Abstract
One carbon metabolism, or methyl transfer, is critical for metabolism in all cells, is involved in the synthesis of purines, pyrimidines, in the methylation of numerous substrates, proteins, DNA and RNA, and in the expression of a number of genes. Serine is the primary endogenous methyl donor to the one carbon pool. Perturbations in methyl transfer due to nutrient and hormonal changes can have profound effect on cell function, growth and proliferation. It is postulated that at critical stages in development, nutrient and environmental influences by their effect on methyl transfer can impair fetal growth, reprogram metabolism and cause long term morbidity in the offspring. The potential for their effects is underscored by the unique gestation related changes in methyl transfer in healthy women, the late expression of transsulfuration cascade in the fetus and the unique metabolism of glycine and serine in the fetus. Dietary protein restriction in animal models and protein malnutrition in humans causes remarkable changes in the methyl transfer in-vivo. Although the specific consequences of perturbation in maternal and fetal methyl transfer remain to be determined, a profound influence is suggested by the demonstrated relationship between maternal folate and B12 insufficiency and metabolic programming.
Introduction
Epidemiological and observational data from studies in human and data from experimental animal models have now established the relationship between impaired fetal growth and long term morbidity in the offspring [1-4]. These data suggest that nutritional and environmental influences at critical times during development can lead to programming of the metabolism of the fetus, cause alterations in growth and the development of chronic non-communicable diseases in adult life (the Developmental Origin of Health and Disease paradigm). Although much has been learnt regarding the molecular, metabolic and physiological associations and mechanisms of the changes in the offspring, the metabolic and physiological changes in the mother that mediate the observed changes in the developing organism have not been studied in detail. Methyl transfer or one carbon metabolism is the key component of cellular metabolism, is involved in synthesis of purines, pyrimidines, and methylation of a number of substrates, proteins, DNA, RNA and indirectly, expression of a number of genes. The non-essential amino acid serine, folate and the essential amino acid methionine constitute the key components of methyl transfer. Since the methionine and folate cycles are ubiquitously present in every cell in the body and participate in key metabolic reactions, perturbation in their metabolism by nutrient deficiency, or by nutrient, hormonal and environmental interactions can have profound impact on the cell function, metabolism, growth and proliferation. Interest in the physiological changes in maternal-fetal and neonatal one carbon metabolism, particularly in humans, has been primarily focused on the consequence of micronutrient deficiencies. Clinical, physiological and molecular data from studies in human and that from animal models suggest that alterations in methyl transfers may be critical contributors to the impaired fetal growth and to the re-programming of the metabolism of developing embryo and the fetus, leading to morbidity in the offspring. In this review some of the data in support of such a concept are presented. Future studies will delineate the hormonal, nutritional and environmental contributors to such perturbations and allow us to develop targeted intervention strategies.
One Carbon Metabolism (Methyl transfers) in-vivo
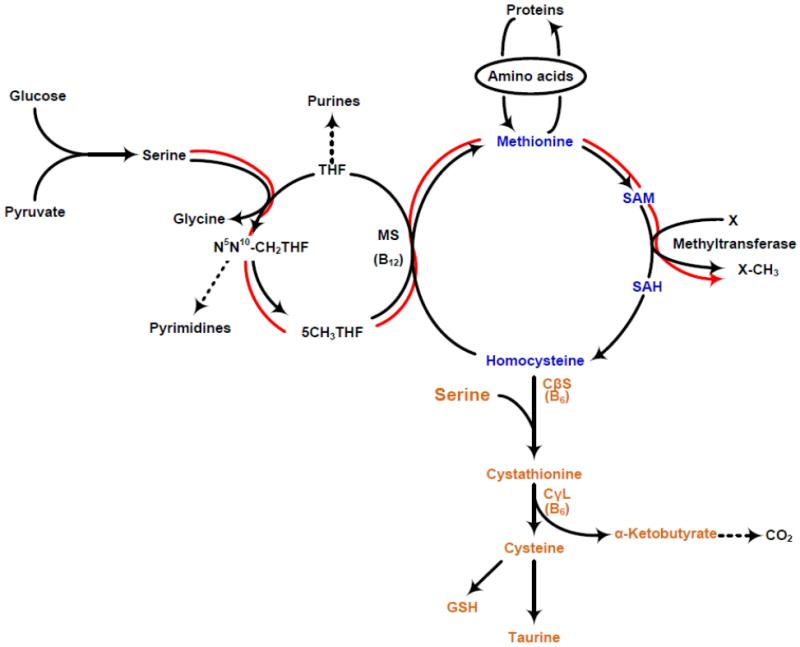
One carbon pool refers to the pool of the methyl groups that are available for the methylation of a variety of compounds, including protein, DNA, RNA etc. This process involves the methyl form of tetrahydrofolate (THF) and is carried out by s-adenosyl methionine (SAM). As shown in figure 1, the transfer of methyl group involves both the folate cycle and the ubiquitous methionine cycle, also called the transmethylation cycle or the “activated” methionine cycle. L-serine, a nutritionally non-essential amino acid, is the primary endogenous methyl donor. Stable isotope tracer studies in young healthy volunteers show that almost 100% of the methyl groups used for whole body remethylation of homocysteine are derived from serine under the conditions of their study [5]. The latter is important, since the tracer studies were done after an overnight fast, however in a fed state, while the subjects were give a protein free isocaloric diet. Previous data in isolated perfused liver preparation had also shown serine to be the major contributor to the methyl groups used for the transmethylation of homocysteine. During fasting, serine is released into the circulation by the kidney and is taken up by most organs, including the skeletal muscle and the liver; the latter being quantitatively the most important consumer of serine [6,7]. As shown in figure 1, the methyl group (beta carbon) of serine is transferred to THF, catalyzed by serine hydroxyl methyl transferase (SHMT), resulting in the formation of glycine and N5-N10methylene THF, which is then converted to 5-methyl THF, catalyzed by methylenetetrahydrofolate reductase (MTHFR). The methylation of homocysteine involves the transfer of methyl group from 5-CH3THF by methionine synthase (MS). Vitamin B12 is a cofactor for this reaction. The other contributor to the methyl groups for methylation of homocysteine, primarily in the liver, is betaine; however,its quantitative contribution has not been measured in-vivo. S-adenosyl methionine (SAM) is the key intermediate of the activated methionine cycle, is the universal bioactive methyl donor and participates in numerous methyl transferase reactions resulting in methylated products (X-CH3). SAM is also the primary allosteric regulator of the methionine metabolism in-vivo. The catabolic pathway of methionine is the transsulfuration cascade. This pathway involves the condensation of homocysteine with serine to form cystathionine, which is then converted to cysteine, α-ketobutyrate and ammonia catalyzed by cystathione γ-lyase (CγL). Cysteine is the precursor of taurine, as well as the component of glutathione, the major intracellular antioxidant.
Figure 1.
One carbon metabolism or methyl transfers in-vivo; shown are folate cycle (black), methionine transmethylation cycle (blue), and the methionine transsulfuration cascade(orange) The flow of methyl groups from serine to methyltransferase is shown in red. THF: tetrahydrofolate; 5 CH3THF: 5 methyl tetrahydrofolate; MS:methionine synthase; SAM: s-adenosyl methionine; SAH: s-adenosylhomocysteine; CβS: cystathionine beta synthase; CγL: cystathionine gamma lyase.
The metabolism of serine, folate and methionine have been studied extensively and discussed in several reviews. It is important to note that three vitamins, folate cobalamine (B12) and pyridoxine (B6) are directly involved in the metabolism of methionine. Insulin and glucagon exert their effect on methionine metabolism by (a) directly affecting the activity of transsulfuration cascade and methionine synthase and (b) indirectly by their effect on whole body protein turnover and therefore effecting methionine flux. In addition, several reactions in the methionine metabolism respond to the change in the cellular redox state.
Methionine and Serine metabolism in human pregnancy
The adaptive changes in methionine metabolism during pregnancy have been examined by us in healthy women studied early and late in gestation and compared them with non-pregnant controls [8]. The data show that the fractional rate and the total rate of transsulfuration of methionine was significantly increased during early gestation with a decrease to that seen in non-pregnant subjects in the third trimester. In contrast the rate of transmethylation, measure of one carbon transfers, was markedly higher in the third trimester of pregnancy. The high rates of transmethylation was speculated to be the consequence of higher methylation demands in the later part of pregnancy.
The rate of appearance of serine was quantified in healthy pregnant women by Kalhan et al using [2-15N13C]serine tracer [9]. Plasma serine concentration and the rate of appearance of serine were lower in pregnant women in both early and in late gestation as compared with healthy non-pregnant women. The rate of appearance of serine was significantly less in the 3rd trimester of pregnancy when compared with early pregnancy (serine Ra: Early (n=12) 123.7 ± 21.5, late (n=8) 102.8 ± 18.2 μmole.kg−1.h−1, mean ± SD). The isotopic tracer used in this study would have recycled between serine and glycine and therefore would have resulted in an underestimation of serine flux. The lower estimation of serine flux would be proportional to the magnitude of tracer recycling. Assuming no significant change in actual serine turnover between early and late gestation, the higher rate of recycling is qualitatively consistent with the data in sheep fetus showing higher rate of serine glycine flux in the fetal compartment (discussed below) and with the higher rate of transmethylation of methionine observed in healthy pregnant women[8].
Serine and glycine metabolism in the placenta and the fetus
The transport of serine and glycine from the mother to the fetus has been examined both in animal models and in humans. The data in humans have been obtained at term gestation. These data show a higher concentration of both serine and glycine in the fetal umbilical vein than in a simultaneously obtained maternal arterial sample [10,11]. In addition, an infusion of amino acid mixture to the mother prior to caesarian section delivery resulted in a significant increase in all amino acids including serine and glycine in the fetus [12]. Bolus infusion studies using tracer isotopes of glycine, leucine and phenylalanine in human pregnancy show a much greater dilution or lower enrichment of glycine tracer in the fetal compartment as compared with leucine or phenylalanine [13]. The authors interpreted these data to suggest a slower transfer rate for glycine when compared with leucine or phenylalanine. However as also suggested by them, these data could indicate a significant production of glycine by the fetus or by the placenta [13]. However as shown by Lewis and colleague a low serine hydroxymethyltransferase activity in the human placenta ( as compared with sheep) would suggest that placental production of glycine from serine may not be a significant source of fetal glycine [14]. Other data in chronically catheterized sheep fetus show a uteroplacental uptake of serine from the mother, no net transport of serine from the mother to the fetus and a virtual equimolar (to serine uptake) release of glycine from the placenta into the fetal compartment [15,16]. These data suggest that the ovine placenta converts large quantities of maternal serine into glycine and releases it into the fetus. Additionally, studies by Cetin and colleagues demonstrated production of serine from glycine by the fetal liver in a chronically catheterized sheep preparation [17,18]. Thus a unique inter-organ cycling of serine and glycine occurs in the sheep fetus and placenta where in glycine is released by the placenta into the fetal circulation, glycine is then taken up by the fetal liver and converted to serine which in turn is taken up by the placenta in substantial quantities. Of significance were remarkably high turnover rates of serine and glycine as compared with leucine, in the fetus (Glycine: ~720 micromoles.kg−1.h−1, Serine: ~2700 micromoles.kg−1..h−1. . Leucine: ~52 micromoles, kg−1.h−1.). Taken together, these studies suggest a high rate of serine and glycine inter-conversion and therefore methyl (one carbon) transfers in the placenta and the fetal liver with some quantitative differences between species. Such studies cannot be done in humans, however, these data are consistent with the higher rates of transmethylation measured in human pregnancy during the third trimester using stable isotopic tracers [8].
One Carbon Metabolism and the Fetus
Effect of dietary protein
Isocaloric protein restriction in pregnancy in the rodents impairs fetal growth and causes programming of the metabolism in the offspring [reviewd in 3,4]. Collectively these data show that protein restriction during various periods of pregnancy, results in fetal growth retardation and is associated with pancreatic dysfunction, impaired glucose, homeostasis, hypertension, changes in circadian rhythm and other metabolic dysfunction in the offspring. The possible mechanisms of these changes in the fetus and the offspring have been reported and discussed in several excellent reviews (83-87). However, there is paucity of data regarding changes in the maternal metabolism that are responsible for the observed responses in the fetus. Petrie and colleagues reported high serum concentrations of homocysteine early in pregnancy (day 4) in rat placed on protein restricted diet [19]. Other changes in maternal metabolic and amino acid patterns have been reported. These data are difficult to interpret because of the differences in time of sampling in relation to gestation, the controls not being pair fed and significant differences in dietary regimens employed. It is also important to recognize the differences in the metabolic responses to isocaloric protein restriction as compared with simple protein restriction. Simple protein, energy restriction could be in part compensated by increase in overall food consumption. In addition, isocaloric restriction results in suppression of adipose tissue and skeletal muscle responses seen with total energy restriction, i.e., mobilization of fatty acids and amino acids from lipolysis and proteolysis.
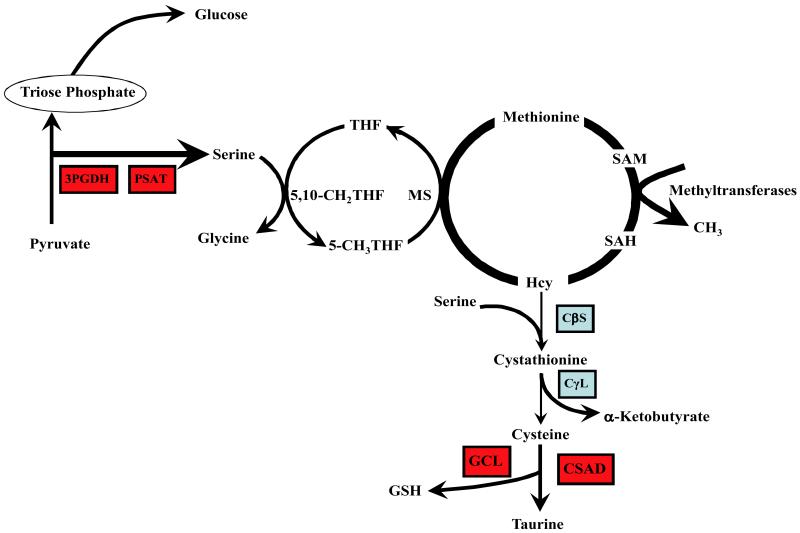
Parimi and colleagues examined changes in plasma amino acids in pair fed pregnant rats on a protein restricted diet [20]. They showed no change in total α-amino nitrogen in the plasma in early gestation and an increase late in pregnancy. The increase in total α-amino nitrogen was primarily due to increase in serine, glycine and glutamine concentration. Rees et al [21] also reported a significant increase in the plasma glycine concentration in response to dietary protein restriction in pregnant rat. Since serine and glycine are primary methyl donors, a change in their levels, along with higher homocysteine levels noted above, suggest change in one carbon or methyl transfer in response to dietary protein restriction . Although such studies cannot be done in human population, an observational study in (non-pregnant) humans showed that subclinical protein malnutrition evidenced by lower transthyretin levels was associated with increase in plasma homocysteine levels, suggesting an impaired methionine-homocysteine metabolism [22]. The plasma concentration of most essential amino acids was lower in the malnourished group, while there was no significant change in the plasma methionine levels, suggesting an independent regulation of methionine levels possibly by down-regulating the transsulfuration cascade. The plasma concentrations of pyridoxal-5-phosphate (vitamin B6) and folate of the malnourished group were not different from controls, while cobalamines (B12) were lower only in the severely malnourished (state III) subjects [22]. Recently, the same investigators have reported hyperhomocysteinemia in a vegetarian, plant-eating population of Chad, who had a lower dietary intake of protein and sulfur amino acids [23]. Their data suggest a direct effect of lower protein intake on methionine-homocysteine and one carbon metabolism. Our group has examined the effect of isocaloric protein restriction in the rat on methyl transfers and methionine metabolism [24]. The data show that dietary protein restriction, in this instance for 7-10 days, resulted in profound change in hepatic metabolism (Fig.2), was associated with differential expression of a number of genes involved in cell cycle, differentiation, transcription, transport, and other metabolic processes. Of importance, there was marked increase in serine biosynthesis and methionine transmethylation and decrease in the activity of transsulfuration cascade (Fig.2) ). All these data underscore the important role of adequate protein intake on methyl transfers, an effect that is independent of the vitamin status. The observed fetal programming effects of maternal protein restriction could be mediated via changes in one carbon metabolism in the mother. Such a hypothesis is supported by the data demonstrating an amelioration of the changes caused by protein restriction when the animals were supplemented with methyl donors like glycine and folate [25].
Figure 2.
Effect of isocaloric protein restriction on methyl transfer in the rat. Rats were placed on a casein based 6% protein diet for 7 to 10 days, controls were on a 24% protein diet and were pair fed. Dietary protein restriction resulted in a marked upregulation (red boxes) of 3-phosphoglycerate dehydrogenase (3PGDH), phosphoserineaminotransferase (PSAT), glutamate-cysteine ligase (GCL),and cysteinesulfinic acid decarboxylase (CSAD).The activity of cystathionine beta synthase (CβS) and cystathionine gamma lyase (CγL) was decreased ( blue boxes). Tracer dilution measured rate of appearance of serine and the rate of transmethylation of methionine ware increased (heavy arrows). Figure reproduced from Kalhan et al, Journal of Biological Chemistry 286:5266-5277,2011.
Folate and Vitamin B12
The clinical evidence relating maternal folate and Vitamin B12 status during pregnancy and the long term consequences to the offspring is discussed by Dr. Yajnik (next chapter). As noted above, folate is an integral part of the transfer of methyl groups from serine or other methyl donors like betaine for the methylation of homocysteine to form methionine (figure 1). Vitamin B12 is a co-factor for the enzyme methionine synthase (homocysteine methyl transferase). Insufficiency of either folate or B12 will affect the methyl transfer by influencing methylation of homocysteine. Iatrogenically induced folate deficiency in healthy human subjects resulted in significant increase in plasma homocysteine levels and attenuation of the rate of methionine synthesis via remethylation of homocysteine [26]. Such kinetic studies have not been done in B12 deficient subjects, however, B12 deficiency by decreasing the activity of methionine synthase will be expected to suppress methylation of homocysteine. These data indicate that both folate and B12 deficiencies can influence maternal-fetal metabolism and cause long term consequences, by effecting methyl transfers in the mother and the fetus. A direct evidence of such a cause and effect relationship is not available as yet. Folate deficiency during pregnancy in the rat has been shown to effect methyl metabolism, however, it did not impact global DNA methylation in the fetus [27]. Whether there were changes in the methylation pattern of specific genes was not determined in that study.
Homocysteine
Alterations in one carbon metabolism as a result of nutrient or other influences result in an increase in plasma concentrations of homocysteine, the demethylated product of methionine and an intermediate amino acid in the methionine transmethylation cycle ( fig.1). Homocysteine does not participate in protein synthesis and an increase in plasma levels of homocysteine reflect altered intracellular homocysteine, and one carbon metabolism. The relationship between elevated homocysteine levels and vascular disease, thrombosis, vascular endothelial dysfunction has been studied extensively and discussed in excellent scientific reviews [28]. A number of studies have shown a pregnancy related decrease in plasma homocysteine concentration in healthy women. Whether homocysteine directly causes impaired fetal growth or via its effect on placental growth and function via its vascular effects has not been delineated in studies in human. An association between elevated levels of homocysteine and pregnancy related disorders such as pre-eclampsia, early pregnancy loss, abruption placentae have been reported [reviewed in 29]. A recent systematic review and meta-analysis by Hogeveen and colleagues concluded that higher maternal total homocysteine concentrations are associated with a small increased risk of small for gestational age offspring. It corresponded to a decrease in birth weight of 31g (95%CI:−13,−51g) for 1-SD increase in maternal total homocysteine [30]. The authors concluded that the small birth weight difference might be of little clinical relevance, but may be of greater importance at the population levels. However the long-term programming consequences of such impairment of growth have not been determined. It is likely, that the attenuated fetal growth, as reflected in lower birth weight is only a crude reflection of the altered metabolic and epi-genomic changes in the growing fetus that may lead to programming and morbidity in later life.
Multinutrient deficiencies
As noted above folate and B12 deficiencies cause a decrease in the transmethylation pathway be impacting the transfer of the methyl groups to homocysteine. In contrast protein malnutrition in humans and in animal models causes an increase in transmethylation, in addition to suppressing the transsulfuration cascade. On the other hand deficiency of B6 did not appear to have any significant effect on activated methionine cycle. Thus the net effect of these opposing responses to multiple nutrient insufficiency, commonly seen in developing societies, is not known. It is speculated that the combined effect of multiple nutrient insufficiency on one carbon metabolism will be the sum of individual affects and could result in a spectrum of responses from low rate of transmethylation (dominant Vitamin B12 and folate deficiency) to high rates of transmethylation (in a predominant protein deficiency state) and modified by the hormonal milieu and the physiological adaptation (as in healthy pregnancy) of the organism. Such effects cannot be discovered from single measurement of biomarkers such as measurement of plasma homocysteine, SAM/SAH ratio or by measurement of single micronutrient. These responses may also explain the lack of significant effect on for example birth weight when nutrient intervention involves a single micro or macronutrient.
Summary.
Methionine, an essential amino acid, along with folic acid are key components of one-carbon metabolism in every cell in the body. Specific changes in the one carbon metabolism have been identified in the mother during pregnancy, in the placenta and in the fetus during development. Methionine and one carbon metabolism can easily be altered by nutrient and hormonal mediators and may cause specific changes in many organs in the mother, placenta and the fetus. We hypothesize that changes in one carbon metabolism as a result of nutrient (vitamins and protein) insufficiency cause fetal growth retardation by nutrient gene interaction and cause permanent changes, leading to adult disease like diabetes, obesity and hypertension.
Acknowledgements
The cited work from the authors laboratory was supported by grants DK079937, HD042154, and RR024989 from the United States National Institutes of Health. Susan Marczewski’s help in preparation of this manuscript is gratefully appreciated.
References
- 1.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg Z, Feil R, Constancia M, et al. Child health, developmental plasticity, and epigenetic programming. Endocr Rev. 2011;32:159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozanne SE, Hales CN. Early programming of glucose-insulin metabolism. Trends Endocrinol Metab. 2002;13:368–73. doi: 10.1016/s1043-2760(02)00666-5. [DOI] [PubMed] [Google Scholar]
- 4.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–47. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 5.Davis SR, Stacpoole PW, Williamson J, et al. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–E279. doi: 10.1152/ajpendo.00351.2003. [DOI] [PubMed] [Google Scholar]
- 6.Brosnan JT, Hall B. Renal serine production in vivo: effects of dietary manipulation of serine status. Can J Physiol Pharmacol. 1989;67:1058–1061. doi: 10.1139/y89-167. [DOI] [PubMed] [Google Scholar]
- 7.Brundin T, Wahren J. Renal oxygen consumption, thermogenesis, and amino acid utilization during iv infusion of amino acids in man. Am. J. Physiol. 1994;267:E648–E655. doi: 10.1152/ajpendo.1994.267.5.E648. [DOI] [PubMed] [Google Scholar]
- 8.Dasarathy J, Gruca L, Bennett C, et al. Methionine metabolism in human pregnancy. Am J Clin Nutr. 2010;91:357–65. doi: 10.3945/ajcn.2009.28457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalhan SC, Gruca LL, Parimi PS, et al. Serine metabolism in human pregnancy. Am J Physiol Endocrinol Metab. 2003;284:E733–E740. doi: 10.1152/ajpendo.00167.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cetin I, Hirst K, Corbetta C, et al. Plasma and erythrocyte amino acids in mother and fetus. Biol Neonate. 1991;60:83–91. doi: 10.1159/000243392. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi S, Sanada K, Sagawa N, et al. Umbilical vein artery differences of plasma amino acids in the last trimester of human pregnancy. Biol Neonate. 1978;34:11–18. doi: 10.1159/000241099. [DOI] [PubMed] [Google Scholar]
- 12.Ronzoni S, Marconi AM, Cetin I, et al. Umbilical amino acid uptake at increasing maternal amino acid concentrations: effect of a maternal amino acid infusate. Am J Obstet Gynecol. 1999;181:477–83. doi: 10.1016/s0002-9378(99)70581-8. [DOI] [PubMed] [Google Scholar]
- 13.Cetin I, Marconi AM, Baggiani AM, et al. In vivo placental transport of glycine and leucine in human pregnancies. Pediatr Res. 1995;37:571–575. doi: 10.1203/00006450-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RM, Godfrey KM, Jackson AA, et al. Low serine hydroxymethyltransferase activity in the human placenta has important implications for fetal glycine supply. J Clin Endocrinol Metab. 2005;90:1594–1598. doi: 10.1210/jc.2004-0317. [DOI] [PubMed] [Google Scholar]
- 15.Chung M, Teng C, Timmerman M, et al. Production and utilization of amino acids by ovine placenta in vivo. Am J Physiol. 1998;274:E13–E22. doi: 10.1152/ajpendo.1998.274.1.E13. [DOI] [PubMed] [Google Scholar]
- 16.Geddie G, Moores R, Meschia G, et al. Comparison of leucine, serine and glycine transport across the ovine placenta. Placenta. 1996;17:619–27. doi: 10.1016/s0143-4004(96)80080-4. [DOI] [PubMed] [Google Scholar]
- 17.Cetin I, Fennessey PV, Quick AN, Jr, et al. Glycine turnover and oxidation and hepatic serine synthesis from glycine in fetal lambs. Am J Physiol. 1991;260:E371–E378. doi: 10.1152/ajpendo.1991.260.3.E371. [DOI] [PubMed] [Google Scholar]
- 18.Cetin I, Fennessey PV, Sparks JW, et al. Fetal serine fluxes across fetal liver, hindlimb, and placenta in late gestation. Am J Physiol. 1992;263:E786–E793. doi: 10.1152/ajpendo.1992.263.4.E786. [DOI] [PubMed] [Google Scholar]
- 19.Petrie L, Duthie SJ, Rees WD, et al. Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br J Nutr. 2002;88:471–477. doi: 10.1079/BJN2002695. [DOI] [PubMed] [Google Scholar]
- 20.Parimi PS, Cripe-Mamie C, Kalhan SC. Metabolic responses to protein restriction during pregnancy in rat and translation initiation factors in the mother and fetus. Pediatr Res. 2004;56:423–31. doi: 10.1203/01.PDR.0000136277.10365.84. [DOI] [PubMed] [Google Scholar]
- 21.Rees WD, Hay SM, Buchana v, et al. The effect of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br J Nutr. 1999;81:243–50. [PubMed] [Google Scholar]
- 22.Ingenbleek Y, Hardilliera E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition. 2002;18:40–46. doi: 10.1016/s0899-9007(01)00783-3. [DOI] [PubMed] [Google Scholar]
- 23.Ingenbleek Y, McCully KS. Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia and atherogenesis. Nutrition. 2012;28:148–153. doi: 10.1016/j.nut.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Kalhan SC, Uppal SO, Moorman JL, et al. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J Biol Chem. 2011;286:5266–5277. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillycrop KA, Phillips ES, Jackson AA, et al. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 26.Cuskelly GJ, Stacpoole PWS, Williams J, et al. Deficiencies of folate and vitamin B6 exert distinct effects on homocysteine, serine, and methionine kinetics. Am J Physiol Endocrinol Metab. 2001;281:E1182–E1190. doi: 10.1152/ajpendo.2001.281.6.E1182. [DOI] [PubMed] [Google Scholar]
- 27.Maloney CA, Hay SM, Rees WD. Folate deficiency during pregnancy impacts on methyl metabolism without affecting global DNA methylation in the rat fetus. Br J Nutr. 2007;97:1090–1098. doi: 10.1017/S0007114507670834. [DOI] [PubMed] [Google Scholar]
- 28.Perla-Kajan J, Twardowski T, Jakubowski H. Mechanism of homocysteine toxicity in humans. Amino Acids. 2007;32:561–572. doi: 10.1007/s00726-006-0432-9. [DOI] [PubMed] [Google Scholar]
- 29.Kalhan SC, Marczewski SE. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev Endocr Metab Disord. 2012 doi: 10.1007/s11154-012-9215-7. in press. [DOI] [PubMed] [Google Scholar]
- 30.Hogeveen M, Blom H, den Heijer M. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr. 2012;95:130–136. doi: 10.3945/ajcn.111.016212. [DOI] [PubMed] [Google Scholar]