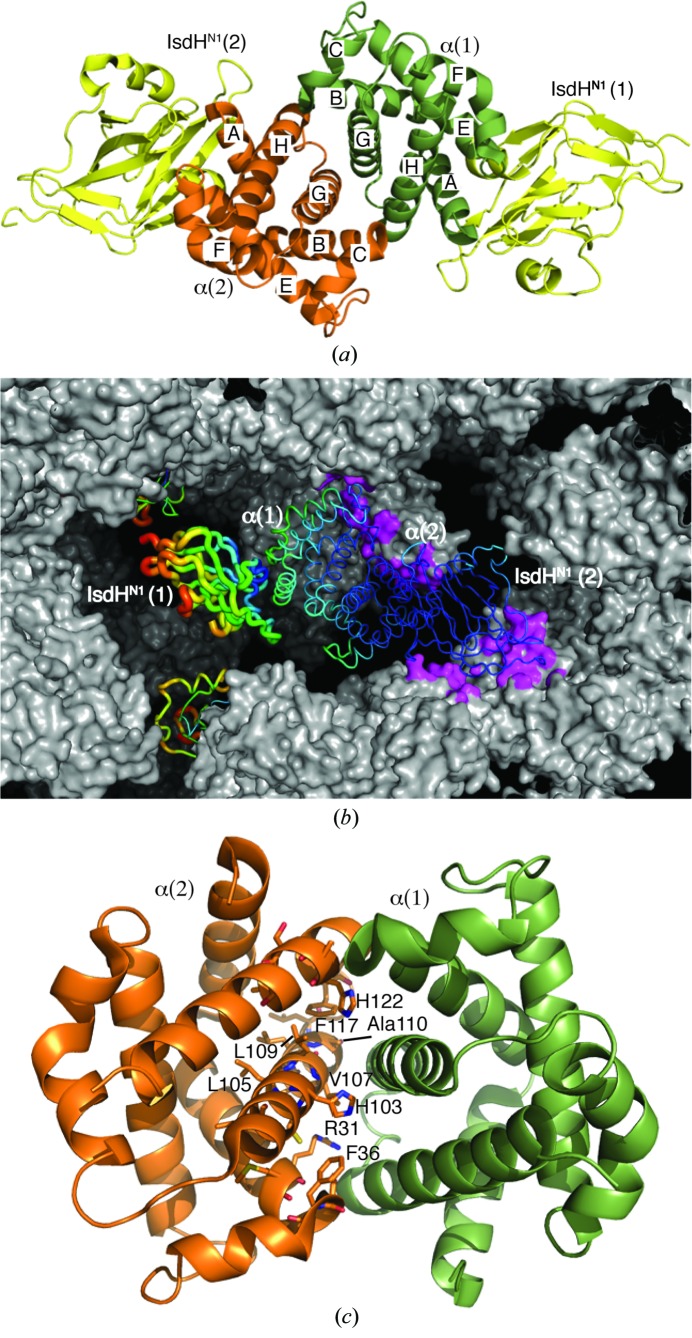
The α chain of haemoglobin (α) has been crystallized as a homodimer bound to a domain from the S. aureus haemoglobin receptor iron-regulated surface determinant H (IsdH). This is the first X-ray crystal structure that captures the weak self-association interface of α.
Keywords: α-haemoglobin, homodimer, HbH, Hb Bart’s, IsdHN1, Staphylococcus aureus
Abstract
Adult haemoglobin (Hb) is made up of two α and two β subunits. Mutations that reduce expression of the α- or β-globin genes lead to the conditions α- or β-thalassaemia, respectively. Whilst both conditions are characterized by anaemia of variable severity, other details of their pathophysiology are different, in part owing to the greater stability of the β chains that is conferred through β self-association. In contrast, α subunits interact weakly, and in the absence of stabilizing quaternary interactions the α chain (α) is prone to haem loss and denaturation. The molecular contacts that confer weak self-association of α have not been determined previously. Here, the first structure of an α2 homodimer is reported in complex with one domain of the Hb receptor from Staphylococcus aureus. The α2 dimer interface has a highly unusual, approximately linear, arrangement of four His side chains within hydrogen-bonding distance of each other. Some interactions present in the α1β1 dimer interface of native Hb are preserved in the α2 dimer. However, a marked asymmetry is observed in the α2 interface, suggesting that steric factors limit the number of stabilizing interactions that can form simultaneously across the interface.
1. Introduction
The major form of adult haemoglobin (HbA) is a tetramer of two α Hb (α) and two β Hb (β) subunits (Perutz et al., 1960 ▶). Each subunit interacts with its non-identical partner through two different interfaces. The α1β1 (and α2β2) interface is extremely stable (K a = ∼5 × 1011 M −1; Valdes & Ackers, 1977b ▶) and remains essentially unchanged by oxygen binding. The α1β2 (and α2β1) interface undergoes a reorganization coupled to oxygen binding involving a ∼15° rotation of the two αβ dimers.
Mutations that reduce expression of the α- or β-globin genes lead to the condition α- or β-thalassaemia, respectively (Nathan & Gunn, 1966 ▶). Insufficiency of α or β production leads to anaemia that can range from mild to fatal (Clegg & Weatherall, 1976 ▶). In addition to the insufficient production of normal Hb, thalassaemia mutations lead to a relative excess of the partner Hb chain, which has cytotoxic effects. Free Hb chains are unstable compared with native Hb owing to the absence of native quaternary interactions. Quaternary interactions reduce chain unfolding and precipitation and reduce haem loss (Hargrove et al., 1997 ▶), which is associated with the production of harmful reactive species catalyzed by haem iron (Rifkind et al., 2004 ▶; Rachmilewitz & Schrier, 2001 ▶). Notably, free globin chains undergo self-association (Bucci et al., 1965 ▶) and differences in the self-association properties of α or β contribute different stabilities and different pathologies in α- or β-thalassaemia.
In α-thalassaemia, excess β self-associates into stable β4 tetramers (HbH). The dimer–tetramer association is more rapid than the monomer–dimer interaction (Philo et al., 1988 ▶), giving the appearance of a monomer–tetramer reaction (K a = ∼4 × 1016 M −3; Valdes & Ackers, 1977a ▶, 1978 ▶). β4 tetramers exhibit noncooperative and high-affinity O2 binding and therefore do not effectively release O2 to the tissues. Formation of β4 achieves a 20-fold stabilization against haem loss compared with β monomers, and consequently HbA and β4 lose haem from the β chains at comparable rates (Hargrove et al., 1996 ▶, 1997 ▶). Nevertheless, β4 protein slowly forms intracellular precipitates, causing oxidative damage to the membrane cytoskeleton of circulating red blood cells, which manifests as haemolytic anaemia (Advani et al., 1992 ▶). In foetal development the foetal β chain, γ, similarly forms γ4 tetramers (Hb Bart’s; Kidd et al., 2001 ▶).
Compared with β, α undergoes comparatively weak self-association to form dimers (K a = ∼5–9 × 103 M −1; Valdes & Ackers, 1977a ▶; Windsor et al., 1992 ▶), coupled with a ~25-fold increase in haem loss compared with HbA or β4 (Hargrove et al., 1997 ▶). As a result, α precipitation and oxidative damage occur early in erythroid cell development (Polliack et al., 1974 ▶). Damage to, and apoptosis of, erythroid precursors leads to compensatory proliferation of erythroid progenitors in the bone marrow, liver and spleen, with consequent disruption to the structure and function of these organs (a condition known as ineffective eythropoiesis; Rachmilewitz & Schrier, 2001 ▶).
In addition to its role in disease, globin chain self-association has significance for the Hb assembly pathway. In normal erythropoiesis, α is produced in a slight excess over β (Vasseur et al., 2011 ▶) and in this way α can act as a chaperone for β by outcompeting the formation of nonfunctional β4 (Miele et al., 2001 ▶). In turn, α is stabilized by its own chaperone, α Hb stabilizing protein (AHSP; Kihm et al., 2002 ▶; Gell et al., 2002 ▶). A moderately strong interaction between AHSP and oxygenated α (K a = 5 × 107 M −1) out-competes weak α2 self-association and prevents α precipitation, while at the same time permitting rapid kinetics of α release to β (Mollan et al., 2012 ▶).
The structures of β4 and γ4 have been determined (Borgstahl et al., 1994a ▶,b ▶; Kidd et al., 2001 ▶), allowing Kidd and coworkers to identify features at the N- and C-termini of β and γ that help to explain why these chains form tetramers but α does not. Here, we present the first crystal structure containing an α2 dimer and identify features at the α2 dimer interface that contribute to weak dimerization. The crystals contain α bound to the first near-iron transporter (NEAT) domain of iron-regulated surface determinant H (IsdH) from Staphylococcus aureus (Pilpa et al., 2006 ▶), and were obtained during investigation of Hb capture by this bacterium.
2. Materials and methods
2.1. Protein production
Hb was purified from blood as reported previously (Gell et al., 2002 ▶). The splitting of carbonmonoxy-liganded Hb into α and β chains was carried out using the well established p-hydroxymercuribenzoate (PMB) method (Bucci & Frontice, 1965 ▶). The resulting αPMB and βPMB chains were separated over DEAE Sepharose resin (GE Healthcare). PMB was removed from α by overnight incubation at 4°C with dithiothreitol followed by purification on an SP Sepharose column and storage at −80°C. For protein crystallography, purified carbonmonoxy α was converted to the oxygenated form by passing a pure stream of oxygen over a protein solution held on ice and illuminated with a focused beam from a 50 W halogen lamp. The oxygenated α was converted to ferric (met) α by addition of excess potassium ferricyanide in 20 mM sodium phosphate pH 7.0. The reaction was monitored to completion by UV–visible spectroscopy and α was isolated using a Sephadex G-25 column. The concentration of α was estimated from the concentration of the associated haem group, measured at 390 nm, from unfolded globin samples in 6 M guanidine hydrochloride (extinction coefficient 37 800 M −1 cm−1).
IsdHN1 (IsdH residues 86–229) from S. aureus strain TCH1516 was cloned into pET-15b (Novagen) for expression with an N-terminal hexa-His tag. IsdHN1 was expressed and purified as described previously (Pilpa et al., 2006 ▶) to yield a final product with the additional N-terminal sequence MGSSHHHHHHSSGLVPRGSHM.
2.2. Crystallization
Ferric α at 5 mg ml−1 (20 mM sodium phosphate pH 7.0) was mixed with one molar equivalent of IsdHN1 prepared in the same buffer and crystallization screening was performed by the hanging-drop vapour-diffusion method in 96-well plates using a Mosquito nanolitre liquid-handling robot (TTP LabTech). Protein complex (400 nl) was mixed with commercially available crystallization screens (The JCSG+, PACT and Classics Suites, Qiagen) in a 1:1 ratio and was incubated at 25°C. Crystals of ferric α–IsdHN1 appeared within a week in 0.2 M sodium sulfate, 0.1 M bis-tris propane, 20% PEG 3350 pH 6.5. On further optimization, single crystals appeared in 0.2 M sodium sulfate, 0.1 M bis-tris propane, 16% PEG 3350 pH 6.5. A single crystal was transferred into a cryoprotectant solution consisting of 30%(v/v) glycerol in the buffer from the crystallization condition and flash-cooled in a nitrogen stream (−173°C).
2.3. Data collection and processing
Data were recorded (over a ϕ range of 180°) to a resolution of 3.05 Å using an ADSC Quantum 315r detector on the MX2 beamline of the Australian Synchrotron at a wavelength of 0.95370 Å using the Blu-Ice control system (McPhillips et al., 2002 ▶). X-ray diffraction data were indexed and scaled using DENZO and SCALEPACK (Otwinowski & Minor, 1997 ▶).
2.4. Structure solution and refinement
Molecular replacement (MR) was performed using the IsdHN1 and α subunit (minus the haem group) of AHSP–α–IsdHN1 (PDB entry 3ovu, D. A. Jacques, K. Krishna Kumar, T. T. Caradoc-Davies, D. B. Langley, J. P. Mackay, J. M. Guss & D. A. Gell, unpublished work) as search models. An unique solution was found by Phaser (McCoy, 2007 ▶) in the tetragonal space group I41 with two molecules of IsdHN1 and two molecules of α in the crystallographic asymmetric unit. The structure was refined using REFMAC5 (Murshudov et al., 2011 ▶), with manual map inspection and model building being performed in Coot (Emsley et al., 2010 ▶). The quality of the model was regularly checked for steric clashes, incorrect stereochemistry and rotamer outliers using MolProbity (Chen et al., 2010 ▶). All structural figures were produced using PyMOL (Schrödinger, http://www.pymol.org). Refined atomic coordinates and experimental structure factors have been deposited in the Protein Data Bank (PDB entry 3s48). Data-collection and refinement statistics are given in Table 1 ▶.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| X-ray source | Australian Synchrotron MX2 |
| Wavelength (Å) | 0.9537 |
| Temperature (°C) | −173 |
| Detector | ADSC Quantum 315r CCD |
| Total rotation range (°) | 180 |
| Space group | I41 |
| Unit-cell parameters (Å) | a = b = 115.4, c = 142.4 |
| Resolution range (Å) | 50.00–3.05 (3.13–3.05) |
| No. of observations | 40834 |
| No. of unique reflections | 17737 |
| Completeness (%) | 99.5 (99.4) |
| Multiplicity | 2.3 (2.2) |
| 〈I/σ(I)〉 | 12.9 (2.1) |
| Wilson B value (Å2) | 119.3 |
| R merge † | 0.265 (0.635) |
| Reflections in working set‡ | 16833 (1244) |
| Reflections in test set | 904 (61) |
| Contents of the asymmetric unit | 2 α + 2 IsdHN1 |
| No. of atoms | |
| Total (non-H) | 4486 |
| Protein | 4398 |
| Water§ | 2 |
| Others (haem) | 86 |
| R cryst | 0.242 (0.358) |
| R free | 0.268 (0.369) |
| R.m.s.d., bond lengths (Å) | 0.004 |
| R.m.s.d., bond angles (°) | 0.639 |
| 〈B〉 (Å2) | |
| IsdHN1(2), chain A | 82 |
| IsdHN1(1), chain B | 249 |
| Chain C | 114 |
| Chain D | 93 |
| Cruickshank’s DPI¶ (Å) | 0.4 |
| PDB code | 3s48 |
R
merge = 
 .
.
Friedel pairs were kept separate for scaling, but were merged by REFMAC5 during structure refinement.
A single water molecule was placed in the distal haem pocket of each globin chain, coordinating the haem FeIII.
Diffraction precision indicator as output from REFMAC5.
2.5. Naming conventions
In addition to the usual convention of numbering amino-acid residues sequentially from the N-terminus of a peptide, it is conventional in the globin field to identify the amino-acid position relative to the structure of sperm whale myoglobin. For example, F8 indicates a residue that is functionally equivalent to the eighth residue in helix F of sperm whale myoglobin (the haem-ligating proximal His). Within the HbA tetramer, interfaces between the four subunits are designated using upper-case numerals according to convention in the field, e.g. the α1 subunit contributes to the α1β1, α1β2 and α1α2 subunit interfaces. The oligomeric state of globin assemblies is designated using subscript numerals by convention, e.g. α2β2 refers to a complex of two α and two β subunits (that is, the HbA tetramer).
3. Results and discussion
3.1. Crystal structure of the α-IsdHN1 complex
The structure of α–IsdHN1 was determined to a resolution of 3.05 Å and refined to an R cryst and R free of 0.242 and 0.268, respectively. The crystallographic asymmetric unit contains an α2 dimer, with each α monomer bound to one molecule of IsdHN1 (Fig. 1 ▶ a). One IsdHN1 domain [IsdHN1(1)] does not make any intermolecular crystal contacts (Fig. 1 ▶ b). Disorder in this region is reflected in substantially higher B factors (Fig. 1 ▶ b and Table 1 ▶, chain B) and the relatively high R merge value.
Figure 1.
The structure of the α–IsdHN1 complex. (a) Two α–IsdHN1 dimers are present in the crystallographic asymmetric unit. (b) The crystal packing is shown. One complete (α–IsdHN1)2 complex in the centre of the figure is represented as a tube (wider tubes and hot colours indicate higher Cα-atom B factors). Crystal contacts are shown as magenta patches on the grey surface of symmetry-related molecules. IsdHN1(1) projects into a cavity in the crystal and has substantially higher B factors. Its nearest neighbours are other symmetry-related IsdHN1(1) subunits (also shown in tube representation). (c) Residues close to the α dimerization interface identified by NMR (Dickson et al., 2013 ▶) are shown in stick representation on the α(2) chain.
The α–IsdHN1 interface is not significantly different from that observed in a previous structure in which IsdHN1 is bound to an αβ dimer (PDB entry 3szk; r.m.s.d. of 0.2 Å for 141 Cα atoms; Krishna Kumar et al., 2011 ▶) and is not discussed further. The structure of the α2 dimer has not previously been observed and reveals that the α2 dimer interface is equivalent to the α1β1 dimer interface in HbA. Residues at, or close to, the α2 dimer interface have previously been identified by NMR chemical shift perturbation (Fig. 1 ▶ c; Dickson et al., 2013 ▶), confirming that the crystal structure accurately represents the α2 interaction in solution. In a recent analysis of 113 crystal structures of protein dimers from the PDB (Chen et al., 2013 ▶), the weakest interaction (between a bromodomain and an acetylated peptide from histone H3) had a K a of ∼1 × 103 M −1 (VanDemark et al., 2007 ▶). A number of electron-transport complexes have successfully been crystallized; these typically display K a in the range 103–106 M −1 and are notable as physiologically important ‘weak’ interactions. The K a of the α2 dimer interface is estimated at 5–9 × 103 M −1 (Valdes & Ackers, 1977a ▶; Windsor et al., 1992 ▶), placing this at the weak-interaction end of the spectrum of crystallized protein complexes.
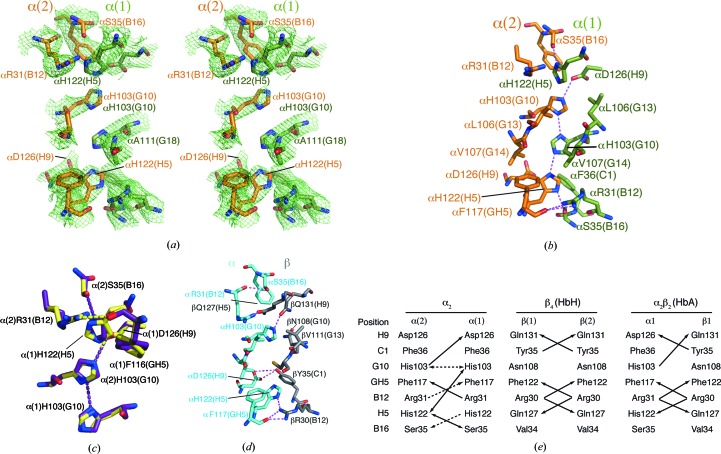
3.2. Buried His residues at the α2 interface
A highly unusual arrangement of four buried His side chains, His103(G10) and His122(H5) from each chain, interdigitate across the α2 dimer interface (Fig. 2 ▶ a). Optimized hydrogen-bonding patterns were investigated using MolProbity (Chen et al., 2010 ▶), PDB2PQR (Dolinsky et al., 2004 ▶) and WHAT IF (Vriend, 1990 ▶). These analyses show hydrogen-bond interactions between Nδ1 of α(2)His103 and Nδ1 of α(1)His103 and between N∊2 of α(1)His103 and N∊2 of α(2)His122, forming a novel ‘histidine-zipper’ arrangement (Fig. 2 ▶ b).
Figure 2.
The α2 dimer interface. (a) The OMIT map electron density of the α2 dimer interface contoured at 1σ (mesh), showing the four buried His side chains. The OMIT map was calculated using the SFCHECK program from CCP4 (Winn et al., 2011 ▶). (b) Hydrogen-bonding interactions at the α2 dimer interface (PDB entry 3s48). (c) Comparison of interfacial residues and hydrogen-bonding interactions in PDB entry 3s48 (violet) and the structure re-refined by PDB_REDO (yellow). (d) Hydrogen-bonding interactions at the α1β1 interface of HbA (PDB entry 2dn3; Park et al., 2006 ▶). (e) A comparison of the hydrogen-bonding interactions at the α1β1-equivalent interfaces of α2, β4 and α2β2. Double-headed arrows indicate hydrogen bonds where the donor/acceptor atoms could potentially be reversed, for example owing to protonation of α(2)His103 under the influence of α(1)Asp126. Dashed lines indicate hydrogen bonds that appear in 3s48 or the PDB_REDO re-refinement of 3s48 but not both.
The N∊2 atom of α(2)His103(G10) is within hydrogen-bonding distance of the β carboxylate of α(1)Asp126(H9). This interaction is predicted to raise the pK a of α(2)His103(G10) such that it may be in the cationic form at physiological pH (PROPKA; Bas et al., 2008 ▶). All other buried His residues are expected to have low pK a (<5.6) and be uncharged. α(2)Asp126(H9) does not make favourable electrostatic interactions and therefore the partial burial of this unpaired negative charge may be a significant factor in destabilization of the α2 interface. In HbA, αAsp126(H9) accepts a hydrogen bond from βTyr35(C1). This interaction appears to contribute significantly to αβ dimer stability, as substitution of βTyr35(C1) for Phe (Hb Philly) produces an unstable Hb (Rieder et al., 1969 ▶).
As part of our structure-validation and interface analysis we also used the PDB_REDO server, which performs an automated iterative re-refinement and model-rebuilding procedure (Joosten et al., 2012 ▶). PDB_REDO re-refinement relaxed the weighting towards standard geometry, compared with the 3s48 refinement, and included noncrystallographic symmetry and B-factor refinement. These differences gave a moderate improvement in R and R free at the expense of a greater number of rotamer, bond-length and Ramachandran outliers (Table 2 ▶). None of the outliers were at the α2 interface, although a number of α2 interface side chains did undergo positional shifts with alternative hydrogen bonding (Fig. 2 ▶ c). Most notably, the guanadinium group of α(2)Arg31 was positioned within hydrogen-bonding distance of the α carbonyl of α(1)Phe117, and the imidazole groups of α(1)His122, α(2)His103 and α(1)His103 were rotated 20–50°, which resulted in the loss of a hydrogen bond between α(1)His103 and α(2)His103 that is present in the 3s48 structure (Fig. 2 ▶ c). Thus, experimental uncertainty in the atomic coordinates is compatible with several hydrogen-bonding patterns, one of these being an extended network involving α(2)His103, α(1)His103 and α(2)His122 (Fig. 2 ▶ b). Overall, the PDB_REDO re-refinement did not improve the model and therefore the results were not deposited.
Table 2. MolProbity (Chen et al., 2010 ▶) evaluation of 3s48 and 3s48 re-refined by PDB_REDO (Joosten et al., 2012 ▶).
| 3s48 | 3s48 re-refined by PDB_REDO | |
|---|---|---|
| R/R free | 0.2420/0.2680 | 0.2218/0.2452 |
| Poor rotamers (goal <1%) | 1 (0.21%) | 47 (9.67%) |
| Ramachandran outliers (goal <0.05%) | 1 (0.18%) | 4 (0.72%) |
| Ramachandran favoured (goal >98%) | 521 (94.04%) | 513 (92.60%) |
| Cβ deviations > 0.25 Å (goal 0) | 0 (0.00%) | 6 (1.11%) |
| Bad backbone bonds (goal 0%) | 0/4611 (0.00%) | 2/4650 (0.04%) |
| Bad backbone angles (goal <0.1%) | 0/6301 (0.00%) | 6/6351 (0.09%) |
A pair of buried His residues, hydrogen bonded through their Nδ1 atoms, is found in the bacterial ammonium transporter (AmtB; Javelle et al., 2006 ▶; Liao et al., 2013 ▶; Zheng et al., 2004 ▶), but we are not aware of structures that have three consecutive hydrogen-bonded His residues, as may occur in the α2 dimer. However, it has previously been speculated that a hydrogen-bonded His-zipper motif occurs at the dimerization face of the bacterial protein RopE (repetitive organellar protein E) from Plasmodium chabaudi (Werner et al., 1998 ▶).
3.3. Asymmetry of the α2 dimer
In native Hb, the largest contribution to subunit interactions, based on structure and thermodynamic measurements, comes from hydrophobic contacts, with a smaller number of electrostatic interactions (Mrabet et al., 1986 ▶; Perutz et al., 1968 ▶; Valdes & Ackers, 1977a ▶). The total surface area buried on formation of the α2 dimer interface is 1473 Å2, which is a reduction of 18% compared with the α1β1 interface in Hb. The reduction in nonpolar buried surface is only slightly greater at 24% (Table 3 ▶). Systematic analyses show a relationship between buried surface area and affinity, but also that dissociation constants can vary over four orders of magnitude for the same buried surface area (Chen et al., 2013 ▶). Remarkably, the stability of the α1β1 and α2 dimers differs by almost eight orders of magnitude indicating that, in this case, buried surface area is an extremely poor indicator of affinity. Analysis of interface packing using the program SC from the CCP4 package (Winn et al., 2011 ▶) indicated lower shape complementarity for the α2 dimerization interface; however, this may reflect the low resolution of data in addition to intrinsic properties of the interface (Lawrence & Colman, 1993 ▶).
Table 3. Comparison of the physicochemical properties of α1β1-equivalent interfaces in α, HbA, β4 and γ4 .
| IsdHN1–α (α2 interface) | HbA | β4 | γ4 | |
|---|---|---|---|---|
| Buried area upon complex formation† (Å2) | 1473 | 1802 | 1698 | 1627 |
| Polar buried area upon complex formation† (Å2) | 995 | 1172 | 1182 | 1109 |
| Nonpolar buried area upon complex formation† (Å2) | 478 | 630 | 516 | 518 |
| No. of residues at the interface† | 38 | 49 | 44 | 45 |
| Shape complementarity by SC ‡ | 0.80 | 0.65 | 0.74 | 0.73 |
The arrangement of the two α chains deviates notably from the C 2 point-group symmetry that is expected for a homodimeric complex: the atoms in one subunit deviate on average by ∼2 Å from the position expected in a truly symmetrical dimer. By comparison the α1β1-equivalent dimers in β4 and γ4 are highly symmetric. At the native α1β1 interface, the G helices of the two chains are in close proximity across the dimer axis, raising the possibility that steric clashes could potentially occur in this region in a homodimeric complex. There is evidence of this in the β4 structures, where the side chains of βCys112(G14) adopt two different rotamers to avoid clashing (Borgstahl et al., 1994a ▶,b ▶). In α, residues at positions G10, G13 and G14 are larger side chains (His, Leu and Val) than their counterparts in β (Asn, Val and Cys), potentially introducing steric clashes that prevent symmetrical packing of the α monomers. It has been shown that substitution of αHis103(G10) with the more bulky Tyr destabilizes the native dimer interface (Hoyer et al., 2002 ▶), indicating that the side chain at G10 does not reorient to relieve the steric clash. Overall, symmetric homodimers have been postulated to be more stable (Blundell & Srinivasan, 1996 ▶).
A comparison of hydrogen-bonding interactions at the α1β1-equivalent interfaces of α2, β4 and HbA highlights the asymmetric nature of the α2 contacts (Fig. 2 ▶ e). The asymmetric interactions of His side chains and unpaired negative charge on α(2)Asp126(H9) have already been discussed. In addition, bilateral interactions of Arg(B12) with the backbone carbonyl of Phe(GH5) occur in β4 and HbA, but only one of these interactions is present in 3s48. Disruption of this interaction in Hb Prato, in which αArg31(B12) is replaced by Ser, leads to an unstable Hb (Marinucci et al., 1979 ▶). The PDB_REDO re-refinement of 3s48 permits bilateral interactions of αArg31(B12), but disrupts symmetrical interactions of αSer35(B12).
4. Conclusion
The structure reveals the α2 dimer interface, through which α self-associates weakly in solution. The α2 dimer interface reveals a novel hydrogen-bonding network involving up to three of the four buried His side chains. To our knowledge, such a ‘histidine zipper’ has not been described previously. Marked asymmetry in the interface contacts suggests that steric factors reduce the number of stabilizing contacts that can be formed by α homodimers compared with β homodimers or αβ heterodimers, with the consequence of reduced α stability in β-thalassaemia.
Supplementary Material
PDB reference: human α-haemoglobin complexed with the first NEAT domain of IsdH from S. aureus, 3s48
Acknowledgments
We thank Dr Joel Mackay (the University of Sydney) for supporting KKK to carry out aspects of this work in his laboratory and Dr Tom Caradoc-Davies at the MX beamlines at the Australian Synchrotron for help with data collection. This work was supported in part by the University of Tasmania (to DAG).
References
- Advani, R., Sorenson, S., Shinar, E., Lande, W., Rachmilewitz, E. & Schrier, S. L. (1992). Blood, 79, 1058–1063. [PubMed]
- Bas, D. C., Rogers, D. M. & Jensen, J. H. (2008). Proteins, 73, 765–783. [DOI] [PubMed]
- Blundell, T. L. & Srinivasan, N. (1996). Proc. Natl Acad. Sci. USA, 93, 14243–14248. [DOI] [PMC free article] [PubMed]
- Borgstahl, G. E., Rogers, P. H. & Arnone, A. (1994a). J. Mol. Biol. 236, 817–830. [DOI] [PubMed]
- Borgstahl, G. E., Rogers, P. H. & Arnone, A. (1994b). J. Mol. Biol. 236, 831–843. [DOI] [PubMed]
- Bucci, E. & Frontice, C. (1965). J. Biol. Chem. 240, PC551–PC552. [PubMed]
- Bucci, E., Fronticelli, C., Chiancone, E., Wyman, J., Antonini, E. & Rossi-Fanelli, A. (1965). J. Mol. Biol. 12, 183–192. [DOI] [PubMed]
- Chen, J., Sawyer, N. & Regan, L. (2013). Protein Sci. 22, 510–515. [DOI] [PMC free article] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Clegg, J. B. & Weatherall, D. J. (1976). Br. Med. Bull. 32, 262–269. [DOI] [PubMed]
- Dickson, C. F., Rich, A. M., D’Avigdor, W. M., Collins, D. A., Lowry, J. A., Mollan, T. L., Khandros, E., Olson, J. S., Weiss, M. J., Mackay, J. P., Lay, P. A. & Gell, D. A. (2013). J. Biol. Chem. 288, 19986–20001. [DOI] [PMC free article] [PubMed]
- Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. (2004). Nucleic Acids Res. 32, W665–W667. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Gell, D., Kong, Y., Eaton, S. A., Weiss, M. J. & Mackay, J. P. (2002). J. Biol. Chem. 277, 40602–40609. [DOI] [PubMed]
- Hargrove, M. S., Barrick, D. & Olson, J. S. (1996). Biochemistry, 35, 11293–11299. [DOI] [PubMed]
- Hargrove, M. S., Whitaker, T., Olson, J. S., Vali, R. J. & Mathews, A. J. (1997). J. Biol. Chem. 272, 17385–17389. [DOI] [PubMed]
- Hoyer, J. D., McCormick, D. J., Snow, K., Kwon, J. H., Booth, D., Duarte, M., Grayson, G., Kubik, K. S., Holmes, M. W. & Fairbanks, V. F. (2002). Hemoglobin, 26, 175–179. [DOI] [PubMed]
- Javelle, A., Lupo, D., Zheng, L., Li, X.-D., Winkler, F. K. & Merrick, M. (2006). J. Biol. Chem. 281, 39492–39498. [DOI] [PubMed]
- Joosten, R. P., Joosten, K., Murshudov, G. N. & Perrakis, A. (2012). Acta Cryst. D68, 484–496. [DOI] [PMC free article] [PubMed]
- Kidd, R. D., Baker, H. M., Mathews, A. J., Brittain, T. & Baker, E. N. (2001). Protein Sci. 10, 1739–1749. [DOI] [PMC free article] [PubMed]
- Kihm, A. J., Kong, Y., Hong, W., Russell, J. E., Rouda, S., Adachi, K., Simon, M. C., Blobel, G. A. & Weiss, M. J. (2002). Nature (London), 417, 758–763. [DOI] [PubMed]
- Krishna Kumar, K., Jacques, D. A., Pishchany, G., Caradoc-Davies, T., Spirig, T., Malmirchegini, G. R., Langley, D. B., Dickson, C. F., Mackay, J. P., Clubb, R. T., Skaar, E. P., Guss, J. M. & Gell, D. A. (2011). J. Biol. Chem. 286, 38439–38447. [DOI] [PMC free article] [PubMed]
- Lawrence, M. C. & Colman, P. M. (1993). J. Mol. Biol. 234, 946–950. [DOI] [PubMed]
- Liao, S.-M., Du, Q.-S., Meng, J.-Z., Pang, Z.-W. & Huang, R.-B. (2013). Chem. Cent. J. 7, 44. [DOI] [PMC free article] [PubMed]
- Marinucci, M., Mavilio, F., Massa, A., Gabbianelli, M., Fontanarosa, P. P., Camagna, A., Ignesti, C. & Tentori, L. (1979). Biochim. Biophys. Acta, 578, 534–540. [DOI] [PubMed]
- McCoy, A. J. (2007). Acta Cryst. D63, 32–41. [DOI] [PMC free article] [PubMed]
- McPhillips, T. M., McPhillips, S. E., Chiu, H.-J., Cohen, A. E., Deacon, A. M., Ellis, P. J., Garman, E., Gonzalez, A., Sauter, N. K., Phizackerley, R. P., Soltis, S. M. & Kuhn, P. (2002). J. Synchrotron Rad. 9, 401–406. [DOI] [PubMed]
- Miele, G., Manson, J. & Clinton, M. (2001). Nature Med. 7, 361–364. [DOI] [PubMed]
- Mollan, T. L., Khandros, E., Weiss, M. J. & Olson, J. S. (2012). J. Biol. Chem. 287, 11338–11350. [DOI] [PMC free article] [PubMed]
- Mrabet, N. T., McDonald, M. J., Turci, S., Sarkar, R., Szabo, A. & Bunn, H. F. (1986). J. Biol. Chem. 261, 5222–5228. [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Nathan, D. G. & Gunn, R. B. (1966). Am. J. Med. 41, 815–830. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Park, S.-Y., Yokoyama, T., Shibayama, N., Shiro, Y. & Tame, J. R. H. (2006). J. Mol. Biol. 360, 690–701. [DOI] [PubMed]
- Perutz, M. F., Muirhead, H., Cox, J. M. & Goaman, L. C. G. (1968). Nature (London), 219, 131–139. [DOI] [PubMed]
- Perutz, M. F., Rossmann, M. G., Cullis, A. F., Muirhead, H., Will, G. & North, A. C. (1960). Nature (London), 185, 416–422. [DOI] [PubMed]
- Philo, J. S., Lary, J. W. & Schuster, T. M. (1988). J. Biol. Chem. 263, 682–689. [PubMed]
- Pilpa, R. M., Fadeev, E. A., Villareal, V. A., Wong, M. L., Phillips, M. & Clubb, R. T. (2006). J. Mol. Biol. 360, 435–447. [DOI] [PubMed]
- Polliack, A., Yataganas, X. & Rachmilewitz, E. A. (1974). Ann. N. Y. Acad. Sci. 232, 261–282. [DOI] [PubMed]
- Rachmilewitz, E. A. & Schrier, S. (2001). Pathophysiology of β-Thalassemia. Cambridge University Press.
- Rieder, R. F., Oski, F. A. & Clegg, J. B. (1969). J. Clin. Invest. 48, 1627–1642. [DOI] [PMC free article] [PubMed]
- Rifkind, J. M., Ramasamy, S., Manoharan, P. T., Nagababu, E. & Mohanty, J. G. (2004). Antioxid. Redox Signal. 6, 657–666. [DOI] [PubMed]
- Valdes, R. Jr & Ackers, G. K. (1977a). J. Biol. Chem. 252, 74–81. [PubMed]
- Valdes, R. Jr & Ackers, G. K. (1977b). J. Biol. Chem. 252, 88–91. [PubMed]
- Valdes, R. Jr & Ackers, G. K. (1978). Proc. Natl Acad. Sci. USA, 75, 311–314. [DOI] [PMC free article] [PubMed]
- VanDemark, A. P., Kasten, M. M., Ferris, E., Heroux, A., Hill, C. P. & Cairns, B. R. (2007). Mol. Cell, 27, 817–828. [DOI] [PMC free article] [PubMed]
- Vangone, A., Spinelli, R., Scarano, V., Cavallo, L. & Oliva, R. (2011). Bioinformatics, 27, 2915–2916. [DOI] [PubMed]
- Vasseur, C., Pissard, S., Domingues-Hamdi, E., Marden, M. C., Galactéros, F. & Baudin-Creuza, V. (2011). Am. J. Hematol. 86, 199–202. [DOI] [PubMed]
- Vriend, G. (1990). Protein Eng. 4, 221–223. [DOI] [PubMed]
- Werner, E. B., Taylor, W. R. & Holder, A. A. (1998). Mol. Biochem. Parasitol. 94, 185–196. [DOI] [PubMed]
- Windsor, W. T., Philo, J. S., Potschka, M. & Schuster, T. M. (1992). Biophys. Chem. 43, 61–71. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Zheng, L., Kostrewa, D., Bernèche, S., Winkler, F. K. & Li, X.-D. (2004). Proc. Natl Acad. Sci. USA, 101, 17090–17095. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: human α-haemoglobin complexed with the first NEAT domain of IsdH from S. aureus, 3s48