Primary central nervous system lymphoma (PCNSL) is a potentially curable brain tumor or at least one in which durable remission can be achieved. We report follow-up study results from NABTT 96-07, a phase II multicenter trial in which 25 non-HIV adult patients with newly diagnosed PCNSL were treated with 8g/m2 of methotrexate intravenously every 2 weeks until a complete response (CR), as determined by radiographic criteria, was achieved or a maximum of 8 induction doses was reached(1). For patients achieving a CR, 2 consolidation cycles were administered every 14 days followed by 11 maintenance cycles (28 days each).
Results
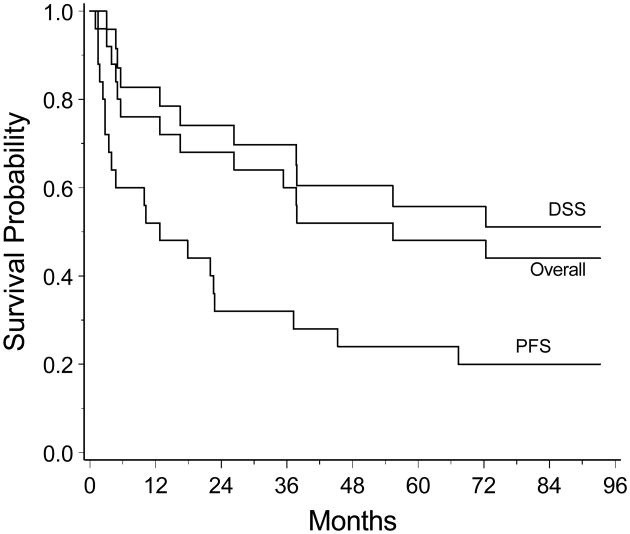
We report updated results of NABTT 96-07 after a minimum of 6.5 years of follow-up. Twelve of the original 25 patients (52%) achieved a CR to methotrexate induction. Five of these 12 patients (40%) who achieved CR have not relapsed after a median follow-up of 6.8 years. Eleven patients died of progressive disease or unknown cause and 3 died from other medical illnesses (2 with cardiac disease, 1 with septic arthritis) for a total of 14 patient deaths. There were no methotrexate-related deaths. Median progression free survival was 12.8 months (95% confidence interval [CI]: 3.5-37.2 months). Multiple salvage regimens, as determined by the treating physicians, were employed for relapses. Thirteen of the relapsed patients received brain irradiation (12 whole brain irradiation (WBI) and 1 radiosurgery). Nine of these 13 died. Six (24%) patients developed disease outside the CNS; 3 of whom died from systemic progression. Median disease specific survival has yet to be reached at 72.3 months (95% CI 37.7 months, not achieved) and median overall survival was 55.4 months (95% CI, 16.5 months, not achieved). Kaplan-Meier survival curves are displayed in the figure.
Figure 1.
Kaplan-Meier curves for overall survival (Overall), disease specific survival (DSS), and progression free survival (PFS) for the 25 patients treated with high dose methotrexate.
Discussion
The optimal therapeutic strategy for patients with newly diagnosed PCNSL has not been defined. Our results support the role of methotrexate as a critical chemotherapeutic agent in the treatment of PCNSL. Moreover, a subset of PCNSL patients may achieve durable remission with methotrexate alone. In contrast, WBI alone is associated with a median overall survival of 11.6 months (2). While it is likely that methotrexate-based combination therapy will achieve superior therapeutic results to methotrexate alone this has not been conclusively demonstrated and the optimal methotrexate-based combination regimen has yet to be defined. The toxicity of methotrexate in our study was modest with only 12 of 25 patients experiencing grade 3 or 4 toxicity after 287 cycles with no episodes of clinically relevant leukoencephalopathy. Methotrexate-based combination chemotherapy regimens are associated with higher frequencies of treatment-related toxicities. Regimens that contain WBI are associated with high rates of neurotoxicity in patients over the age of 60. In patients who achieve CR to methotrexate it remains to be determined what the optimal duration of therapy should be.
In another trial of methotrexate using the same dose as our study, a CR proportion of only 29.7% was achieved, less than the CR proportion of 52% observed in our study(3). However, the maximum number of induction cycles allowed in this study was 6 whereas in our study the maximum number of induction cycles was 8 and the median number of cycles to achieve CR was 6. Thus, more than half of the complete responses were achieved after receiving a 7th or 8th induction cycle in our study and it is possible that the CR proportion would have exceeded 29.7% in the other study if more induction cycles had been delivered. Further consideration of the optimal number of induction cycles is warranted.
Progression-free survival in our study was shorter than that reported for some combination regimens in patients with newly diagnosed PCNSL (4). Moreover, the proportion of patients who experienced progression outside the CNS was higher than anticipated. However, salvage therapy was successful in many of our patients as demonstrated by the prolonged median overall survival which compares favorably to combination and WBI-containing regimens. High-dose methotrexate alone or in combination with other therapies is the most effective treatment available for PCNSL. Future studies are necessary to identify the optimal methotrexate-based combination regimen that will produce maximal efficacy and acceptable toxicity.
Supplementary Material
Footnotes
Statistical analysis was done by Kathryn A. Carson, Sc.M.
Disclosures: The authors have nothing to disclose. All authors have agreed to the conditions in the Authors Disclosure Form.
Disclosure: The authors report no conflicts of interest.
References
- 1.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiation therapy: a report of the NABTT 96-07. Journal of Clinical Oncology. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 3.Herrlinger U, Schabet M, Burgger W, et al. German cancer society neuro-oncology working group NOA-3 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma: final report. Ann Neurol. 2002;51:247–252. doi: 10.1002/ana.10102. [DOI] [PubMed] [Google Scholar]
- 4.DeAngelis L, Seiferheld W, Schold S, Fisher B, Schultz C. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. Journal of Clinical Oncology. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.