Abstract
In this review we propose that the fear extinction model can be used as an experimental tool to cut across symptom dimensions of multiple anxiety disorders to enhance our understanding of the psychopathology of these disorders, and potentially facilitate the detection of biomarkers for the same. We evaluate evidence for this proposition from studies examining the neurocircuitry underlying fear extinction in rodents, healthy humans and clinical populations. Furthermore, we assess the potential use of the fear extinction model to predict vulnerability for anxiety and treatment response, and to improve existing, or lead to developing novel, treatments. Finally, we suggest potential directions for future research that will help to further validate extinction as a biomarker for anxiety across diagnostic categories, and that will help to bridge the gap between basic neuroscience and clinical practice.
Fear is an adaptive response that has evolved to provide protection from potential harm in the environment. But when fear is excessive and disproportionate to the situation, it could lead to the development of an anxiety disorder. The World Health Organization [1] predicts that by the year 2020 anxiety and depressive disorders combined will be the second most burdensome illness in the world, with current lifetime prevalence rates for anxiety disorders being 25% in developed countries [2]. Even more concerning, despite an increase in the rate of individuals receiving treatment, there has been no decrease in prevalence rates [3]. Traditional drug treatments for anxiety, such as benzodiazepines or selective serotonin reuptake inhibitors, offer symptom relief but relapse is very common post-treatment. Cognitive behavioral therapy (CBT) is the most effective evidence-based psychological treatment for anxiety disorders. A major component of CBT is exposure therapy, which involves gradually exposing the individual to the feared stimulus or outcome in the absence of any danger. However, CBT has limitations including high treatment dropout and refusal rates, and relapse following treatment termination does occur [4;5]. Furthermore it is time consuming and costly. This has prompted the comment that “a therapeutic impasse” has been reached, and that further progress in enhancing current treatments will only be made with a deeper understanding of the neural mechanisms underlying fear and its reduction [5].
In line with this view, the National Institute of Mental Health (NIMH) has recently proposed that mental health disorders should be viewed as disorders of brain circuitry, biomarkers of which may be detected using current and emerging tools in clinical neuroscience. It is the hope of NIMH that conceptualizing mental health disorders in this way will foster advances in the early detection of vulnerability to such disorders, as well as advances in predicting treatment response. Furthermore, it is hoped that treatments will eventually be tailored to meet the specific idiosyncrasies (both biological and psychological) of the individual. To meet this aim, NIMH proposes that rather than adhering to strict diagnostic categories (and determining research samples using this method) research should focus on identifying the fundamental underlying mechanisms of dysfunction across the various mental health disorders [6]. To this end, anxiety disorders would be conceptualized not as distinct diagnostic categories but as disorders of fear circuitry, and/or disorders of fear extinction/inhibition.
Advances in neuroimaging have permitted increasing specificity in the investigation of the neural basis of anxiety disorders. Such studies have investigated neural activity in individuals with anxiety disorders during a variety of conditions, including resting state, symptom provocation, and cognitive activity in a range of mental tasks. In general, research has implicated the amygdala, hippocampus, insula, anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (vmPFC) as regions of interest across anxiety disorders [7;8;9]. However, results regarding the specific neural regions implicated, as well as the direction of difference in neural activity in such regions, have been disparate both across and within diagnostic categories [8]. The disparity is likely due, at least in part, to the fact that different studies employ widely different tasks during functional neuroimaging that tap into a variety of functions (i.e., emotion detection, affect regulation, fear processing, fear inhibition, etc).
In this review we will give a brief overview of the neural circuits of fear conditioning and extinction and summarize some recent findings on fear extinction in psychiatric disorders. We will then go on to propose that fear extinction is a good candidate model that can be used to examine the fundamental underlying mechanism of dysfunction across anxiety disorders. To support this proposition, we provide an overview of the advantages of using the fear extinction model to this end. We then assess how well the fear extinction model meets the criteria outlined by NIMH regarding its potential as a means to identify a fundamental biomarker of anxiety that can be used as a diagnostic tool, to predict vulnerability and treatment response, and to translate laboratory findings into clinical practice.
Why Fear Extinction?
In the laboratory, fear is acquired when a neutral conditioned stimulus (CS, e.g., a light or tone) is paired with an aversive unconditioned stimulus (US, such as a mild shock). After several such pairings the subject learns that the CS predicts the US and subsequent presentation of the CS elicits a variety of fear responses, including freezing in the rodent and skin conductance responses (SCR) in the human. Once acquired, fear to the CS can be extinguished by repeatedly exposing the subject to the CS in the absence of any aversive outcome. During extinction training the subject's fear responses gradually decline, and when tested the following day the subject typically exhibits long-term extinction recall.
The fear conditioning and extinction model has been used extensively to examine the neurobiology underlying fear processes in non-human animals and more recently in humans. We argue that the fear extinction model is suited to examine potential biomarkers of anxiety for several reasons. Firstly, anxiety is characterized above all as a failure to appropriately inhibit, or extinguish, fear [10]. Individuals with anxiety disorders avoid fear-provoking situations and stimuli, or endure them by employing a range of different “safety behaviors” that are designed to protect the individual from harm. Avoidance and the use of safety behaviors prevent the individual from challenging his/her unrealistic beliefs and so prevent fear extinction. Hence, the fear extinction model provides a direct measure of what is widely accepted to be a central underlying dysfunction in anxiety disorders. As such, the measurement of neural activity during extinction may provide a sensitive measure of the neural circuitry involved in the maintenance of anxiety disorders.
Secondly, it was noted above that exposure therapy is a dominant component of CBT. Even though anxiety disorders are characterized by dysfunctional cognitive processes (e.g., overestimation of the probability and cost of negative outcomes), research has shown that exposure therapy without explicit cognitive intervention is just as effective, and invokes just as much cognitive change, as comprehensive CBT that combines behavioral with cognitive interventions [11]. Exposure therapy was based on the extinction procedure used in animal studies of fear inhibition. Thus in addition to potentially detecting the neural basis for the underlying dysfunction in anxiety disorders, extinction can also be used as a valid model of the most effective psychological treatment for anxiety disorders.
A third advantage of the fear extinction model is that comparison of animal studies with human neuroimaging studies suggests considerable similarity between the neural structures involved in extinction in the rodent and in the human, as reviewed in more detail below. The cross-species validity of the extinction model permits rodents to be used to address questions that are not possible to address using human subjects, such as trialing the effects of novel drugs on extinction and subsequent relapse, with the assurance that these findings are readily translatable to the human population.
The fear acquisition and fear extinction circuitries
The neurobiology of fear acquisition is well characterized in rodents and humans [12]. Briefly, is widely accepted that the basolateral complex of the amygdala (BLA) is the main neural structure in which information about the CS and the US converge. This has been supported by studies in rodents using lesions, pharmacological inactivation, electrophysiology, and drug antagonists, which together have demonstrated that interfering with normal functioning of the BLA disrupts the acquisition and expression of fear conditioning [13]. There is also evidence from rodent studies that the prelimbic (PL) division of the medial prefrontal cortex (mPFC) is involved in regulating the expression of learned fear. Inactivation of PL reduces the expression of cued and contextual fear [14], while microstimulation of PL increases conditioned freezing and reduces extinction [15], prompting the assertion that PL regulates fear expression via activating the amygdala. This is supported by findings that PL neurons potentiate their response to a tone CS, and that extinction failure is associated with a persistence of PL neuronal responding following extinction training [16]. Using functional magnetic resonance imaging (fMRI), it has been determined that humans show robust increases in activity in the amygdala and dorsal anterior cingulated (dACC, which appears to be functionally analogous to the PL) during fear acquisition and expression [17;18]. More recently, it has been demonstrated in healthy humans that cortical thickness of the dACC is positively correlated with SCR during fear conditioning acquisition, and that dACC activation during acquisition of conditioning increases in response to a CS paired with shock (CS+), relative to a CS not paired with shock (CS-) [19]. It should be noted that a recent study has failed to replicate the correlation between the cortical thickness of the dACC and fear acquisition levels in healthy subjects [20]. In addition to the amygdala and dACC, other regions have been also implicated in fear expression in humans including the insula, thalamus, and brainstem regions such as the PAG.
Fear extinction, on the other hand, involves interactions between the infralimbic (IL) region of the mPFC, the BLA, and the hippocampus. It is proposed that when an extinguished cue is presented in the extinction training context the hippocampus activates the IL, which in turn activates inhibitory interneurons in the BLA that inhibit the output neurons in the central amygdala (CeA), thus preventing conditioned responding. In contrast, when the extinguished cue is presented in a context other than the extinction training context, the hippocampus does not activate IL and CeA activity is not inhibited, thus conditioned responding returns [21].
Subsequent analysis of the neurobiology underlying fear extinction in humans using fMRI has revealed remarkable preservation of this circuitry across species. Specifically, earlier fMRI studies demonstrated that the amygdala exhibits increased activation to the CS+ relative to the CS- during early extinction training, and this activation decreases across extinction training [22]. It was also demonstrated that the amygdala and orbitofrontal cortex (part of the vmPFC) exhibit increased activity during extinction training for an olfactory cue [23], and another study demonstrated differences in amygdala and hippocampal activity during extinction training in comparison to a non-extinguished control group [17]. Later studies focused on the neurobiology of extinction recall. These studies have consistently demonstrated that extinction recall is associated with increased activity in the vmPFC [18;24;25], and it has been proposed that the vmPFC is the human homologue of the rat IL. Furthermore, it has been shown using structural MRI that extinction recall is positively correlated with the thickness of the vmPFC [20;26;27].
Several studies have also demonstrated evidence for increased hippocampal activity during extinction recall [17;25]. Furthermore, one study reported increased hippocampal and vmPFC activity during recall in the extinction context, but not in the original conditioning context [24]. These findings support the notion that the hippocampus modulates when and where extinction is expressed depending on the contextual information. Hence, there is much evidence to support the notion that a distinct neural circuitry involving interactions between the amygdala, vmPFC, and hippocampus underlies the ability to extinguish fear, and that this circuitry has been preserved across evolution (Figure 1).
Figure 1.
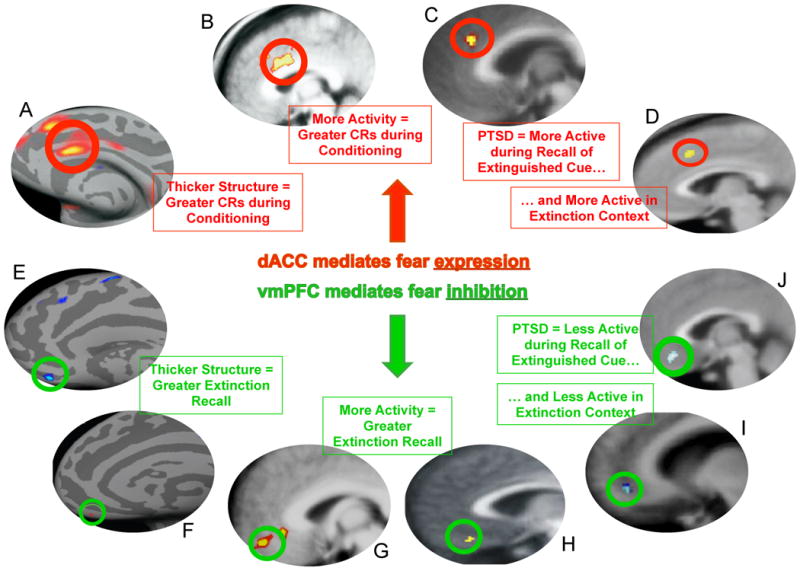
Summary of neuroimaging research demonstrating that the dACC and vmPFC regulate fear expression and fear inhibition, and evidence that these areas are dysfunctional in anxiety disorders. A; B [19]; C [32]; D [37]; E [24]; F [26] G [32]; H [22]; I [32]; J [37].
Is fear extinction, or the fear extinction circuitry, altered in clinically anxious populations?
It is widely accepted that anxiety is maintained due to a failure to appropriately extinguish fear. This section addresses two questions: 1) Do clinically anxious populations exhibit alterations in the neural circuitry that mediates normal extinction and, 2) does the extinction model provide a sensitive measure of this dysfunction in clinical populations?
Evidence from Neuroimaging Studies using Symptom Provocation
In general, the neural structures thought to mediate fear extinction have also been identified as structures of interest in symptom provocation studies, in which fear-relevant stimuli are presented to anxious and non-anxious participants. For example, one study reported decreases in medial frontal gyrus blood flow in PTSD participants exposed to trauma reminders compared to trauma-exposed controls, and medial frontal gyrus blood flow was inversely correlated with changes in amygdala blood flow [28]. This study also reported a positive correlation between changes in amygdala blood flow and symptom severity, and a negative correlation between changes in medial frontal gyrus blood flow and symptom severity. Heightened amygdala [29;30] and diminished vmPFC activity [30] have also been reported in PTSD subjects whilst viewing fearful faces during fMRI, compared to trauma-exposed controls.
Similar findings have been reported for specific phobia as for PTSD. For example, spider phobics exhibited increased amygdala, insula, ACC, and dorsolateral PFC activation when viewing spider-related compared to neutral images, a finding that was absent in non-phobic controls [31]. One recent study [32] examined brain activity using fMRI in spider phobics who were asked to voluntarily up- and down-regulate their emotions elicited by spider imagery, and by non-phobic but generally aversive imagery, using a cognitive reappraisal strategy. Spider phobics exhibited increased dACC and insula activity, but reduced vmPFC activity, when attempting to regulate emotional responses to spider imagery, while no such changes were observed during regulation towards aversive, phobic-irrelevant, imagery. This suggests that the same neural circuitry may regulate both automatic fear-inhibition tasks (i.e., laboratory fear extinction, where no explicit instruction to regulate emotions is given) and effortful fear-inhibition tasks (i.e., where an explicit instruction to regulate emotions is given). Furthermore, this suggests that there may be a deficit in this circuitry in spider phobic populations.
Evidence from Neural Connectivity Studies
More recent studies have used imaging techniques to measure the strength of connectivity between the vmPFC and amygdala and correlate this with anxious traits. For example, using diffusion tensor imaging it was shown that the strength of the reciprocal connections between the amygdala and PFC predicts trait levels of anxiety, such that the weaker the pathway, the greater the level of trait anxiety [33]. A later study reported that amygdala resting state activity was positively coupled to vmPFC activity in low anxious subjects, and negatively coupled to vmPFC activity in high-anxious subjects [34]. Together, these studies suggest that dysfunctions in vmPFC-amygdala connectivity may mediate susceptibility to anxiety.
Evidence from Neuroimaging Studies on Treatment Outcome
Another way to address the question of whether dysfunctions in the neural circuitry of extinction underlie anxiety disorders is to determine whether successful recovery from anxiety is correlated with changes in this neural circuitry. Indeed, one session of intensive exposure therapy has been shown to reduce amygdala, dACC, and insula hyperactivation in response to viewing phobic-relevant stimuli in individuals with spider phobia, as measured two weeks post-exposure [35]. Another study reported reduced hyperactivity in the ACC and insula post-CBT treatment for spider therapy in comparison to a waitlist control group [36]. Post-CBT decreases in ACC blood flow, and increases in vmPFC blood flow, have been reported to occur in Panic Disorder [37]. These effects do not appear to be restricted to CBT, as similar neural changes have also been reported to occur following pharmacological treatment for social phobia [38]. This latter study reported a comparable decrease in regional cerebral blood flow in the amygdala and hippocampus following successful treatment with citalopram or CBT. This suggests that successful pharmacological and psychological treatments may in some cases target the same underlying dysfunction in the neural circuitry underlying extinction.
Evidence from Psychophysiological and Behavioral Studies
Several studies have directly measured fear inhibition ability in anxious populations using laboratory extinction tasks. These studies have consistently demonstrated that anxious patients exhibit deficits in fear extinction. For example, individuals with panic disorder exhibit larger SCRs during extinction training and rate the extinguished CS as more unpleasant, despite showing no differences from healthy controls in SCRs or valance ratings during or following conditioning [39]. Enhanced resistance to extinction has also consistently been reported in the PTSD population, as indexed by larger differential SCRs to the CS+ versus the CS- [40], larger heart rate responses [41], and stronger on-line valence and expectancy ratings [42] in comparison to trauma-exposed or healthy control groups. We have reported that individuals with PTSD exhibit deficits in extinction recall, despite there being no differences in conditioning or within-session extinction training, as indexed by enhanced SCR during recall but not conditioning or extinction training [43;44]. Furthermore, we have also reported a negative correlation between symptom severity in PTSD and extinction recall [44]. One recent study reported enhanced conditioning combined with impairments in fear extinction in PTSD compared to trauma-exposed controls, and there was a positive correlation between symptom severity and both the enhanced conditioning and impaired extinction [45]. PTSD impairment in fear inhibition has also been reported using a model of inhibition that isolates the inhibitory component of extinction [46], and this effect was not detected in a cohort of depressed people [47].
Evidence from Neuroimaging Studies using Fear Extinction
The data discussed above indicate that anxiety disorders are associated with deficiencies in the neural circuitry of extinction. However, these deficiencies in neural circuitry have not been thoroughly examined in the context of fear inhibition. Indeed, only few studies have investigated the neural activity during fear extinction in anxious patients. The first study to investigate this using PET demonstrated that fear acquisition is associated with increased resting metabolic activity in the left amygdala, and fear extinction is associated with decreased resting metabolic activity in the vmPFC, in PTSD subjects compared to healthy controls [48]. We extended these results to examine extinction recall the day after extinction training using fMRI. We found that PTSD participants exhibited reduced activity in the vmPFC and hippocampus, but heightened dACC activity during extinction recall [45]. There was also a positive correlation between the magnitude of extinction recall with vmPFC and hippocampal activity across all participants. This suggests that hyperactivity within the dACC, and hypoactivity within the vmPFC, may contribute to the impairment in extinction observed in PTSD. A subsequent study from our group demonstrated that during extinction recall PTSD subjects showed both reduced vmPFC activity, and heightened dACC activity, in response to the extinction context [49], suggesting that hyperactivity within the dACC and hypoactivity within the vmPFC may also mediate an inability to use contextual cues to predict safety (Figure 2). The function of the neural circuitry of extinction recall across the different anxiety disorders beyond PTSD remains to be examined, however the studies described suggest that investigating neural activity during fear extinction tasks may be a useful means of understanding the psychopathology underlying anxiety disorders.
Can the extinction model be used to predict vulnerability to anxiety disorders?
It is possible that impairments in extinction and alterations in the corresponding neural circuitry are a consequence of anxiety, rather than being central to the underlying pathology. Results from studies that have addressed this question using twins have been mixed. For example, one study reported that genes accounted for 35-45% of the variance associated with conditioning and extinction rates [50], suggesting that dysfunctions in extinction may be transmitted via heredity. In contrast, in a small sample of monozygotic twins, we reported impaired extinction retention in PTSD participants relative to their monozygotic, non-trauma exposed co-twin, and relative to non-PTSD trauma-exposed participants and their co-twin [43]. That is, we found no evidence to support the pre-existing presence of impaired extinction retention in PTSD participants.
If it is the case that impairments in fear extinction are secondary to the development of PTSD, then there should be no evidence of impairment in extinction prior to symptom onset, and the extinction model would not be able to predict subsequent development of anxiety or treatment response. To our knowledge, only one study has directly assessed the potential of the extinction model to predict vulnerability to anxiety disorders [51], in which the extinction of SCR and corrugator electromyogram (EMG) responses to an aversively conditioned stimulus (colored circles) in firefighters during cadet training was examined. Participants were reassessed for PTSD 24 months post-training following trauma exposure. Reduced extinction of EMG responses during extinction training at the time of cadet training accounted for 31% of the variance associated with subsequent PTSD symptomatology two years post-cadet training, supporting the premise that impaired extinction may be a precursor to anxiety, and that early detection of this impairment may be used to predict vulnerability to anxiety.
No studies have investigated whether alterations in the functional activity of the neural circuitry involved in extinction can predict subsequent development of PTSD. However, studies that have investigated differences in brain morphology using structural MRI have consistently demonstrated that PTSD is associated with decreased hippocampal volume, although, it remains controversial whether or not alterations in brain morphology are a precursor to or a consequence of PTSD development. At least one study supports the former notion that a smaller hippocampal volume is predictive of PTSD development [52]. This study demonstrated that monozygotic twins discordant for trauma exposure (in whom the trauma-exposed twin developed PTSD) exhibited smaller hippocampal volumes than monozygotic twins discordant for trauma exposure (in whom the trauma-exposed twin did not develop PTSD). Furthermore, symptom severity in the PTSD participants was negatively correlated with their own hippocampal volume as well as that of their co-twin. On the other hand, a later study using the same subjects found reduced gray matter density in the ACC of PTSD subjects compared to their combat-unexposed co-twins, and to combat-exposed twins without PTSD and their co-twins, suggesting that neural abnormalities in this region may be a consequence of PTSD [53]. A more recent study examined resting state activity using PET in dizygotic twins, one of whom had PTSD, compared to twins, one of whom had been exposed to trauma but did not develop PTSD [54]. This study reported higher resting state in the dACC and midcingulate cortex in participants with PTSD and their co-twins, relative to trauma-exposed participants and their co-twins, suggesting that alterations in the neural circuitry underlying conditioning may be a risk factor for subsequent development of PTSD. Clearly, more work is required to elucidate exactly how much pre-existing dysfunctions in extinction ability and the neural circuitry underlying extinction contribute to subsequent development of PTSD and other anxiety disorders.
Can the extinction model be used to predict treatment response?
As noted previously, even the most successful treatments for anxiety disorders are associated with relapse, and a certain percentage fail to respond at all. At this point, the factors that predict responsiveness to treatment remain largely elusive. One possibility is that the extinction model may be employed to predict the likelihood of responding to CBT-based treatments that primarily use extinction procedures. To our knowledge, no studies have investigated extinction ability prior to treatment and correlated the magnitude of extinction retention with the success of treatment response. However, two studies have examined neural activity in the circuitry mediating extinction prior to treatment. One examined resting metabolic activity in participants with OCD prior to receiving behavioral therapy or fluoxetine treatment [55]. In the behavioral therapy group positive treatment response was correlated with higher pre-treatment metabolism in the left frontal orbital cortex, while the reverse was the case for positive response to the medication treatment (i.e., treatment response was negatively correlated with pre-treatment left frontal orbital cortex metabolism). The second study examined the pattern of neural activation in response to fearful and neutral faces in individuals with PTSD prior to CBT [56]. They found that poor treatment response, as measured 6 months post-treatment, was associated with greater amygdala and ventral ACC region activation. Although these findings are preliminary, they suggest that pre-treatment measurement of neural activity in the extinction circuitry could provide important information regarding the intensity, duration, and type of therapy required to prevent, or reduce, subsequent relapse.
Can the extinction model be used to investigate ways of improving current treatments OR to test the efficacy of novel therapies?
As exposure therapy is based on extinction, laboratory investigations of the neurobiology of extinction in the rodent and the human have proved fruitful in providing ways of enhancing CBT for anxiety disorders. Without doubt, the most successful of these investigations has been that of the effect of d-cycloserine (DCS) on the extinction of conditioned fear. DCS was initially demonstrated to enhance extinction of conditioned fear in rats, and to reduce stress-precipitated relapse [57;58]. Since then, DCS has been shown to enhance exposure therapy in humans with a range of anxiety disorders [59]. Investigations of DCS and extinction have influenced a new wave of thinking in pharmacotherapy for anxiety disorders, whereby rather than developing drugs that merely mask the symptoms of anxiety (and often interfere with the effectiveness of psychological therapies) scientists are investigating the potential of drugs that can be used to augment the underlying therapeutic mechanisms of CBT. Numerous novel pharmacological enhancers of extinction are currently being investigated in preclinical and clinical research [60].
Other studies have focused on directly stimulating the neural structures that have been proposed to be dysfunctional in anxiety. Repetitive transcranial magnetic stimulation (rTMS) is a procedure in which cortical neurons are stimulated in a noninvasive way without the side-effects often associated with electroconvulsive therapy. rTMS of the lateral prefrontal cortex has been shown to reduce compulsive urges in people with OCD as measured eight hours post-stimulation, an effect that was not found when the midoccipital region was stimulated [61]. A more recent study reported that 10 sessions of high frequency stimulation to the dorsolateral PFC significantly reduced PTSD symptoms up to 3 months post-treatment [62]. This suggests that mere stimulation of neural regions underlying extinction can be therapeutic.
Results from rTMS studies raise the possibility that extinction procedures may be combined with brain stimulation in order to enhance the effectiveness of such treatments. Indeed, this has already been investigated at a preclinical level in rodents. It has been shown that combining CS presentations with IL stimulation reduced conditioned freezing during extinction training in rats. Furthermore, this reduction in freezing remained evident during extinction recall, an effect that was absent when the stimulation was not paired with the CS presentation [63]. As rTMS is already being examined in clinical trials, this provides the potential for translating this finding from the rodent to clinical populations. Importantly, it should be noted that the recent advances made in enhancing current treatments for anxiety reviewed here all stemmed from preclinical investigations of extinction in non-human animals, thus illustrating the validity of extinction as a model of treatment.
Caveats of the Fear Extinction Model
Several limitations to the fear extinction model, and its proposed underlying neural circuitry, have been raised. For example, it was recently reported that PTSD was less prevalent in Vietnam War veterans who had experienced damage to the vmPFC and/or the amygdala; a finding that appears contradictory to the model suggesting that anxiety results from a failure of vmPFC inhibition over amygdala activity [64]. It is important to note that the average lesion area in this study was not limited to the vmPFC; large portions of the surrounding anterior, lateral and dorsal prefrontal cortices were included. Future studies with focal lesions that are more confined to the vmPFC should be conducted to specifically examine the relationship between vmPFC lesions and PTSD prevalence.
Another limitation to the extinction model is that neuroimaging studies of anxiety have not always produced results that are consistent with the current neural model of fear extinction. For example, some studies have reported no difference in amygdala activation during the presentation of trauma-related reminders in PTSD subjects [65;66]. In addition, some studies have reported hyper-activation in the vmPFC during symptom provocation in PTSD subjects compared to healthy [67] and trauma-exposed [68] controls, or no difference in vmPFC activation between groups [69]. The reason underlying this discrepancy is unclear, but it may be due to symptom provocation and fear extinction tasks measuring slightly different functions (i.e., fear processing versus fear inhibition).
Finally, it is clear that the extinction model does not capture all aspects of clinical anxiety; in particular cognitive components like anticipatory anxiety [9]. Similarly, it does not capture all aspects of any given anxiety disorder. For example, the underlying pathogenesis of OCD, which is characterized by intrusive thoughts and rituals, is not well-modeled by extinction and may be regulated by an entirely different neural circuit to that of extinction [9]. Despite these limitations, the previous data indicate that the fear extinction model can be used to understand the psychopathology of anxiety disorders, and to determine similarities and differences between the various diagnostic categories.
Future Directions
In this review, we have argued that a) the extinction model is advantageous because it models the commonly accepted underlying dysfunction in anxiety disorders, it models the most widely used treatment of anxiety, and there is much evidence for the cross-species validity of the model, b) a basic neural circuitry that regulates fear extinction has been identified via rodent and human research, and there is moderate evidence to suggest that this neural network is dysfunctional in anxiety disorders, c) there is preliminary evidence to suggest that the extinction model may potentially be used to detect vulnerability to anxiety disorders, and to predict likelihood of treatment response, and, d) there is considerable evidence to suggest that the extinction model can be used as a means to investigate ways of enhancing existing treatments for anxiety. We argue that these factors make the extinction model, and its underlying neural circuitry, an excellent candidate for a biomarker of anxiety disorders, and a useful tool to understand the psychopathology of anxiety. However, there is further work to be done to help validate the extinction model as a biomarker of anxiety disorders across diagnostic categories, and to ensure that the model continues to lead to developments in treatment for anxiety. We have summarized what we believe to be the most important future directions below.
Investigate the Neural Circuitry underlying Fear Extinction Retention across the Diagnostic Categories
In order to use the extinction model as a biomarker for anxiety disorders across the board, it is clear that more research needs to be conducted to clarify whether or not similar dysfunctions exist in the neural circuitry of extinction in populations with anxiety disorders other than PTSD, as measured using imaging techniques during laboratory extinction tasks. In order to foster comparisons across these studies, it will be important for future research to employ a standardized extinction paradigm. Earlier studies of fear extinction have examined fear conditioning and extinction acquisition in a one-day paradigm, and have not examined subsequent extinction retention [23;42;48]. We argue that later studies that have examined extinction retention in a two-day paradigm may be more clinically relevant (and may be more sensitive to detecting behavioral and neural differences between clinical and healthy populations) given that the impairment in fear extinction in anxious populations may be specific to the retention of extinction memories over time, rather than in the acquisition of such memories. This is supported by recent studies that have reported no differences in fear conditioning or extinction acquisition, but significant differences in extinction retention, in clinical populations [43;44], and by the findings that anxious populations tend to show recovery of extinguished fear over time.
Focus on less Studied Factors and Populations
If future research were to focus on anxiety as a dimension rather than the specific categories, this may also open up the possibility of examining historically under-studied factors and populations that may be critical to furthering advancements in treatment. For example, very little research has examined the role of sex hormones on fear extinction, or sex differences in extinction, despite the fact that women are twice as likely to develop anxiety compared to men [2]. Indeed, sex differences in emotional memories have consistently been documented in rodents and humans [70]. Furthermore, stress differentially affects fear acquisition in male and female rodents, and this appears to be modulated by estradiol [71]. In addition, damage to the vmPFC differentially affects men and women, with unilateral right damage producing severe defects in men while unilateral left damage produced severe defects in women [72]. Emerging evidence in both rodents and naturally cycling women suggests that fluctuations in the menstrual cycle alters extinction retention [73;74], and furthermore, that exogenous estradiol administration may be a novel enhancer of extinction retention [73;75]. These recent findings strongly suggest that further research should examine the effect of hormones on fear extinction, or at least consider sex as an important variable.
Another relatively neglected factor is the effect of sleep on extinction. Rodent research has demonstrated that fear conditioning reduces rapid eye movement (REM) sleep, while fear extinction restores normal levels of REM sleep [76], except when extinction recall is disrupted [77]. In healthy humans sleep enhances extinction recall [78]. Furthermore, sleep disturbance is associated with poor treatment outcome in PTSD, and activity in the amygdala and PFC modulate sleep [79]. However, the relationship between sleep and extinction is not well defined, particularly in human clinical samples. Future investigation into the effect of sleep on extinction may lead to new insights into methods of augmenting exposure therapy by modulating sleep.
Using a dimensional diagnostic system to recruit research samples may also make it easier to examine fear extinction in special populations, including children, adolescents, and the elderly. Preclinical research in the rodent has revealed that developing rats exhibit relapse-resistant extinction that depends on different molecular and neural circuitry to those which mediate extinction in the adult rat [80]. Extinction also appears to be altered during adolescence, a time during which the PFC undergoes rapid reorganization, and is associated with high rates of relapse and altered molecular activity within the IL [81;82]. Studies that examine extinction across development may identify the specific point during development at which the dysfunction in neural circuitry emerges (which may be several years prior to overt symptom onset). For example, it has recently been shown that temperament measured at 4 months of age is predictive of orbital and vmPFC structure at 18 years of age [83]. This kind of longitudinal research may lead to insight into how to protect against the development of anxiety.
Conclusion
The advent and the continuous development of neuroimaging tools has allowed us to form a solid base of knowledge regarding the neural dysfunctions across anxiety disorders. The recent explosion of interest in understanding the neural substrates of fear extinction in rodents reflects the importance of the neurobiology of fear inhibition and its relevance to anxiety disorders. Research using healthy human subjects has confirmed the cross-species validity of this model, and has demonstrated that the behavioral and neural mechanisms underlying fear extinction have been strongly preserved across evolution. Our current knowledge base from these two fields should allow us to move forward and merge the two together in a translational fashion using a multimodal across-species approach. While this experimental model is not perfect as it does not capture all aspects of any given anxiety disorder, we argue that it is a good model to understand the neural circuits underlying learning not to fear per se, which then can be directly translated to understanding these disorders and can eventually be useful to improving treatment outcomes for these patients.
Acknowledgments
This work was supported by Institute of Mental Health Grants K01MH080346 and 1R01MH081975-01 (MGH subcontract) to MRM, and an American Australian Association Neurological Fellowship to BMG.
Footnotes
Disclosures: BMG reports no competing interests. MRM has received fees from MircoTransponder Inc. in a project not related to the present work.
References
- 1.World Health Organization: WHO. The world health report 2001: mental health: new understanding, new hope. Geneva: World Health Organisation; 2001. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005b;352:2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann SG, Smits JAJ. Cognitive-behavioural therapy for adult anxiety disorders: a meta- analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychcol Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 7.Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Curr Opin Psychiatry. 2008;22:96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- 8.Etkin A, Wagner TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto MW, Smits JAJ, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(Supp5):34–41. [PubMed] [Google Scholar]
- 11.Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 13.LeDoux J. The amygdala. Curr Biol. 2007;17:868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognit Affect Behav Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- 18.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortexin fear expression. Biol Psychiatry. 2007a;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 20.Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cort. 2011 doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quirk GJ. :56–72. [Google Scholar]
- 22.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 23.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 2004;7:1145–1153. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 24.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007b;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Milad MR, Quinn BT, Pitam RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. NeuroReport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- 28.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 29.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 30.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 31.Straube T, Mentzel HJ, Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4:257–267. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MJ, Whalen PJ. The structural integrity of an amygdala–prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JM, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cort. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goossens L, Sunaert S, Peeters R, Griez EJ, Schruers KR. Amygdala hyperfunction in phobic fear normalizes after exposure. Biol Psychiatry. 2007;62:1119–1125. doi: 10.1016/j.biopsych.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Straube T, Glauer M, Dilger S, Mentzel HJ. Effects of cognitive behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, Ohnishi T, Matsuda H, Yasuda A, Sato A, Diksic M, Kubokia T. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage. 2006;33:218–226. doi: 10.1016/j.neuroimage.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 39.Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: enhanced resistance to extinction. J Abnorm Psychol. 2007;116:612–617. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- 40.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 41.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 42.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LS, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse related post- traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez- Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Arch Gen Psychiatry. 2003;60:702–708. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- 51.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 52.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2001;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat- related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin LM, Lasko NB, Macklin ML, Karpf RD, Milad MR, Orr SP; Goetz JM, Fischman AJ, Rauch SL, Pitman RK. Resting metabolic activity in cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brody AL, Saxena S, Schwartz JM, Stoessel PW, Maidment K, Phelps ME, Baxter LR. FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Res. 1998;84:1–6. doi: 10.1016/s0925-4927(98)00041-9. [DOI] [PubMed] [Google Scholar]
- 56.Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med. 2008;38:555–561. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- 57.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ledgerwood L, Richardson R, Cranney J. d-cycloserine and the Facilitation of Extinction of Conditioned Fear: Consequences for Reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 59.Norberg MM, Krystal JH, Tolin DF. A Meta-analysis of D-Cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Graham BM, Langton JM, Richardson R. Pharmacological enhancement of fear reduction: preclinical models. Br J Pharmacol. 2011 doi: 10.1111/j.14765381.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenberg BD, George MS, Martin JD, Benjamin J, Schlaepfer TE, Altemus M, Wassermann EM, Post RM, Murphy DL. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry. 1997;154:867–869. doi: 10.1176/ajp.154.6.867. [DOI] [PubMed] [Google Scholar]
- 62.Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanhã C, Ferreira-Santos E, Meleiro A, Corchs F, Zaghi S, Pascual-Leone A, Fregni F. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 64.Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 67.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta MA, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 68.Zubieta J, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I. Medial frontal cortex involvement in PTSD symptoms: a SPECT study. J Psychiatr Res. 1999:259–264. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 69.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 70.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 71.Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J Neurosci. 2010;30:16188–16196. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- 73.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang Y, Yang C, Liang Y, Yeh C, Huang C. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus. :1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- 76.Wellman LL, Yang L, Tang X, Sanford LD. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep. 2008;31:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 77.Deschaux O, Thevenet A, Spennato G, Arnaud C, Moreau JL, Garcia R. Low-frequency stimulation of the hippocampus following fear extinction impairs both restoration of rapid eye movement sleep and retrieval of extinction memory. Neuroscience. 2010;170:92–98. doi: 10.1016/j.neuroscience.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 78.Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- 79.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010;67:279–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 81.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: Effects of d-cycloserine. Neuropsychopharmacology. 2010;35:2134–2142. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cort. 2011;21:530–538. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- 83.Schwartz CE, Kunwar PS, Greve DN, Moran LR, Viner JC, Covino JM, Kagan J, Stewart SE, Snidman NC, Vangel MG, Wallace SR. Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age. Arch Gen Psychiatry. 2010;67:78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]


