therapeutic effects of helminth infection. Trichinella spiralis infection of dark agouti rats results in increased frequencies of CD4+CD25+Foxp3+ Tregs (Gruden-Movsesijan and others 2010). Similarly, Walsh and others demonstrate that, along with amelioration of EAE, Fasciola hepatica infection mediates induction of IL-10-producing Tr1 cells and Foxp3+ Tregs capable of producing IL-10 and TGF-β (Walsh and others 2009).
Microbiota-Derived Antigens Enhance Foxp3+ Treg Frequency and Function
Our published studies on B. fragilis polysaccharide A (PSA) demonstrate that commensal-mediated therapeutic effects in EAE can be achieved using a single commensal component in the place of generalized bacterial modification. Although 8 kinds of capsular polysaccharides are found on the surface of B. fragilis, the capacity for the organism to successfully promote certain T cell responses, lymphoid structure organization, and mediate protection against EAE is dependent on the presence of PSA (Surana and Kasper 2011). Of potential clinical interest, oral gavage administration of purified PSA on its own has the capacity to drive these effects precluding complications, which may arise from therapeutic use of live commensal organisms. Interestingly, re-colonization of oral antibiotic-treated EAE-resistant mice with a strain of B. fragilis lacking PSA (ΔPSA B. fragilis) promotes susceptibility to EAE, whereas use of PSA-expressing wild-type B. fragilis maintains protection against disease, suggesting that PSA alone has the capacity to influence resistance (Ochoa-Repáraz and others 2010a). While PSA is associated with induction of Th1 responses in GF animals, in the context of experimental autoimmune conditions such as TNBS-mediated colitis, investigators report enhanced Treg frequencies and IL-10 production (Mazmanian and others 2008). We previously demonstrate that oral gavage administration of PSA induces a population of IL-10-producing CD4+CD25+Foxp3+ Tregs. Toll-like receptor (TLR) 2 recognition as well as IL-10 production is central to PSA-mediated protection against EAE, as PSA administration to either IL-10 KO or TLR2-deficient mice does not protect from disease. Furthermore, adoptive transfer of CD4+CD25+ cells from PSA-treated mice protects recipient mice from disease (Ochoa-Repáraz and others 2010c).
In the context of EAE, PSA induces the accumulation of DCs expressing the gut-homing marker CD103 in the cervical lymph nodes of EAE-afflicted mice. These DCs have the potential to convert conventional CD4+ T cells into IL-10-producing Foxp3+ Tregs; however, CD103+ DCs from mice treated with ΔPSA B. fragilis demonstrate a diminished capacity to do so (Ochoa-Repáraz and others 2010a). Surprisingly, PSA-expressing (WT) B. fragilis potentiates both the capability of conventional CD4+ T cells to be converted into Foxp3+ Tregs, as well as the regulatory function of CD4+CD25+ cells, which includes those expressing Foxp3+. Indeed, Tregs converted from conventional CD4s derived from WT B. fragilis produce significantly more TGF-β and IL-10, compared with those generated from ΔPSA B. fragilis–treated mice. Adoptive transfer of converted Foxp3+ Tregs from WT B. fragilis–treated mice protected from EAE, whereas those generated from ΔPSA B. fragilis–treated mice failed to do so. Another bacterial compound Escherichia coli colonization factor antigen 1 (CFA/I) also shows potential to modulate EAE in this manner. Delivery of CFA/I via an attenuated Salmonella enterica serovar Typhimorium vector results in IL-4-, IL-10-, and IL-13-producing Th2 cells and induction of TGF-β-secreting Foxp3+ Tregs (Ochoa-Repáraz and others 2007). While both cell types contribute to protection against EAE, TGF-β-producing Tregs are particularly apt in suppressing Th1 and Th17 responses (Jun and others 2012).
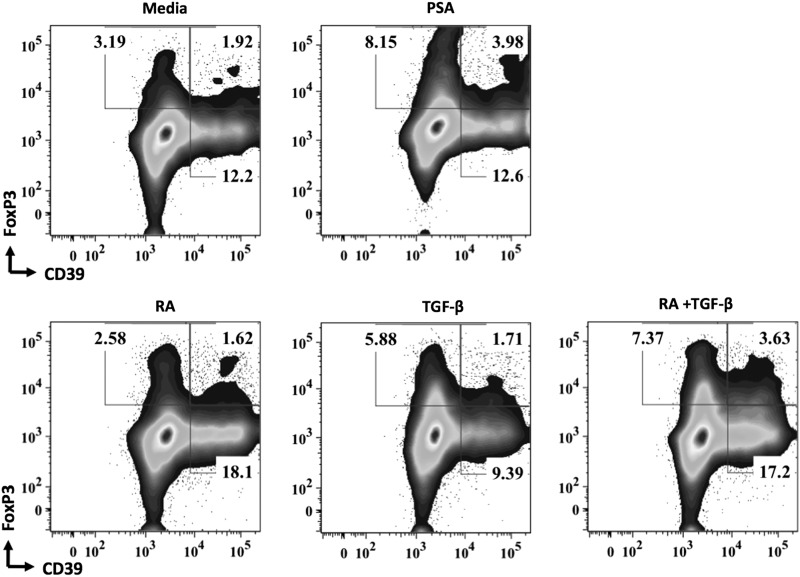
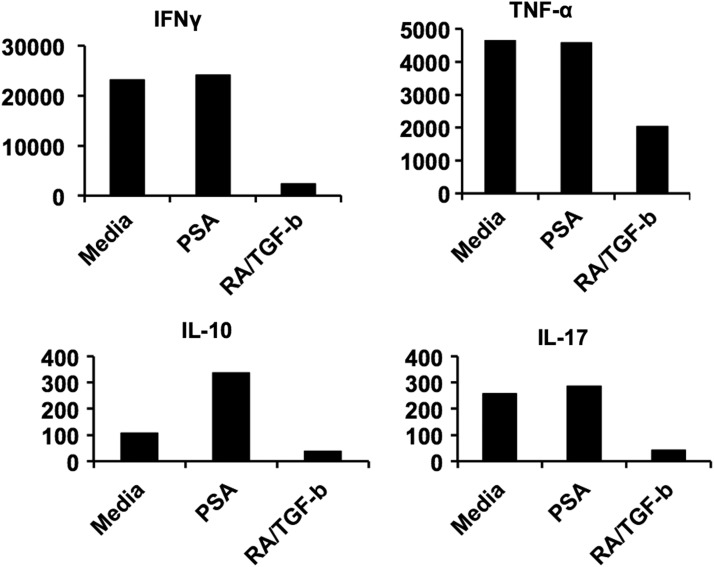
There are few examples of commensal agents impacting the various human Treg subsets implicated in MS dysfunction. Kreisman and Cobb (2011) demonstrate that PSA cultured in vitro with sorted human PBMCs does not result in Foxp3+ Treg induction, as might be expected based on murine studies. To the contrary, repeated exposure of human PBMCs to PSA in vitro yields IL-10-producing Tr1 cells. In contrast to these observations, we have now demonstrated that CD39+Foxp3+ Tregs may be generated from human PBMCs with the addition of TCR stimulation, CD80/86 costimulation, and exogenous IL-2 (Fig. 1). The discrepancies observed might be caused by culture conditions; however, additional studies are underway to clarify these differences. IL-10 produced by these cells is central to their capacity to suppress effector activities. Blocking antibody against IL-10 abrogated their in vitro suppressive function (Kreisman and Cobb 2011). In agreement with Kreisman and Cobb (2011), we have demonstrated PSA's capacity to enhance IL-10 production (Fig. 2); however, the specific cellular source of this enhanced IL-10 is under investigation. Of further note, production of effector cytokines IFNγ, TNF-α, and IL-17 was not significantly altered (Fig. 2). In the helminth observational study by Correale and Farez (2007), described previously in this review, CD4+CD25+Foxp3+ Treg frequencies were significantly heightened compared with uninfected individuals. Finally, a moderate increase in the frequency of CD4+CD25+Foxp3+ Tregs was reported for 2 of the 5 individuals during the HINT-1 study (Fleming and others 2011). The association between oral TSO administration and shift in the gut microbiota colonization has not been evaluated to date.
FIG. 1.
PSA enhances the frequency of human CD39+Foxp3+ Tregs in vitro. Representative flow cytometry plot of peripheral blood mononuclear cells cultured for 5 days with 100 U/mL recombinant IL-2 and αCD3/CD28 beads (cell-to-bead ratio 4:1) in the presence or absence of 100 μg/mL of PSA. For comparison, induced Foxp3+ Tregs were generated using 5 ng/mL of recombinant TGF-β and 4 nM of RA. PSA, polysaccharide A; RA, retinoic acid; Tregs, T regulatory cells.
FIG. 2.
PSA promotes IL-10 production by human peripheral blood mononuclear cells in vitro. Cytokine production (pg/mL) by human peripheral blood mononuclear cells after culture for 5 days with 100 U/mL recombinant IL-2 and αCD3/CD28 beads (cell-to-bead ratio 4:1) in the presence or absence of 100 μg/mL of PSA, 5 ng/mL of recombinant TGF-β, and 4 nM of RA.
B Cell-Driven T Cell Responses Are Implicated in MS Pathology
Capable of secreting immunoglobulins (Igs) and pro- and anti-inflammatory cytokines, and driving T cell responses as APCs, B cells are important multifunctional lymphocytes during immune responses. Igs facilitate persistent memory against recurrent pathogens, and provide a point of synergy between the adaptive and innate immune systems via opsonization. B cells have been shown to drive Th1 effector function through secretion of IL-12, and may produce several cytokines associated with Th17 polarization (IL-1β, IL-6, IL-23, and TGF-β) (Pistoia 1997; Appel and others 2004; Agrawal and Gupta 2011; Ramgolam and others 2011); however, B cell induction of Th17 cells remains to be demonstrated in an experimental setting.
B cell activity is implicated both at the earliest stages of MS immune pathogenesis before the transition from clinically isolated syndrome to definite MS, as well as during progressive stages when ectopic germinal center-like follicles may be detected within the meninges. In addition, B cells as well as plasma cells may be detected in association with MS lesions. Indeed, the view MS as a strictly T cell-driven disease is all but obsolete; rather, there are increasingly clear indications for a central role of B cell cytokine production, antigen presentation, and resulting Th polarization in MS. For instance, B cells derived from MS patients promote greater Th1 responses against MOG and MBP stimulation compared with healthy individuals (Harp and others 2010). Depleting inflammatory B cells using CD20-specific antibody therapy reduces T cells in the CSF (Cross and others 2006) as well as harmful inflammatory cytokines such as IL-6 produced by B cells and IL-17 produced by T cells (Barr and others 2012). Podoplanin-expressing Th17 cells are key in the formation of B cell containing ectopic lymphoid follicles in the CNS of EAE-afflicted mice (Peters and others 2011). These secondary-lymphoid-like structures are believed to play important roles in perpetuating aggressive immune responses within the CNS characteristic of progressive stages of MS. Taken together, B cells producing the Th17-polarizing cytokine IL-6 may contribute to the formation of Th17 cells, which in turn may perpetuate B cell-mediated inflammation within the CNS.
Conversely, suppressive IL-10-producing B cells may be important for regulating inflammatory responses in MS either directly or by inducing Th2 rather than Th1 and Th17 responses. Several studies report deficient B cell IL-10 production in individuals with MS compared with healthy individuals (Duddy and others 2007; Hirotani and others 2010; Knippenberg and others 2011). Adoptive transfer of IL-10-producing CD5+CD1d+ B cells attenuates EAE pathology (Matsushita and others 2008), and is associated with enhanced levels of brain-derived neurotrophic factor in the CNS, implying that these cells may promote a level of neuroprotection. GA treatment of EAE-afflicted mice yields decreased B cell production of IL-6, IL-12, and IFNγ levels, while enhancing Th2-associated cytokines IL-4, IL-13, and IL-10 (Begum-Haque and others 2010), suggesting that these B cells may promote anti-inflammatory Th2 responses. In agreement with these observations, Kala and others (2010) show that adoptively transferred B cells from GA-treated mice limit Th1 and Th17 responses and enhance B cell IL-10 production. Similarly, in IFNβ-1-treated patients, B cell production of IL-1β and IL-23 is diminished, while secretion of IL-10 is enhanced. Finally, both GA- and IFNβ-1-treated patients exhibit reduced expression of costimulatory molecules CD40 and CD80 expression, limiting their capacity to activate T cells (Kala and others 2010; Ramgolam and others 2011).
Although commensal influence on B cell activity is perhaps best known for maintenance of IgA isotype secretion in the intestine, for instance, by facilitating appropriate maturation of intestinal plasma cells (Fritz and others 2013), commensal-mediated promotion of IL-10-producing B cells is an increasingly prevalent finding. Inducing a broad shift among the microbiota via delivery of combination antibiotics treatment as described in our studies results in generation of an anti-inflammatory population of CD19+CD5+ B cells. These cells produce IL-10 when stimulated with lipopolysaccharide, and confer a level of protection against EAE when adoptively transferred to EAE-inflicted mice. Mice receiving antibiotic-induced B cells yield enhanced IL-13 and IL-10, as well as reduced IFNγ and IL-17, in response to in vitro stimulation with MOG compared with recipients of conventional CD19+CD5+ cells (Ochoa-Repáraz and others 2010b). Wilson and others (2010) report induction of protective regulatory CD19+CD23Hi B cells by Heligmosomoides polygyrus infection. Upon transfer to EAE mice, these cells significantly reduce disease severity in an IL-10-independent manner (Wilson and others 2010).
The observational helminth study by Correale and others (2008) revealed that MS patients with naturally occurring helminth infections possess B cells with heightened IL-10-producing capacity compared with uninfected individuals. Furthermore, while B cells from infected individuals and healthy donors attenuate MBP-specific Th1 responses in an IL-10-dependent manner, B cells from uninfected patients were unable to do so. Finally, B cells from infected patients secreted brain-derived neurotropic factor and nerve growth factor, suggesting contribution of these cells to neuroprotection and dampening of inflammation. Interestingly, these observed anti-inflammatory effects were limited to extracellular helminth infections, as patients infected with intracellular parasite Trypanosoma cruzi did not exhibit these protective activities.
Conclusions: Commensal-Based Therapy in MS
Before the introduction of high-dose IFNβ-1b, there were no effective therapies to treat RRMS. Since that time there have been an increasing number of new therapies with novel targets that have been added to the treatment repertoire. Despite advancements in disease-modifying therapies, there remains a need for additional therapeutic options as treatments vary in efficacy, and can be associated with discomforting and adverse side effects that may affect adherence to treatment regimen by patients. Targeting the gut microbiome or helminths represents a potential new paradigm in the treatment of a large range of human diseases, including autoimmune disease and human MS. Currently, our understanding of both the biology of the gut microbiome and the immunoregulatory potential of helminths is still in its infancy. The microbiota possesses the capacity to determine susceptibility to CNS demyelinating disease, demanding further investigation of underlying complex commensal–host interactions. Nonetheless, it is an exciting prospect that the gut may well harbor critical environmental factors, which, in association with the appropriate genetic predisposition, may render individuals susceptible to MS. Consistent with this are the observations of anti-inflammatory responses and protection from disease during helminth infection in MS. Such findings add credence to the role of the hygiene hypothesis in which excessive hygiene results in the lack of exposure to these potentially protective agents and their corresponding effect on the gut microbiome. In this review we described the impact of bacterial agents (and derived antigens) as well as helminths on key aspects of both experimental models of MS and human disease. The mechanisms that determine pathogenic or regulatory T cell commensal-induced responses are not yet well understood. Polarizing cytokines and other soluble factors produced by APCs such as GALT DCs or intestinal epithelial cells appear central, and a role for various TLRs is frequently reported. Whether future therapeutic approaches to MS will employ commensal-based products depends on nuanced understanding of these underlying mechanisms. Selection and utilization of specific organisms responsible for the desired therapeutic outcome by colonization or the administration of novel antigens, such as B. fragilis–derived PSA as reviewed in this report, will be resolved over time.
Author Disclosure Statement
Dr. Kasper has received honoraria from Teva Neuroscience, Genzyme, EMD Serono, Biogen/Idec, and Bayer. Kiel Telesford and Dr. Ochoa-Reparaz have nothing to disclose.
References
- Afzali B, Mitchell PJ, Edozie FC, Povoleri GAM, Dowson SE, Demandt L, Walter G, Canavan JB, Scotta C, Menon B, Chana PS, Khamri W, Kordasti SY, Heck S, Grimbacher B, Tree T, Cope AP, Taams LS, Lechler RI, John S, Lombardi G. 2013. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. Eur J Immunol 43(8):2043–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Gupta S. 2011. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J Clin Immunol 31(1):89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. 2003. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci USA 100(24):14157–14162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Teitelbaum D, Sela M, Arnon R. 1997. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 94(20):10821–10826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J, Harris M, Wells S, Mills G, Chalamidas K, Ganta V, McGee J, Jennings M, Gonzalez-Toledo E, Minagar A. 2010. Alterations in serum MMP-8, MMP-9, IL-12p40 and IL-23 in multiple sclerosis patients treated with interferon-1b. Mult Scler J 16(7):801–809 [DOI] [PubMed] [Google Scholar]
- Appel H, Neure L, Kuhne M, Braun J, Rudwaleit M, Sieper J. 2004. An elevated level of IL-10- and TGFbeta-secreting T cells, B cells and macrophages in the synovial membrane of patients with reactive arthritis compared to rheumatoid arthritis. Clin Rheumatol 23(5):435–440 [DOI] [PubMed] [Google Scholar]
- Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa-Atta L, Solé D, Carvalho EM. 2000. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol 123(2):145–148 [DOI] [PubMed] [Google Scholar]
- Asano M, Toda M, Sakaguchi N, Sakaguchi S. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 184(2):387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. 2006. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest 116(12):3252–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O'Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209(5):1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum-Haque S, Sharma A, Christy M, Lentini T, Ochoa-Repáraz J, Fayed IF, Mielcarz D, Haque A, Kasper LH. 2010. Increased expression of B cell-associated regulatory cytokines by glatiramer acetate in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 219(1–2):47–53 [DOI] [PubMed] [Google Scholar]
- Begum-Haque S, Sharma A, Kasper IR, Foureau DM, Mielcarz DW, Haque A, Kasper LH. 2008. Downregulation of IL-17 and IL-6 in the central nervous system by glatiramer acetate in experimental autoimmune encephalomyelitis. J Neuroimmunol 204(1–2):58–65 [DOI] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Al Rasbi Z, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. 2012. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479(7374):538–541 [DOI] [PubMed] [Google Scholar]
- Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. 2010. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol 184(8):4317–4326 [DOI] [PubMed] [Google Scholar]
- Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. 1999. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 21(4):169–176 [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M. 2007. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol 61(2):97–108 [DOI] [PubMed] [Google Scholar]
- Correale J, Farez M, Razzitte G. 2008. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol 64(2):187–199 [DOI] [PubMed] [Google Scholar]
- Correale J, Farez MF. 2011. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol 233(1–2):6–11 [DOI] [PubMed] [Google Scholar]
- Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons J-A. 2006. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 180(1–2):63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. 2007. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 178(10):6092–6099 [DOI] [PubMed] [Google Scholar]
- Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, Eid P, Novelli F. 2009. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann Neurol 65(5):499–509 [DOI] [PubMed] [Google Scholar]
- Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr., Weinstock JV. 2003. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 284(3):G385–G391 [DOI] [PubMed] [Google Scholar]
- Fleming J, Isaak A, Lee J, Luzzio C, Carrithers M, Cook T, Field A, Boland J, Fabry Z. 2011. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler J 17(6):743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KHG. 2009. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183(11):7602–7610 [DOI] [PubMed] [Google Scholar]
- Fritz JH, Rojas OL, Simard N, McCarthy DD, Hapfelmeier S, Rubino S, Robertson SJ, Larijani M, Gosselin J, Ivanov II, Martin A, Casellas R, Philpott DJ, Girardin SE, McCoy KD, Macpherson AJ, Paige CJ, Gommerman JL. 2013. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature 481(7380):199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, Krammer P, Linderkamp O, Suri-Payer E. 2006. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood 108(10):3371–3378 [DOI] [PubMed] [Google Scholar]
- Gran B, Zhang G-X, Yu S, Li J, Chen X-H, Ventura ES, Kamoun M, Rostami A. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol 169(12):7104–7110 [DOI] [PubMed] [Google Scholar]
- Gruden-Movsesijan AN, Mostaricia-Stojkovic N, Stosic-grujicic S, Milic M, Sofronic-Milosavljevic L. 2010. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol 32(6):450–459 [DOI] [PubMed] [Google Scholar]
- Haas J, Fritzsching B, Trübswetter P, Korporal M, Milkova L, Fritz B, Vobis D, Krammer PH, Suri-Payer E, Wildemann B. 2007. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol 179(2):1322–1330 [DOI] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PIW, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. 2007. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357(9):851–862 [DOI] [PubMed] [Google Scholar]
- Harp CT, Ireland S, Davis LS, Remington G, Cassidy B, Cravens PD, Stüve O, Lovett-Racke AE, Eagar TN, Greenberg BM, Racke MK, Cowell LG, Karandikar NJ, Frohman EM, Monson NL. 2010. Memory B cells from a subset of treatment-naïve relapsing-remitting multiple sclerosis patients elicit CD4+ T-cell proliferation and IFN-γ production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur J Immunol 40(10):2942–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani M, Niino M, Fukazawa T, Kikuchi S, Yabe I, Hamada S, Tajima Y, Sasaki H. 2010. Decreased IL-10 production mediated by Toll-like receptor 9 in B cells in multiple sclerosis. J Neuroimmunol 221(1–2):95–100 [DOI] [PubMed] [Google Scholar]
- Hong J, Li N, Zhang X, Zheng B, Zhang JZ. 2005. Induction of CD4+ CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA 102(18):6449–6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV. 2004. Bacterial contributions to mammalian gut development. Trends Microbiol 12(3):129–134 [DOI] [PubMed] [Google Scholar]
- Hug A, Korporal M, Schröder I, Haas J, Glatz K, Storch-Hagenlocher B, Wildemann B. 2003. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J Immunol 171(1):432–437 [DOI] [PubMed] [Google Scholar]
- Huttenhower C. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486(7402):207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Boppana S, Huang H, Dhib-Jalbut S, Ito K. 2011. Gastrointestinal tract is the place where encephalitogenic T cells develop in the spontaneous EAE mice [Abstract]. J Immunol 186:101.11 [Google Scholar]
- Ito T, Hanabuchi S, Wang Y-H, Park WR, Arima K, Bover L, Qin FX-F, Gilliet M, Liu Y-J. 2008. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28(6):870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139(3):485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee Y, Piao WH, Liu R, Bai XF, Rhodes S, Rodebaugh R, Campagnolo DI, Shi FD, Vollmer TL. 2007. CD4(+)CD25(+) regulatory T cells contribute to the therapeutic effects of glatiramer acetate in experimental autoimmune encephalomyelitis. Clin Immunol 125(1):34–42 [DOI] [PubMed] [Google Scholar]
- Jun S, Ochoa-Repáraz J, Zlotkowska D, Hoyt T, Pascual DW. 2012. Bystander-mediated stimulation of proteolipid protein-specific regulatory T (Treg) cells confers protection against experimental autoimmune encephalomyelitis (EAE) via TGF-β. J Neuroimmunol 245(1–2):39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala M, Rhodes SN, Piao W-H, Shi F-D, Campagnolo DI, Vollmer TL. 2010. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp Neurol 221(1):136–145 [DOI] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, Nunez G. 2013. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13(5):321–335 [DOI] [PubMed] [Google Scholar]
- Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. 2006. Intestinal helminths protect in a murine model of asthma. J Immunol 177(3):1628–1635 [DOI] [PubMed] [Google Scholar]
- Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, Hupperts R, Damoiseaux J. 2011. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 239(1–2):80–86 [DOI] [PubMed] [Google Scholar]
- Kreisman LSC, Cobb BA. 2011. Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem 286(11):8810–8818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B. 2010. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 5(2):e9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 108(Suppl. 1):4615–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. 2002. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 8(5):500–508 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4(6):478–485 [DOI] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. 2008. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118(10):3420–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122(1):107–118 [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. 2008. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453(7195):620–625 [DOI] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. 2009. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30(6):899–911 [DOI] [PubMed] [Google Scholar]
- Nepom GT. 2005. Tetramer analysis of human autoreactive CD4-positive T cells. Adv Immunol 88:51–71 [DOI] [PubMed] [Google Scholar]
- Neuhaus O, Farina C, Yassouridis A, Wiendl H, Then Bergh F, Dose T, Wekerle H, Hohlfeld R. 2000. Multiple sclerosis: comparison of copolymer-1- reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci USA 97(13):7452–7457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. 2000. Multiple sclerosis. N Engl J Med 343(13):938–952 [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. 2010a. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide A expression. J Immunol 185(7):4101–4108 [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. 2009. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol 183(10):6041–6050 [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. 2010b. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes 1(2):103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. 2010c. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol 3(5):487–495 [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. 2007. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol 178(3):1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. 1987. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Am Acad Neurol 37(7):1097–1102 [DOI] [PubMed] [Google Scholar]
- Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. 2013. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 121(14):2647–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, Bettelli E, Kuchroo VK. 2011. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 35(6):986–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistoia V. 1997. Production of cytokines by human B cells in health and disease. Immunol Today 18(7):343–350 [DOI] [PubMed] [Google Scholar]
- Ramgolam VS, Sha Y, Marcus KL, Choudhary N, Troiani L, Chopra M, Markovic-Plese S. 2011. B cells as a therapeutic target for IFN-β in relapsing-remitting multiple sclerosis. J Immunol 186(7):4518–4526 [DOI] [PubMed] [Google Scholar]
- Rubio JP, Stankovich J, Field J, Tubridy N, Marriott M, Chapman C, Bahlo M, Perera D, Johnson LJ, Tait BD, Varney MD, Speed TP, Taylor BV, Foote SJ, Butzkueven H, Kilpatrick TJ. 2008. Replication of KIAA0350, IL2RA, RPL5 and CD58 as multiple sclerosis susceptibility genes in Australians. Genes Immun 9(7):624–630 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. 2010. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10(7):490–500 [DOI] [PubMed] [Google Scholar]
- Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol 7(9):796–804 [DOI] [PubMed] [Google Scholar]
- Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. 2003. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol 15(1):59–69 [DOI] [PubMed] [Google Scholar]
- Sommer F, Bäckhed F. 2013. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11(4):227–238 [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. 2005. Immunology of multiple sclerosis. Annu Rev Immunol 23(1):683–747 [DOI] [PubMed] [Google Scholar]
- Surana NK, Kasper DL. 2011. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunol Rev 245(1):13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Biagioli T, Rosati E, Ballerini C, Mazzanti B, Ben-Nun A, Massacesi L, Vergelli M. 2001. IL-7-enhanced T-cell response to myelin proteins in multiple sclerosis. J Neuroimmunol 121(1–2):111–119 [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Broekmans T, Hensen K, Rummens J-L, Stinissen P. 2008a. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol 180(9):6411–6420 [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Liblau R, Stinissen P. 2010. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med 16(2):58–68 [DOI] [PubMed] [Google Scholar]
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens J-L, Medaer R, Hupperts R, Stinissen P. 2008b. Compromised CD4+CD25 highregulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123(1):79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta V. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med 199(7):971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KP, Brady MT, Finlay CM, Boon L, Mills KHG. 2009. Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. J Immunol 183(3):1577–1586 [DOI] [PubMed] [Google Scholar]
- Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, Maizels RM. 2010. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol 40(6):1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. 1994. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med 179(3):973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Hu X, Zhou G, Lu Z, Qiu W, Bao J, Dai Y. 2008. Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J Neuroimmunol 194(1–2):107–114 [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. 2010. Differentiation of effector CD4 T cell populations. Annu Rev Immunol 28(1):445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]