SUMMARY
CD4+ T cells play a critical role in determining the disease outcome in murine cutaneous leishmaniasis, and selective usage of T-cell receptor (TCR) is implied in promoting Leishmania major infection. However, little information is available on TCR usage in Leishmania-specific, IFN-γ-producing CD4+ T cells. In this study, we investigated the TCR diversity and activation of CD4+ T cells in a nonhealing model associated with L. amazonensis (La) infection and a self-healing model associated with L. braziliensis (Lb) infection. While marked expansion in the absolute number of several subsets was observed in Lb-infected mice, the percentages of TCR Vβ+ CD4+-cell subsets were comparable in draining LN- and lesion-derived T cells in two infection models. We found that multiple TCR Vβ CD4+T cells contributed collectively and comparably to IFN-γ production and that the overall levels of IFN-γ production positively correlated with the control of Lb infection. Moreover, pre-infection with Lb parasites provided cross-protection against secondary La infection, owing to an enhanced magnitude of T-cell activation and IFN-γ production. Collectively, this study suggests that the magnitude of CD4+ T-cell activation, rather than the TCR diversity, is the major determining factor for the outcome of Leishmania infection.
Keywords: leishmania, protozoan parasites, T-cell activation, TCR diversity
INTRODUCTION
In murine cutaneous leishmaniasis, resistance to Leish-mania major in the majority of inbred strains of mice is associated with the development of a IFN-γ-producing Th1 response, while susceptibility in a few strains (such as BALB ⁄ c mice) is attributed to a IL-4-producing Th2 response (1). However, most, if not all, mouse strains are genetically susceptible to L. amazonensis (La, a New World species), and this generalized susceptibility in mice is attributed to an impaired or weak Th1-cell response rather than to increased IL-4 production (2–4). In contrast, L. braziliensis (Lb, another New World species) induces self-healing skin lesions in most tested mouse strains, including BALB/c mice that are highly susceptible to L. major presumably owing to the induction of strong innate and Th1 responses during the infection (5,6) and to the relatively high sensitivity of Lb parasites to TNF-α-and nitric oxide–based parasite killing (7–9). Thus, the findings from these murine models clearly indicate that the outcome of infection depends both on the parasite species involved and on the nature of host immune responses to Leishmania antigen. Therefore, it is not surprising that the adoptive transfer of L. major-specific Th1 or Th2 cell lines to immunodeficient mice can confer resistance or susceptibility in L. major infection (10,11) and that adoptive transfer of La-specific Th1- or Th2-cell lines to competent mice can alter host susceptibility to L. amazonensis infection (4,12). The critical role of CD4+ T cells in La-induced, nonhealing disease has also been confirmed in MHC II– deficient mice (13); however, the immunological characteristics of parasite-specific Th subsets and the mechanisms responsible for differentiation of these disparate Th populations remain largely unexplored.
Upon its encounter with foreign antigens, the germ line– encoded β chain of T-cell receptor (TCR Vβ) through recombination establishes Ag specificity and diversity of cellular immunity (14,15). Several studies have shown that the oligoclonal expansion of Ag-specific, Vβ-expressing T cells is associated with the emergence of immune escape and disease progression in viral infections (16), tumours (17) and autoimmune diseases (18). Studies in L. major– infected BALB/c mice have identified TCR Vα8+ Vβ4+ CD4+ T cells as the major source of early IL-4 production by recognizing the Leishmania antigen LACK (Leishmania homologue of receptors for activated C kinase) (19,20), although such T cells appeared to be primed by cross-reactive antigens derived from the gut flora (21). Even in L. major–infected resistant C57BL/6 mice, LACK-specific T cells were also found to be the source of early IL-4 production when mice were given anti-IFN-γ or anti-IL-12 at the onset of infection (22). Thus far, there is little information on the characterization of TCR usage in Leish-mania-specific, IFN-γ-producing Th1 cells.
In this study, we used C57BL/6 mice and investigated the TCR diversity of CD4+ T cells from a nonhealing model associated with La infection and a self-healing disease model associated with Lb infection. Furthermore, we characterized IFN-γ-producing Th1 cells based on TCR usage during primary infection with these two parasite species, respectively, and during secondary La infection following pre-exposure to Lb parasites. Our results support a view that the magnitude of CD4+ T-cell activation, rather than the TCR diversity, is the main determining factor for the outcome of Leishmania infection.
MATERIALS AND METHODS
Mice
Female C57BL/6J (B6) mice, at 6~8 weeks old from the Jackson Laboratory (Ben Harbor, ME), were used in this study. Mice were maintained under specific pathogen-free conditions and used for experimentation, according to protocols approved by the institutional Animal Care and Use Committees.
Antibodies
The following mAbs were purchased from eBioscience (San Diego, CA) unless stated otherwise: FITC- or PE-conjugated anti-IFN-γ (XMG1.2); PerCP Cy5.5-conjugated anti-IL-17 (eBio17B7); APC anti-CD4 (GK1.5) and PE-Cy7 anti-CD3 (145-2C11), as well as isotype control Abs, including FITC-conjugated rat IgG1, PE-conjugated rat IgG1 and PerCP Cy5.5-conjugated rat IgG2a. The Mouse Vβ TCR screening panel kit (Abs conjugated with FITC) and PE-conjugated TCR Vβ4 (KT4), Vβ6 (RR4-7), Vβ7 (TR310) and Vβ8 (F23.1) were purchased from BD Biosciences (San Jose, CA, USA).
Parasite culture and Ag preparation
Infectivity of L. amazonensis (MHOM/BR/77/LTB0016) was maintained by regular passage through BALB/c mice (Harlan Sprague-Dawley, Indianapolis, IN, USA) and L. braziliensis (MHOM/BR/79/LTB111) by regular passage through Syrian golden hamsters (Harlan Sprague-Dawley). Promastigotes were cultured at 23°C in Schneider's Drosophila medium (Invitrogen, Carlsbad, CA, USA), pH 7.0, supplemented with 20% FBS (Sigma, St. Louis, MO, USA), 2 mm L-glutamine, and 50 μg/mL gentamicin. Stationary promastigote cultures of less than five passages were used for animal infection. To prepare promastigote lysates, parasites (2 × 108/mL in PBS) were subjected to six freeze-thaw cycles and a 15-min sonication. The soluble parasite antigens were stored in aliquots at −20°C until use.
TCR Vβ analysis via flow cytometry
B6 mice (five per group) were subcutaneously (s.c.) infected with L. amazonensis or L. braziliensis stationary promastigotes (2 × 106 in PBS) in the right hind foot. At indicated time of infection, we collected popliteal draining LN cells and splenocytes from individual mice. To ensure sufficient cells for staining and subsequent analyses, we conveniently pooled draining LN cells within the group into two sample sets, such as three draining LNs into one set and the other two draining LNs into the other set. Cells were then stimulated with a PMA/ionomycin/Golgi Plug (BD Biosciences) for 6 h. Cells were first stained for surface markers, including CD3, CD4 and individual TCR Vβ. Then, the intracellular IFN-γ production was stained following cytofixation/permeabilization with a Cytofix/Cytoperm Kit (BD Biosciences). The percentages of CD4+ TCR Vβ+ cells gated on CD3+ cells and TCR Vβ+ IFN-γ+ cells gated on CD4+ cells were analysed on the FACScan (BD Biosciences), and results were analysed using FlowJo software (TreeStar, Ashland, OR, USA). To obtain the absolute cell number of CD4+Vb+ cells, we first got an averaged cell number per draining LN from each sample set. We then calculated the absolute cell number of CD3+ CD4+ TCR Vβ+ cells by multiplying the averaged absolute cell number per LN by their corresponding percentages of positively stained cells (CD3, CD4 and the individual TCR Vβ in CD4 cells).
For TCR Vβ analysis of lesion-derived cells, foot lesional tissues were collected and pooled as mentioned earlier and digested in the complete Iscove's modified Dulbecco's medium containing 10% FBS, 1 mm sodium pyruvate, 50 μm 2-ME, 50 μg/mL gentamicin and 100 U/mL penicillin, as well as collagenase/dispase (100 μg/mL) and DNase I (100 U/mL; Roche), for 2 h at 37°C. After passage through the cell strainer (40 μm; BD Biosciences), the single-cell suspension was on the top of 40% and 70% Percoll solution (Sigma). After centrifugation for 25 min at room temperature, the purified cells from a 40/70% layer of Percoll were collected and stained with CD3, CD4 and TCR Vβ Abs. The percentages of TCR Vβ+ cells gated on CD3+ CD4+ cells were analysed by FACS.
TCR Vβ analysis via RT-PCR
B6 mice were infected with 2 × 106 La or Lb promastigotes for 4 weeks. Draining LN cells were restimulated with the corresponding La or Lb antigens for 3 day, and CD4+ T cells were purified via positive selection. Na ve CD4+ T cells were used as controls. TCR Vβ repertoire clonality for purified CD4+ T cells was analysed by RT-PCR and gel-based assays using specially designed Super-TCRExpress™ kits by scientists in BioMed Immunotech Incorporation (Tampa, FL, USA).
In vivo evaluation of infection
Leishmania braziliensis stationary promastigotes (2 × 106) were injected subcutaneously (s.c.) in the right hind foot. After the healing of lesions at 8 or 24 weeks, some of the mice were injected with stationary promastigotes of La (2 × 106) in the left hind foot. Naïve mice were similarly infected and used as controls. Lesion sizes in the left hind foot were monitored weekly with a digital caliper (Control Company, Friendswood, TX, USA), and tissue parasite loads at 8 weeks post-La infection were measured via a limiting dilution assay.
Preferred TCR Vβ analysis in secondary infection
After 8 or 24 weeks of primary infection with Lb parasites in the right hind foot, healed mice as well as control mice were infected with La parasites in the left hind foot. At 1 week post-infection with La, draining LN and spleen cells were collected and briefly (6 h) stimulated with PMA/ionomycin/GolgiPlug. The intracellular IFN-γ and IL-17 production from CD4+ T cells as well as IFN-γ production from several tested TCR Vβ+ (Vβ4, 6, 7, and 8) CD4+ cells was analysed by FACS.
Evaluation of T-cell intracellular cytokine profile
At 4 weeks post-infection, draining LN cells from naïve, La- or Lb-infected mice were restimulated with PMA/ionomycin/GolgiPlug for 6 h. The intracellular cytokines (IFN-γ, IL-10, IL-17, IL-2 and TNF-α) from CD4+ CD44+ cells were analysed by FACS.
ELISA
Individual draining LN cells (4 × 106/mL) were collected from naïve, La- or Lb-infected B6 mice (four per group) at 4 weeks and then restimulated with either La or Lb soluble Leishmanial antigen for 72 h. Cytokines (IFN-γ, IL-10, IL-6) in the supernatants were measured by ELISA following the protocol from eBiosciences.
Statistical analysis
The distributions of the outcome variables were first examined. As the sample sizes were too small to ascertain normality and homogeneity of variance, the nonparametric Kruskal–Wallis tests were used for overall significance test. If the overall test was significant, then the Mann– Whitney tests were used for pairwise comparisons. Multiple comparisons were made using a Bonferroni adjustment method. For experiments that used pooled samples, each experiment for the pooled sample was used as the unit of analysis. For experiments that used individual animals, each animal was used as the unit of analysis. The statistical analyses were conducted using GraphPad Prism, version 4.00, for Windows (GraphPad Software, San Diego, CA, USA) and SAS® 9.2 software (SAS® Institute Inc., Cary, NC, USA). Statistically significant values are referred to as follows: *P < 0.05; **P < 0.01.
RESULTS
The profile of TCR Vβ usage in primary infection with La or Lb parasites
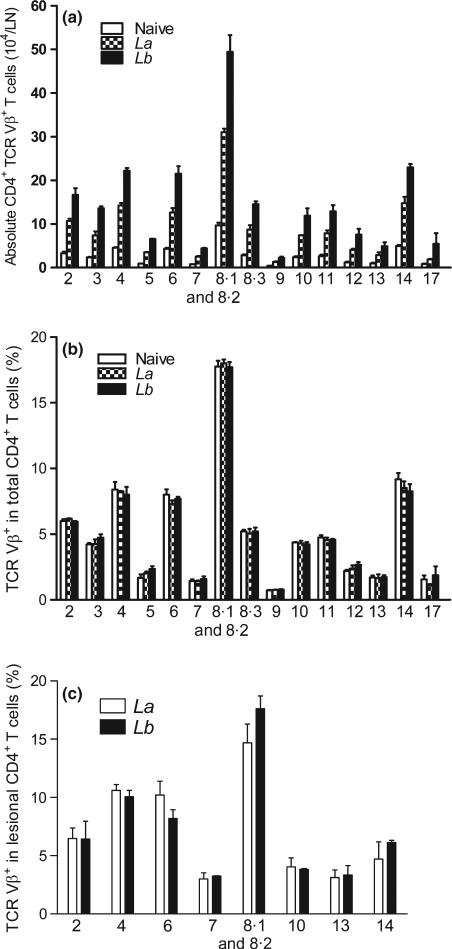
To investigate the profile and magnitude of T-cell activation in nonhealing or self-healing cutaneous Leishmaniasis, we infected B6 mice with La or Lb parasites in the hind foot. At 4 weeks post-infection, we examined TCR Vβ usage in both draining LN and lesional CD4+ T cells. As shown in Figure 1(a), while infection with both parasites markedly stimulated the expansion of CD4+ T cells in draining LN when compared to naive controls, Lb-infected mice showed a stronger increase in the absolute numbers in nearly all tested subsets of Vβ+ CD4+ T cells than did La-infected mice. However, the percentages of Vβ-bearing CD4+ T cells were similar in draining LNs of naïve, La- and Lb-infected mice, in which the cells bearing Vβ8, Vβ4, Vβ6 and Vβ14 represented more than 60% of the LN CD4+ T cells (Figure 1b). We then examined the TCR Vβ usage in lesion-derived CD4+ T cells, focusing on the major Vβ types. As shown in Figure 1(c), the percentages of the tested Vβ types were similar in CD4+ T cells derived from La or Lb lesions and comparable to those in the draining LN (Figure 1b). Therefore, neither La nor Lb infection significantly altered TCR Vβ diversity in draining LN- and lesion-derived CD4+ T cells, although Lb infection showed greater increase in cell numbers.
Figure 1.
The percentages of TCR Vβ usage in draining LN and lesional CD4+ T cells in primary infection. C57BL/6 mice (5 per group) were infected with 2 × 106 La or Lb promastigotes, and draining LN cells or lesions were collected at 4 weeks. To ensure sufficient LN cells for staining and subsequent analyses, the samples within groups were pooled and divided into two sets (see Materials and Methods). The absolute number of CD4+ TCR Vβ+ T cell per LN (a) and the percentages of indicated TCR Vβ usage in LN CD4+ T cells (b) were analysed by FACS via a Vβ TCR screening panel kit. (c) The percentages of indicated TCR Vβ usage in lesional CD3+ CD4+ T cells were analysed by FACS using a Vβ TCR screening panel kit. The results are shown as mean ± SD (pooled from these four sets of results from two independent repeats), and the bars represent the maximum and minimum values.
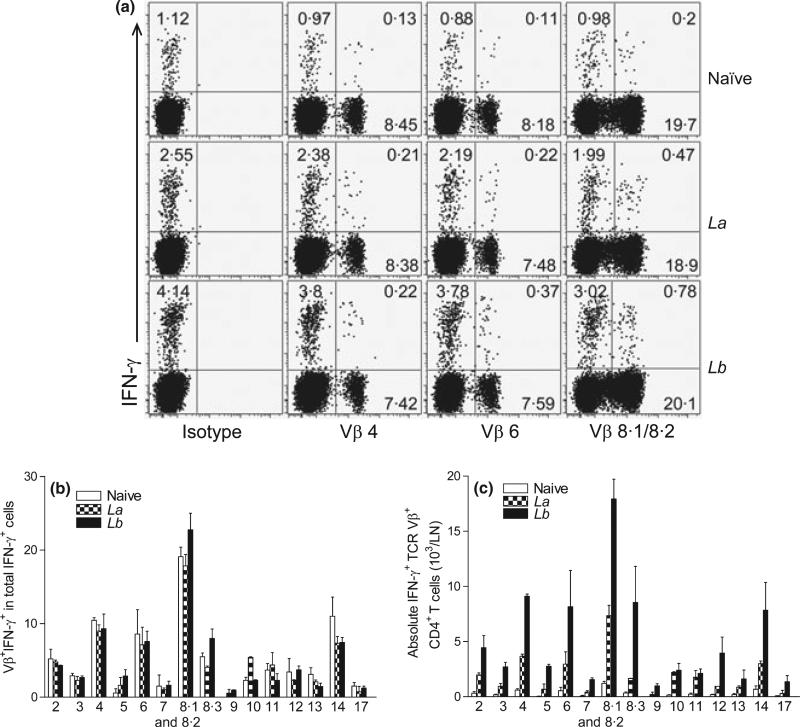
Because the percentages of IFN-γ-producing CD4+ T cells correlate with the disease outcomes in La- and Lb-infected mice (5), we collected draining LN cells at 4 weeks post-infection and performed intracellular IFN-γ staining, gating on each TCR Vβ+ subpopulation. It was evident that Lb infection triggered significantly stronger IFN-γ responses than did La infection, as judged by their frequencies of Vβ4-, 6- and 8.1/8.2–bearing IFN-γ+ CD4+ T cells. For example, 0.78% of Vβ8.1/8.2–bearing CD4+ T cells produced IFN-γ in Lb-infected mice, whereas only 0.47% of these cells produced IFN-γ in La-infected mice (Figure 2a). However, neither La nor Lb infection changed the relative frequencies of Vβ+ IFN-γ+ cells among total IFN-γ+ cells (Figure 2b), and Vβ 8.1/8.2–and Vβ4-bearing cells contributed to ~20% and ~8% of total IFN-γ production in all three groups of mice, respectively. Notably, draining LN from Lb-infected mice contained higher numbers of IFN-γ-producing TCR Vβ+ CD4+ T subsets than those from La-infected mice (Figure 2c). Therefore, although the relative contributions of individual Vβ+ CD4+ T cells to total IFN-γ production were comparable in both infection models, Lb infection apparently induced a higher magnitude of CD4+ T-cell activation and IFN-γ production than did La infection.
Figure 2.
The percentages of IFN-γ production in TCR Vβ+ CD4+ T cells derived from draining LN. C57BL/6 mice (five per group) were infected with 2 × 106 La or Lb promastigotes for 4 weeks, and draining LN cells were collected and pooled into two sample sets. After a brief (6 h) treatment with PMA/ionomycin/GolgiPlug, intracellular IFN-γ production from CD4+ TCR Vβ+ T cells was analysed together with Vβ TCR screening panel by FACS. (a) Shown are the percentages of IFN-γ-producing Vβ4, Vβ6 and Vβ8.1/8.2 cells, respectively. Data are representative results from four repeats with similar trends. (b) Shown are the ratios/percentages of IFN-γ-producing TCR Vβ among total IFN-γ+ CD4+ T cells. (c) Shown are the absolute numbers of IFN-γ-producing Vβ+ CD4+ T cells per LN. The results are shown as mean ± SD (pooled from these four sets of results from two independent repeats), and the bars represent the maximum and minimum values.
To confirm these flow cytometric data, we analysed the oligoclonalities in the CDR3 region of 22 individual TCR Vβ chains by RT-PCR-based assays, in which multiple PCR primer sets were uniquely designed for specific amplification of the Vβ, Dβ or Jβ genes. We found that when compared to naïve controls, CD4+ T cells purified from La- and Lb-infected mice displayed multiple TCR Vβ clonalities based on VDJ rearrangement in the CDR3 region and that TCR Vβ clonalities were evident and strong in CD4+ T cells of Lb-infected mice (Supplemental Figure S1). Our FACS- and PCR-based studies suggest that in contrast to viral infection (23), primary infection with La or Lb parasites does not show a highly focused, selective expansion of particular Vβ population.
The status of TCR Vβ diversity and T-cell activation in secondary La and Lb infection
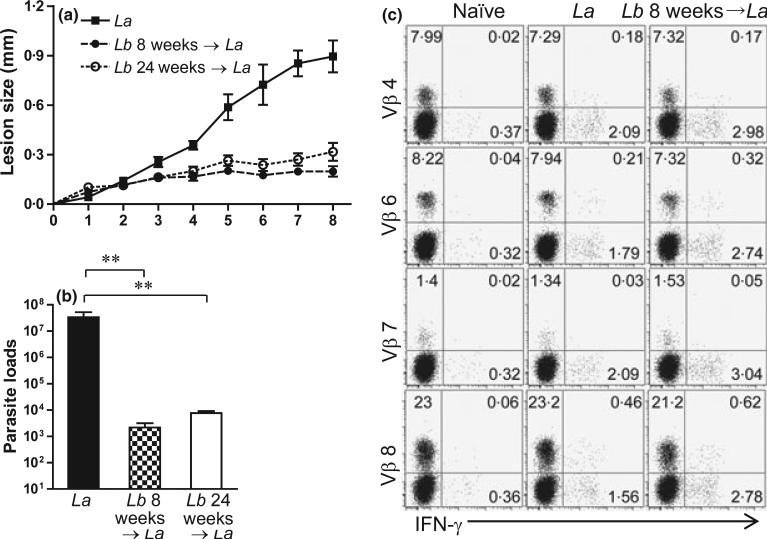
We have previously reported that Lb infection in B6 mice is self-healing, with no signs of disease and detectable tissue parasites at 8 weeks (5). To test whether pre-infection with Lb could enhance CD4+ T-cell activation and protect mice against La infection, we infected mice with Lb parasites in one foot for 8 weeks (short-term) or 24 weeks (long-term) and then challenged these healed mice with La parasites in another foot. As shown in Figure 3, pre-infection with Lb for either 8 or 24 weeks provided efficient protection against a secondary infection with La parasites, as judged by the significant reduction in lesion sizes and tissue parasite loads. To analyse the role of CD4+ T subsets in this protection, we took two approaches. First, we compared CD4+ T-cell activation and TCR Vβ diversity from draining LN at 1 week post-infection with La alone versus La infection following pre-infection with Lb for 8 weeks (short-term). We focused on IFN-γ production in Vβ8, Vβ4 and Vβ6 (because of their relatively high frequencies) and used Vβ7 as an example of low-frequency types (Figure 1). Compared to La infection alone, pre-infection with Lb increased IFN-γ production from total CD4+ T cells, as well as from Vβ6- and Vβ8-bearing CD4+ T cells (Figure 3c).
Figure 3.
Pre-infection with Leishmania braziliensis (Lb) can cross-protect mice against L. amazonensis (La) infection. C57BL/6 mice (five per group) were infected with 2 × 106 Lb promastigotes in the left hind foot. After the healing of the primary infection at 8 weeks (short-term) or 24 weeks (long-term), some mice and their age-matched controls were given 2 × 106 La promastigotes in the right hind foot. (a) Lesion sizes (mm) were monitored weekly for an additional 8 weeks following La infection. (B) Parasite loads in La-infected foot were determined at 8 weeks post-infection using a limiting dilution assay. **P < 0.01 (by Kruskal–Wallis test) indicates statistical significances among these three groups. (c) Lb-exposed mice (infected and healed at 8 weeks) as well as age-matched controls were given 2′ 106 La promastigotes in the right hind foot. One week later, draining LN cells were collected and pooled into two sample sets. The percentages of IFN-γ production from indicated Vβ+ CD4+ T cells were measured. Shown are representative results from four repeats with similar trend.
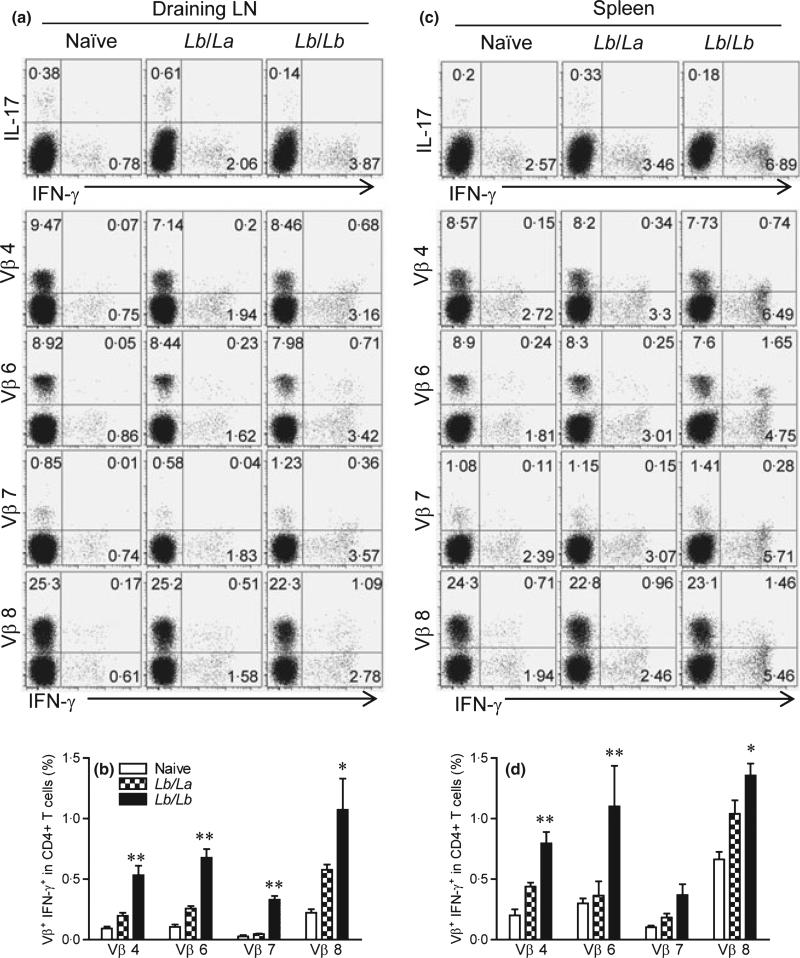
Second, we compared CD4+ T-cell activation and TCR Vβ diversity from draining LN and the spleen at 1 week post-infection with La versus Lb parasites in mice that were pre-infected with Lb for 24 weeks (long-term). As shown in Figure 4(a), the secondary infection with Lb (the Lb/Lb group) consistently showed higher IFN-γ but lower IL-17 production from draining LN CD4+ T cells than did the La counterparts (the Lb/La group). For the tested Vβ-bearing CD4+ T-cell subsets (Vβ4, 6, 7, and 8), the Lb/Lb groups displayed 2.1- to 9-fold higher frequencies of IFN-γ-producing cells in draining LNs. It was evident in Figure 4(b) that the Lb/Lb groups showed high frequencies of IFN-γ-producing cells in the tested T-cell subsets. Likewise, the similar trends were observed for cells obtained from the spleen (Figure 4c,d). Collectively, our results indicate that repeated exposures to Lb parasites (the Lb/Lb groups) preferentially stimulate the expansion of IFN-γ-producing cells among multiple Vβ-bearing CD4+ T-cell subsets and that such responses contribute to the protection against a secondary infection with La parasites.
Figure 4.
IFN-γ production from several preferred memory TCR Vβ CD4+ T cells from draining LN and spleen. C57BL/6 mice (five per group) were infected with 2 × 106 Lb promastigotes in the left hind foot. After they healed from the primary infection (at 24 weeks), mice were injected with 2 × 106 La or Lb promastigotes in the right hind foot. One week later, draining LN cells were collected and pooled into two sample sets. Splenocytes were prepared from individual mice. (a, c) Cells were briefly (6 h) treated with PMA/ionomycin/GolgiPlug and subjected for FACS analysis for the percentages of cytokine-producing CD4+ T cells, as well as intracellular IFN-γ from indicated subsets of Vβ+ CD4+ T cells. (b, d) The percentages of Vβ+ IFN-γ+ cells among total CD4+ T cells were pooled from two independent repeats and shown as mean ± SD, and the bars represent the maximum and minimum values. *P < 0.05 and **P < 0.01 (by Mann–Whitney test) indicate statistical significances between the Lb/La and Lb/Lb groups.
Enhanced magnitude of T-cell activation and Th1 cytokine production in cross-protected mice
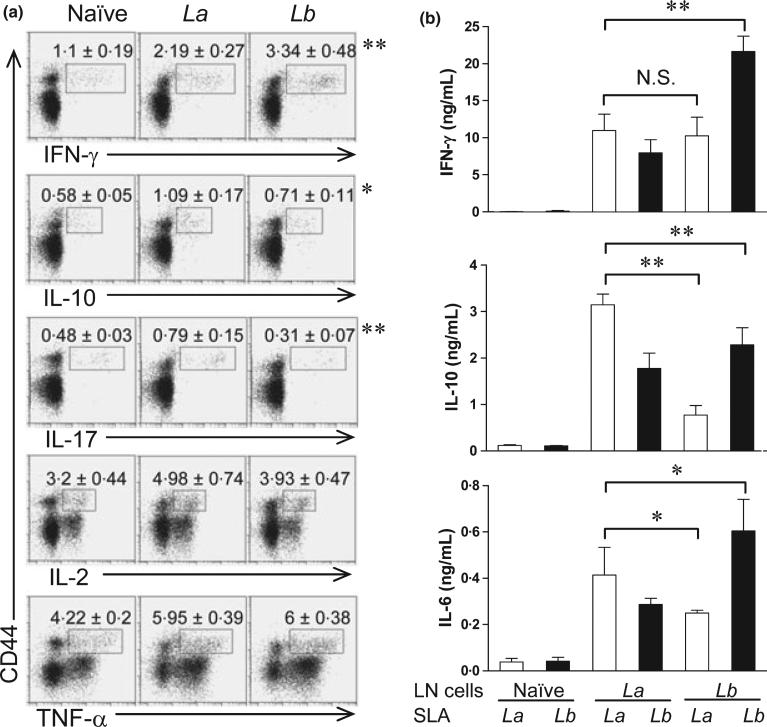
To further characterize CD4+ T-cell activation during the primary and secondary infections, we collected draining LN cells at 4 weeks post-infection with La or Lb and stimulated cells briefly (6 h) with PMA/ionomycin, The ex vivo production of intracellular cytokines (IFN-γ, IL-10, IL-17, IL-2 and TNF-α) in CD4+ CD44+ T cells was analysed by FACS. As shown in Figure 5(a), CD4+ CD44+ T cells from Lb-infected mice contained higher frequencies of IFN-γ-producing cells, but lower frequencies of IL-10-and IL-17-producing cells than did the counterparts from La-infected mice. On average, the ratios of IFN-γ- vs. IL-10-producing cells in Lb-, La- and noninfected mice were 4.7, 2.0 and 1.7, respectively. The frequencies of IL-2- and TNF-α-producing CD4+ CD44+ T cells were comparable in two infection models. Therefore, CD4+ T cells derived from Lb-infected mice were highly activated with a strong Th1 phenotype.
Figure 5.
Intracellular cytokines from naïve and infected CD4+ CD44+ T cells and cytokine production from draining LN cells restimulated with SLA. C57BL/6 mice (four per group) were infected with 2 × 106 La or Lb promastigotes in the left hind foot for 4 weeks. (a) Individual draining LN cells were collected and briefly (6 h) treated with PMA/ionomycin/GolgiPlug, and then analysed by FACS for the intra-cellular cytokine productions gated on CD4+ CD44+ T cells. The percentages of intracellular cytokines are shown as mean ± SD, and data were pooled from two independent repeats. The percentages from isotype control groups were less than 0.01%. *P < 0.05 and **P < 0.01 (by Mann–Whitney test) indicate statistically significant differences between the La and Lb groups. (b) Individual draining LN cells were collected and restimulated with soluble Leishmanial antigen (SLA) for 72 h. Cytokines in culture supernatants were measured by an ELISA. Data are shown as mean ± SD (pooled from two independent repeats), and the bars represent the maximum and minimum values. *P < 0.05 and **P < 0.01 (by Kruskal–Wallis test) indicate statistical significances among these four groups.
Next, we designed a cross-stimulation experiment, in which draining LN cells from La- or Lb-infected mice were restimulated in vitro with La or Lb antigens, and vice versa. The ELISA measurement for the levels of IFN-γ, IL-10 and IL-6 in culture supernatants showed several interesting trends (Figure 5b). In the homologue reactivation setting, Lb-LN cells restimulated with Lb antigens produced significantly higher levels of IFN-γ and IL-6, but lower levels of IL-10 than did La-LN cells restimulated with La antigens. On average, the IFN-γ/IL-10 ratios were 10:1 in the Lb/Lb cells, but only 3 : 1 in the La/La cells. In the cross-activation setting, however, Lb-LN cells restimulated with La antigens produced relatively low levels of tested cytokines, but these cells still displayed a Th1-favoured response (with ~10 ng/mL of IFN-γ vs. ~1 ng/mL of IL-10). We also performed cell transfer experiments, in which 5 × 106 of CD4+ T cells purified either from the spleen of naïve mice or draining LN of Lb-infected mice (at 4 weeks) were adoptively transferred into naïve C57BL/6 mouse 1 day prior to infection with La parasites. Similar to a previously reported cross-infection study (24), we found no major differences in disease development between the infection control and cell-transferred groups (data not shown). Collectively, the data presented here expend our previous findings (5), confirming a strong expansion of Th1-type cells during Lb infection and a relatively weak Th1-type response during La infection.
DISCUSSION
In this study, we have attempted to define the role of CD4+ T cells using several approaches, including the analysis of TCR Vβ usage and cytokine-producing cells in non-healing versus self-healing models following infection with two New World species of Leishmania, as well as the comparative analyses of these CD4+ T cells in primary versus secondary Leishmania infections. The most important finding in this study is that the magnitude of CD4+ T-cell activation rather than TCR diversity is the main determining factor for disease outcome in murine cutaneous Leishmaniasis. This conclusion is based upon the observations that multiple TCR Vβ CD4+ T cells contributed collectively and comparably to IFN-γ production and that the overall levels of IFN-γ production positively correlated with the control of the infection.
In the Leishmania research field, a well-studied example of parasite-specific T cells is the LACK-specific, TCR Vα8+ Vβ4+ CD4+ T cells, which are capable of producing high levels of IL-4 at an early stage of infection and instructing Th2 development in L. major-infected susceptible BALB/c mice (20). The identification of such pathogenic T-cell subsets felicitates the understanding of mouse susceptibility to L. major infection via multiple approaches, including the use of antagonist LACK peptides, the depletion of LACK-specific T cells and the test of LACK-based immunization regimens (25–27). At present, there is little information on TCR repertoires in CD4+ T cells specific to other Leishmania species or to protective antigens. This lack of information on the signature of pathogenic versus protective immunity hampers the development of an anti-Leishmania vaccine. In an attempt to address these issues, we conducted the present study. It was somewhat surprising to us that CD4+ T cells derived from both nonhealing (La) and self-healing (Lb) models displayed comparable TCR diversity in either draining LN- or lesion-derived CD4+ T cells (Figure 1). Furthermore, we found that the production of IFN-γ appeared to be evenly contributed by multiple rather than one or two dominant Vβ+ CD4+ T cells during La or Lb infection, which is different from the report with the dominant IL-4 production by Vβ4+ CD4+ T cells in L. major infection (20). Of note, the relative contribution of individual Vβ cells to the total IFN-γ production appeared comparable between La and Lb infection (Figure 2). Therefore, IFN-γ-producing CD4+ T cells in Leishmania infection are not directly related to TCR Vβ diversity.
The TCR diversity-related studies are well advanced in viral and bacterial infection in mouse models and humans. For example, several reports have shown the conserved TCR repertoire expansion in primary and memory CD8+ T-cell responses to lymphocytic choriomeningitis virus or influenza virus epitopes in mice (23,28). With regard to murine infection with intracellular bacteria Listeria monocytogenes, although the narrowed ‘private’ TCR Vβ repertoire was found within rechallenged individual mice, the antigen-specific T cells detected by a tetramer-based approach revealed a relatively diverse TCR Vβ repertoire in primary and memory CD8+ T-cell populations (29,30). Likewise, diverse TCR Vβ usages in CD4+ and CD8+ T cells were reported during pulmonary Cryptococcus neoformans infection in mice (31). Because protozoan parasites contain relatively large genome sizes and complex protein profiles but replicate relatively slow in vivo, our findings of a diverse rather than focused TCR Vβ repertoire in FACS analyses of CD4+ T cells during Leishmania infection may not be surprising.
The potential concerns of this FACS-based approach include its biological relevance and detection limit. We took two approaches to address these issues. First, we performed detailed analyses for IFN-γ production among several major Vβ subsets (Vβ4, 6 and 8) and a minor Vβ subset (Vβ7). The interesting findings are (1) in comparison with La infection counterparts, Lb infection showed higher percentages of IFN-γ-producing cells in each of the tested individual TCR Vβ subsets in primary (Figure 2) and secondary infection (Figures 3 and 4) and (2) for a given Vβ subset, its relative contribution to IFN-γ production appeared comparable in La versus Lb infection, judged by the percentages of IFN-γ+ cells within its Vβ+ cells. These functional analyses again suggest a diverse rather than focused TCR Vβ repertoire in Leishmania infection. Second, we examined the CDR3 region of individual TCR Vβ by PCR- and gel-based techniques, because PCR-based spectratyping is a powerful tool to analyse the sizes of TCR CDR3 regions of the oligoclonal expansion of T cells (16–18). It was consistent that multiple clonalities in Ag-restimulated CD4+ T cells from Lb- and La-infected mice and that CD4+ cells from Lb-infected mice showed a tendency of high values in multiplication factor index, suggesting a strong magnitude of T-cell responses (Supplemental Figure S1). Because FACS- and PCR-based analyses examine T-cell clonality from different aspects, the future development of tetramer-based FACS on Leishmania Ag-specific CD4+ T cells would be helpful for accurate assessment. Nevertheless, results from these studies clearly indicate the magnitude of CD4+ T-cell activation induced by different Leishmania species correlates with infection outcome.
Having demonstrated strong T-cell activation and IFN-γ production in Lb infection, we then examined whether pre-infection with Lb could provide cross-protection against secondary La infection via an enhanced T-cell activation. We showed that this cross-protection correlated nicely with the increased T-cell activation and IFN-γ production from CD4+ T cells (Figure 3), a finding consistent with previous studies on L. major pre-infection followed with a secondary infection with La or L. mexicana parasites (24,32). Again, the tested 4 Vβ subset contributed comparably to IFN-γ production. Because the quality or multifunctional capacity of CD4+ T cells is a crucial determinant in vaccine-mediated protection against L. major (33) and malaria (34), we investigated the production of several cytokines from CD4+ CD44+ effector T cells in La- or Lb-infected mice. It was evident that CD4+ CD44+ T cells derived from Lb-infected mice tended to produce high levels of IFN-γ, but low levels of IL-10 and IL-17 (Figure 5a), and that this Th1-favoured response was maintained even when cells were stimulated in vitro with La antigen (Figure 5b). Therefore, the healing from control of Leishmania infection requires sequential events that include efficient dendritic cell activation (5), adequate innate responses (12) and activated Th1-type responses to a relatively broad spectrum of parasite antigens. Notably, the adoptive transfer of Lb-specific CD4+ T cells into naïve mice failed to protect mice against the subsequent La infection (data not shown), a finding consistent with a previous report showing the lack of protection against L. mexicana infection following the adoptive transfer of L. major-specific CD3+ T cells (24). The reasons for this lack of cross-protection by cell transfer alone may include the maintenance of effective Th1 responses and cell recruitment in the recipients, as well as the unique features of L. amazonensis and L. mexicana parasites (35).
In summary, our comparative analyses of CD4+ T cells in different models of cutaneous Leishmaniasis indicate that Leishmania infection does not change the diversity of the TCR Vβ repertoire in either self-healing or nonhealing model and that multiple TCR Vβ CD4+ T cells contribute collectively and comparably to IFN-γ production. It is clear in this study that the healing of Leishmania infection is positively correlated with the magnitude of CD4+ T-cell activation and overall levels of IFN-γ production, rather than to the TCR diversity among CD4+ T cells. The lack of a focused expansion of particular TCR-bearing CD4+ T cells in the primary and secondary infection models also suggests to us that multiple (rather than dominant) parasite antigens are recognized by the host. This study provides important information for the control of Leishmania infection.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mardelle Susman and Dr Jiaren Sun for critical reading of this manuscript, Dr Zhong Kou from the Bio-Med Immunotech for insightful discussion and TCR analyses and Dr Alai Tan for statistical analyses. This research was supported by National Institutes of Health Grants AI043003 to L. Soong.
Footnotes
Disclosures: None.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 2.Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–372. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- 3.Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun. 2002;70:2151–2158. doi: 10.1128/IAI.70.4.2151-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am J Trop Med Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 5.Vargas-Inchaustegui DA, Xin L, Soong L. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J Immunol. 2008;180:7537–7545. doi: 10.4049/jimmunol.180.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas-Inchaustegui DA, Tai W, Xin L, Hogg AE, Corry DB, Soong L. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect Immun. 2009;77:2948–2956. doi: 10.1128/IAI.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeKrey GK, Lima HC, Titus RG. Analysis of the immune responses of mice to infection with Leishmania braziliensis. Infect Immun. 1998;66:827–829. doi: 10.1128/iai.66.2.827-829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Moura TR, Novais FO, Oliveira F, et al. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect Immun. 2005;73:5827–5834. doi: 10.1128/IAI.73.9.5827-5834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha FJ, Schleicher U, Mattner J, Alber G, Bogdan C. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect Immun. 2007;75:3823–3832. doi: 10.1128/IAI.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holaday BJ, Sadick MD, Wang ZE, et al. Reconstitution of Leishmania immunity in severe combined immunodeficient mice using Th1- and Th2-like cell lines. J Immunol. 1991;147:1653–1658. [PubMed] [Google Scholar]
- 12.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soong L, Chang CH, Sun J, et al. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J Immunol. 1997;158:5374–5383. [PubMed] [Google Scholar]
- 14.Schatz DG. V(D)J recombination. Immunol Rev. 2004;200:5–11. doi: 10.1111/j.0105-2896.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 15.Gras S, Kjer-Nielsen L, Burrows SR, McCluskey J, Rossjohn J. T-cell receptor bias and immunity. Curr Opin Immunol. 2008;20:119–125. doi: 10.1016/j.coi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Kou ZC, Puhr JS, Wu SS, Goodenow MM, Sleasman JW. Combination antiretroviral therapy results in a rapid increase in T cell receptor variable region beta repertoire diversity within CD45RA CD8 T cells in human immunodeficiency virus-infected children. J Infect Dis. 2003;187:385–397. doi: 10.1086/367674. [DOI] [PubMed] [Google Scholar]
- 17.Stuge TB, Holmes SP, Saharan S, et al. Diversity and recognition efficiency of T cell responses to cancer. PLoS Med. 2004;1:e28. doi: 10.1371/journal.pmed.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim G, Tanuma N, Kojima T, et al. CDR3 size spectratyping and sequencing of spectra-type-derived TCR of spinal cord T cells in autoimmune encephalomyelitis. J Immunol. 1998;160:509–513. [PubMed] [Google Scholar]
- 19.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 20.Launois P, Maillard I, Pingel S, et al. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 21.Julia V, McSorley SS, Malherbe L, et al. Priming by microbial antigens from the intestinal flora determines the ability of CD4+ T cells to rapidly secrete IL-4 in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:5637–5645. doi: 10.4049/jimmunol.165.10.5637. [DOI] [PubMed] [Google Scholar]
- 22.Launois P, Gumy A, Himmelrich H, Locks-ley RM, Rocken M, Louis JA. Rapid IL-4 production by Leishmania homolog of mammalian RACK1-reactive CD4+ T cells in resistant mice treated once with anti-IL-12 or -IFN-gamma antibodies at the onset of infection with Leishmania major instructs Th2 cell development, resulting in nonhealing lesions. J Immunol. 2002;168:4628–4635. doi: 10.4049/jimmunol.168.9.4628. [DOI] [PubMed] [Google Scholar]
- 23.Sourdive DJ, Murali-Krishna K, Altman JD, et al. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu AC, Scott P. Leishmania mexicana infection induces impaired lymph node expansion and Th1 cell differentiation despite normal T cell proliferation. J Immunol. 2007;179:8200–8207. doi: 10.4049/jimmunol.179.12.8200. [DOI] [PubMed] [Google Scholar]
- 25.Pingel S, Launois P, Fowell DJ, et al. Altered ligands reveal limited plasticity in the T cell response to a pathogenic epitope. J Exp Med. 1999;189:1111–1120. doi: 10.1084/jem.189.7.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 27.Gurunathan S, Sacks DL, Brown DR, et al. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedzierska K, Turner SJ, Doherty PC. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc Natl Acad Sci U S A. 2004;101:4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch DH, Pilip I, Pamer EG. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J Exp Med. 1998;188:61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huleatt JW, Pilip I, Kerksiek K, Pamer EG. Intestinal and splenic T cell responses to enteric Listeria monocytogenes infection: distinct repertoires of responding CD8 T lymphocytes. J Immunol. 2001;166:4065–4073. doi: 10.4049/jimmunol.166.6.4065. [DOI] [PubMed] [Google Scholar]
- 31.Lindell DM, Ballinger MN, McDonald RA, Toews GB, Huffnagle GB. Diversity of the T-cell response to pulmonary Cryptococcus neoformans infection. Infect Immun. 2006;74:4538–4548. doi: 10.1128/IAI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanloubbeeck Y, Jones DE. Protection of C3HeB/FeJ mice against Leishmania amazonensis challenge after previous Leishmania major infection. Am J Trop Med Hyg. 2004;71:407–411. [PubMed] [Google Scholar]
- 33.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 34.Huaman MC, Mullen GE, Long CA, Mahanty S. Plasmodium falciparum apical membrane antigen 1 vaccine elicits multi-functional CD4 cytokine-producing and memory T cells. Vaccine. 2009;27:5239–5246. doi: 10.1016/j.vaccine.2009.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynn MA, McMaster WR. Leishmania: conserved evolution–diverse diseases. Trends Parasitol. 2008;24:103–105. doi: 10.1016/j.pt.2007.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.