Abstract
Background : Exercise-induced arterial hypertension (EIAH) leads to myocardial hypertrophy and is associated with a poor prognosis. EIAH might be related to the “cardiac fatigue” caused by endurance training. The goal of this study was to examine whether there is any relationship between EIAH and left ventricular hypertrophy in Ironman-triathletes.
Methods: We used echocardiography and spiroergometry to determine the left ventricular mass (LVM), the aerobic/anaerobic thresholds and the steady-state blood pressure of 51 healthy male triathletes. The main inclusion criterion was the participation in at least one middle or long distance triathlon.
Results: When comparing triathletes with LVM <220g and athletes with LVM >220g there was a significant difference between blood pressure values (BP) at the anaerobic threshold (185.2± 21.5 mmHg vs. 198.8 ±22.3 mmHg, p=0.037). The spiroergometric results were: maximum oxygen uptake (relative VO 2max) 57.3 ±7.5ml/min/kg vs. 59.8±9.5ml/min/kg (p=ns). Cut-point analysis for the relationship of BP >170 mmHg at the aerobic threshold and the probability of LVM >220g showed a sensitivity of 95.8%, a specificity of 33.3%, with a positive predictive value of 56.8 %, a good negative predictive value of 90%. The probability of LVM >220g increased with higher BP during exercise (OR: 1.027, 95% CI 1.002-1.052, p= 0.034) or with higher training volume (OR: 1.23, 95% CI 1.04 -1.47, p = 0.019). Echocardiography showed predominantly concentric remodelling, followed by concentric hypertrophy.
Conclusion: Significant left ventricular hypertrophy with LVM >220g is associated with higher arterial blood pressure at the aerobic or anaerobic threshold. The endurance athletes with EIAH may require a therapeutic intervention to at least prevent extensive stiffening of the heart muscle and exercise-induced cardiac fatigue.
Introduction
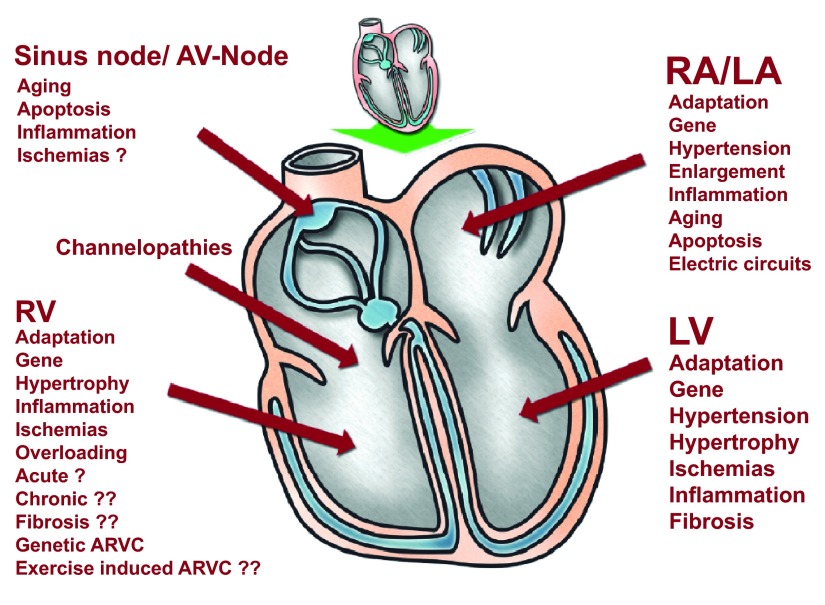
Myocardial hypertrophy in hypertensive patients has a negative influence on long-term prognosis 1, cardiac arrhythmias and mortality 2, 3. Myocardial hypertrophy in otherwise healthy, non-hypertensive individuals can be caused by exercise-induced arterial hypertension (EIAH) 4– 6 and may also result in poor prognosis 7. Moreover, exercise-induced hypertrophy may cause sudden cardiac death in athletes 8. However, myocardial hypertrophy induced by extensive exercise presents so called “normal diastolic” function, which might be a result of “physiological” adaptation 9, 10. EIAH or elevated blood pressure values during exercise might have a “negative” impact on cardiac function in athletes and might be one of the important factors causing “exercise-induced” cardiac fatigue ( Figure 1). Our hypothesis is provocative, but this suggestion might become important for many professional and leisure athletes ( Figure 2).
Figure 1. Factors which affect cardiac structures and function during exercise.
This figure shows the factors with possible negative influence on myocardium like inflammation, fibrosis etc. It demonstrates the possible complexity of different actions.
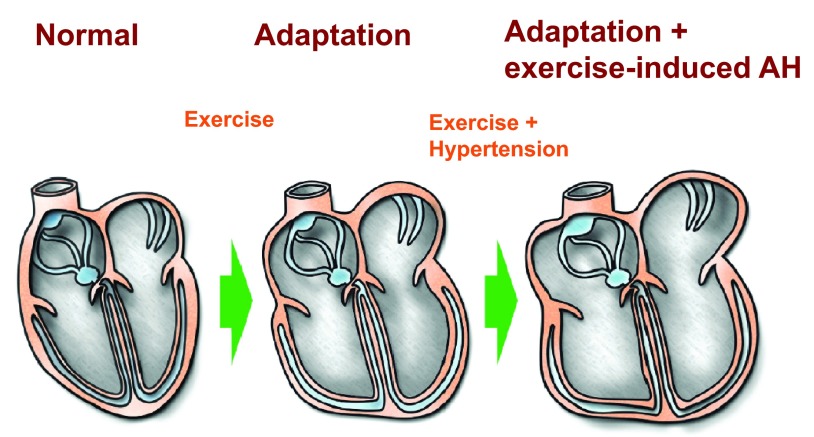
Figure 2. Scheme of possible adaptation of cardiac cavities in endurance sport and possible pathological enlargement/hypertrophy in case of exercise-induced arterial hypertension.
Right and left atrium have more connective tissue construct as muscular ventricular chambers and more affinity for pathological enlargement in case of pressure overload.
Physical activity in the general population is of fundamental importance 11, 12. The role of EIAH in normotensive adult athletes 13 or healthy men is currently under discussion 6, 14. It is unclear how far endurance sport can influence a “negative remodelling” of the athlete’s heart 15. The dosage of exercise bouts which causes cardiac injury 18, 19, and the “true pathologic values” of EIAH are unknown or under debate 6.
Endurance sport is linked to cardiac injury 20. In individual cases, long term training might lead to arrhythmias 21, atrial fibrillation 22, 23 or myocardial fibrosis 16, 24 and early sudden cardiac death 24– 26, female athletes are less commonly affected 26, 27.
The type of sport discipline has also an influence on the type of hypertrophy. Some authors distinguish the strength-trained heart and an endurance-trained heart 10, 28. Further factors that might influence exercise-induced hypertrophy are genetic factors 29, 30, gender 31, environmental factors 32, endocrine factors 33 and arterial hypertension 34.
In this study, we examined the impact of EIAH on cardiac hypertrophy in 51 normotensive (at rest) healthy Ironman athletes with long daily training times.
Materials and methods
The influence of EIAH on cardiac hypertrophy was examined in 51 male triathletes (mean age 37.2, Table 1) who finished an Ironman 70.3 (n=17, 1.9km swim, 90km bicycle ride and 21,1km run) or Ironman full distance (n=34/3.8km swim, 180km bicycle ride and/42.2km run). The training habits were similar for both the 70.3 and long distance Ironman. The minimum training-time was two years. All triathletes have been examined by spiroergometry and echocardiography. There is no consensus about the value of systolic BP that determines EIAH 6. According to the literature, EIAH is described as systolic BP >210mmHg for males and >190mmHg for females, as maximal values during exercise 5. In our study, the absolute values primarily were not defined. Odds ratios analysis was used to calculate the probability of elevated blood pressure and hypertrophy. The estimation of sensitivity/specificity to detect the boundaries of the EIAH and the positive or negative predictive values for the blood pressure boundaries should be performed during the study. Our interest was directed to the aerobic and anaerobic threshold, because this is a constant level of blood pressure maintained during training or competitions.
Table 1. Anthropometric and echocardiographic data.
The blue coloured area shows the anthropometric data of the two groups with different LVM. The orange coloured data are echocardiographic data of the left ventricle. The green coloured data are the Doppler-flow data. The last two lines are the data of the right ventricle.
| LVM <220g | LVM >220g | p-value | |||||
|---|---|---|---|---|---|---|---|
| n | mean | sd | n | mean | sd | Mann-Whitney-U-Test | |
| Age (years) | 27 | 37.2 | 10.7 | 24 | 38.3 | 13.5 | 0.947 |
| Weight (kg) | 27 | 182.1 | 6.30 | 24 | 182.5 | 7.1 | 0.932 |
| Size (cm) | 27 | 75.0 | 6.20 | 24 | 78.2 | 11.3 | 0.231 |
| BMI (kg/m 2) | 27 | 22.6 | 1.60 | 24 | 23.4 | 2.0 | 0.206 |
| BSA (m 2) | 27 | 1.95 | 0.11 | 24 | 1.99 | 0.18 | 0.395 |
| %body fat | 27 | 12.3 | 3.4 | 24 | 12.4 | 3.9 | 0.828 |
| Aorta (cm) | 27 | 2.9 | 0.4 | 24 | 3.0 | 0.3 | 0.236 |
| Left atrium (cm) | 27 | 2.46 | 0.27 | 24 | 2.64 | 0.27 | 0.020 |
| LAESV* (ml) | 27 | 27.7 | 8.00 | 24 | 30.7 | 7.5 | 0.098 |
| IVS diastolic (cm) | 27 | 1.16 | 0.10 | 24 | 1.31 | 0.12 | 0.000 |
| IVS systolic (cm) | 27 | 1.57 | 0.13 | 24 | 1.78 | 0.16 | 0.000 |
| PWD diastolic (cm) | 27 | 1.13 | 0.08 | 24 | 1.32 | 0.13 | 0.000 |
| PWD systolic (cm) | 27 | 1.59 | 0.1 | 24 | 1.83 | 0.14 | 0.000 |
| Relative wall thickness | 27 | 0.48 | 0.06 | 24 | 0.53 | 0.07 | 0.055 |
| LVEDD (cm) | 27 | 4.7 | 0.4 | 24 | 5.0 | 0.3 | 0.003 |
| LVESD (cm) | 27 | 4.7 | 0.4 | 24 | 5.0 | 0.3 | 0.003 |
| LVM (g) | 27 | 185.3 | 19.3 | 24 | 254.1 | 27.0 | 0.000 |
| LVM (g/m 2) | 27 | 95.3 | 10.1 | 24 | 128.8 | 17.6 | 0.000 |
| LVEDV (ml) | 27 | 132.7 | 18.8 | 24 | 145.0 | 24.4 | 0.086 |
| LVESV (ml) | 27 | 50.6 | 7.9 | 24 | 55.0 | 11.4 | 0.234 |
| SV (ml) | 27 | 82.0 | 11.9 | 24 | 89.9 | 15.3 | 0.059 |
| EF (%) | 27 | 62.5 | 2.0 | 24 | 61.6 | 2.9 | 0.212 |
| LVOT V max (m/s) | 27 | 0.80 | 0.13 | 24 | 0.80 | 0.13 | 0.917 |
| MV E max (m/s) | 27 | 0.54 | 0.1 | 24 | 0.51 | 0.09 | 0.196 |
| MV A max (m/s) | 27 | 0.37 | 0.07 | 24 | 0.36 | 0.05 | 0.857 |
| MV E/A Ratio | 27 | 1.51 | 0.35 | 24 | 1.43 | 0.27 | 0.313 |
| RV parasternal | 27 | 3.1 | 0.1 | 24 | 3.30 | 0.1 | 0.000 |
| RV AFC% | 27 | 32.8 | 1.8 | 24 | 34.2 | 2.4 | 0.047 |
Mean = mean value. sd = standard deviation. BMI = body mass index. BSA = body surface area. LAESV = left atrial endsystolic volume. IVS = interventricular septum. PWD = diastolic left ventricular posterior wall thickness. RWT: relative wall thickness: (2xPWD/LVEDD). LVEDD = left ventricular end-diastolic diameter. LVM = left ventricular mass. LVEDV: left ventricular enddiastolic volume. LVESV: left ventricular endsystolic volume. SV: Stroke volume. EF: Ejection fraction in %. LVOT = left ventricular outflow tract. MV: Mitral valve. parasternal: right ventricular diameter in 2D parasternal view. RV AFC%: right ventricular area fractional change.
A Vivid 7 model echocardiograph manufactured by general Electric was used for the examinations. The Ergobike 8I manufactured by Daum and the Metalizer 3B produced by Cortex were used for the spiroergometric examination 35.
The assessment of each triathlete was performed in 2011 and 2012 on the same day with the echocardiography first followed by spiroergometry. The spiroergometry was performed as follows: the stress test (exercise bike) was conducted in stages after successful gas and volume calibration: 50W for 3 minutes, 100W for further 3 minutes and thereafter increased by another 30W for 3 minutes (ramp-test). The test ended when the subject could no longer maintain the predefined rpm of 90 or if the subject was exhausted.
The echocardiographic analysis was conducted according to general recommendations 36, 37. The formula recommended by the American Society of Echocardiography (ASE) was used for calculate the muscle mass. Enddiastolic LV-volume (EDV) and Endsystolic LV-volume (ESV) were determined monoplane after the modified Simpson method 36.
The spiroergometric analyses were conducted according to previously published protocols 38, 39: VAT (ventilatory aerobic threshold) was determined as the first non-linear increase of the ventilatory equivalent for oxygen without simultaneous increase of the ventilatory equivalent for CO 2, and RCP (respiratory compensation point: anaerobic threshold) was determined as simultaneous non-linear increase of both ventilatory equivalents according to previous recommendations 38, 39.
VO 2max was registered as the highest average value of oxygen absorption over 30 seconds.
Statistical analysis
The entire statistical analysis plan was designed as follows: Stata/IC 13.1 for Windows was used for data preparation and statistical analysis. The Mann-Whitney-U-Test was used to compare the groups with LVM >220g and LVM <220g. Odds Ratios were calculated to measure the association between blood pressure, training habits and the probability of LVM >220g. Since these exposure variables are quantitative variables, an approximate estimate of the log odds-ratio for a one-unit increase in exposure and a 1-degree-of-freedom test for trend were calculated. All statistical tests were two-sided with a signficance level of 0.05.
In addition, sensitivity, specificity, positive and negative predictive values as well as the proportion of correctly classified participants were calculated for each possible cut-point of blood pressure to describe the performance of blood pressure as a “diagnostic test” for LVM >220g.
Results
Anthropometry and echocardiography
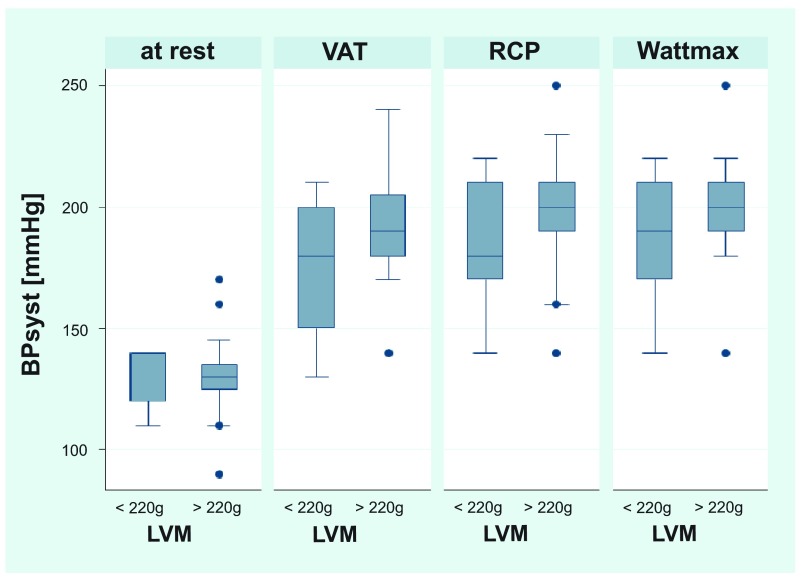
Anthropometric baseline data of triathletes are listed in Table 1. The blood pressure values of the two groups (LVM <220g and LVM >220g) are visualized in Figure 3 (for exact values see Table 2). In Figure 3 one can see that triathletes with LVM >220g have higher blood pressure values at ventilatory aerobic threshold (VAT) and anaerobic threshold (RCP) and at maximum achieved Watt-level (Wattmax). The results showed myocardial hypertrophy in most participants and were classified as according to Lang et al. 36. Normal morphology was found in three triathletes, eccentric hypertrophy was shown in one athlete, concentric remodelling was observed in 26 triathletes and concentric hypertrophy in 21. Right ventricular remodelling or other pathological findings of the right ventricle were not found in any of the athletes. Left ventricular function was good in all triathletes (EF >55%). All relevant echocardiographic values are shown in Table 1. All further parameters are shown in Table 2, sorted according to the p-value.
Figure 3. Blood pressure values at rest and at different exercise levels in two groups of triathletes with different LVM.
The group with LVM >220 shows significant higher systolic blood pressure (BP) values at the aerobic threshold (VAT), anaerobic threshold (RCP) and at the maximum exercise-level (Wattmax).
BP = Blood pressure. LVM = left ventricular mass. VAT = ventilatore aerobic threshold.
RCP = respiratory compensation point. Wattmax = Maximum exercise-level.
Table 2. Performance, BP and training parameters depending on LVM, sorted according to p-value.
Triathletes in the group with LVM >220g have significant longer training times and distances on bike, longer overall training times ( Mann-Whitney-U-Test).
| Further parameters | LVM <220g | LVM >220g | p-value | ||||
|---|---|---|---|---|---|---|---|
| n | Mw | SD | n | Mw | SD | Mann-Whitney-U-Test | |
| abs. VO 2 AerobicThreshold | 27 | 3.2 | 0.5 | 24 | 3.7 | 0.5 | 0.001 |
| abs.VO 2 AnaerobicThreshold | 27 | 3.6 | 0.5 | 23 | 4.2 | 0.7 | 0.001 |
| Tr-distance bike/week | 27 | 190.3 | 65.8 | 24 | 250.2 | 60 | 0.004 |
| Watt AnaerobicThreshold | 27 | 295.6 | 43.5 | 23 | 332.2 | 51.3 | 0.014 |
| rel. VO 2 AerobicT. ml/kg/min | 27 | 42.5 | 7.8 | 24 | 48.2 | 7.9 | 0.017 |
| Watt AerobicThreshold | 27 | 265.6 | 46.6 | 24 | 301.3 | 53.4 | 0.023 |
| %VO 2max AnaerobicThreshold | 27 | 85 | 10.5 | 23 | 90.6 | 9 | 0.026 |
| Tr-time bike | 27 | 7 | 2.2 | 24 | 8.6 | 2.4 | 0.034 |
| Tr-time overall | 27 | 15.7 | 2.7 | 24 | 17.8 | 3.3 | 0.035 |
| BPs AnaerobicThreshold | 27 | 185.2 | 21.5 | 24 | 198.8 | 22.3 | 0.037 |
| Watt max | 27 | 336.7 | 41.9 | 24 | 363.8 | 56.6 | 0.042 |
| %VO 2max AerobicThreshold | 27 | 74.3 | 12.1 | 24 | 81 | 8.9 | 0.046 |
| Tr-time swim/week | 27 | 3.2 | 1.2 | 24 | 3.8 | 1.4 | 0.049 |
| rel.VO 2 AnaerobicThreshold | 27 | 48.4 | 7.2 | 23 | 54.4 | 9.9 | 0.054 |
| BPs Wattmax | 27 | 188.1 | 20.4 | 24 | 199.6 | 19.9 | 0.055 |
| BPs AerobicThreshold | 27 | 178 | 24.6 | 24 | 192.9 | 20.5 | 0.056 |
| abs. VO 2max | 27 | 4.3 | 0.5 | 24 | 4.6 | 0.8 | 0.059 |
| Tr-distance swim/week | 26 | 6.9 | 3.5 | 24 | 8.7 | 4.2 | 0.090 |
| BPs Rest | 27 | 125.4 | 10.8 | 24 | 130.8 | 15.7 | 0.105 |
| Triathlon since years | 27 | 7.4 | 4.8 | 24 | 11 | 7.7 | 0.142 |
| rel. VO 2max ml/min/kg | 27 | 57.3 | 7.5 | 24 | 59.8 | 9.5 | 0.281 |
| HR rest | 27 | 60.3 | 5.5 | 24 | 58.9 | 8 | 0.328 |
| Tr-distance run/week | 27 | 51.4 | 14.6 | 24 | 53.8 | 12.1 | 0.355 |
| HR max | 27 | 179.4 | 10.6 | 24 | 176.2 | 11.5 | 0.385 |
| Watt max/kg | 27 | 4.5 | 0.6 | 24 | 4.7 | 0.7 | 0.433 |
| HR AerobicThreshold | 27 | 150 | 14.8 | 24 | 152.7 | 12.6 | 0.503 |
| IVRT | 27 | 101.3 | 23.3 | 24 | 103.1 | 16.5 | 0.515 |
| HR AnaerobicThreshold | 27 | 162.7 | 12.5 | 23 | 163.7 | 12 | 0.599 |
| BPd Wattmax | 27 | 79.8 | 9.2 | 24 | 79.8 | 10.7 | 0.891 |
| BPdiastol RCP | 27 | 78 | 7.9 | 24 | 78.3 | 11.3 | 0.913 |
| Tr-time run/week | 27 | 4.9 | 1.5 | 24 | 4.9 | 1.2 | 1.000 |
| BPd AerobicThershold | 27 | 78.5 | 9.1 | 24 | 78.8 | 10.8 | 1.000 |
Tr = training. BP = Blood Pressure
BPs AnaerobicThreshold = systolic blood pressure at the anaerobic threshold
BPs Wattmax = systolic blood pressure at the maximum power output time
rel. VO 2RCP = relative oxygen uptake at the anaerobic threshold
rel. VO 2max ml/min/kg = relative maximal oxygen uptake
IVRT = Isovolumetric relaxation time
Watt max = maximum power output
Spiroergometry/physiological performance/blood pressure values
Oxygen uptake, ergometer performance and heart rate with VAT, RCP and at peak capacity are shown in Table 3. Participants with LVM >220g achieved at all thresholds and at maximum level higher power output values. Relative oxygen uptake values were slightly higher in the group with LVM >220g but not significant different at the maximum stage of loading. Spiroergometric maximum oxygen uptake (relVO 2max) was 57.3±7.5 ml/min/kg vs. 59.8±9.5 ml/min/kg (p=n.s.) for LVM <220g vs. >220g, respectively.
Table 3. Heart rate, oxygen uptake and performance in both groups of triathletes with different LVM.
The table is divided in three main blocks: the first block reflects data at the aerobic threshold, the second one at the anaerobic threshold and the last one at the maximum exercise stage.
| LVM <220g | LVM >220g | p-value | |||||
|---|---|---|---|---|---|---|---|
| n | Mv | sd | n | Mv | sd | Mann-Whitney-U-Test | |
| VAT (ventilatory aerobic threshold) | |||||||
| HR | 27 | 150.0 | 14.8 | 24 | 152.7 | 12.6 | 0.503 |
| aVO 2 | 27 | 3.2 | 0.5 | 24 | 3.7 | 0.5 | 0.001 |
| rVO 2 | 27 | 3.2 | 0.5 | 24 | 3.7 | 0.5 | 0.001 |
| %VO 2max | 27 | 74.3 | 12.1 | 24 | 81.0 | 8.9 | 0.046 |
| Watt | 27 | 265.6 | 46.6 | 24 | 301.3 | 53.4 | 0.023 |
| RCP (respiratory compensation point = anaerobic threshold) | |||||||
| HR | 27 | 162.7 | 12.5 | 24 | 163.7 | 12.0 | 0.599 |
| aVO 2 | 27 | 3.6 | 0.5 | 24 | 4.2 | 0.7 | 0.001 |
| rVO 2 | 27 | 48.4 | 7.2 | 24 | 54.4 | 9.9 | 0.054 |
| %VO 2max | 27 | 85.0 | 10.5 | 24 | 90.6 | 9.0 | 0.026 |
| Watt | 27 | 295.6 | 43.5 | 24 | 332.2 | 51.3 | 0.014 |
| Peak capacity | |||||||
| HR | 27 | 179.4 | 10.6 | 24 | 176.2 | 11.5 | 0.385 |
| aVO 2 | 27 | 4.3 | 0.5 | 24 | 4.6 | 0.8 | 0.059 |
| rVO 2 | 27 | 57.3 | 7.5 | 24 | 59.8 | 9.5 | 0.281 |
| Watt | 27 | 336.7 | 41.9 | 24 | 363.8 | 56.6 | 0.042 |
Mv = Mean value;
sd = standard deviation,
aVO2 = absolute oxygen uptake in L/min,
rVO2 = relative oxygen uptake in ml/min/kg,
% point of the overall exercise-test
HR = heart rate,
Watt = power output
Table 4 shows the cut-point analysis for blood pressure values and the probability of development of LVM >220g. BP values over 180mmHg at the aerobic threshold might define the athletes at risk of developing LVM >220g and a possible further cardiac fatigue.
Table 4. Cut-point analysis for the relationship of blood pressure at the aerobic threshold and the probability of LVM >220g.
| BP | Sens.% | Spec. % | PPV % | NPV % | Correct % |
|---|---|---|---|---|---|
| 130 | 100 | 0 | 47.1 | - | 47.1 |
| 140 | 100 | 3.7 | 48 | 100 | 49 |
| 145 | 95.8 | 7.4 | 47.9 | 66.7 | 49 |
| 150 | 95.8 | 11.1 | 48,9 | 75 | 51 |
| 160 | 95.8 | 25.9 | 53.5 | 87.5 | 58.8 |
| 170 | 95.8 | 33.3 | 56.1 | 90 | 62.7 |
| 180 | 87.5 | 40.7 | 56.8 | 78.6 | 62.7 |
| 190 | 62.5 | 55.6 | 55.6 | 62.5 | 58.8 |
| 200 | 45.8 | 66.7 | 55 | 58.1 | 56.9 |
| 210 | 25 | 85.2 | 60 | 56.1 | 56.9 |
| 220 | 12.5 | 100 | 100 | 56.3 | 58.8 |
| 240 | 4.2 | 100 | 100 | 54 | 54.9 |
Left ventricular hypertrophy
According to the values reported by Devereux et al. 40 and Bove et al. 41, we divided the triathletes in two groups: group 1 (LVM >220g) and group 2 (LVM <220g) to assess the possible reasons for left ventricular hypertrophy. The significant differences between the two groups are shown in Table 1 and Table 2. In summary, left ventricular mass (<220g vs. >220g) is associated with significantly different blood pressure values at the anaerobic threshold (185.2±21.5mmHg vs. 198.8±22.3mmHg, p=0.037).
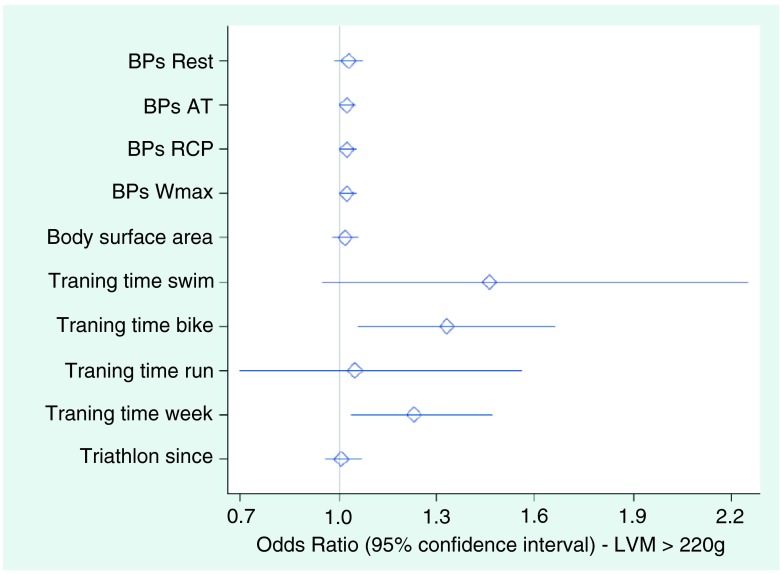
The probability of dependent factors for LV-hypertrophy was calculated by odds ratios ( Table 5). Odds ratios analysis showed a significant relationship between the arterial pressure values during exercise (significant p-values at the aerobic and anaerobic threshold in Table 5). The significant values are bold in Table 5. A further relationship was found between bike training times and overall training times and LVM >220g ( Figure 4). Values above 1.0 show this significant relationship.
Figure 4. Odds ratios analysis for probability of LVM.
This figure is based on the values reported in Table 5. Values above 1.0 show a significant relationship between bike training times and overall training times and LVM >220g.
Table 5. Odds Ratios with 95% confidence intervals (CI) for probability of LVM >220g.
The significant p-values at the aerobic and anaerobic threshold are in bold.
| LVM <220g (n=27) | LVM >220g (n=24) | |||||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | OR | 95%-CI | p-value | ||
| BPs Rest | 125.4 | 10.8 | 130.8 | 15.7 | 1.031 | 0.989 | 1.074 | 0.148 |
| BPs AerobicT | 178.0 | 24.6 | 192.9 | 20.5 | 1.027 | 1.003 | 1.051 | 0.025 |
| BPs AnaerobicT | 185.2 | 21.5 | 198.8 | 22.3 | 1.027 | 1.002 | 1.052 | 0.034 |
| BPs Wattmax | 188.1 | 20.4 | 199.6 | 19.9 | 1.027 | 1.000 | 1.054 | 0.050 |
| BSA | 194.7 | 10.6 | 198.7 | 17.9 | 1.02 | 0.98 | 1.06 | 0.328 |
| Tr-time swim | 3.2 | 1.2 | 3.8 | 1.4 | 1.46 | 0.95 | 2.25 | 0.081 |
| Tr-time bike | 7.0 | 2.2 | 8.6 | 2.4 | 1.33 | 1.06 | 1.66 | 0.015 |
| Tr-time run | 4.9 | 1.5 | 4.9 | 1.2 | 1.05 | 0.70 | 1.56 | 0.823 |
| Tr-time overall | 15.7 | 2.7 | 17.8 | 3.3 | 1.23 | 1.04 | 1.47 | 0.019 |
| Triathlon since years | 14.5 | 9.0 | 15.7 | 10.3 | 1.01 | 0.96 | 1.07 | 0.654 |
mean = mean value.
BSA = Body Surface Area
Tr = Training
BPs = systolic blood pressure
AerobicT = Aerobic threshold
AnaerobicT = Anaerobic Threshold
Anthropometry parameters, training habits, echocardiography and spirometry data of 51 healthy male triathletes who completed an Ironman 70.3 or an Ironman full distance race are shown. D = Ironman Distance, LD Long distance / MD = Ironman 70.3, A= Age, G= gender, We = Weight, H = Height, BSA = body surface area, %BF = %body fat, Tts = Training time swim, Tds= Training distance swim, Ttb= Training time bike, Tdb= Training distance bike, Ttr= Training time run, Tdr= Training distance run, Ttt= Total training time, Ts= Triathlon since, sbT= Sport before Triathlon, sbTs= Sport before triathlon since, HRmax= Heart Rate at exertion, HRVAT= Heart rate at ventilator anaerobic threshold, HRRCP= Heart rate at respiratory compensation point, HRLAM= Heart rate at Lactate threshold 4,0mmol/l (Mader), HRLAD= Heart rate at Lactate threshold according to Dickhuth, HRLAI= Heart rate at first nonlinear increase of blood lactate, Abs VO2max= Maximum oxygen uptake L/min, Abs. VO2VAT= Oxygen uptake at ventilatory anaerobic threshold L/min, Abs. VO2RCP= Oxygen uptake at respiratory compensation point L/min, Rel VO2max= Maximum oxygen uptake relative to body weight mlL/min/kg, Rel VO2VAT= Oxygen uptake at ventilator anaerobic threshold relative to body weight mlL/min/kg, Rel VO2RCP= Oxygen uptake at respiratory compensation point relative to body weight mlL/min/kg, %VO2maxAT= Oxygen uptake at ventilator anaerobic threshold as percentage of maximum oxygen uptake, %VO2maxRCP= Oxygen uptake at respiratory compensation point as percentage of maximum oxygen uptake, VEmax= Maximum minute ventilation, O2HFmax= Maximum O2 Pulse, RERmax= Maximum Respiratory Exchange Ratio, BLCmax= Blood lactate concentration at exertion, Wmax= Maximum ergometer performance (Watt), WAT= Ergometer performance at ventilator anaerobic threshold, WRCP= Ergometer performance at respiratory compensation point, WLAM= Ergometer performance at Lactate threshold 4,0mmol/l (Mader), WLAD= Ergometer performance at Lactate threshold according to Dickhuth, WLAI= Ergometer performance at first nonlinear increase of blood lactate, Wmax/kg= Maximum ergometer performance in relation to body weight (Watt/kg), BPsRest= Systolic blood pressure at rest, BPdRest= Diastolic blood pressure at rest, BPsVAT= Systolic blood pressure at ventilator anaerobic threshold, BPdVAT= Diastolic blood pressure at ventilator anaerobic threshold, BPsRCP= Systolic blood pressure at respiratory compensation point, BPdRCP= Diastolic blood pressure at respiratory compensation point, BPsWmax= Systolic blood pressure at exertion, BPdWmax= Diastolic blood pressure at exertion, Ao= Aortic root dimension, LA= Left atrial diameter, IVSd= Inter-ventricular septum in diastole, LVIDd= Left ventricular internal diameter in diastole, LVPWd= Left ventricular posterior wall in diastole, %IVS= Percentage of thickening of the inter-ventricular septum form diastole to systole, LVIDs= Left ventricular internal diameter in systole, %FS= Fractional shortening, LVPWs= Left ventricular posterior wall in systole, LV Mass (ASE) = left ventricular mass according to the ASE recommended formula, LVEDV MOD A4c ml= Left ventricular end-diastolic volume calculated according to the Simpson method in apical 4 chamber view, LVESV MOD A4c ml= Left ventricular end-systolic volume calculated according to the Simpson method in apical 4 chamber view, LVEF MOD A4c= Left ventricular ejection fraction calculated according to the Simpson method in apical 4 chamber view, SV Mod A4C ml= Stroke volume calculated according to the Simpson method in apical 4 chamber view, EF Biplan= Left ventricular ejection fraction calculated according to the Simpson method in apical 4 and 2 chamber view, LVEDV MOD BP ml= Left ventricular end-diastolic volume calculated according to the Simpson method in apical 4 and 2 chamber view, LVESV MOD BP ml= Left ventricular end-systolic volume calculated according to the Simpson method in apical 4 and 2 chamber view, MV E Max m/s= Mitral valvular E-Wave m/s, MV A Max m/s= Mitral valvular A-Wave m/s, MV E/A Ratio= Mitral valvular E/A Ratio, LVOT Vmax= Left ventricular outflow tract maximum velocity in PW-Doppler, HR Rest= Heart frequency at rest, RWT= Relative wall thickness, RV parasternal= Right ventricular diameter in the parasternal long axis, RVDA = Right venrticular area in diastole, RVSA= Right ventricular area in systole, RV FAC= Area change fraction RVSAx100/RVDA.
Discussion
The most interesting finding of this study is that myocardial hypertrophy depends on exercise-induced arterial hypertension. This confirms the results described by Douglas et al. 13 and Longas-Tejero et al. 42, who found a hypertensive response to exercise in eight of 37 healthy athletes (18 soccer players, 12 mountain climbers and seven canoeists). In this cited study, athletes with EIAH showed higher LVM (205g/m 2) compared to those without exaggerated blood pressure response to exercise (143g/m 2). There is no consensus about the value of systolic blood pressure that constitutes EIAH 6. According to our study, it seems that a systolic BP value >180mmHg at the aerobic threshold indicates exercise-induced arterial hypertension. So far, the boundaries for EIAH have never been estimated. In this study, we have chosen the aerobic threshold as the measuring point because the majority of the triathlete’s training is carried out at this level. When the hypertensive BP value is reached, we should analyse whether a careful low dosage treatment might be beneficial (for example with ACE inhibitors or AT 1-blockers). The goal of such therapy would be to cut the blood pressure peaks (bouts) during training or competitions and avoid an increase of stiffness of the aorta 43 or LV-hypertrophy in people at risk. Raised BP bouts can lead to pathological enlargement of atrial dimensions in athletes ( Figure 2 and Figure 5). Enlargement of the left atrium may lead to atrial fibrillation and higher activity of electric circuits. There are no clear statements or guidelines regarding the role of EIAH in the daily practice of sports medicine 44. This manuscript may encourage a discussion about this important issue. The possible impact of EIAH on cardiac structures in triathletes is shown in Figure 2. A specific case of cardiac remodelling is shown in Figure 5. In this Figure are shown normal heart cavities of a triathlete with EIAH in 2011 and massive atrial enlargement in 2014. Exercise-induced hypertension was often discussed in the 1990s 4, 5, 7 reflecting the results of the Framingham Study 5. The negative role of EIAH in non-athletic men is relatively clear 6, but the impact on athletes needs to be discussed and the “pathological range” of EIAH should be evaluated. Exercise-induced hypertension promotes myocardial hypertrophy 4 and increases cardiovascular risks 7 in normotensive men. Athletes with EIAH are in similar way “persons at risk” and may develop a pathological cardiac chamber enlargement and atrial fibrillation, but have less “cardiovascular risk” because of the healthier life style and the positive impact of sport in the development of arteriosclerotic complications.
Figure 5. Pathological cardiac remodelling (especially of right and left atrium) of a 48 years old triathlete with EIAH.
In 2011, the participant showed a normal size of the right and left atrium. An atrial enlaragement occured 2014 (arrows) after a period of high intensity training (echocardiography in 4 chamber view during atrial fibrillation). Even after cardioversion (2014) into sinus rhythm (7 days in sinus rhythm) he retains larger atrial cavities as in 2011.
Cardiac adaptation to exercise, left ventricular hypertrophy and sudden cardiac death
The specific endurance training of triathletes leads to physiological changes of performance parameters 45 and also results in changes in cardiac function or heart structures 46. This adaptation is linked to the nature and magnitude of the physical exercise 31. The physiological adaptation is a “harmonic increase in size” of a healthy heart caused by physical activity 47. The term “athlete’s heart” 9, 31 has been known since 1899 48. Cardiovascular adaptations to exercise have been systematically defined according to the type of endurance training.
Concentric hypertrophy in triathletes has already been described 49, 50. Douglas et al. 50 suggested that athletes develop hypertrophy possibly due to the systolic blood pressure increase under exercise, which could be explained by the frequency of the training. Diastolic function was shown to be normal under those conditions.
In the present study, odds ratio analysis showed a significant relationship of myocardial thickening to exercise-induced blood pressure. It can be assumed that training over an extended period with exercise-induced blood pressure elevation can lead to hypertrophy in a similar way to that found in pathological conditions with arterial hypertension. Concentric remodelling was found in 26 cases and concentric hypertrophy in 21 cases. Concentric remodelling and concentric hypertrophy occurs more often in male athletes 31. Different authors have concluded that strength training predominantly leads to concentric hypertrophy and endurance training to eccentric hypertrophy 28. In this study concentric remodelling was observed most frequently. George et al. 31 reported that the expected pattern of eccentric enlargement was replaced by a pattern of concentric or symmetric enlargement in groups of highly trained athletes. Generally, the adaptation of the cardiac mass seems not to depend on the type of conditioning 31. In 1989, Douglas et al. 13 published a comparison of 36 triathletes with 17 normal controls and 15 arterial hypertension patients. They determined that triathletes undergo cardiac adaptations similar to pressure overload of the left ventricle and they described a relative wall thickness (RWT) of 0.41. The authors concluded that the relation of myocardial hypertrophy to exercise training is strengthened further by exercise blood pressure. However, unlike the pathologic hypertrophy resulting from hypertension, the triathlete’s heart would show “normal” diastolic LV-function.
The difference between triathletes and racing cyclists is that the thriathlete’s training does not only take place under strength/endurance conditions, but also under running conditions. Modified strength training also results in different changes in the cardiac structures of triathletes in comparison to professional racing cyclists 51. In a study including 14 professional cycle racers it was shown that the left ventricular muscle mass resulted in eccentric hypertrophy compared to concentric hypertrophy as also shown in our study. Therefore, the functional changes found in the cardiac structures for triathletes resemble the changes in runners 52. Sudden cardiac death of athletes is more common in male athletes 27, 53. The different causes of sudden cardiac death are silent coronary disease 54, hypertrophic cardiomyopathy 55 and arrhythmogenic right ventricular cardiomyopathy 56 ( Table 6). Maron et al. 55 described hypertrophic cardiomyopathy as common cause of sudden cardiac death (36%) in young athletes and 8% were presented with indeterminate LV-hypertrophy (possible HCM). The probability of the negative role of hypertrophy in athletes 10 and the problem of qualitative and quantitative relevance are under discussion 57, 58.
Table 6. Causes of sudden cardiac death in young athletes <35 years in %.
| Causes of sudden cardiac
death |
Maron 2007 55 | Corrado 2003 56 | Solberg 2010 54 | Marijon 2011 27 |
|---|---|---|---|---|
| Aortic rupture | 2.2 | 1.8 | 4.3 | 2 |
| Aortic stenosis/cong. HD | 1.8 | 4.3 | 6 | |
| ARVC | 4 | 22 | 4 | |
| Channelpathies (QT, WPW) | 3 | 1.8 | 8.7 | 12 |
| Coronary artery anomalies | 24 | 11 | 3.3 | |
| Coronary disease | 3 | 18 | 48 | 6 |
| Dilatative CM | 2 | 1.8 | 4 | |
| Hypertrophic CM | 36 | 1,8 | 4.3 | 10 |
| MVP | 4 | 7.3 | 2 | |
| Myocarditis | 5.4 | 9 | 22 | 4 |
| Possible HCM | 8 | 4 | ||
| Riva muscle bridge | 2.2 | 3.6 | 2 | |
| Unclear | 1.8 | 36 | ||
| n = 1049 | n = 55 | n = 22 | n = 50 |
cong. HD = Congenital Heart Disease. ARVC = Arrhythmogenic Right Ventricular Cardiomyopathy. QT = QT-Syndrome: Romano-Ward Syndrome, and Jervell-Lange-Nielsen-Syndrome. WPW: Wolff-Parkinson-White Syndrome. CM = Cardiomyopathy. HCM = hypertrophic Cardiomyopathy. MVP = Mitral Valve Prolapse.
Left ventricular “fatigue”
Some papers have reported that excessive endurance training may cause negative remodelling of cardiac structures 15, 59. Predominantly marathons and Ironman-distance triathlons can cause a transient overload of the right ventricle 59. Fibrosis of the left ventricle in older runners was described as a possible cause of death 24, 25. Numerous investigations regarding the increase in bio-markers (mainly Troponin cTnI and NTproBnP) in runners of marathons 60 as well as triathlon 61 competitions have been conducted. A significant increase in bio-markers after the race was found in all those studies. Uniformly, this was considered as a proof of possible injuries to the heart muscle 62. Overall, the increase in bio-markers in athletes with intensive muscle work should not necessarily be interpreted as heart specific 63, because it also depends on the athlete’s weight 64 and may be associated with the myolysis (creatine kinase up to 10000 U/l after long-term running) 65. The discussion on this issue is ongoing 17, 20, 66.
Limitations and future directions
The cross-sectional design of this study does not allow a causality regarding the negative role of EIAH in athletes to be established. Although our data suggest that left ventricular hypertrophy might be related to EIAH beyond the normal exercise-induced adaptation, confirmatory longitudinal work is necessary.
The results of this study support the authors’ subjective impression of daily practice and engagement in sports medicine over 15 years. We observe rhythm disorders in many cyclists and triathletes around the age of 50, and many of them have elevated blood pressure values during exercise. The probability of increasing stiffness of the aorta as an aging process supported by EIAH remains to be discussed. The present study attempts to analyse the probability of LVM and EIAH and should stimulate further follow-up investigations. It is a very important aim to prevent a potential fibrosis of the left atrium 67 or left ventricular myocardium in athletes 16 in order to avoid “negative cardiac remodelling” induced by exercise and to preserve the positive effects of physical activity 12. Approximately two million people participate in long-distance races in the United States annually 68 and there are only limited data regarding their exercise-induced blood pressure, which might be one of the main factors triggering cardiac events 69, 70.
Conclusions
The relationship between myocardial hypertrophy and arterial blood pressure during exercise remains an open issue. The literature 13, 42 seems to suggest a clear relationship. The relevance of EIAH has to be examined in the future in consideration of serious reports 8, 57, 58. The cited authors suggested the isolated (without EIAH) exercise-induced hypertrophy as a substrate for sudden cardiac death or rhythm disorders. EIAH may enhance the “physiological” exercise-induced hypertrophy in a pathological way. Accordingly, the blood pressure values or EIAH should be thoroughly examined during routine or pre-event check-up.
The long training-times for Ironman-distances of triathletes with EIAH can lead to additional enlargement of the heart cavities ( Figure 2) and may trigger possible sudden cardiac death during triathlon competitions 71.
There is strong evidence that athletes have higher incidence of atrial fibrillation and bradyarrhythmias increasing with age 21– 23. We don’t know the definitive reasons for this, but EIAH and LVM might be one of the factors. Cases of early death in individual cases due by myocardial fibrosis are possible 24, 25. However, the general prevalence or incidence of EIAH in athletes is unknown. The problem of EIAH seems to be linked more to competitive athletes with vigorous training and mainly to males. It is known that low-intensity training 72 and aerobic exercise have a positive impact on blood pressure lowering 73– 75. The hypertensive or non-hypertensive response to exercise seems to be related to hereditary factors 76, to aging or to the individual arterial stiffness 43. It is crucial to define the people at risk and possibly start therapy 77. In our daily practice we treat the athletes at risk with low-dose ACE-inhibitors or AT 1-blockers before training or competition. The dosage should be tested using an exercise test. Possible therapies for the prevention of fibrosis or atrial fibrillation have already been discussed 23, 78.
Further international, prospective, longitudinal studies on possible negative cardiac remodelling caused by EIAH and sport should be conducted. These studies could help to avoid the adverse effects of sport in people at risk. The overlap of EIAH and exercise-induced hypertrophy has the potential for increased QT-dispersion 79 and is a ticking clock for cardiac fatigue especially for middle aged men. Independent of all the competitive sporting activities with an enormous importance for hobby-athletes, media and industry, physical activity in general population is of fundamental importance 11, 12.
Consent
All athletes provided written informed consent to voluntary testing of the performance and using the data for the study. Triathletes underwent their annual medical check-up or examination for planning their training, which would have been carried out in clinical routine in any case. A special approval by an ethics committee was not mandatory because of the study independent character of the examinations. The examinations were a part of clinical routine support of the triathletes. Pharmaceutical interventions in the triathletes were not affected by the study.
Data availability
figshare: Data of exercise-induced arterial hypertension in triathletes, doi: http://dx.doi.org/10.6084/m9.figshare.1010160 80
Acknowledgements
Thanks Frank Blumberg for the graphical preparation of Figure 1 and Figure 2.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
v1; ref status: indexed
References
- 1.Verdecchia P, Schillaci G, Borgioni C, et al. : Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97(1):48–54 10.1161/01.CIR.97.1.48 [DOI] [PubMed] [Google Scholar]
- 2.Mensah GA, Pappas TW, Koren MJ, et al. : Comparison of classification of the severity of hypertension by blood pressure level and by World Health Organization criteria in the prediction of concurrent cardiac abnormalities and subsequent complications in essential hypertension. J Hypertens. 1993;11(12):1429–1440 [DOI] [PubMed] [Google Scholar]
- 3.McLenachan JM, Henderson E, Morris KI, et al. : Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N Engl J Med. 1987;317(13):787–792 10.1056/NEJM198709243171302 [DOI] [PubMed] [Google Scholar]
- 4.Gottdiener JS, Brown J, Zoltick J, et al. : Left ventricular hypertrophy in men with normal blood pressure: relation to exaggerated blood pressure response to exercise. Ann Intern Med. 1990;112(3):161–166 10.7326/0003-4819-112-3-161 [DOI] [PubMed] [Google Scholar]
- 5.Lauer MS, Levy D, Anderson KM, et al. : Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham Heart Study. Ann Intern Med. 1992;116(3):203–210 10.1097/00042752-199207000-00025 [DOI] [PubMed] [Google Scholar]
- 6.Schultz MG, Otahal P, Cleland VJ, et al. : Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am J Hypertens. 2013;26(3):357–366 10.1093/ajh/hps053 [DOI] [PubMed] [Google Scholar]
- 7.Allison TG, Cordeiro MA, Miller TD, et al. : Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol. 1999;83(3):371–375 10.1016/S0002-9149(98)00871-6 [DOI] [PubMed] [Google Scholar]
- 8.Hart G: Exercise-induced cardiac hypertrophy: a substrate for sudden death in athletes? Exp Physiol. 2003;88(5):639–644 10.1113/eph8802619 [DOI] [PubMed] [Google Scholar]
- 9.Lauschke J, Maisch B: Athlete’s heart or hypertrophic cardiomyopathy? Clin Res Cardiol. 2009;98(2):80–88 10.1007/s00392-008-0721-2 [DOI] [PubMed] [Google Scholar]
- 10.Fagard R: Athlete’s heart. Heart. 2003;89(12):1455–1461 10.1136/heart.89.12.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warburton DE, Nicol CW, Bredin SS: Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danaei G, Ding EL, Mozaffarian D, et al. : The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058 10.1371/journal.pmed.1000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas PS: Cardiac considerations in the triathlete. Med Sci Sports Exerc. 1989;21(5 Suppl):S214–218 [PubMed] [Google Scholar]
- 14.Michelsen S, Knutsen KM, Stugaard M, et al. : Is left ventricular mass in apparently healthy, normotensive men correlated to maximal blood pressure during exercise? Eur Heart J. 1990;11(3):241–248 [DOI] [PubMed] [Google Scholar]
- 15.Oxborough D, Birch K, Shave R, et al. : “Exercise-induced cardiac fatigue”--a review of the echocardiographic literature. Echocardiography. 2010;27:1130–1140 [DOI] [PubMed] [Google Scholar]
- 16.Wilson M, O’Hanlon R, Prasad S, et al. : Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol (1985). 2011;110(6):1622–1626 10.1152/japplphysiol.01280.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Keefe JH, Harshal RP, Lavie CJ, et al. : Potential adverse cardiovascular effects from excessive endurance exercise. Mayo Clin Proc. 2012;87(6):587–595 10.1016/j.mayocp.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blair SN, Kohl HW, Gordon NF, et al. : How much physical activity is good for health? Annu Rev Public Health. 1992;13:99–126 10.1146/annurev.pu.13.050192.000531 [DOI] [PubMed] [Google Scholar]
- 19.Eaton SB, Eaton Iii SB: An evolutionary perspective on human physical activity: implications for health. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):153–159 10.1016/S1095-6433(03)00208-3 [DOI] [PubMed] [Google Scholar]
- 20.Leischik R: Endurance Sport and Cardiac Injury. Polish Heart Journal, Kardiologia Polska.Ahead of Print. 2014–04–08.2014. Reference Source [DOI] [PubMed] [Google Scholar]
- 21.Andersen K, Farahmand B, Ahlbom A, et al. : Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. 2013;34(47):3624–3631 10.1093/eurheartj/eht188 [DOI] [PubMed] [Google Scholar]
- 22.Calvo N, Brugada J, Sitges M, et al. : Atrial fibrillation and atrial flutter in athletes. Br J Sports Med. 2012;46(Suppl 1):i37–43 10.1136/bjsports-2012-091171 [DOI] [PubMed] [Google Scholar]
- 23.Leischik R, Littwitz H: Slow recovery of the right and left ventricular deformation after conversion from atrial fibrillation. American Journal of Sports Science. 2014;2:13–16 Reference Source [Google Scholar]
- 24.Whyte G, Sheppard M, George K, et al. : Post-mortem evidence of idiopathic left ventricular hypertrophy and idiopathic interstitial myocardial fibrosis: is exercise the cause? BMJ Case Rep. 2009;2009: bcr08.2008.0758. 10.1136/bcr.08.2008.0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowe WJ: A world record marathon runner with silent ischemia without coronary atherosclerosis. Chest. 1991;99(5):1306–1308 10.1378/chest.99.5.1306 [DOI] [PubMed] [Google Scholar]
- 26.Leischik R, Spelsberg N, Littwitz H, et al. : Physiological performance and cardiac function in female ironman-triathletes. American Journal of Sports Science. 2014;2:41–47 Reference Source [Google Scholar]
- 27.Marijon E, Tafflet M, Celermajer DS, et al. : Sports-related sudden death in the general population. Circulation. 2011;124(6):672–681 10.1161/CIRCULATIONAHA.110.008979 [DOI] [PubMed] [Google Scholar]
- 28.Pluim BM, Zwinderman AH, van der Laarse A, et al. : The athlete’s heart a meta-analysis of cardiac structure and function. Circulation. 2000;101(3):336–344 10.1161/01.CIR.101.3.336 [DOI] [PubMed] [Google Scholar]
- 29.Bray MS, Hagberg JM, Perusse L, et al. : The human gene map for performance and health-related fitness phenotypes: the 2006–2007 update. Med Sci Sports Exerc. 2009;41(1):35–73 10.1249/01.mss.0000233789.01164.4f [DOI] [PubMed] [Google Scholar]
- 30.Alvarez R, Terrados N, Ortolano R, et al. : Genetic variation in the renin-angiotensin system and athletic performance. Eur J Appl Physiol. 2000;82(1–2):117–120 10.1007/s004210050660 [DOI] [PubMed] [Google Scholar]
- 31.George KP, Wolfe LA, Burggraf GW: The ‘athletic heart syndrome’. A critical review. Sports Med. 1991;11(5):300–330 10.2165/00007256-199111050-00003 [DOI] [PubMed] [Google Scholar]
- 32.Adams TD, Yanowitz FG, Fisher AG, et al. : Heritability of cardiac size: an echocardiographic and electrocardiographic study of monozygotic and dizygotic twins. Circulation. 1985;71(1):39–44 10.1161/01.CIR.71.1.39 [DOI] [PubMed] [Google Scholar]
- 33.Cumming DC, Wall SR, Galbraith MA, et al. : Reproductive hormone responses to resistance exercise. Med Sci Sports Exerc. 1987;19(3):234–238 10.1249/00005768-198706000-00009 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan NM, Deveraux RB, Miller HS, Jr: 26th Bethesda conference: recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities. Task Force4: systemic hypertension. J Am Coll Cardiol. 1994;24(4):885–888 [DOI] [PubMed] [Google Scholar]
- 35.Roalstad MS: Physiologic testing of the ultraendurance triathlete. Med Sci Sports Exerc. 1989;21(5 Suppl):S200–204 10.1249/00005768-198910001-00013 [DOI] [PubMed] [Google Scholar]
- 36.Lang RM, Bierig M, Devereux RB, et al. : Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108 10.1016/j.euje.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 37.Nagueh SF, Appleton CP, Gillebert TC, et al. : Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193 10.1093/ejechocard/jep007 [DOI] [PubMed] [Google Scholar]
- 38.Beaver WL, Wasserman K, Whipp BJ: A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985). 1986;60(6):2020–2027 [DOI] [PubMed] [Google Scholar]
- 39.Wasserman K, Whipp BJ, Koyl SN, et al. : Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35(2):236–243 [DOI] [PubMed] [Google Scholar]
- 40.Devereux RB, Alonso DR, Lutas EM, et al. : Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458 10.1016/0002-9149(86)90771-X [DOI] [PubMed] [Google Scholar]
- 41.Bove KE, Rowlands DT, Scott RC: Observations on the assessment of cardiac hypertrophy utilizing a chamber partition technique. Circulation. 1966;33(4):558–568 10.1161/01.CIR.33.4.558 [DOI] [PubMed] [Google Scholar]
- 42.Longas Tejero MA, Casanovas Lenguas JA: [Prevalence of hypertensive response to exercise in a group of healthy young male athletes. Relationship with left ventricular mass and prospective clinical implications]. Rev Esp Cardiol. 1996;49(2):104–110 [PubMed] [Google Scholar]
- 43.Laurent S, Cockcroft J, Van Bortel L, et al. : Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 44.Pelliccia A, Fagard R, Bjørnstad HH, et al. : Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study group of Sports Cardiology of the Working group of Cardiac Rehabilitation and Exercise Physiology and the Working group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(14):1422–1445 10.1093/eurheartj/ehi325 [DOI] [PubMed] [Google Scholar]
- 45.Suriano R, Bishop D: Physiological attributes of triathletes. J Sci Med Sport. 2010;13(3):340–347 10.1016/j.jsams.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 46.Atchley AE, Jr, Douglas PS: Left ventricular hypertrophy in athletes: morphologic features and clinical correlates. Cardiol Clin. 2007;25(3):371–382, v 10.1016/j.ccl.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 47.Dickhuth H, Hipp A, Niess A, et al. : Differenzialdiagnostik der physiologischen Herzhypertrophie (Sportherz). Deutsche Zeitschrift für Sportmedizin. 2001;52:205–210 Reference Source [Google Scholar]
- 48.Henschen S: Skilanglauf und Skiwettlauf. Eine medizinische Sportstudie. Mitt Med Klin Upsala. 1899;2:74 [Google Scholar]
- 49.Douglas PS, O'Toole ML, Katz SE, et al. : Left ventricular hypertrophy in athletes. Am J Cardiol. 1997;80(10):1384–1388 [DOI] [PubMed] [Google Scholar]
- 50.Douglas PS, O'Toole ML, Hiller WD, et al. : Left ventricular structure and function by echocardiography in ultraendurance athletes. Am J Cardiol. 1986;58(9):805–809 10.1016/0002-9149(86)90358-9 [DOI] [PubMed] [Google Scholar]
- 51.Bekaert I, Pannier JL, Van de Weghe C, et al. : Non-invasive evaluation of cardiac function in professional cyclists. Br Heart J. 1981;45(2):213–218 10.1136/hrt.45.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fagard R, Aubert A, Staessen J, et al. : Cardiac structure and function in cyclists and runners. Comparative echocardiographic study. Br Heart J. 1984;52(2):124–129 10.1136/hrt.52.2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maron BJ, Pelliccia A: The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114(15):1633–1644 10.1161/CIRCULATIONAHA.106.613562 [DOI] [PubMed] [Google Scholar]
- 54.Solberg EE, Gjertsen F, Haugstad E, et al. : Sudden death in sports among young adults in Norway. Eur J Cardiovasc Prev Rehabil. 2010;17(3):337–341 10.1097/HJR.0b013e328332f8f7 [DOI] [PubMed] [Google Scholar]
- 55.Maron BJ, Thompson PD, Ackerman MJ, et al. : Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115(12):1643–1655 10.1161/CIRCULATIONAHA.107.181423 [DOI] [PubMed] [Google Scholar]
- 56.Corrado D, Basso C, Rizzoli G, et al. : Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42(11):1959–1963 10.1016/j.jacc.2003.03.002 [DOI] [PubMed] [Google Scholar]
- 57.McCann GP, Muir DF, Hillis WS: Athletic left ventricular hypertrophy: long-term studies are required. Eur Heart J. 2000;21(5):351–353 10.1053/euhj.1999.1783 [DOI] [PubMed] [Google Scholar]
- 58.Shephard RJ: The athlete's heart: is big beautiful? Br J Sports Med. 1996;30(1):5–10 10.1136/bjsm.30.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.La gerche A, Burns AT, Mooney DJ, et al. : Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33(8):998–1006 10.1093/eurheartj/ehr397 [DOI] [PubMed] [Google Scholar]
- 60.Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. : Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation. 2006;114(22):2325–2333 10.1161/CIRCULATIONAHA.106.647461 [DOI] [PubMed] [Google Scholar]
- 61.Rifai N, Douglas PS, O'Toole M, et al. : Cardiac troponin T and I, echocardiographic [correction of electrocardiographic] wall motion analyses, and ejection fractions in athletes participating in the Hawaii Ironman Triathlon. Am J Cardiol. 1999;83(7):1085–1089 [DOI] [PubMed] [Google Scholar]
- 62.Shave RE, Dawson E, Whyte G, et al. : Evidence of exercise-induced cardiac dysfunction and elevated cTnT in separate cohorts competing in an ultra-endurance mountain marathon race. Int J Sports Med. 2002;23(7):489–494 10.1055/s-2002-35069 [DOI] [PubMed] [Google Scholar]
- 63.Tanindi A, Cemri M: Troponin elevation in conditions other than acute coronary syndromes. Vasc Health Risk Manag. 2011;7:597–603 10.2147/VHRM.S24509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shave R, George KP, Atkinson G, et al. : Exercise-induced cardiac troponin T release: a meta-analysis. Med Sci Sports Exerc. 2007;39(12):2099–2106 10.1249/mss.0b013e318153ff78 [DOI] [PubMed] [Google Scholar]
- 65.Kim YJ, Shin YO, Lee JB, et al. : The effects of running a 308km ultra-marathon on cardiac markers. Eur J Sport Sci. 2014;14 Suppl 1:S92–97 10.1080/17461391.2011.654267 [DOI] [PubMed] [Google Scholar]
- 66.Leischik R: Ugly duckling or Nosferatu? - marathon running and cardiovascular function How to screen the athletes?2014. Reference Source [Google Scholar]
- 67.Burstein B, Nattel S: Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–809 10.1016/j.jacc.2007.09.064 [DOI] [PubMed] [Google Scholar]
- 68.Kim JH, Malhotra R, Chiampas G, et al. : Cardiac arrest during long-distance running races. N Engl J Med. 2012;366(2):130–140 10.1056/NEJMoa1106468 [DOI] [PubMed] [Google Scholar]
- 69.Shaper AG, Wannamethee G, Walker M: Physical activity, hypertension and risk of heart attack in men without evidence of ischaemic heart disease. J Hum Hypertens. 1994;8(1):3–10 [PubMed] [Google Scholar]
- 70.Kurl S, Laukkanen JA, Rauramaa R, et al. : Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32(9):2036–2041 10.1161/hs0901.095395 [DOI] [PubMed] [Google Scholar]
- 71.Harris KM, Henry JT, Rohman E, et al. : Sudden death during the triathlon. Jama. 2010;303(13):1255–7 10.1001/jama.2010.368 [DOI] [PubMed] [Google Scholar]
- 72.Rogers MW, Probst MM, Gruber JJ, et al. : Differential effects of exercise training intensity on blood pressure and cardiovascular responses to stress in borderline hypertensive humans. J Hypertens. 1996;14(11):1369–1375 [DOI] [PubMed] [Google Scholar]
- 73.Kelley GA, Sharpe Kelley K: Aerobic exercise and resting blood pressure in older adults: a meta-analytic review of randomized controlled trials. J Gerontol A Biol Sci Med Sci. 2001;56(5):M298–303 10.1093/gerona/56.5.M298 [DOI] [PubMed] [Google Scholar]
- 74.Whelton SP, Chin A, Xin X, et al. : Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136(7):493–503 10.7326/0003-4819-136-7-200204020-00006 [DOI] [PubMed] [Google Scholar]
- 75.Cornelissen VA, Fagard RH: Effects of endurance training on blood pressure, blood pressure–regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46(4):667–75 10.1161/01.HYP.0000184225.05629.51 [DOI] [PubMed] [Google Scholar]
- 76.Walker AJ, Bassett DR, Jr, Duey WJ, et al. : Cardiovascular and plasma catecholamine responses to exercise in blacks and whites. Hypertension. 1992;20(4):542–8 10.1161/01.HYP.20.4.542 [DOI] [PubMed] [Google Scholar]
- 77.Vanhees L, Fagard R, Lijnen P, et al. : Effect of antihypertensive medication on endurance exercise capacity in hypertensive sportsmen. J Hypertens. 1991;9(11):1063–8 10.1097/00004872-199111000-00013 [DOI] [PubMed] [Google Scholar]
- 78.Ehrlich JR, Hohnloser SH, Nattel S: Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27(5):512–8 10.1093/eurheartj/ehi668 [DOI] [PubMed] [Google Scholar]
- 79.Zoghi M, Gurgun C, Yavuzgil O, et al. : QT dispersion in patients with different etiologies of left ventricular hypertrophy: the significance of QT dispersion in endurance athletes. Int J Cardiol. 2002;84(2–3):153–9 [DOI] [PubMed] [Google Scholar]
- 80.Leischik R, Spelsberg N, Niggermann H: Data of exercise-induced arterial hypertension in triathletes. Figshare. 2014. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]