Abstract
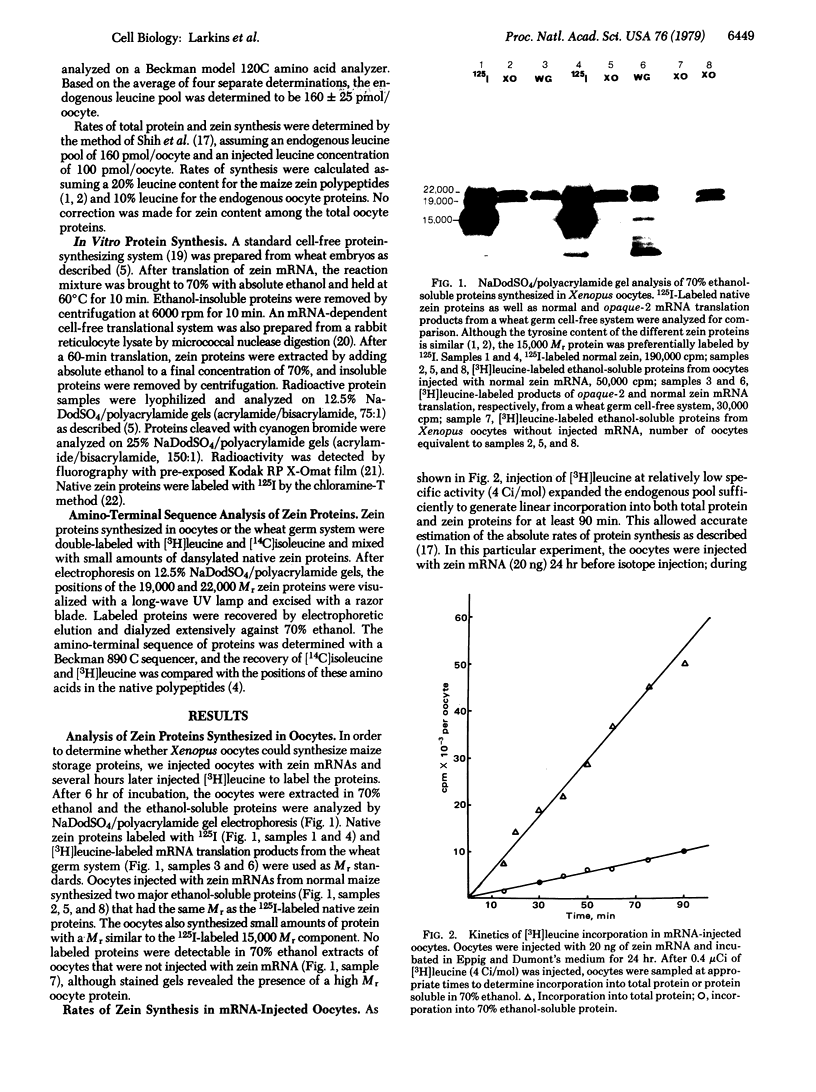
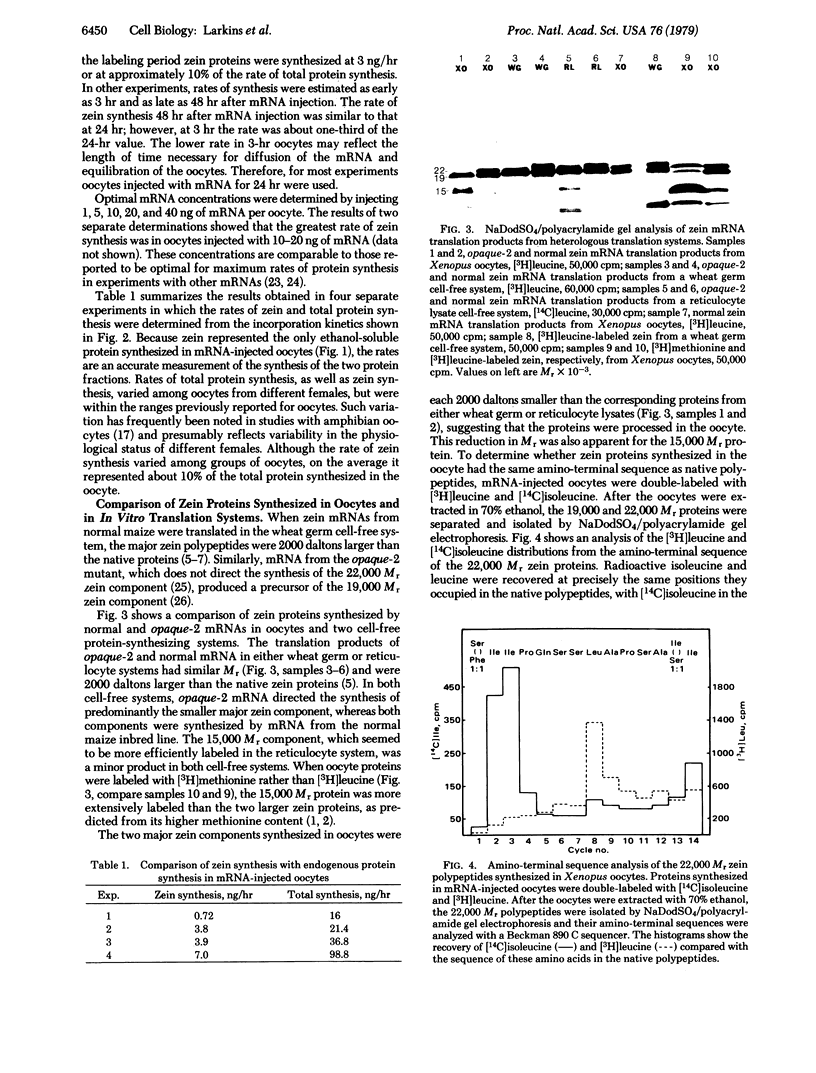
Xenopus oocytes injected with zein mRNAs efficiently synthesized maize storage proteins for prolonged periods. Under optimal conditions, zein was synthesized at 3 ng/hr and represented approximately 10% of the total protein synthesized in the oocyte. The mRNA from the normal maize inbred line directed synthesis of all the major zein components; however, products of mRNA from the opaque-2 mutant did not contain the largest zein component. Zein proteins synthesized in the oocyte were 2000 daltons smaller than proteins synthesized by cell-free translation of mRNAs in the wheat germ and reticulocyte systems. This result, which suggested that the oocyte processed prezein polypeptides into native zein proteins, was confirmed by amino-terminal sequence analysis of zein proteins from the oocytes. Cyanogen bromide cleavage of translation products from oocytes and the wheat germ system confirmed the existence of several proteins within each of the major zein components. However, we were unable to detect the presence of signal sequences on zein peptides by this technique.
Keywords: signal peptide, seed protein, zein mRNA
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A., Rubenstein I., Simon M. N. Purification and translation of zein messenger RNA from maize endosperm protein bodies. Proc Natl Acad Sci U S A. 1978 Feb;75(2):696–700. doi: 10.1073/pnas.75.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J. J., Jr, Dumont J. N. Amino acid pools in developing oocytes of Xenopus laevis. Dev Biol. 1972 Jul;28(3):531–536. doi: 10.1016/0012-1606(72)90036-x. [DOI] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H., Gurdon J. B. Studies of the injection of poly(A)+ protamine mRNA into Xenopus laevis oocytes. Exp Cell Res. 1978 Dec;117(2):325–334. doi: 10.1016/0014-4827(78)90146-5. [DOI] [PubMed] [Google Scholar]
- Jensen J. N., Merkle J. R., Rasmussen D. I. Locus number and multiple hemoglobins in Eutamias and Peromyscus. Biochem Genet. 1976 Aug;14(7-8):541–545. doi: 10.1007/BF00485833. [DOI] [PubMed] [Google Scholar]
- Jones R. A., Larkins B. A., Tsai C. Y. Reduced synthesis of zein in vitro by a high lysine mutant of maize. Biochem Biophys Res Commun. 1976 Mar 22;69(2):404–410. doi: 10.1016/0006-291x(76)90536-2. [DOI] [PubMed] [Google Scholar]
- Kindas-Mügge I., Lane C. D., Kreil G. Insect protein synthesis in frog cells: the translation of honey bee promelittin messenger RNA in Xenopus oocytes. J Mol Biol. 1974 Aug 15;87(3):451–462. doi: 10.1016/0022-2836(74)90096-5. [DOI] [PubMed] [Google Scholar]
- LaMarca M. J., Smith L. D., Strobel M. C. Quantitative and qualitative analysis of RNA synthesis in stage 6 and stage 4 oocytes of Xenopus laevis. Dev Biol. 1973 Sep;34(1):106–118. doi: 10.1016/0012-1606(73)90342-4. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Gurdon J. B., Partington G. A. Protein synthesis in oocytes of Xenopus laevis is not regulated by the supply of messenger RNA. Cell. 1977 Jun;11(2):345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher U. In Vitro Synthesis of a Precursor to the Methionine-rich Polypeptide of the Zein Fraction of Corn. Plant Physiol. 1979 Feb;63(2):354–358. doi: 10.1104/pp.63.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A., Thiele B. J., Prehn S., Marbaix G., Cleuter Y., Hubert E., Huez G. Synthesis of carp proinsulin in Xenopus oocytes. Eur J Biochem. 1978 Jun 15;87(2):229–233. doi: 10.1111/j.1432-1033.1978.tb12370.x. [DOI] [PubMed] [Google Scholar]
- Shih R. J., O'Connor C. M., Keem K., Smith L. D. Kinetic analysis of amino acid pools and protein synthesis in amphibian oocytes and embryos. Dev Biol. 1978 Sep;66(1):172–182. doi: 10.1016/0012-1606(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Van der Donk J. A. Translation of plant messengers in egg cells of Xenopus laevis. Nature. 1975 Aug 21;256(5519):674–675. doi: 10.1038/256674a0. [DOI] [PubMed] [Google Scholar]
- Vassart G., Brocas H., Lecocq R., Dumont J. E. Thyroglobulin messenger RNA: translation of a 33-S mRNA into a peptide immunologically related to thyroglobulin. Eur J Biochem. 1975 Jun 16;55(1):15–22. doi: 10.1111/j.1432-1033.1975.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Zehavi-Willner T., Lane C. Subcellular compartmentation of albumin and globin made in oocytes under the direction of injected messenger RNA. Cell. 1977 Jul;11(3):683–693. doi: 10.1016/0092-8674(77)90085-x. [DOI] [PubMed] [Google Scholar]