Abstract
Purpose
Prognostic factor analyses have proven useful in predicting outcome in patients with newly diagnosed malignant glioma. Similar analyses in patients with recurrent glioma could substantially affect the design and conduct of clinical trials.
Patients and Methods
Between 1995 and 2002, 333 adults with recurrent gliomas were enrolled on 10 phase I or II trials of systemic or local therapy. The studies had similar inclusion criteria and were conducted within the NABTT CNS Consortium. Ninety-three percent of the patients have died. Cox proportional hazards (PH) regression and recursive partitioning analysis (RPA) were performed to identify prognostic factors.
Results
Factors associated with an increased risk of death were increased age, lower Karnofsky performance status (KPS), initial and on-study histologies of glioblastoma multiforme (GBM), corticosteroid use, shorter time from original diagnosis to recurrence, and tumor outside frontal lobe. The final PH model included initial histology of GBM (relative risk (RR)=2.01), 10 year increase in age (RR=1.23), KPS<80 (RR=1.54), and corticosteroid use (RR=1.49). RPA resulted in 7 classes. Median survival time was poorest in non-GBM patients with KPS<80 or GBM patients, age≥50, taking corticosteroids (4.4 mo; 95% confidence interval (CI)=3.6-5.4), best in patients with initial histology other than GBM with KPS≥80 and tumor confined to the frontal lobe (25.7 mo; 95% CI=18.7-52.5), and was 7.0 mo (95% CI=6.2-8.0) for all patients.
Conclusion
Initial histology, age, KPS, and corticosteroid use are prognostic for survival in recurrent glioma patients. To allow comparisons across Phase II trials, enrollment criteria may need to be restricted.
Introduction
The prognosis for patients with recurrent glioma is poor and few therapies have been found to be efficacious.1-3 Novel therapies for this patient population are frequently tested in uncontrolled or historically controlled phase II trials with the assumption that the patients in these trials are homogeneous. However, the patients may differ on several characteristics, including age, performance status, initial or on-study histology, time from initial diagnosis to recurrence, location of tumor and whether it is resectable, number and type of prior therapies, and use of concomitant medications (e.g., corticosteroids, anti-convulsants).
Prognostic factor analyses have proven useful in predicting outcome in patients with newly diagnosed malignant glioma. Factors such as age, Karnofsky performance status (KPS), and extent of resection have been shown to be prognostic for survival in newly diagnosed patients in the RTOG recursive partitioning analysis (RPA).4 As these prognostic factors have more impact on survival than the currently available therapies, randomized prospective clinical trials in newly diagnosed high grade gliomas are now stratified to account for these differences. Likewise, phase II studies in this patient population are analyzed using published RPA data so results can be fairly compared with other uncontrolled trials. If this were not done, studies entering patients with more favorable prognostic factors would appear superior even if the therapy was ineffective.
Few studies have examined prognostic factors in patients with recurrent high grade gliomas and the findings have not been consistent. As most novel agents and approaches are studied in patients with recurrent high grade gliomas before they are given to patients who have just been diagnosed, a similar RPA analysis in patients with recurrent disease would be extremely important in deciding which novel therapies should be pursued and which should be abandoned. The objective of our study was to determine whether baseline demographic and clinical characteristics are prognostic for survival in patients with recurrent glioma utilizing RPA.
Patients and Methods
Between 1995 and 2002, 333 adult patients were enrolled on 10 phase I or II clinical trials for the treatment of recurrent glioma conducted within the New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium.5 These trials are described in the Online Table. Six of the trials involved systemic chemotherapy treatment; 9-aminocamptothecin (9-AC)6, suramin7, phenylbutyrate8, aprinocarsen9, irinotecan (CPT-11)10,11 and oral procarbazine.12 The other four were trials of local chemotherapy or brachytherapy; Gliadel®13, the Gliasite Radiation Therapy System (RTS)14, Onyx-01515, and Gliadel® and intravenous O6-benzylguanine.16 The trials were approved by the Cancer Therapy Evaluation Program at the National Cancer Institute and the institutional review boards at each NABTT CNS Consortium participating site. The sites that participated in at least one of these trials were: the University of Alabama, Brown University, the Cleveland Clinic, Columbia University, Emory University, Henry Ford Hospital, the Johns Hopkins University, Massachusetts General Hospital, Moffett Cancer Center, Northwestern University, the University of Pennsylvania, the University of Texas at San Antonio, Wake Forest University, and Washington University. None of the treatments in the phase II trials were found to be efficacious.
The studies had similar inclusion criteria. Eligible patients were at least 18 years of age, had histologically proven malignant glioma (anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), or glioblastoma multiforme (GBM)), had progression or recurrence, and had a KPS ≥ 60. Three of the studies (9-AC, RTS, and phenylbutyrate) did not require previous radiation therapy (RT), and there was no minimum elapsed time between RT and enrollment for Gliadel®, only 28 days for phenylbutyrate, and two months for 9-AC. The other studies required prior RT and ≥ 3 months since completion. The phenylbutyrate, Gliadel®, RTS, and O6-benzylguanine protocols did not limit the number of prior chemotherapies allowed, but the other protocols limited them to one or two. Other eligibility criteria included: ≥ 3 weeks since last dose of non-nitrosourea chemotherapy, ≥ 6 weeks since a nitrosourea chemotherapy, and hematologic and biochemical parameters within an acceptable range. Additionally, patients could not be pregnant or breast-feeding and needed to have a minimum life expectancy of ≥ 2 months. All patients gave informed consent.
Prognostic Factors
Patient prognostic factor data were collected at the baseline visit as part of each study. Demographic factors were age at entry, sex, and race. Clinical factors assessed were KPS, initial histology, time from initial histology until study entry, histology at study entry, site of tumor, and hemoglobin level. Treatment factors included steroid use, anticonvulsant use, surgical resection as part of study, and number of prior chemotherapies, surgeries, and radiation therapies.
Twelve patients were enrolled in two of the studies and are included in the analysis twice. However, the demographic, clinical characteristics and survival data are specific to the respective study, and therefore these are treated as independent observations. Though this may not be ideal, excluding the patients' data from either or both of the studies also could introduce a bias.
Survival
All enrolled patients, even if off treatment or off study, were routinely followed for survival. Overall survival time was calculated from date of start of treatment on the present study until the date of death from any cause. Patients still alive or lost to follow-up were censored at the date of last follow-up. At the time of analysis, 308 of the 333 patients (93%) had died.
Statistical Considerations
Continuous data were summarized with medians and ranges and categorical data with frequencies and percents. Some factors were recoded into dichotomies because of infrequent values. These included: race, recoded as white and non-white; histology (both initial and on-study), recoded as GBM and non-GBM; prior chemotherapies, as <1 and ≥1, and also <2 and ≥2; prior surgeries, as <2 and ≥2; prior RT courses, as <2 and ≥2. Tumor site was dichotomized into tumor confined to the frontal lobe and at least some tumor outside of the frontal lobe because previous studies have shown a reduced risk of death when the tumor is confined to the frontal lobe.17,18 Age and KPS were analyzed as continuous measures and were also dichotomized. Age was dichotomized at the median (50 years) and also at 40 and 60 years. KPS was dichotomized at <80 and at <90.
The comparability of survival times for each protocol was assessed in two ways. First, the hazard rate, calculated as the number of deaths divided by the total patient-years of follow-up, and 95% confidence interval (CI) were computed for each protocol. Then Cox proportional hazards (PH) regression analysis19 was used to compare survival from each protocol to the combined data from the other protocols.
Univariate PH regression analysis was performed to identify possible prognostic factors associated with an increased risk of death. Factors identified as being possible predictors in the univariate analysis, defined as a p value < 0.20, were then included in a backward stepwise multivariate analysis. The least significant factor was dropped from the model, and the model was refit. This process was repeated until only significant factors (p<0.05) remained. Because some factors had missing data, a forward analysis was also performed to confirm the final model.
RPA was performed based on the log-likelihood ratios from the PH regressions.20 Data were partitioned on the factor with the greatest log likelihood, then univariable PH regressions were refit on the partitioned data. This process continued until either no more factors were significant at the p<0.05 level, or the remaining sample size was less than 20. Kaplan-Meier survival estimates21 (KM) were obtained for each of the RPA classes, and tested for inequality with the Wilcoxon test. Amalgamation of classes with similar survival times was then performed and the curves were plotted.
Confidence intervals were calculated using standard methods. Analyses were performed using SAS version 9 (SAS Institute, Cary, NC). All reported p values are two-sided.
Results
Demographic and clinical characteristics of the patients by trial are presented in Table 1 and for all patients combined in Table 2. The hazard rate and 95% CI for each protocol are shown in Figure 1. Only one protocol was significantly different than the others for survival on PH regression analysis. The phase II study of aprinocarsen exhibited an increased risk when compared to the combined data from the other trials (risk ratio (RR) = 1.62, 95% CI=(1.02, 2.56)). Grossman and colleagues propose that the integrity of the blood brain barrier could be a possible reason for this increased risk of death.9 However, there were only 21 patients in this trial and differences in known prognostic factors, such as KPS and histology cannot be ruled out as the cause. Therefore, we included these patients in our primary analysis reported here. We then excluded these patients, repeated the analysis, and obtained nearly identical results.
Table 1.
Baseline demographic and clinical characteristics by New Approaches to Brain Tumor Therapy CNS Consortium (NABTT) trial.
| NABTT Trial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9-AC | Suramin | Phenyl | Aprino | CPT-11 | Procarb | Gliadel® | Gliasite | O6-BG | Onyx | |
| Gender, % male | 60 | 100 | 67 | 71 | 59 | 76 | 72 | 71 | 67 | 71 |
| Race, % non-white | 7 | 0 | 19 | 0 | 9 | 8 | 2 | 29 | 14 | 0 |
| Age, median years | 47 | 55 | 51 | 46 | 48 | 56 | 48 | 48 | 52 | 52 |
| KPS, median | 90 | 80 | 80 | 80 | 90 | 80 | 80 | 80 | 90 | 90 |
| KPS, % 60-80 | 49 | 64 | 61 | 67 | 45 | 65 | 57 | 62 | 45 | 33 |
| KPS, % 60-70 | 37 | 27 | 33 | 38 | 28 | 29 | 24 | 33 | 29 | 25 |
| Initial histology, % GBM | 56 | 73 | 58 | 67 | 72 | 56 | 70 | 71 | 81 | 73 |
| On-study histology, % GBM | 62 | 91 | 76 | 76 | 79 | 70 | 77 | 86 | 90 | 83 |
| Time from initial dx, median months | 12.8 | 10.7 | 10.2 | 9.4 | 11.0 | 11.5 | 9.1 | 9.5 | 10.3 | 13.6 |
| Tumor outside frontal lobe, % yes | 80 | 91 | 89 | 95 | 91 | 81 | 85 | 67 | 80 | 70 |
| Corticosteroid use, % yes | 60 | 82 | 84 | 75 | 70 | 58 | 81 | 86 | 71 | 61 |
| Anticonvulsant use, % yes | 69 | 91 | 84 | 81 | 72 | 86 | 12 | 67 | 88 | 68 |
| Hemoglobin, median | 14.0 | 13.8 | 12.7 | 12.9 | 13.2 | 14.2 | 14.1 | * | 13.8 | 14.5 |
| Prior Chemotherapy, % >0 | 69 | 91 | 74 | 86 | 88 | 71 | 56 | 57 | 69 | 91 |
| Prior Chemotherapy, % >1 | 10 | 36 | 37 | 38 | 7 | 4 | 17 | 14 | 21 | 32 |
| Prior Surgery, % >1 | 71 | 64 | 47 | 52 | 47 | 51 | 30 | 29 | 38 | 9 |
| Prior RT, % >1 | 19 | 27 | 21 | 5 | 3 | 0 | 0 | 14 | 0 | 5 |
Abbreviations: KPS, Karnofsky performance status; GBM, glioblastoma multiforme; dx, diagnosis; RT, radiation therapy
No hemoglobin data available for this study.
Table 2.
Baseline demographic and clinical characteristics for all patients.
| Total N | N (%) or Median (range) | |
|---|---|---|
| Gender, male | 333 | 228 (68.5) |
| Race, non-white | 333 | 29 (8.7) |
| Age, years | 333 | 49.8 (18.5-75.0) |
| KPS | 321 | 80 (60-100) |
| KPS, 60-80 | 321 | 171 (53.3) |
| KPS, 60-70 | 321 | 95 (29.6) |
| Initial histology, GBM | 325 | 219 (67.4) |
| On-study histology, GBM | 329 | 255 (77.5) |
| Time from initial dx, months | 325 | 10.7 (3.2-231.1) |
| Tumor outside frontal lobe | 307 | 255 (83.1) |
| Corticosteroid use | 322 | 227 (70.5) |
| Anticonvulsant use | 328 | 227 (69.2) |
| Hemoglobin | 246 | 13.8 (9.6-17.5) |
| Prior Chemotherapy, > 0 | 326 | 241 (73.9) |
| Prior Chemotherapy, > 1 | 326 | 55 (16.9) |
| Prior Surgery, > 1 | 328 | 146 (44.5) |
| Prior RT, > 1 | 328 | 22 (6.7) |
| Surgical Protocol | 333 | 130 (39.0) |
Abbreviations: KPS, Karnofsky performance status; GBM, glioblastoma multiforme; dx, diagnosis; RT, radiation therapy
Figure 1.
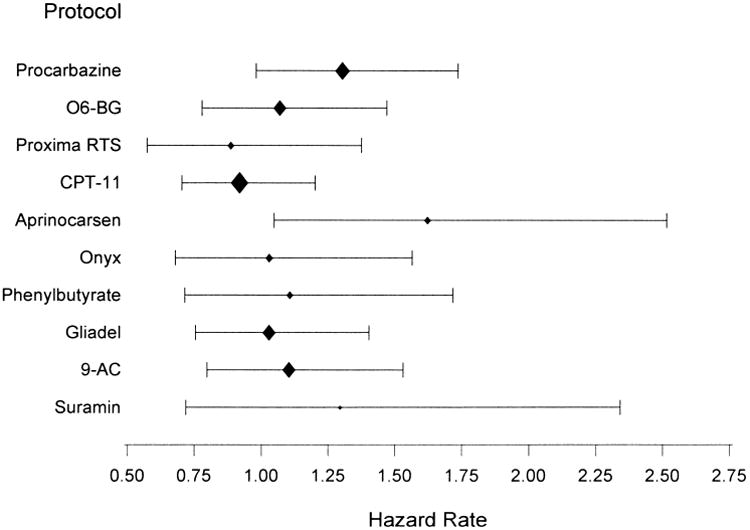
The hazard rate and 95% confidence interval for the 10 protocols included in this study. The size of the diamond is proportional to the number of patients in the trial.
The results of the univariate PH regression analysis are in Table 3. The factors that were at least marginally associated with an increased risk of death (p < 0.20) included increased age, lower KPS, initial and on-study histologies of GBM, corticosteroid use, shorter time from initial diagnosis, and tumor located outside of the frontal lobe. These factors were included in the multivariate PH regression analysis. The final multivariate regression model included: initial histology of GBM vs other (RR=2.01; 95% CI=(1.50, 2.70); p<0.0001), decade increase in age (RR=1.23; 95% CI=(1.10, 1.37); p=0.0002), KPS of 60-70 vs 80-100 (RR=1.54; 95% CI=(1.18, 2.00); p=0.001), and corticosteroid use (RR=1.49; 95% CI=(1.13, 1.96); p=0.005).
Table 3.
Results of univariable proportional hazards regression analysis.
| Risk Ratio | 95% Confidence Interval | P | |
|---|---|---|---|
| Gender, male vs female | 1.10 | 0.86-1.40 | 0.45 |
| Race, non-white vs white | 0.87 | 0.58-1.31 | 0.51 |
| Age, decade increase | 1.29 | 1.17-1.42 | <0.0001 |
| Age, ≥ 40 vs < 40 yrs | 1.59 | 1.20-2.11 | 0.001 |
| Age, ≥ 50 vs < 50 yrs | 1.65 | 1.31-2.08 | <0.0001 |
| Age, ≥ 60 vs < 60 yrs | 1.71 | 1.30-2.23 | <0.0001 |
| KPS, 10 pt increase | 1.33 | 1.21-1.46 | <0.0001 |
| KPS, 60-80 vs 90-100 | 1.68 | 1.33-2.11 | <0.0001 |
| KPS, 60-70 vs 80-100 | 1.98 | 1.54-2.55 | <0.0001 |
| Initial histology, GBM vs other | 2.36 | 1.81-3.09 | <0.0001 |
| On-study histology, GBM vs other | 2.44 | 1.80-3.30 | <0.0001 |
| Time from initial dx, 1 yr increase | 0.93 | 0.89-0.98 | 0.005 |
| Tumor outside frontal lobe, yes vs no | 1.88 | 1.36-2.60 | 0.0001 |
| Corticosteroid use, yes vs no | 1.65 | 1.28-2.12 | 0.0001 |
| Anticonvulsant use, yes vs no | 0.98 | 0.77-1.25 | 0.87 |
| Hemoglobin, 1 unit increase | 1.07 | 0.98-1.17 | 0.11 |
| Prior Chemotherapy, > 0 vs 0 | 0.95 | 0.73-1.22 | 0.67 |
| Prior Chemotherapy, > 1 vs 0-1 | 0.84 | 0.61-1.14 | 0.25 |
| Prior Surgery, > 1 vs 0-1 | 1.02 | 0.82-1.28 | 0.85 |
| Prior RT, > 1 vs 0-1 | 0.97 | 0.62-1.53 | 0.91 |
| Surgical Protocol, yes vs no | 0.86 | 0.68-1.08 | 0.18 |
Abbreviations: KPS, Karnofsky performance status; GBM, glioblastoma multiforme; dx, diagnosis; RT, radiation therapy
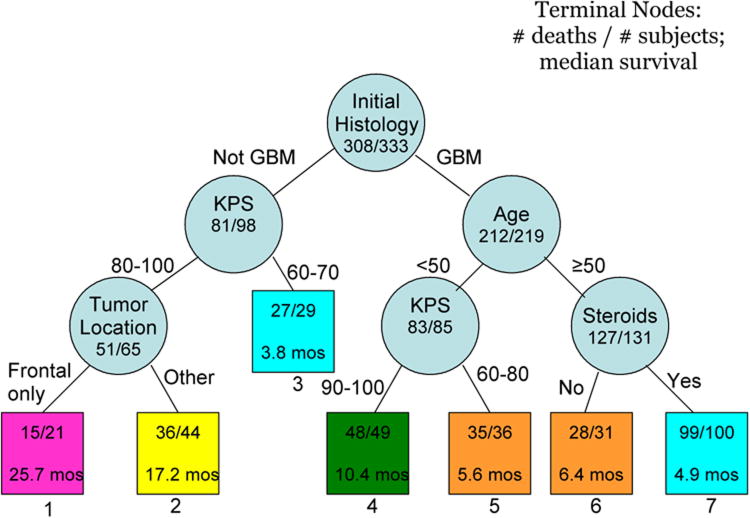
The RPA, displayed in Figure 2, resulted in 7 terminal nodes (or classes). The first partition was initial histology. For the non-GBM patients, the next split was KPS, dichotomized at < 80 and ≥ 80. For the partition with poorer KPS, no further variables were prognostic for survival. For the better KPS group, an additional partition of tumor location was prognostic. For the GBM patients, age dichotomized as < 50 and ≥ 50 was the next split. KPS, dichotomized as < 90 and ≥ 90 was the final partition for patients < 50 years of age. For patients who were at least 50 years of age, corticosteroid use was the final partition.
Figure 2.
Results of the recursive partitioning analysis (RPA). The terminal nodes are represented by squares, which contain the number of deaths out of the number of patients in those nodes, as well as the median survival time in months. The RPA classes are numbered below the squares.
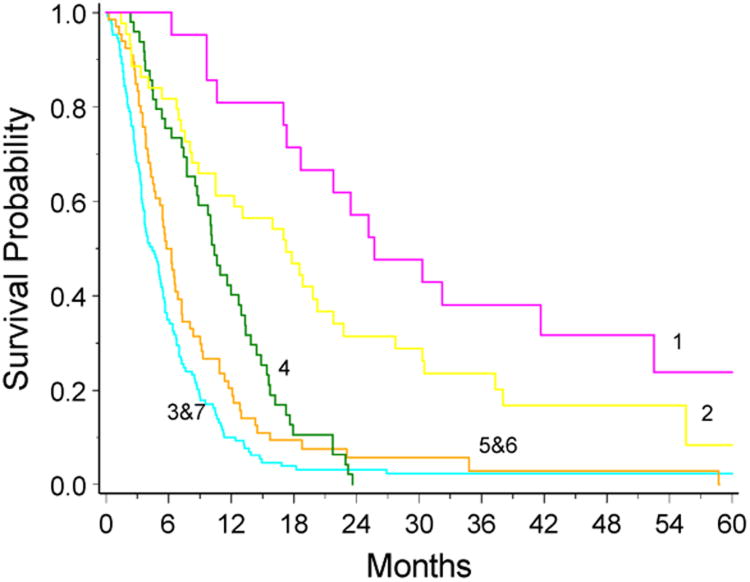
Median survival time for these 7 RPA classes ranged from 3.8 months to 25.7 months. The survival times for class 3 compared to class 7 and for class 5 compared to class 6 were not significant on the Wilcoxon test for inequalities, so these classes were combined and the resulting KM survival curves were plotted (Figure 3). The median overall survival for all patients was 7.0 months (95% CI=6.2, 8.0). Median survival was poorest in non-GBM patients with KPS < 80 or GBM patients, age ≥ 50, taking corticosteroids (4.4 months; 95% CI=3.6, 5.4) and best in patients with initial histology other than GBM with KPS ≥ 80 and tumor confined to the frontal lobe (25.7 months; 95% CI=18.7, 52.5).
Figure 3.
Kaplan-Meier survival curves for the 5 different classes from the RPA. The curves were truncated at 60 months.
The RPA analysis selected different prognostic factors for the GBM and non-GBM strata. To validate this finding, we stratified the patients by initial histology and ran separate PH models on each stratum. For the non-GBM stratum, the final multivariate PH model included KPS of 60-70 vs 80-100 (RR=3.38; 95% CI=1.94-5.86; p<0.0001), age ≥ 60 vs < 60 (RR=2.45; 95% CI=1.16-5.17; p=0.02) and tumor outside frontal lobe vs confined to frontal lobe (RR=2.04; 95% CI=1.09-3.82); p=0.03). This model contains all of the factors that were significant in the RPA and the additional prognostic factor of age dichotomized at 60 years. For the GBM stratum, the final PH model included age ≥ 50 vs < 50 (RR=1.51; 95% CI=1.13-2.03; p=0.006), 10 point increase in KPS (RR=1.13; 95% CI=1.01-1.27; p=0.04) and steroid use (RR=1.42; 95% CI=1.01-1.99; p=0.04). The factors in this model were the same as in the RPA, though the cut points were different for age and KPS.
Tumor grade was available for 94 of the 98 patients that had initial histology other than GBM; 17 (18%) were low grade and 77 (82%) were high grade gliomas. All 17 of the patients with low grade glioma had died and 47% of them had on-study histology of GBM. The percent of patients with low grade glioma at initial histology in RPA class 1 was 26%, in RPA class 2 was 16% and in RPA class 3 was 18%.
Though a large number of factors were tested in the RPA analysis, the significance level was set at p < 0.05. If a more stringent requirement of p < 0.01 were used, most of the partitions would remain. Only the partition of tumor location (p=0.03) in the non-GBM stratum and the partition of KPS (p=0.03) for the GBM, age < 50 stratum would not have been made. The comparison of the RPA nodes would reflect these same results; RPA classes 1 and 2 would not be significantly different (p=0.03), and the RPA classes of 2 and 4 would not be significantly different (p=0.02).
Discussion
Our data suggest that patients with recurrent gliomas entering clinical trials have widely variable outcomes based on baseline demographic and clinical characteristics. The most significant of these prognostic factors are initial histology, KPS, age and corticosteroid use. The differences seen in the median survival time between our 7 RPA classes are larger than the treatment effects being evaluated in many studies. RPA classes 1 and 2, where the initial histology was other than GBM and KPS was ≥ 80, consisted of 20% of the patients in our database. Their median survival time was greater than 17 months. Compared to the 7.0 month median survival time for our entire cohort of patients this survival difference is quite large. Restricting the analysis to those patients whose initial diagnosis was GBM, the median survival times for the nodes varies from 4.9 months to 10.4 months.
Many phase II studies in recurrent glioma restrict enrollment to GBM or GBM and AA, but they do not restrict enrollment to those that had an initial diagnosis of high grade glioma. Our results suggest that this may be inappropriate if the goal is to have a relatively homogeneous population that could be compared to other similarly constructed trials. Though a few of the studies in the present analysis allowed on-study diagnoses of AO, there were relatively few patients with this diagnosis. Excluding these patients from the analysis did not reduce the median survival time for RPA nodes 1 and 2 by more than 0.3 months.
The finding of age, KPS, and extent of resection being prognostic for survival in recurrent glioma is not unexpected. They have been shown to be powerful prognostic factors for survival in newly diagnosed patients using RPA.4 Other studies suggest that corticosteroid dose was prognostic for survival in newly diagnosed gliomas.22-24 The study by Hohwieler Schloss and colleagues included patients with all histological grades and found that the 15 patients with less corticosteroid dependency had a median survival time of 29 months compared to 5 months for the 29 patients with greater corticosteroid dependency.
Nevertheless, very few studies have directly addressed prognostic factors in recurrent gliomas. Wong and colleagues25 performed RPA in recurrent glioma, using histology at recurrence as a prognostic factor and limiting the sample to those with recurrent GBM or AA. Similar to our study, histology was the first split in their RPA, and for the AA subgroup, the next split was KPS. However, they had no further significant splits in their analysis. Perhaps that is due to the younger age of their patients (median 45 years compared to median 50 years in the present study), and that they were testing age dichotomized at 40 years. A second study found no association of tumor grade, and only KPS to be prognostic for survival in recurrent glioma.26.
Our study focused on prognostic factors related to survival rather than on progression-free survival (PFS). There are several reasons for this. First, the NABTT CNS Consortium studies included in this analysis had formal endpoints that were either response rate or survival. Second, the survival endpoint is unequivocal in contrast to the frequently used 6-month PFS. This measure uses bi-monthly MRIs which are a measure of blood-brain barrier dysfunction rather than true tumor size. Furthermore, it is subjective, susceptible to manipulations of glucocorticoid dose or therapies which may increase or decrease blood-brain barrier dysfunction, and has an artificial 6-month cut-off even if in retrospect that patient had been slowly progressing. Finally, given the huge effect that prognostic factors have on survival in patients with recurrent gliomas as shown in this study, it is highly likely that the same results would apply to PFS as Wong and colleagues25 reported in their study.
There are a few limitations to our study. First, the resulting algorithm has not been validated in a different dataset. We plan to validate the algorithm when sufficient patients have been treated on other phase I and II NABTT recurrent glioma trials. Secondly, no data were collected on subsequent therapies that patients received after going off the study included here. However, this is frequently the case in many trials, and thus contributes to the generalizability of the results. Data from both phase I and II studies were included. However, none of the phase II studies were found to be efficacious and we first compared the survival times for each study. Lastly, there is some variation in the patient eligibility criteria between studies.
Phase II trials play a critical role in the assessment of novel therapeutic approaches in patients with high grade gliomas. As it would be far too costly and inefficient to conduct large randomized prospective trials of all novel treatment concepts, estimated response rates, 6 month PFS, and overall survival from phase II trials are compared in order to decide which therapies should be studied further. From the data presented in this manuscript, it is clear that patients with recurrent gliomas have strikingly different prognoses depending on their initial histology, age, KPS, and corticosteroid use. The RPA data from this study will permit investigators to design studies with more homogeneous patient populations or to retrospectively adjust outcome data thereby improving investigators' abilities to appropriately compare outcomes across phase II studies.
Supplementary Material
Online Table. Description of New Approaches to Brain Tumor Therapy CNS Consortium (NABTT) trials included in this study
Acknowledgments
Financial Support: NABTT grant CA62475.
Footnotes
Parts of this study were presented at ASCO 2005, Orlando, FL
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Huncharek M, Muscat J. Treatment of recurrent high grade astrocytoma; results of a systematic review of 1,415 patients. Anticancer Res. 1998;18:1303–1311. [PubMed] [Google Scholar]
- 3.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas the polymer-brain tumor treatment group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 4.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a north central cancer treatment Group/Radiation therapy oncology Group/Eastern cooperative oncology group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 5.Grossman SA, Fisher JD, Piantadosi S, et al. The New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium: Organization, objectives, and activities. Cancer Control. 1998;5:107–114. doi: 10.1177/107327489800500201. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg F, Grossman SA, Mikkelsen T, et al. NABTT CNS consortium. Lack of efficacy of 9-aminocamptothecin in adults with newly diagnosed glioblastoma multiforme and recurrent high-grade astrocytoma. Neuro-oncol. 2000;2:29–33. doi: 10.1093/neuonc/2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman SA, Phuphanich S, Lesser G, et al. Toxicity, efficacy, and pharmacology of suramin in adults with recurrent high-grade gliomas. J Clin Oncol. 2001;19:3260–3266. doi: 10.1200/JCO.2001.19.13.3260. [DOI] [PubMed] [Google Scholar]
- 8.Phuphanich S, Baker SD, Grossman SA, et al. Oral sodium phenylbutyrate in patients with recurrent malignant gliomas: A dose escalation and pharmacologic study. Neuro-oncol. 2005;7:177–182. doi: 10.1215/S1152851704000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman SA, Alavi JB, Supko JG, et al. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C-alpha delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro-oncol. 2005;7:32–40. doi: 10.1215/S1152851703000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- 11.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: Final report of NABTT 97-11. Neuro-oncol. 2004;6:21–27. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossman SA, Carson KA, Batchelor TT, et al. The effect of enzyme-inducing antiseizure drugs on the pharmacokinetics and tolerability of procarbazine hydrochloride. Clin Cancer Res. 2006;12:5174–5181. doi: 10.1158/1078-0432.CCR-06-0932. [DOI] [PubMed] [Google Scholar]
- 13.Olivi A, Grossman SA, Tatter S, et al. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: A New Approaches to Brain Tumor Therapy CNS Consortium trial. J Clin Oncol. 2003;21:1845–1849. doi: 10.1200/JCO.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 14.Tatter SB, Shaw EG, Rosenblum ML, et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite radiation therapy system) for treatment of recurrent malignant glioma: Multicenter safety and feasibility trial. J Neurosurg. 2003;99:297–303. doi: 10.3171/jns.2003.99.2.0297. [DOI] [PubMed] [Google Scholar]
- 15.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Weingart J, Grossman SA, Carson KA, et al. Phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-benzylguanine in adults with recurrent malignant glioma: New Approaches to Brain Tumor Therapy CNS Consortium trial. J Clin Oncol. 2007;25:399–404. doi: 10.1200/JCO.2006.06.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro-oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieder C, Nestle U, Niewald M, et al. Hyperfractionated reirradiation for malignant glioma. Front Radiat Ther Oncol. 1999;33:150–157. doi: 10.1159/000061231. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.Ciampi A, Negassa A, Lou Z. Tree-structured prediction for censored survival data and the cox model. J Clin Epidemiol. 1995;48:675–689. doi: 10.1016/0895-4356(94)00164-l. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Hohwieler Schloss M, Freidberg SR, Heatley GJ, et al. Glucocorticoid dependency as a prognostic factor in radiotherapy for cerebral gliomas. Acta Oncol. 1989;28:51–55. doi: 10.3109/02841868909111181. [DOI] [PubMed] [Google Scholar]
- 23.Odrazka K, Petera J, Kohlova T, et al. Prognostic impact of hemoglobin level prior to radiotherapy on survival in patients with glioblastoma. Strahlenther Onkol. 2003;179:615–619. doi: 10.1007/s00066-003-1097-x. [DOI] [PubMed] [Google Scholar]
- 24.Gundersen S, Lote K, Hannisdal E. Prognostic factors for glioblastoma multiforme--development of a prognostic index. Acta Oncol. 1996;35(Suppl 8):123–127. doi: 10.3109/02841869609098530. [DOI] [PubMed] [Google Scholar]
- 25.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 26.Eagan RT, Scott M. Evaluation of prognostic factors in chemotherapy of recurrent brain tumors. J Clin Oncol. 1983;1:38–44. doi: 10.1200/JCO.1983.1.1.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table. Description of New Approaches to Brain Tumor Therapy CNS Consortium (NABTT) trials included in this study