Abstract
Background and Purpose
Endoglin (ENG) deficiency causes hereditary hemorrhagic telangiectasia-1 (HHT1) and impairs myocardial repair. Pulmonary arteriovenous malformations (AVM) in HHT1 patients are associated with a high incidence of paradoxical embolism in the cerebral circulation and ischemic brain injury. We hypothesized that ENG deficiency impairs stroke recovery.
Methods
Eng heterozygous (Eng+/−) and wild-type (WT) mice underwent permanent distal middle cerebral artery occlusion (pMCAO). Pial collateral vessels were quantified before pMCAO. Infarct/atrophic volume, vascular density and macrophages were quantified in various days after pMCAO; and behavioral function was assessed using corner and adhesive removal tests on days 3, 15, 30 and 60 after pMCAO. The association between ENG 207G>A polymorphism and brain AVM rupture and surgery outcome was analyzed using logistic regression analysis in 256 ruptured and 157 unruptured patients.
Results
After pMCAO, Eng+/− mice showed larger infarct/atrophic volumes at all time points (P<0.05), and worse behavior performance (p<0.05) at 15, 30 and 60 days compared to WT mice. Eng+/− mice had fewer macrophages on day 3 (P=0.009) and more macrophages on day 60 (P=0.02) in the peri-infarct region. Although Eng+/− and WT mice had similar numbers of pial collateral vessels before pMCAO, Eng+/− mice had lower vascular density in the peri-infarct region (p=0.05) on day 60 after pMCAO. In humans, ENG 207A allele has been associated with worse outcomes after AVM rupture or surgery of unruptured AVM patients.
Conclusions
ENG deficiency impairs brain injury recovery. Reduced angiogenesis, impaired macrophage homing, and delayed inflammation resolution could be the underlying mechanism.
Keywords: angiogenesis, ENG polymorphism, hereditary hemorrhagic telangiectasia, ischemic stroke, macrophage
Introduction
Endoglin (ENG, CD105) is an auxiliary receptor for the TGF-β receptor complex. Upregulated ENG expression has been reported during wound healing and tumor vascularization, and in inflammatory tissues and developing embryos.1–3 Mutations in ENG cause type 1 hereditary hemorrhagic telangiectasia (HHT1), a disease characterized by arteriovenous malformation (AVM) in multiple organs and telangiectasia (small AVM) in the skin, mucous membranes.4 Further, a common genetic polymorphism in ENG (207G>A) has been associated with increased risk of sporadic brain AVM.5 Although this polymorphism is synonymous (does not alter encoded amino acid), the A allele reduces the predicted binding score of SRp40, a splicing protein,6 which could influence ENG protein production.
HHT1 patients have a higher prevalence of lung AVMs than other HHT patients.7 One of the major consequences of lung AVM is the increased risk of paradoxical embolism in the systemic circulation through arteriovenous shunting, which can cause embolic ischemic brain injury. The impact of ENG mutation on recovery from stroke and brain surgery is not clear.
In this study using a mouse ischemic stroke model, we tested the hypothesis that ENG deficiency weakens brain injury repair through impaired angiogenesis and macrophage function. We further analyzed the association between a functional polymorphism in ENG and outcomes after brain AVM rupture and surgical resection of unrupture AVM in sporadic AVM patients.
Materials and Methods
The methods and materials are described in detail in the online-only Data Supplement.
Animals
Animal experimental procedures were approved by the Institution of Animal Care and Use Committees of the University of California, San Francisco (UCSF) and Duke University, and conformed to NIH Guidelines for the use of animals in research. Mice were fed standard rodent food and water ad libitum, and were housed (5 per cage) in sawdust-lined cages in an air-conditioned environment with 12-hour light/dark cycles. Adult Eng+/− mice8 and their wild-type (WT) littermates were used. Tail genomic DNA was used to identify mouse genotype through PCR using primers: R(R9b): 5’-tgagcctgacgggaaactg-3’, and F(F9a): 5’-accatcttgtcctgagtagcg-3’. Eng expression in the brain of Eng+/− mouse is reduced to about 59% of the level in WT mouse.9
Human Study
Eligible AVM patients with modified Rankin Scale (mRS) outcome data and DNA for genetic studies were identified from the UCSF Brain AVM Study Project, an ongoing prospective longitudinal cohort study. Patients (245) who presented with an intracerebral hemorrhage (ICH) and had outcome data available after hemorrhage but prior to treatment were referred to as the natural history cohort. Patients (353) who underwent microsurgical excision of a brain AVM between 2000 and 2011 with outcome data available before and after treatment were referred to as the surgical cohort. All patients signed informed consent to participate and the study was approved by the Committee for Human Research at UCSF.
Statistical Analysis
One-way ANOVA was performed followed by Bonferroni posthoc to analyze the difference of the means of infarct/atrophic volume, vessel density and the number of CD68+ cells at the peri-infarct region among groups.
For analyses of the behavior test, Gaussian distribution was tested using d’Agostino and Pearson omnibus normality test. Equalities of variances were tested using the F-test. For dual comparisons, t-tests (Student, Mann-Wittney for non Gaussian distribution and Welch’s correction for unequal variances) were used when appropriate. To test the effect of genotype (WT vs Eng+/−) in the acute phase (day 3 after pMCAO), we performed a Student t-test. To test the effect of genotype in the post-acute phase (days 7–60 after pMCAO), we used a mixed-effects linear regression model (taking into account the genotype and the number of days since the insult, after checking two-way interactions involving the genotype) to predict the specified behavior outcome.
Sample sizes were: 10 per group for the behavior test; 13 Eng+/− and 12 WT mice for infarct volume measurement on day 1; 7 per group for infarct/atrophic volume on days 3 and 60; 3-week old and (6 WT and 6 Eng+/−) and 11-week-old (8 WT and 4 Eng+/−) mice for collateral quantification, and 6 per group for vessel density and CD68+ cell quantification. Data are presented as mean ± SD. A two-tailed P value < 0.05 was considered statistically significant.
For human data, univariable and multivariable logistic regression analyses of outcomes were performed. Results are reported as odds ratios (OR) and 95% confidence intervals (95% CI). Prior to constructing multivariate models, we tested whether the effect of ENG 207G>A genotype on outcomes was modified by other predictors (i.e., interactions) using likelihood-ratio testing (LRT). A significant interaction (p=0.05) was observed in the surgical cohort between ENG 207G>A genotype and hemorrhagic presentation; thus all surgical results are presented stratified as ruptured versus unruptured. Predictors in the multivariable models included ENG 207G>A genotype, age at presentation, gender, race/ethnicity (Caucasians vs. non-Caucasians), AVM size, exclusively deep venous drainage, and eloquent location.
Results
Eng Deficiency Delayed Functional Recovery
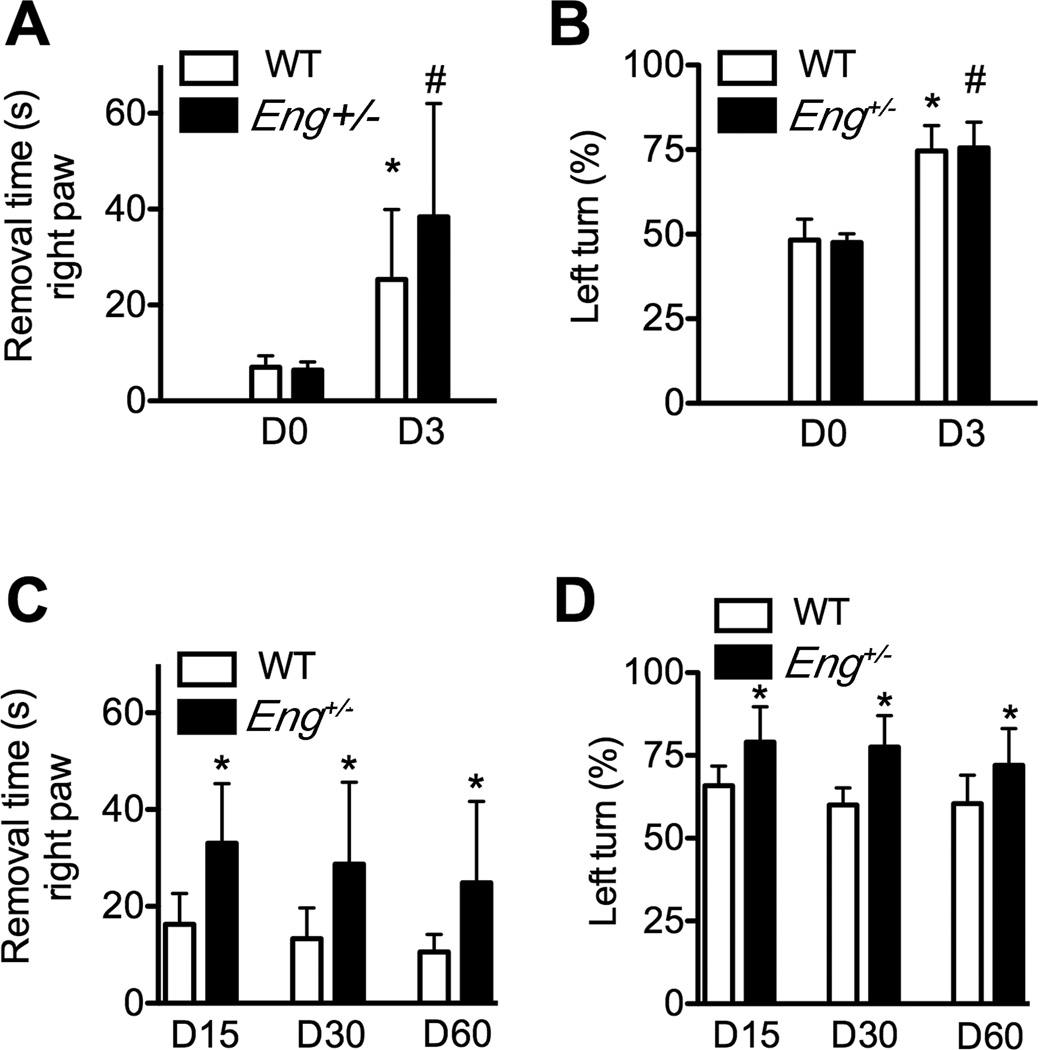
Adhesive removal and corner tests were used. After the training phase, the baseline behavior results tests were similar in all groups. At the acute phase of stroke (day 3 of pMCAO), both WT mice and Eng+/− mice took longer to remove the adhesive from their right paws (WT: 25±15 seconds [s] vs. 7±2s, P=0.001; Eng+/−: 38±7s vs. 6±1s, Figure 1A) and made more left turns (WT: 75±8% vs 48±6%, p<0.001, Eng+/−: 76±8% vs.47±3%, P<0.001, Figure 1B) compared to their baseline performance. The adhesive removal time from the left paw was not affected (p=0.73, Supplementary Figure IA). There is not difference between Eng+/− and WT mice (adhesive removal: p=0.13 and corner tests: p=0.78, Figure 1A–1B).
Figure 1. Behavior dysfunction.
A. Quantification of the adhesive removal test from the right paw. *p=0.001 vs. WT on day 0; #: P=0.13 vs. WT on day 3 B. Quantification of the corner test on day 0 and day 3. *: P<0.001 vs WT on day 0. #: P=0.78 vs. WT on day 3. C. Quantification of the adhesive removal test from the right paw on days 15, 30 and 60 after pMCAO. *: P<0.05 vs. corresponding WT groups. D. Quantification of the corner test on days 15, 30 and 60. *: P<0.05 vs. corresponding WT group. D0, D3, D15, D30 and D60 are 0, 3, 15, 30 and 60 days, respectively, after pMCAO.
In the post-acute phase (days 15–60 post pMCAO), Eng+/− mice took longer to remove tapes from the right paw on day 15 (16±6s vs 33±12s, p=0.002), day 30 (13±6s vs 29±17s, P=0.02) and day 60 (11±4s vs 25±17s, P=0.03, Figure 1C), and made more left turns (lesion site) than WT mice on day 15 (66±6% vs 79±11%, P=0.002), 30 (60±5% vs 77±10%, P<0.001) and day 60 (60±9 vs 72±11; P=0.01, Figure 1D) after pMCAO. Both genotype (OR: 15.5; 95% CI: 8.0; 11.5) and the timing post pMCAO (OR: 0.14; 95% CI: 0.05–0.24) influenced performance in removing adhesive from the right paw and in the corner test (genotype: OR: 14.1; 95% CI: 8.5; 19.7; P<0.001 and timing: OR: 0.13; 95% CI: 0.05–0.20; P=0.001). Left paw adhesive removal was not affected (Supplementary Figure IB).
Eng+/− Mice Had More Severe Brain Injury
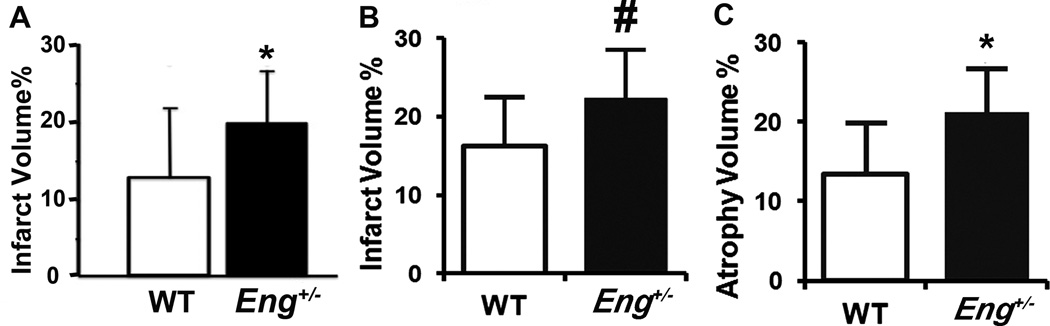
Eng+/− mice exhibited larger infarct volume than WT mice (19.7±6.5 mm3 vs. 12.6±8.9 mm3, P=0.03) on day 1 (Figure 2A & Supplementary Figure IIA) and day 3 (22±6% vs. 16±6%, P=0.04, Figure 2B & Supplementary Figure IIB); and larger atrophic volume than WT mice on day 60 after pMCAO (21.27±5% vs. 13.4±6%, P=0.03, Figure 2C & Supplementary Figure IIC). These data indicate that ischemic insult caused more severe brain damage in Eng+/− mice.
Figure 2. Infarct/atrophic volumes.
A. Infarct volume on day 1. n=13 (Eng+/−) and n=12 (WT mice). *: P=0.03. B. Infarct volume on day 3. #: P= 0.04. n= 7. C. Atrophic volume on day 60. *: P=0.03. n=7. Bars=2 mm.
Eng Deficiency Impairs CD68+ Macrophage Recruitment or Clearance
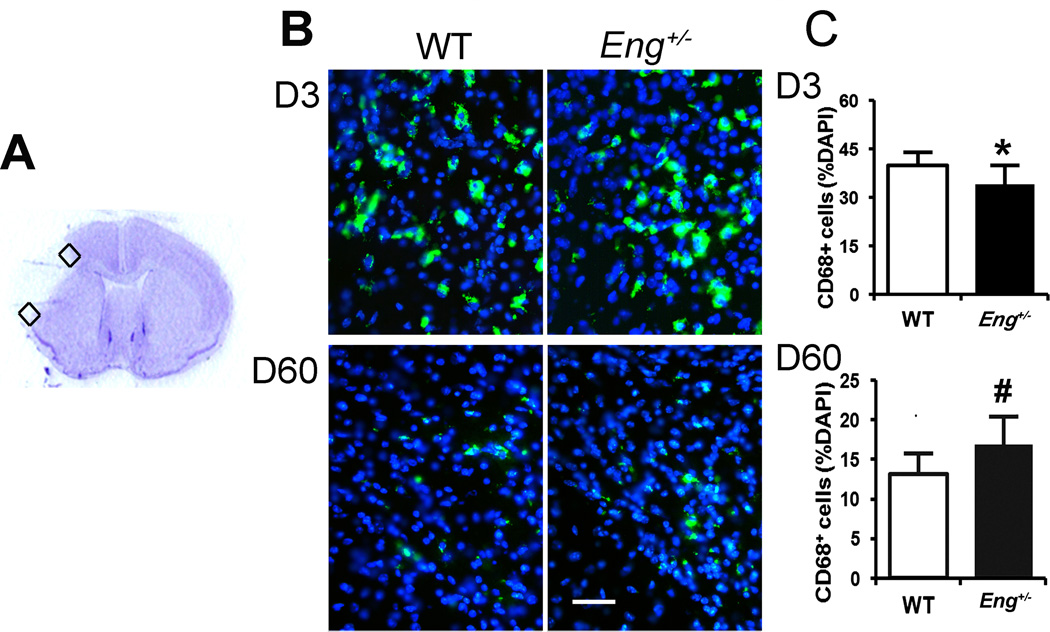
CD68+ macrophages were quantified in the peri-infarct region right outside the infarct border on days 3 and 60 after pMCAO. Eng+/− mice had fewer microphages on day 3 (34±6% of total cells vs 40±4%, P=0.009) and more macrophages on day 60 (17±4% vs 13±3%, P=0.02) compared to WT mice (Figure 3), indicating that Eng deficiency delayed macrophage homing and clearance or promoted macrophage homing at the post-acute stage of ischemic stroke.
Figure 3. CD68+ cells in the peri-infarct area.
A. Coronal section showing the areas (squares) used for quantification. B. Representative images of CD68 antibody-stained (green) section. The nuclei were counterstained with DAPI (blue). Bar=50µm. C. Quantification of CD68+ cells in the peri-infarct area 3 (D3) and 60 days (D60) after pMCAO. *: P=0.009, #: P=0.02. N=10.
Eng Deficiency Reduced Angiogenic Response to Ischemic Brain Injury
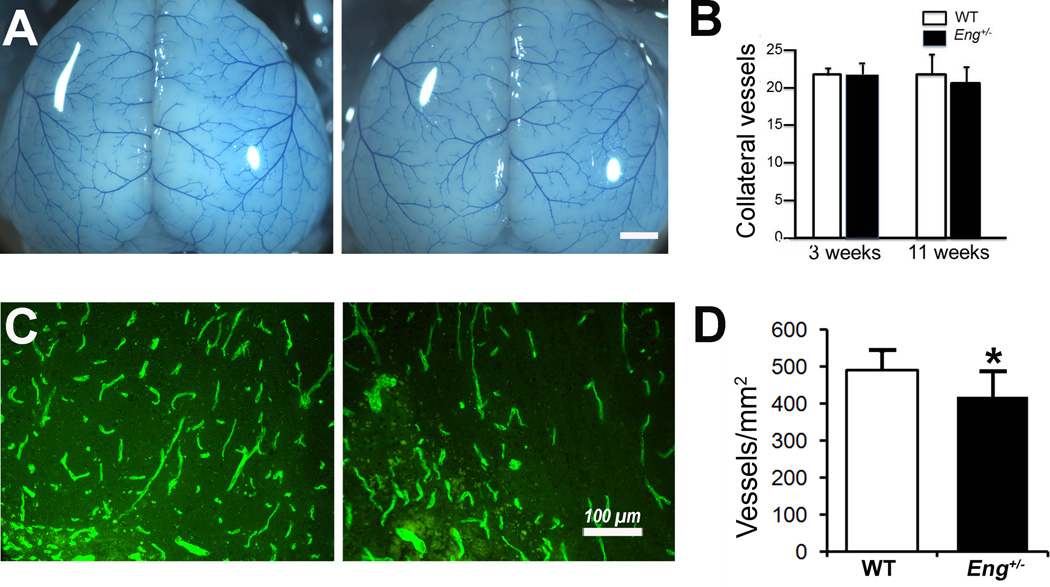
Among the factors determining infarct volume after stroke, collateral vessel anatomy (especially the collateral vessel number between the anterior cerebral artery [ACA] and the middle cerebral artery [MCA]) has been proposed as a major factor determining infarct volume.10 We quantified the collateral vessel numbers before pMCAO, and found no difference between the Eng+/− and WT mice at the age of 3 (21±1.8 vs. 21±0.8 vessels/mm2, P=0.56) and 11 weeks (20±2.1 vs. 21±2.9, p=0.6, Figure 4A & B). However, microvascular density in the peri-infarct area was lower in Eng+/− mice than WT mice (417±69 vs 490±52 vessels/mm2, p=0.05, Figure 4C & D) on day 60 after pMCAO, indicating that Eng deficiency reduced post-ischemic angiogenesis.
Figure 4. Pial collateral vessels at baseline and vessel density in the peri-infarct cortex.
A. Representative photographs of collateral vessels of 3-week-old WT and Eng+/− mice. Bar=1 mm. B. Quantification of collateral vessel number of 3-week-old mice (WT: N=6, Eng+/−: N=6) and 11-week-old mice (WT: N=8, Eng+/−: N=4). C. Representative images of CD31 antibody-stained sections. Bar=100µm. D. Quantification of vessel density. *P=0.05, N=6.
ENG 207G>A Allele is Associated with Poor Outcome After Brain AVM Surgical Resection in Unruptured Patients
Characteristics of patients with good and bad outcomes, stratified by hemorrhage, are presented in Supplementary Table I. In the unruptured surgical group, patients with poor outcome had higher Spetzler-Martin scores (p=0.05). Those with poor outcome were also more likely to be carriers of the A allele of ENG 207G>A (P=0.01), and were at higher risk of poor outcome compared to unruptured patients carrying only the G allele (univariate OR=3.57, 95% CI=1.22–10.43, P=0.02). A corresponding effect was not seen in ruptured AVM surgical patients. Multivariable logistic regression analysis confirmed the association between the A allele and poor functional outcome, independent of other risk factors, among unruptured (OR=3.65, 95% CI=1.15–11.54, P=0.03) but not ruptured patients (OR=0.67, 95% CI=0.18–2.51, P=0.55) (Table 1). A sensitivity analysis restricted to Caucasian subjects (n=139) yielded similar results (data not shown).
Table 1.
Impact of ENG 207G>A Genotype and Risk Factors on Outcome Post-BAVM Resection
| Hemorrhagic presentation n=137 | ||||
| Univariate | Multivariable | |||
| Variable | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| A allele | 3.57 (1.22–10.43) | 0.02 | 3.65 (1.15–11.54) | 0.03 |
| Age, decade | 1.11 (0.89–1.40) | 0.39 | 1.19 (0.92–1.54) | 0.19 |
| Female gender | 0.65 (0.32–1.36) | 0.26 | 0.68 (0.31–1.49) | 0.34 |
| Non-white ethnicity | 1.55 (0.74–3.25) | 0.24 | 2.12 (0.90–4.99) | 0.09 |
| BAVM size, cm | 1.23 (0.93–1.63) | 0.15 | 1.22 (0.89–1.67) | 0.21 |
| Deep venous drainage | 1.33 (0.62–2.87) | 0.47 | 0.90 (0.37–2.18) | 0.81 |
| Eloquent location | 1.92 (0.91–4.01) | 0.09 | 1.71 (0.75–3.89) | 0.20 |
| Length of follow up | 0.92 (0.68–1.24) | 0.58 | 0.88 (0.64–1.22) | 0.45 |
| Nonhemorrhagic presentation n=119 | ||||
| Univariate | Multivariable | |||
| Variable | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| A allele | 0.67 (0.18–2.42) | 0.54 | 0.67 (0.18–2.51) | 0.55 |
| Age, decade | 1.20 (0.92–1.57) | 0.18 | 1.34 (0.97 −1.83) | 0.07 |
| Female gender | 0.56 (0.22–1.46) | 0.24 | 0.60 (0.20–1.83) | 0.37 |
| Non-white ethnicity | 0.90 (0.35–2.30) | 0.82 | 0.82 (0.56–2.59) | 0.74 |
| BAVM size, cm | 1.29 (0.97–1.72) | 0.08 | 1.16 (0.80–1.68) | 0.43 |
| Deep venous drainage | 2.83 (1.01–7.89) | 0.05 | 2.01 (0.60–6.81) | 0.26 |
| Eloquent location | 1.84 (0.69–4.96) | 0.23 | 1.43 (0.46–4.44) | 0.54 |
| Length of follow up | 0.58 (0.44–0.80) | <0.01 | 0.60 (0.45–0.81) | <0.01 |
ENG 207G>A Allele is Associated with Increased Risk of Poor Neurological Status in the Natural Course of Brain AVM Rupture
Poor outcome patients were also more likely to carry the A allele (P=0.05), and had shorter latencies between hemorrhage and treatment (P<0.01). There were no notable differences in patient age, race/ethnicity, Spetzler-Martin distribution, AVM size, drainage pattern, or location (Supplementary Table II) for those included in the natural history cohort.
Logistic regression results (Table 2) show that the A allele of ENG 207G>A was associated with having an mRS>2 following AVM rupture after adjustment for baseline mRS and time between hemorrhage and mRS assessment (OR=2.88, 95% CI=1.10–7.75, P=0.03). Multivariable analysis showed this effect to be independent of other risk factors (OR=3.21, 95% CI=1.19–8.68, P=0.02).
Table 2.
Impact of ENG 207G>A Genotype and Other Risk Factors on Risk of Poor Outcome in the Natural Course of BAVM Rupture
| Reduced Model | Full Model | |||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| A allele | 2.88 (1.10–7.55) | 0.03 | 3.21 (1.19–8.68) | 0.02 |
| Baseline mRS | 1.09 (0.44–2.69) | 0.85 | 0.99 (0.38–2.58) | 0.98 |
| Days after rupture | 1.00 (1.00–1.00) | 0.04 | 1.00 (1.00–1.00) | 0.03 |
| Age, decade | 0.98 (0.81–1.18) | 0.78 | ||
| Female gender | 1.84 (0.90–3.78) | 0.10 | ||
| Non-white ethnicity | 0.72 (0.35–1.49) | 0.38 | ||
| BAVM size, cm | 1.04 (0.82–1.33) | 0.75 | ||
| Deep venous drainage | 0.79 (0.37–1.71) | 0.55 | ||
| Eloquent location | 1.47 (0.71–3.03) | 0.30 | ||
Discussion
In this study, we demonstrated that Eng deficiency impairs brain ischemic injury repair. Eng+/− mice had more severe functional defects and larger atrophic volume than WT mice, which is associated with delayed macrophage homing and clearance or enhanced macrophage homing at the sub-acute stage. Although Eng+/− and WT mice had a similar number of collaterals between ACA and MCA at baseline, Eng+/− mice had lower vessel density at the peri-infarct region than WT mice 60 days after pMCAO, which suggests that Eng deficiency leads to reduced response to angiogenic stimulation. Further we found that in humans, there was an association between functional polymorphism in ENG and outcome of brain AVM rupture and surgery.
ENG is highly expressed in proliferating vascular endothelial cells,11 and is elevated in the settings of inflammation and wound healing.12 After ischemia-reperfusion injury and myocardial infarction, endoglin is upregulated in the ischemic area and border zone.13 Microvascularity within the infarct zone was strikingly lower in Eng+/− mice than WT mice. We also found that Eng+/− mice had lower vessel density in the peri-infarct region after pMCAO. VEGF expression is reduced in cultured Eng+/− endothelial cells.14 Both VEGFR 1 and 2 are reduced in Eng+/− macrophages at baseline and after VEGF stimulation.15 Eng+/− macrophages and WT macrophages express similar level of Mmp9 at baseline. Mmp9 expression is increased in WT, but not in Eng+/−, macrophages after VEGF (50 ng/ml) treatment.15 In the brain, VEGF-induced upregulation of VEGFR2 expression is greatly impaired in Eng+/− mice.16 Together, these data suggest that reduced angiogenesis in response to injury is one of the mechanisms for impaired tissue repair in Eng+/− mice after pMCAO.
Interestingly, Eng+/− mice had more severe brain injury than WT mice even on day 1 after pMCAO, which could not be explained by impairment of tissue repair. Hypoxia induces endothelial Eng expression,17 and Eng prevents apoptosis in hypoxia endothelial cells.18 Hence, vascular damage in Eng+/− mice could be more severe than in WT mice after ischemic insult. Eng haploinsufficiency has been associated with reduced nitric oxide (NO) production and increased endothelial NOS-derived superoxide production19. Bioavailability of NO is lower in Eng+/− mice than WT mice.20 NO produced by endothelial cell induces vascular relaxation.21 Thus, reduced vessel relaxation and increased vascular damage, together with oxidative stress (more superoxide production), in Eng+/− mice enhance brain injury during the acute stage of ischemic stroke.
In addition to endothelial cells, several other cell types also express ENG. For example, ENG is present in monocytes and is upregulated during the monocyte to macrophage transition.22 Recruitment of human monocytes to the infarcted murine heart and subsequent vessel formation was severely impaired when HHT1 monocytes were used.13 Eng deficiency in endothelial cell reduced leukocyte adhesion and transmigration23 and impaired the endothelial-autonomous capacity to upregulate SDF-1 expression in response to hindlimb ischemic injury.24 In this study, we found that Eng+/− mice had fewer CD68+ cells in the peri-infarct area at 3 days and more CD68+ cells at 60 days after pMCAO. Taken together, Eng deficiency appears to impair monocyte adhesion and migration. Future studies are needed to determine whether increased macrophage in Eng+/− mice at the post-acute stage is the result of delayed clearance or prolonged/enhanced macrophage homing at this stage.
The roles of post-ischemic inflammation are bi-directional.25 The postischemic inflammatory response contributes to secondary brain injury, and inflammation can be detrimental, as the influx of inflammatory cells amplifies brain cell death. During recovery, however, inflammation may be construed as plastic forms of tissue remodeling.26 How the impaired macrophage homing and clearance impair brain injury recovery need to be explored in future studies.
ENG is a causative gene of HHT1. The prevalence of brain AVM in HHT1 patients is 1000-fold higher than the prevalence in the general population (10/100,000).27 A common genetic polymorphism in ENG (207G>A) has also been associated with increased risk of sporadic brain AVM.5 Further, HHT1 patients have a high prevalence of lung AVMs,7 which increases the risk of embolic ischemic brain injury. An insertion/deletion polymorphism in endoglin has also been associated with sporadic primary intracranial hemorrhage (ICH).28 Our study is the first to demonstrate that ENG polymorphisms are associated with recovery after brain AVM rupture or surgery in patients. Thus, ENG may play a crucial role in all types of injury repair, including ischemic stroke, hemorrhagic stroke, or iatrogenic injury. Using an ischemic stroke mouse model, we showed that reduced angiogenesis, impaired macrophage homing, and delayed inflammation resolution could be the underlying mechanism. Further studies are needed to determine whether the same mechanism applies to all brain injuries.
Conclusion
We have demonstrated in this study that Eng deficiency exacerbates ischemic brain injury and delays neurobehavioral recovery in mice, which is associated with reduced angiogenesis, impaired CD68+ cells homing, and delayed inflammation resolution. ENG may also play a role in surgical and hemorrhagic recovery in human brain AVM patients. Understanding how ENG is involved in different kinds of brain injury could provide new therapeutic opportunities to improve outcomes in patients.
Supplementary Material
Acknowledgments
We thank Voltaire Gungab for assistance with manuscript preparation, and members of the UCSF BAVM Study Project (http://avm.ucsf.edu) for their support.
Sources of Funding
This study was supported by grants from the National Institutes of Health: R01NS027713 and P01NS044155 to H.S., R01NS034949 and P01NS044155 to H.K., and R01HL097281 to D.M. Additional support was provided by a grant to W.L.Y. from the Michael Ryan Zodda Foundation.
Disclosures
Dr. Maze is a Board Member of the Foundation for Anesthesia Education and Research (FAER), Smarttots, and the Society for Anesthesia and Sleep Medicine, and is supported by a grant from the National Institutes of Health.
References
- 1.Bernabeu C, Conley BA, Vary CP. Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem. 2007;102:1375–1388. doi: 10.1002/jcb.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintanilla M, Ramirez JR, Perez-Gomez E, Romero D, Velasco B, Letarte M, et al. Expression of the TGF-beta coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis. Oncogene. 2003;22:5976–5985. doi: 10.1038/sj.onc.1206841. [DOI] [PubMed] [Google Scholar]
- 3.Dallas NA, Gray MJ, Xia L, Fan F, van Buren G, 2nd, Gaur P, et al. Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin Cancer Res. 2008;14:8052–8060. doi: 10.1158/1078-0432.CCR-08-1520. [DOI] [PubMed] [Google Scholar]
- 4.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 5.Pawlikowska L, Tran MN, Achrol AS, Ha C, Burchard EG, Choudhry S, et al. Polymorphisms in transforming growth factor-B-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke. 2005;36:2278–2280. doi: 10.1161/01.STR.0000182253.91167.fa. [DOI] [PubMed] [Google Scholar]
- 6.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: A web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourdeau A, Faughnan ME, Letarte M. Endoglin-deficient mice, a unique model to study hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med. 2000;10:279–285. doi: 10.1016/s1050-1738(01)00062-7. [DOI] [PubMed] [Google Scholar]
- 8.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 9.Satomi J, Mount RJ, Toporsian M, Paterson AD, Wallace MC, Harrison RV, et al. Cerebral vascular abnormalities in a murine model of hereditary hemorrhagic telangiectasia. Stroke. 2003;34:783–789. doi: 10.1161/01.STR.0000056170.47815.37. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–934. doi: 10.1038/jcbfm.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, et al. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer. 1999;81:568–572. doi: 10.1002/(sici)1097-0215(19990517)81:4<568::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Torsney E, Charlton R, Parums D, Collis M, Arthur HM. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res. 2002;51:464–470. doi: 10.1007/pl00012413. [DOI] [PubMed] [Google Scholar]
- 13.van Laake LW, van den Driesche S, Post S, Feijen A, Jansen MA, Driessens MH, et al. Endoglin has a crucial role in blood cell-mediated vascular repair. Circulation. 2006;114:2288–2297. doi: 10.1161/CIRCULATIONAHA.106.639161. [DOI] [PubMed] [Google Scholar]
- 14.Jerkic M, Rodriguez-Barbero A, Prieto M, Toporsian M, Pericacho M, Rivas-Elena JV, et al. Reduced angiogenic responses in adult Endoglin heterozygous mice. Cardiovasc Res. 2006;69:845–854. doi: 10.1016/j.cardiores.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Choi EJ, Walker EJ, Degos V, Jun K, Kuo R, Su H, et al. Endoglin deficiency in bone marrow is sufficient to cause cerebrovascular dysplasia in the adult mouse after vascular endothelial growth factor stimulation. Stroke. 2013;44:795–798. doi: 10.1161/STROKEAHA.112.671974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, et al. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24:237–244. doi: 10.1097/01.WCB.0000107730.66603.51. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Sun Y, Xie L, Jin K, Sheibani N, Greenberg DA. Hypoxic induction of endoglin via mitogen-activated protein kinases in mouse brain microvascular endothelial cells. Stroke. 2003;34:2483–2488. doi: 10.1161/01.STR.0000088644.60368.ED. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, et al. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci. 2003;116:2677–2685. doi: 10.1242/jcs.00470. [DOI] [PubMed] [Google Scholar]
- 19.Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–692. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Gomez E, Jerkic M, Prieto M, del Castillo G, Martin-Villar E, Letarte M, et al. Impaired wound repair in adult endoglin heterozygous mice associated with lower NO bioavailability. J Invest Dermatol. 2014;134:247–255. doi: 10.1038/jid.2013.263. [DOI] [PubMed] [Google Scholar]
- 21.Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature Lond. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 22.Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, et al. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992;22:393–397. doi: 10.1002/eji.1830220216. [DOI] [PubMed] [Google Scholar]
- 23.Rossi E, Sanz-Rodriguez F, Eleno N, Duwell A, Blanco FJ, Langa C, et al. Endothelial endoglin is involved in inflammation: role in leukocyte adhesion and transmigration. Blood. 2013;121:403–415. doi: 10.1182/blood-2012-06-435347. [DOI] [PubMed] [Google Scholar]
- 24.Young K, Conley B, Romero D, Tweedie E, O'Neill C, Pinz I, et al. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120:4263–4273. doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Marchuk DA, Pawlikowska L, Chen Y, Su H, Yang GY, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. doi: 10.1007/978-3-211-09469-3_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberts MJ, Davis JP, Graffagnino C, McClenny C, Delong D, Granger C, et al. Endoglin gene polymorphism as a risk factor for sporadic intracerebral hemorrhage. Ann Neurol. 1997;41:683–686. doi: 10.1002/ana.410410519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.