Abstract
The pharmacokinetics of dantrolene and its active metabolite, 5-hydroxydantrolene, after a single oral dose of either 5 mg/kg or 10 mg/kg of dantrolene were determined. The effects of exposure to dantrolene and 5-hydroxydantrolene on activated whole blood gene expression of the cytokines interleukin-2 (IL-2) and interferon-γ (IFN-γ) were also investigated. When dantrolene was administered at a 5 mg/kg dose, peak plasma concentration (Cmax) was 0.43 µg/ml, terminal half-life (t1/2) was 1.26 hrs, and area under the time-concentration curve (AUC) was 3.87 µg hr/mL. For the 10 mg/kg dose, Cmax was 0.65 µg/ml, t1/2 was 1.21 hrs, and AUC was 5.94 µg hr/mL. For all calculated parameters, however, there were large standard deviations and wide ranges noted between and within individual dogs: t1/2, for example, ranged from 0.43 to 6.93 hrs, Cmax ratios ranged from 1.05 to 3.39, and relative bioavailability (rF) values ranged from 0.02 to 1.56. While activated whole blood expression of IL-2 and IFN-γ as measured by qRT-PCR was markedly suppressed following exposure to very high concentrations (30 µg/mL and 50 µg/mL, respectively) of both dantrolene and 5-hydroxydantrolene, biologically and therapeutically relevant suppression of cytokine expression did not occur at the much lower drug concentrations achieved with oral dantrolene dosing.
Keywords: Dantrolene, 5-hydroxydantrolene, pharmacokinetics, pharmacodynamics, dog
Introduction
Dantrolene is a direct-acting skeletal muscle relaxant that exerts its effects by dissociating excitation-contraction coupling in muscle via inhibition of the release of calcium from intracellular storage sites within the sarcoplasmic reticulum. Dantrolene does not, however, have any effect on the electrical excitability of the muscle or on the neuromuscular junction (Ellis & Bryant, 1972; Ellis & Carpenter, 1972). The ryanodine receptor (RYR) is the important dantrolene-binding site within skeletal muscle and serves as the major intracellular calcium release channel (Fruen, Mickelson et al., 1997).
Dantrolene is metabolized in the liver by oxidative and reductive pathways. Through the oxidative pathway, the hydantoin ring is hydroxylated and forms 5-hydroxydantrolene, and through the reductive pathway, nitro-moiety and acetylation of the benzene ring forms the reduced acetylated derivative of dantrolene. In humans, dantrolene is excreted in both urine and bile, with 79% excreted as 5-hydroxydantrolene, 17% as a reduced acetylated derivative, and 4% as unchanged drug (Inan & Wei, 2010). In dogs, dantrolene is also excreted through the urinary and biliary systems, primarily as 5-hydroxydantrolene (Wuis, Vree et al., 1990).
Clinically, dantrolene is most commonly known for its use as a life-saving antidote to the muscle hyperexcitability associated with malignant hyperthermia, and has a well-documented efficacy in the treatment of this condition in humans, horses, dogs, and swine (Flewellen & Nelson, 1980; Waldron-Mease, Klein et al., 1981; Ward, Chaffman et al., 1986; Nelson, 1991). Dantrolene was initially used in humans as a muscle relaxant for the long term treatment of skeletal muscle spasticity (Krause, Gerbershagen et al., 2004). In dogs, dantrolene has been used in the treatment of rhabdomyolysis (Wells, Sedacca et al., 2009). Dantrolene has also been investigated in research dogs, both for its potentially beneficial influence on the function of failing cardiomyocytes, and for its effects on urethral sphincter tone (Kobayashi, Yano et al., 2009).
Recently, potential therapeutic uses of dantrolene beyond its effects on muscle have attracted attention. Dantrolene appears to have significant inhibitory effects on T-cells via RYR receptor antagonism, introducing the intriguing possibility that dantrolene might have a role as an immunosuppressive agent. One study revealed that dantrolene suppressed the lipopolysaccharide-induced plasma levels of interleukin-12 and interferon-γ levels independently of the increased release of interleukin-10 in endotoxemic mice (Nemeth, Hasko et al., 1998). Another in vitro study revealed that dantrolene had a strong inhibitory effect on interleukin-2-dependent proliferation of CTLL-2 T-cells (Conrad, Hanniman et al., 2004). Currently, there are only a limited number of immunosuppressive drugs that are available for use in the treatment of immune-mediated diseases in the dog. Compared to many immunosuppressive drugs, the oral form of dantrolene is relatively inexpensive and carries less risk of detrimental side effects. A new and effective immunosuppressive agent with a milder side effect profile and lower cost than currently available pharmaceuticals would be very beneficial in both human and veterinary medicine.
The pharmacokinetic profile of oral dantrolene in the dog has not been thoroughly evaluated, which hinders the ability to develop accurate and species-specific dosing protocols. The pharmacokinetics of intravenously administered dantrolene in the dog have been previously described (Wuis, Vree et al., 1990). While intravenous administration of dantrolene is the primary method of treatment for acute conditions such as malignant hyperthermia, the preferred method of administering dantrolene for the long term treatment of more chronic conditions such as immune-mediated diseases is via the oral route. In addition, the intravenous form of dantrolene is cost prohibitive for long term treatment in veterinary medicine.
The purposes of this study were to obtain crucial pharmacokinetic information regarding the use of oral dantrolene in dogs, including standard compartment and moment pharmacokinetic parameters, and to determine the in vitro pharmacodynamic effects of dantrolene and its metabolite 5-hydroxydantrolene on activated whole blood gene expression of the cytokines interleukin-2 (IL-2)1 and interferon-γ (IFN-γ)2. This study was planned as the first step in the potential development of dantrolene for use as an immunosuppressive agent in dogs.
Materials and Methods
Pharmacokinetic Study
Animals
Six healthy unmedicated adult intact female Walker hound dogs, with a mean weight of 22.5 kg and mean age of 3 years, were studied. Water was available ad libitum while food was withheld for 12 hours prior to drug administration and for 12 hours after drug administration. Prior to the commencement of this study and seven days prior to drug administration, all dogs were determined to be clinically healthy and free of disease by thorough physical examination, complete blood count, serum biochemistry panel, and urinalysis. Blood and urine analyses were performed by the Diagnostic Laboratory Service of the Mississippi State University College of Veterinary Medicine using standard protocols. The dogs were not involved in any additional studies during the duration of this study, and did not receive any other medications for at least two weeks prior to the commencement of the study. The dogs were closely observed during drug administration for possible side effects, and monitored post-treatment by repeat complete blood count, serum biochemistry, and urinalysis. The study was approved by the Mississippi State University Institutional Animal Care and Use Committee.
Study Design
A balanced two-way cross-over design was used for this study. Half of the study population received a 5 mg/kg dose and the other half received a 10 mg/kg dose of oral dantrolene, to the nearest whole capsule. The precise dose given ranged from 4.44 to 5.49 mg/kg and from 9.62 to 10.42 mg/kg, respectively. The capsules were delivered directly into the posterior portion of the oral cavity, and the dog was monitored post administration to confirm that the entire dose was received and that no vomiting occurred. Following a 2 week washout period, the groups were reversed and dogs were given the alternate dose of oral dantrolene using the same protocol as the prior trial. The 25 mg dantrolene capsules, Dantrium®, were purchased from JHP Pharmaceuticals, LLC and stored according to label instructions until administration.
Blood Collection for Pharmacokinetic Analysis
Blood samples were collected at time 0 (immediately prior to drug administration) and at 15, 30, and 45 minutes, and 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 18, 24, 36, 48, 72, and 96 hours post-drug administration. Blood samples were obtained from the jugular vein via venipuncture using a 10 mL syringe and a 20 gauge needle. The 10 mL of whole blood were placed into an EDTA blood tube (Kendall/Tyco Healthcare, Mansfield, MA, USA) and immediately placed on ice. The blood tubes were then centrifuged at 4000 RPM for 10 minutes. The supernatant plasma was separated and transferred into storage vials (Sarstedt Inc., Newton, NC, USA) and stored at –80 degrees Celsius for approximately 6 months, until analysis.
Drug Analysis
A stock solution of dantrolene sodium (cat. D9175, 100mg; Sigma, St. Louis, MO, USA) was prepared at a concentration of 1 mg/mL in acetonitrile. 5-Hydroxydantrolene (cat. H825175, 5 mg) was purchased from Toronto Research Chemicals (North York, ON, Canada). Nitrofurazone (cat. 31706, 100ng/mL) was purchased from Sigma-Aldrich (St. Louis, MO, USA), and was utilized as the internal standard (IS)3. Liquid chromatography mass spectrometry (LC-MS) 4 grade acetonitrile, water and methanol were purchased from Fisher Scientific (Waltham, MA, USA). A LC-MS assay was developed in order to measure dantrolene and its primary metabolite, 5-hydroxydantrolene, in canine plasma. This assay was confirmed to be accurate and repeatable by spiking known quantities of dantrolene into drug-free (blank) canine plasma, processing the samples as described below, and analyzing the spiked samples by LC-MS/MS analysis.
To prevent degradation of dantrolene and its metabolites, each sample of canine plasma was protected from excessive exposure to ultraviolet light during thawing and sample preparation. After thawing the stored frozen dog plasma, 500 µL aliquots of plasma were added to glass tubes followed by the addition of nitrofurazone (2.5 nmol, 5 µL of a 500 µM stock prepared in acetonitrile) and 3 mL of acetonitrile. The samples were vortexed for one minute and mixed on a shaker for 10 minutes at room temperature, followed by centrifugation (3,500 × g, 10 minutes, at room temperature). The supernatants were transferred to clean glass tubes, dried under N2, and re-dissolved in 200 µL of 10:90 (v/v) methanol:water containing 0.1% (v/v) formic acid. After filtration (0.1 µm), 10 µL of the extract was injected onto an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm) equipped with a pre-column (2.1 × 5 mm, 1.7 µm). The mobile phase was a blend of solvent A (0.1% acetic acid in water) and solvent B (0.1% acetic acid in methanol). Analytes were eluted with the following gradient program: 0 min (95% A, 5% B), 1.5 min (95% A, 5% B), 8 min (100% B), 10 min (100% B), 11.5 min (95% A, 5% B), 15 min (95% A, 5% B). The flow rate was 0.3 mL/min and the entire column eluate was directed into a Thermo Quantum Access Max triple-quadrupole mass spectrometer using heated electrospray ionization in negative ion mode. Data acquisition was performed using Thermo Xcalibur 2.0 software and quantitation of analytes performed using Quan Browser. The following single-reaction monitoring (SRM)5 transitions were monitored for quantification: dantrolene (m/z 313>199; m/z 313>227), 5-hydroxydantrolene (m/z 328>229), and nitrofurazone (m/z 197>197). The retention time for the internal standard (nitrofurazone), dantrolene, and 5-hydroxydantrolene were 4.34 min, 6.24 min, and 6.22 min, respectively.
Drug-free (blank) dog plasma was used for the generation of calibrators, negative control and quality control (QC)6 samples. Calibrators and QC samples were prepared by spiking drug-free dog plasma with known quantities of working standards of 5-hydroxydantrolene, dantrolene, and the internal standard. Final concentrations for calibrators were 10, 25, 50, 100, 200 and 400 ng/mL. The extraction of calibrators was carried out in the same manner as described above for the unknown samples. Calibration curves for both dantrolene and 5-hydroxydantrolene were generated by weighted (1/X) linear regression analysis using the ratio of analyte peak area to the IS peak area with correlation coefficients (r2) >0.99. The limit of quantitation of the analysis was 10 ng/mL for both dantrolene and 5-hydroxydantrolene, which is the concentration of the lowest calibration standard. The recovery of dantrolene and 5-hydroxydantrolene from plasma was typically >60%. Extraction recoveries of both analytes were automatically corrected for because fixed volumes of drug-free (blank) canine plasma (500 µL) were spiked with increasing amounts of dantrolene and 5-hydroxydantrolene to prepare calibration standards. The calibrators were then fortified with the same amount of internal standard (nitrofurazone) as the unknown plasma samples and extracted in the exact manner as the unknowns. Intra-run coefficients of variation for dantrolene and 5-hydroxydantrolene were 5% and 7%, respectively; whereas, inter-run coefficients of variation were typically <20% for both compounds.
Pharmacokinetic and Statistical Analysis
Compartmental analysis of the single-dose plasma concentration-time data of dantrolene was performed using the pharmacokinetic computer program WinSAAM (Simulation Analysis and Modeling, version 3.0.7, www.winsaam.com) with an equal weighting of data points. Data for the 5 mg/kg and the 10 mg/kg doses were separately fit to a one or two-compartment extravascular model, each with and without a lag time in absorption (Tlag)7. The compartmental model with the lowest Akaike Information Criterion (AIC)8 was selected as the best fit. Mean residence time (MRT)9, area under the curve (AUC0-∞)10, and total body clearance divided by fraction of drug absorbed (CL/F)11 were determined using the linear trapezoidal method and standard moment kinetic equations (Farrier, 2010). Cmax and Tmax were observational values. A compartmental analysis was not performed on the primary metabolite, 5-hydroxydantrolene; however, Cmax and Tmax are reported. The relative bioavailability of the 10 mg/kg dose compared to the 5 mg/kg dose was calculated using the formula Relative F (rF)12 = (AUC0-∞10 / Dose5) × (AUC0-∞5 / Dose10). The Cmax ratio assigned the numerator to the value from the 10 mg/kg Cmax and the denominator from the 5 mg/kg Cmax.
In Vitro Cytokine Gene Expression Study
Blood Sample Collection and Drug Exposure
Approximately 20 mL of whole blood was collected from the jugular vein of a healthy intact female Walker hound using a Vacutainer collection system into seven 3 mL sodium heparin blood tubes (Kendall/Tyco Healthcare, Mansfield, MA, USA). 1.2 mL samples of whole blood were then exposed to dantrolene (Sigma) at the following concentrations for one hour: 0.1, 1, 10, 20, 30, and 50 µg/mL. A 500 ng/mL cyclosporine-treated sample (Sigma, St. Louis, MO, USA), also with a one hour drug exposure time, was used as a positive control for suppression of cytokine gene expression, and an untreated blood sample was used as a negative control. In a subsequent similar study evaluating the effects of the dantrolene metabolite 5-hydroxydantrolene, approximately 15 mL of whole blood was collected from the jugular vein of a healthy intact female Walker hound using a needle and syringe into five 3 mL sodium heparin blood tubes (Kendall/Tyco Healthcare, Mansfield, MA, USA). 1.2 mL samples of whole blood were then exposed to 5-hydroxydantrolene (Sigma, St. Louis, MO, USA) at the following drug concentrations for one hour: 0.01, 0.05, 0.1, 0.5, 1, 10, and 50 µg/mL). Again, a 500 ng/ml cyclosporine-treated sample (Sigma, St. Louis, MO, USA) and an untreated sample were run in parallel as a positive control and negative control respectively.
Activation
One hour after addition of drug, 1.2 ml whole blood samples were activated using a combination of 1.5µl phorbol myristate (12.5 ng/mL) (Sigma, St. Louis, MO) and 1.92µl ionomycin (0.8 µM) (Sigma, St. Louis, MO). The samples were incubated in a 5% CO2 atmosphere at 37°C for 5 hours.
RNA Isolation
Total RNA was isolated from 1 mL of heparinized whole blood using a QIAamp RNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions and then stored at −80°C until use. An on-column DNase (27.27 Kunitz units) treatment (Qiagen, Valencia, CA, USA) was performed on all samples to remove genomic DNA. RNA was quantified at room temperature using a Nanodrop ND-1000 spectrophotometer using the ND-1000 V3.3.0 software.
Cytokine Gene Expression Quantification
The expression of cytokines IL-2 and IFN-γ and normalizer gene GAPDH were compared via quantitative reverse transcription (qRT)13-PCR using a SuperScript™ III Platinum® SYBR® Green One-Step qRT-PCR kit with Rox used as a reference dye (Invitrogen, Grand Island, NY, USA). Primers for IL-2, IFN-γ, and GAPDH were based on reported GenBank nucleotide sequences as previously published by Kobayashi et al. (Kobayashi, Momoi et al., 2007). All reactions were run on an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA) using 7500 software v2.0.6 for analysis. The qRT-PCR was performed with a final volume of 20 µL containing 15 ng of RNA and 200 nM of each primer. Thermal cycling parameters were as follows: 50°C for 3 min, 95°C for 5 min, 40 cycles of 95°C for 15 sec and 60°C for 30 sec followed by a melting curve analysis by 1 cycle of 95°C for 15 sec, 60°C for 1 min, 95°C for 30 sec and 60°C for 15 sec. All samples were run in triplicate while non-template controls were run in duplicate. To calculate the relative change in gene expression for all samples the 2−ΔΔCt method was employed using GAPDH as a reference gene where ΔΔCt = (CtGOI – Ctnorm)treated – (CtGOI – Ctnorm)untreated where GOI is the gene of interest and norm is the reference gene (Livak & Schmittgen, 2001).
Results
Animals
Dantrolene appeared to be well tolerated after oral administration. All dogs remained bright, alert, and responsive throughout the duration of the study, and no gastrointestinal effects were observed. A complete blood count, serum biochemistry, and urinalysis performed prior to initiating the study and two weeks after completion of the study revealed no significant abnormalities.
Pharmacokinetics
The plasma concentration-time profiles for the 5 and 10 mg/kg dantrolene doses are displayed in Figures 1 and 2, respectively. Data from both doses were best fit by a one compartment extravascular open model. There was however no clear distinction as to whether a Tlag was appropriate, with four of six dogs in the 5 mg/kg group best fit without a Tlag, and four of six dogs in the 10 mg/kg group best fit with a Tlag. When no clear advantage for one model versus another exists, it is common to choose the simpler of the two. Table 1 therefore contains the parameters for the model without a Tlag. Moment parameters and the relative bioavailability results for all of the dogs are shown in Table 2 to demonstrate the variability both between and within each dog. Table 3 contains the mean Cmax and Tmax values for the primary metabolite, 5-hydroxydantrolene.
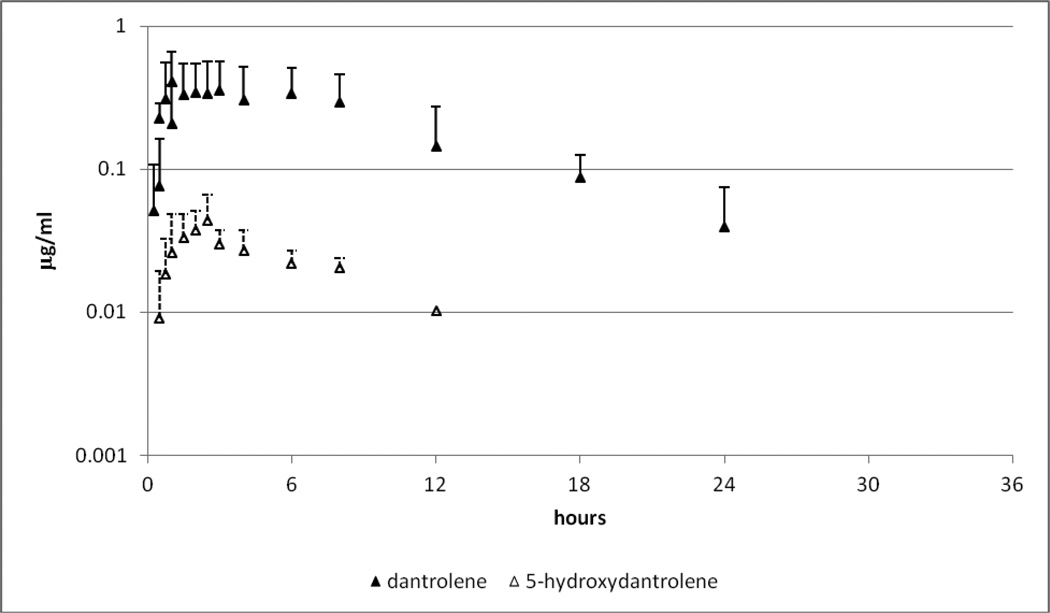
Figure 1.
Mean and standard deviation (SD) of plasma dantrolene (closed triangle) and 5-hydroxydantrolene (open triangle) following administration of a single oral dose of dantrolene at 5 mg/kg.
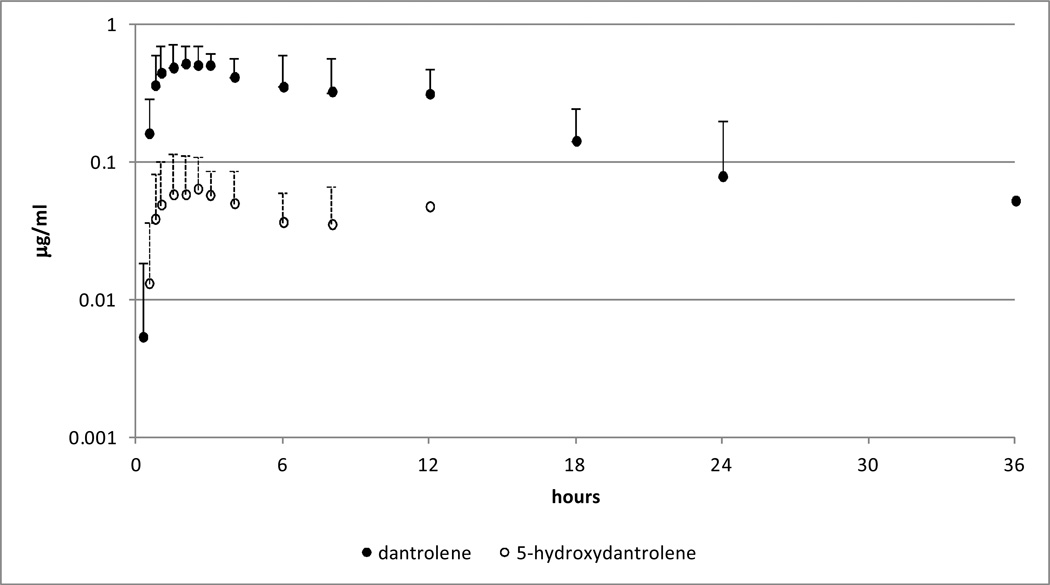
Figure 2.
Mean and standard deviation (SD) of plasma dantrolene (closed circle) and 5-hydroxydantrolene (open circle) following administration of a single oral dose of dantrolene at 10 mg/kg.
Table 1.
Mean ± SD of compartmental pharmacokinetic parameters of dantrolene following administration of a single oral dose of dantrolene at 5 and 10 mg/kg. The range is shown in parenthesis.
| Dose | 5 mg/kg | 10 mg/kg |
|---|---|---|
| λz (/hr) |
0.549 ± 0.6216 (0.109 – 1.618) |
0.574 ± 0.5606 (0.1 – 1.572) |
| t1/2 (hrs) |
1.26 (0.43 – 6.36) |
1.21 (0.44 – 6.93) |
| Ka (/hr) |
0.67 ± 0.3072 (0.219 – 1.154) |
0.557 ± 0.2644 (0.255 – 1.012) |
| Vd/F (L/kg) |
6.18 ± 0.707 (5.409 – 7.309) |
8.39 ± 1.9543 (5.22 – 10.127) |
Table 2.
Noncompartmental pharmacokinetic parameters of dantrolene following administration of a single oral dose of dantrolene at 5 and 10 mg/kg.
| Dog number | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dose mg/kg |
1 | 2 | 3 | 4 | 5 | 6 | Mean ± SD (range) |
|
|
AUC0-∞ (µg hr / mL) |
5 | 4.755 | 0.907 | 7.123 | 2.495 | 7.445 | 0.494 | 3.87 ± 3.04 (0.494 – 7.445) |
| 10 | 4.335 | 8.387 | 7.059 | 2.223 | 11.409 | 2.223 | 5.94 ± 3.67 (2.223 – 11.409) |
|
| Relative F | 0.46 | 0.17 | 1.04 | 0.12 | 1.56 | 0.02 | 0.56 ± 0.61 (0.02 – 1.56) |
|
|
MRT0-∞ (hr) |
5 | 7.37 | 4.22 | 7.72 | 6.16 | 10.95 | 2.18 | 6.43 ± 3.03 (2.18 – 10.95) |
| 10 | 8.15 | 8.72 | 8.13 | 3.14 | 15.07 | 3.14 | 7.73 ± 4.41 (3.14 – 15.07) |
|
|
Cmax (µg/ml) |
5 | 0.461 | 0.22 | 0.762 | 0.372 | 0.583 | 0.199 | 0.43 ± 0.22 (0.199 – 0.762) |
| 10 | 0.485 | 0.745 | 0.854 | 0.598 | 0.618 | 0.598 | 0.65 ± 0.13 (0.485 – 0.854) |
|
| Cmax ratio | 1.05 | 3.39 | 1.12 | 1.61 | 1.06 | 3.01 | 1.87 ± 1.06 (1.05 – 3.39) |
|
|
Tmax (hr) |
5 | 3 | 1.5 | 0.75 | 3 | 2 | 0.75 | 1.83 ± 1.02 (0.75 – 3) |
| 10 | 3 | 6 | 2.5 | 1.5 | 2 | 1.5 | 2.75 ± 1.7 (1.5 – 6) |
|
|
CL/F (L/kg hour) |
5 | 0.9 | 5 | 0.7 | 1.8 | 0.7 | 11.1 | 3.37 ± 4.13 (0.7 – 11.1) |
| 10 | 2.3 | 1.2 | 1.4 | 4.5 | 0.9 | 4.3 | 2.4 ± 1.59 (0.9 – 4.5) |
|
Table 3.
Mean ± SD of Cmax and Tmax for 5-hydroxydantrolene following administration of a single oral dose of dantrolene at 5 and 10 mg/kg. The range is shown in parenthesis.
| Dose | 5 mg/kg | 10 mg/kg |
|---|---|---|
| Cmax (µg/ml) |
0.055 ± 0.0242 (0.031 – 0.087) |
0.073 ± 0.0512 (0.033 – 0.169) |
| Tmax (hr) |
3.67 ± 2.295 (1.5 – 8) |
2.17 ± 0.816 (1 – 3) |
Pharmacodynamics
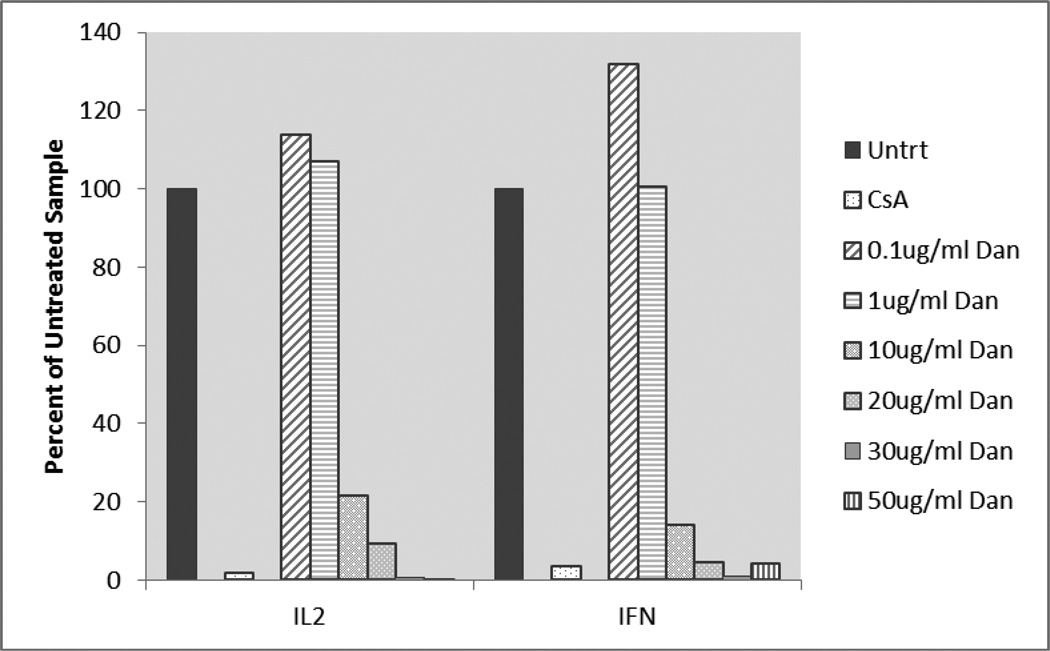
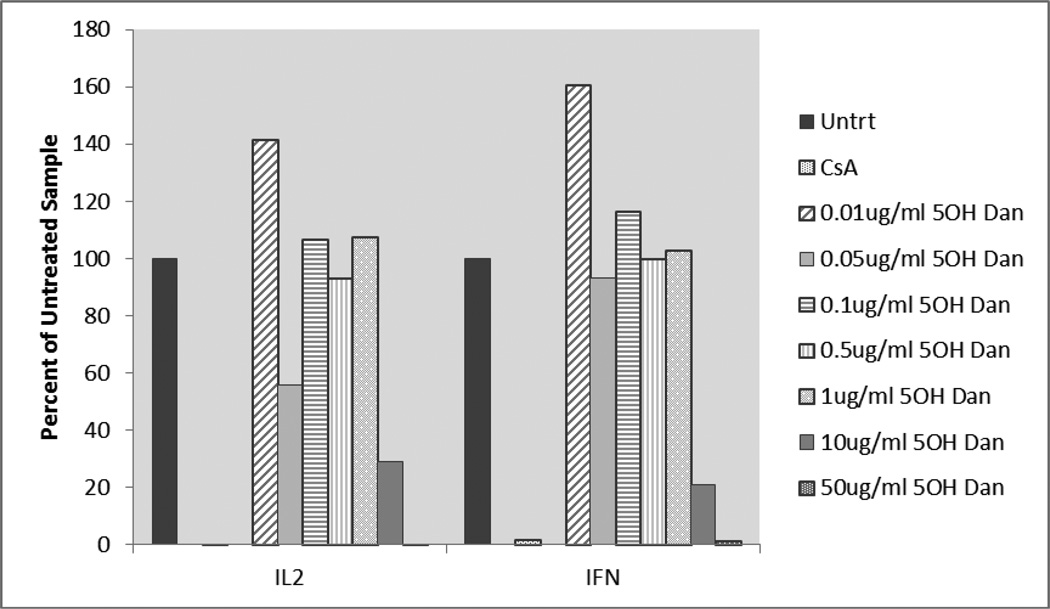
qRT-PCR data was analyzed by comparing dantrolene-exposed and 5-hydroxydantrolene-exposed samples to an untreated control. The delta Ct was calculated relative to the normalizer gene (GAPDH) and the delta delta Ct was calculated relative to the untreated sample. By using the 2−ΔΔCt method, the relative change in expression of IL-2 and IFN-γ after dantrolene and 5-hydroxydantrolene exposure was revealed. Cytokine gene expression was presented as a percentage where the untreated control sample represented 100% expression of the genes coding for IL-2 and IFN-γ. Dantrolene and 5-hydroxydantrolene exposure results are given graphically in Figures 3 and 4. Slightly increased IL-2 and IFN- γ gene expression was observed when blood was exposed to dantrolene at a concentration of 0.1 and 1 µg/ml, while concentration-dependent decreased gene expression was seen with dantrolene treatment at concentrations of 10, 20, 30, and 50 µg/ml. Cytokine gene expression only decreased to levels comparable to or below the level of expression associated with exposure to cyclosporine at the two highest concentrations of dantrolene, 30 and 50 µg/ml. Slightly increased IL-2 and IFN- γ gene expression was observed when blood was exposed to 5-hydroxydantrolene at a concentration of 0.01, 0.1 and 1 µg/ml, while concentration-dependent decreased cytokine gene expression was seen with 5-hydroxydantrolene exposure at concentrations of 10 and 50 µg/ml. A slight decrease in IL-2 and IFN-γ gene expression was observed in the sample exposed to 0.5 µg/ml 5-hydroxydantrolene, with a moderate decrease in IL-2 expression and a slight decrease also observed in IFN-γ expression in the sample exposed to 0.05 µg/ml compared to the untreated control. Cytokine gene expression only decreased to levels comparable to the level of expression associated with exposure to cyclosporine, however, at the highest concentration of 5-hydroxydantrolene, 50 µg/ml.
Figure 3.
Activated whole blood IL-2 and IFN-γ gene expression in dantrolene-exposed samples as percentage of cytokine expression in unexposed samples. Untrt = Unexposed, CsA = Cyclosporine, Dan = Dantrolene, IL2 = Interleukin-2, IFN = Interferon-γ
Figure 4.
Activated whole blood IL-2 and IFN-γ gene expression in 5-hydroxydantrolene-exposed samples as percentage of cytokine expression in unexposed samples. Untrt = Unexposed, CsA = Cyclosporine, 5OH Dan = 5-hydroxydantrolene IL2 = Interleukin-2, IFN = Interferon-γ
Discussion
Following oral administration of dantrolene in dogs, the drug is rapidly absorbed and the primary metabolite, 5-hydroxydantrolene, is produced. The mean dantrolene terminal half-life was 1.26 hours for the 5 mg/kg dose and 1.21 hours for the 10 mg/kg dose. Wuis et al. reported a similar terminal half-life of 1.2 hours in his study using intravenously administered dantrolene at a dose of 2.6–2.8 mg/kg in three dogs (Wuis, Vree et al., 1990). The volume of distribution and absolute bioavailability of dantrolene cannot be determined without an accompanying intravenous study; however, Wuis et al. reported a Vss of 0.79 to 1.35 L/kg and a total body clearance of 0.45 to 1.32 L/Kg hr after intravenous administration (Wuis, Vree et al., 1990). The extremely large Vd/F and CL/F values obtained in our study may be attributable to poor oral bioavailability, protein binding, or another unknown process. If future research identifies that oral dantrolene can be used in a clinical situation as an effective treatment, another study could be performed to determine the drug’s volume of distribution and absolute bioavailability. For the purpose of our study, we found it to be more important to determine dose linearity with oral drug administration, since intravenous dantrolene is cost prohibitive in clinical veterinary medicine.
Two doses were compared in this study to determine if dose linearity exists for oral dantrolene in the dog. The determination of dose linearity is based on comparison of Cmax values and the relative bioavailability (rF). With dose linearity Cmax values should increase proportionally. In this study doubling the dose should result in a Cmax ratio of 2. As for rF, because the formula normalizes the AUC for the doses being compared, when dose linearity exists the value should be 1.0. A lack of linearity can occur with either a lower rF (e.g., saturation of the absorptive process) or a higher rF (e.g., saturation of an elimination process). If one looks at only the mean values for the Cmax ratio (1.8) and rF (0.56) it would appear that the former argues for reasonable dose linearity while the latter implies a lack of linearity, with the larger dose less bioavailable than the smaller dose. The low rF for dantrolene observed in our study suggests that the reduced bioavailability seen at higher oral drug doses may be due to saturation of drug intestinal absorption. A lack of dose linearity with oral dantrolene has also been reported in human patients receiving dantrolene. In one study in human patients receiving oral dantrolene as a treatment for spasticity, mean dantrolene blood levels increased in an approximately linear fashion as oral dose rates were increased from low to medium dosages, but then failed to continue to increase in a linear fashion when oral dose rates were increased further (Meyler, Bakker et al., 1981). The authors speculated that the lack of dose linearity at higher dantrolene doses might have been due to capacity-limited intestinal absorption. The same phenomenon may explain the low relative bioavailability (rF) observed in our study.
Perhaps the most important observations from our study were the large standard deviations and the wide ranges noted between individual dogs for all calculated parameters. For example, the range of values for rF was 0.02 to 1.56, indicating that, in individual dogs, it cannot be determined whether an increase or a decrease in rF dominates. There was also a very wide range in Cmax ratios, ranging from 1.05 to 3.39, and t1/2, ranging from 0.43 to 6.93 hours. This interindividual variability has also been seen in horses and human with oral dosing of dantrolene (McKenzie, Valberg et al., 2004; Meyler, Bakker et al., 1981). Whether dose linearity exists when dosing oral dantrolene in dogs may be somewhat of a moot point, as the extreme variability in pharmacokinetic values within each dog precludes achievement of consistently predictable plasma concentrations at any standard oral dose.
The results of our study suggest that the pharmacokinetic profile of oral dantrolene is markedly different than that reported in humans, where blood levels of the drug following oral dosing appear to be consistently higher for a much more sustained period. In one study evaluating the use of preoperative oral dantrolene in humans to determine if protective blood levels could be reached at the time of induction of anesthesia and in the postoperative period, patients were given a total dose of 5 mg/kg of oral dantrolene in three or four divided doses every 6 hours, with the last dose 4 hours preoperatively. In this study, all 10 evaluated patients had plasma dantrolene levels greater than 2.8 µg/ml at the time of induction of anesthesia, for at least 6 hours after induction, and, in three patients, up to 18 hours after induction. The mean elimination half-life was 15.8 hours (+/−6.0 hours). The specific oral dantrolene regimen utilized in this preoperative study actually resulted in longer protective plasma levels than did a standard intravenous dantrolene protocol in people (Allen, Cattran et al., 1988). In our study, in contrast, administration of oral dantrolene to dogs only achieved a mean plasma dantrolene level of 0.65 µg/ml, with an elimination half-life of far less than 2 hours, even at a total oral drug dose rate of double that used in the human study. The relatively low plasma drug levels attained in our study are in fact comparable to the plasma levels achieved by much lower dose rates in people: in one study in adult patients receiving oral dantrolene at a total dose of only 25 mg four times daily, for example, a mean plasma drug level of 0.59 µg/ml three hours post-dosing was attained (Meyler, Bakker et al., 1981).
Oral dantrolene certainly appears to be clinically effective for the treatment of certain conditions in other species at doses comparable to the doses used in the dogs in our study. Premedication with oral dantrolene at 5 mg/kg 24 hours before anesthesia, for example, has been shown to have a preventative effect on the development of malignant hyperthermia in swine (O'Brien P, Cribb et al., 1983). Oral dantrolene has also been used to effectively treat spasticity in people, both in adults and in children. In adults with spasticity, some patients show marked improvement at a total daily dose of 100 mg daily (25 mg four times daily), whereas others fail to improve at four times that dose, even if comparable blood drug levels are achieved (Meyler, Bakker et al., 1981). Neither oral dose rate nor blood drug levels predict response to therapy in such patients, suggesting that oral dantrolene must be individualized by dosing ‘to effect’ based on resolution of clinical signs. Oral dantrolene in affected children is generally started at a low dose and titrated upward ‘to effect’ every 5 to 7 days as tolerated, with final doses as high as 12 mg/kg/day (Krach, 2001).
Given the marked animal-to-animal variation observed in the pharmacokinetic profile of oral dantrolene in the dogs in our study, similarly individualizing therapy by titrating doses upwards ‘to effect’ based on resolution of clinical signs has the potential to be effective in individual dogs. This approach, however, would only be likely to be effective in dogs with conditions where a clinical response to treatment could be easily evaluated, such as the use of dantrolene to alleviate urethral spasm. One experimental study of the effects of dantrolene sodium on the urethral pressure profile in healthy anesthetized dogs revealed that, at intravenous doses of dantrolene ranging from 1 mg/kg to 15 mg/kg, there was a dose-dependent depressive effect on the urethral pressure profile associated with the musculature of the distal urethra, with subtle effects first becoming detectable at a dose of 3 mg/kg, and with the highest dose (15 mg/kg) reducing urethral pressures to approximately half that of untreated control dogs (Khalaf, Foley et al., 1979). The intravenous doses of dantrolene needed to affect urethral function appear to be approximately comparable to doses needed to affect skeletal muscle function, since another study in healthy anesthetized dogs found that, with intravenous dantrolene, the effective dose (ED50) for inhibition of skeletal muscle contractions was 4.5 mg/kg (Ellis, Wessels et al., 1976). Although neither of these two intravenous dantrolene studies in dogs measured drug blood concentrations, a later intravenous dantrolene pharmacokinetic study by Wuis et al. demonstrated that a single dose of 2.8 mg/kg attained peak drug concentrations of approximately 2 µg/ml in the dog (Wuis, Vree et al., 1990). When considered together, the results of these three intravenous dantrolene studies suggest that blood drug concentrations of at least 2 µg/ml are needed to exert significant measurable effects on canine musculature. Since our pharmacokinetic study has confirmed that oral dantrolene, even at escalating doses, only achieves blood drug concentrations that are only approximately 25% of the levels that appear to be needed to affect canine muscle function, the results of our study prompt concerns that the standard recommended oral doses of dantrolene may not in fact be effective for treating conditions such as urethral spasm in many dogs.
The main long term goal of our pharmacokinetic study was to determine blood drug concentrations that could be achieved with oral dantrolene, with the intention of exploring the possibility of utilizing dantrolene as an immunosuppressive agent in dogs. Several previous studies in other species have documented that dantrolene suppresses T-cell production of cytokines such as IL-2 and IFN-γ (Nemeth, Hasko et al., 1998; Conrad, Hanniman et al., 2004). Suppression of T-cell production of nuclear factor of activated T-cells (NFAT)-regulated cytokines, including IL-2 and IFN-γ, via inhibition of the enzyme calcineurin, is the established mechanism of action of several potent immunosuppressive agents, including cyclosporine. Since dantrolene has been reported to suppress T-cell production of the same cytokines, it is reasonable to assume that dantrolene may exert immunosuppressive effects that are similar to those exerted by calcineurin inhibitors. Using a quantitative reverse transcription PCR assay that was modeled on assays used to determine the effects of calcineurin inhibitors in human patients, and that has been validated in our laboratory for use in dogs, we established that at high concentrations dantrolene markedly suppresses activated whole blood gene expression of IL-2 and IFN-γ to a degree that is very comparable to the effects of cyclosporine at therapeutic drug concentrations (Giese, Zeier et al., 2004; Archer, Fellman et al., 2009; Kuzuya, Kobayashi et al., 2009; Fellman, Stokes et al., 2011; Riggs, Archer et al., 2012). However, at dantrolene concentrations of 1 µg/mL or less, the drug had no detectable suppressive effect on activated whole blood cytokine gene expression. While our assay does not specifically measure T-cell cytokine expression since whole blood is used rather than isolated T-cells, this method of cytokine expression measurement is well accepted in the human literature as a marker of the effects of immunosuppressive drugs on T-cell production of the NFAT-regulated cytokines (Giese, Zeier et al., 2004; Hartel, Schumacher et al., 2004; Konstandin, Sommerer et al., 2007; Kuzuya, Kobayashi et al., 2009). Our technique has some advantages over others including decreased sample preparation time and minimization of artifacts induced by cell separation. Based on the results of our in vitro gene expression study, it is likely that blood dantrolene concentrations of much higher than 1 µg/mL would be needed to inhibit T-cell production of IL-2 and IFN-γ. Unfortunately, blood dantrolene concentrations as high as 1 µg/mL were not achieved in any dog in our study, even at the higher oral dose rate. Given our finding that higher oral doses of dantrolene were less bioavailable than lower doses, suggesting the possibility that intestinal absorption of the drug is a saturable process, in our opinion it is unlikely that merely increasing the oral dose of dantrolene even further will reliably attain the range of blood dantrolene concentrations required to suppress T-cell function. Following completion of our dantrolene study, we repeated the in vitro study using the same PCR assay and exposure to 5-hydroxydantrolene rather than dantrolene. We evaluated 5-hydroxydantrolene to ensure that it was not this primary metabolite, rather than dantrolene, that was responsible for suppression of T-cell function. As with our prior dantrolene study, we established that at high drug concentrations 5-hydroxydantrolene markedly suppresses activated whole blood gene expression of IL-2 and IFN-γ, and that the concentration of 5-hydroxydantrolene needed to significantly suppress cytokine expression, 10 µg/mL, is over 100 times the mean peak blood concentration of 5-hydroxydantrolene (0.073 µg/mL) attained with the higher oral dose of dantrolene. Although expression of IL-2 was also somewhat suppressed at a 5-hydroxydantrolene concentration of 0.05 µg/mL, the degree of suppression was not comparable to that caused by cyclosporine, and not of a magnitude generally considered to be of therapeutic significance (greater than 50% suppression compared to untreated samples). Furthermore, since the suppression of IL-2 expression at a 0.05 µg/mL 5-hydroxydantrolene concentration did not seem to occur in a concentration-dependent fashion, and was not observed at drug concentrations both immediately above and immediately below 0.05 µg/mL, the result is likely to be of minimal biologic or therapeutic significance.
One limitation of our study was that the dogs studied were all of a single breed, and it is therefore possible that the conclusions drawn from our study may not be completely applicable to other dog breeds.
Based on the results of our study, we believe that it may be advisable to revisit the origins of the standard published recommended oral doses of dantrolene in dogs. Canine recommended oral dose rates are similar to those recommended in children and, given that we can identify no previous published paper outlining the pharmacokinetics of oral dantrolene in dogs, it is reasonable to assume that dosing recommendations in dogs may have been derived by extrapolation of human dosing recommendations. Given our finding that the pharmacokinetics of oral dantrolene in dogs are markedly different than in people, and that the drug appears to attain markedly lower blood levels at comparable oral dose rates, it seems highly likely that currently recommended oral doses of dantrolene in dogs will often be ineffective in individual dogs.
Unfortunately, given the short half-life, low blood levels, and marked individual-to-individual variability in drug pharmacokinetics observed in our study, we cannot suggest an oral dosing regimen for dantrolene that is likely to be effective in most dogs. When dantrolene is used to treat a disease with a readily observable clinical effect, for example treatment of urethral spasm, clinicians could consider escalating the drug dose rate ‘to effect’ based on resolution of clinical signs. However, when the desired effects of the drug are not immediately clinically obvious, the continued use of oral dantrolene is not recommended. Given the results of our study, the use of oral dantrolene as an immunosuppressive agent is unlikely to be clinically feasible, since therapeutically relevant suppression of the NFAT-regulated cytokines IL-2 and IFN-γ only occurred at drug concentrations that are not feasible with oral dantrolene dosing.
Footnotes
IL-2 interleukin-2
IFN-γ interferon-γ
IS internal standard
LC-MS liquid chromatography mass spectrometry
SRM single-reaction monitoring
QC quality control
Tlag lag time in absorption
AIC Akaike Information Criterion
MRT Mean residence time
AUC0-∞ area under the curve
CL/F total body clearance divided by fraction of drug absorbed
rF Relative bioavailability
qRT quantitative reverse transcription
References
- Allen GC, Cattran CB, Peterson RG, Lalande M. Plasma levels of dantrolene following oral administration in malignant hyperthermia-susceptible patients. Anesthesiology. 1988;69(6):900–904. doi: 10.1097/00000542-198812000-00016. [DOI] [PubMed] [Google Scholar]
- Archer TM, Fellman CL, Stokes JV, Pinchuk LM, Lunsford KV, Pruett SB, Langston VC, Mackin AJ. Pharmacodynamic Monitoring of Canine T-Cell Cytokine Responses to Oral Cyclosporine. J Vet Intern Med. 2011;25(6):1391–1397. doi: 10.1111/j.1939-1676.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- Conrad DM, Hanniman EA, Watson CL, Mader JS, Hoskin DW. Ryanodine receptor signaling is required for anti-CD3-induced T cell proliferation, interleukin-2 synthesis, and interleukin-2 receptor signaling. J Cell Biochem. 2004;92(2):387–399. doi: 10.1002/jcb.20064. [DOI] [PubMed] [Google Scholar]
- Ellis KO, Bryant SH. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- Ellis KO, Carpenter JF. Studies on the mechanism of action of dantrolene sodium. A skeletal muscle relaxant. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):83–94. doi: 10.1007/BF00505069. [DOI] [PubMed] [Google Scholar]
- Ellis KO, Wessels FL, Carpenter JF. Effects of intravenous dantrolene sodium on respiratory and cardiovascular functions. J Pharm Sci. 1976;65(9):1359–1364. doi: 10.1002/jps.2600650925. [DOI] [PubMed] [Google Scholar]
- Farrier DS. [Accessed 3/1/2010];PK Solutions 2: Noncompartmental Pharmacokinetics Data Analysis. 2009 Available at www.SummitPK.com.
- Fellman CL, Stokes JV, Archer TM, Pinchuk LM, Lunsford KV, Mackin AJ. Cyclosporine A affects the in vitro expression of T cell activation-related molecules and cytokines in dogs. Vet Immunol Immunopathol. 2011 doi: 10.1016/j.vetimm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Flewellen EH, Nelson TE. Dantrolene dose response in malignant hyperthermia-susceptible (MHS) swine: method to obtain prophylaxis and therapeusis. Anesthesiology. 1980;52(4):303–308. doi: 10.1097/00000542-198004000-00003. [DOI] [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Louis CF. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J Biol Chem. 1997;272(43):26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- Giese T, Zeier M, Meuer S. Analysis of NFAT-regulated gene expression in vivo: A novel perspective for optimal individualized doses of calcineurin inhibitors. Nephrol Dial Transplant. 2004;19:55–60. doi: 10.1093/ndt/gfh1043. [DOI] [PubMed] [Google Scholar]
- Hartel C, Schumacher N, Fricke L, Ebel B, Kirchner H, Muller-Steinhardt M. Sensitivity of whole-blood T lymphocytes in individual patients to tacrolimus (FK 506): impact of interleukin-2 mRNA expression as surrogate measure of immunosuppressive effect. Clin Chem. 2004;50(1):141–151. doi: 10.1373/clinchem.2003.024950. [DOI] [PubMed] [Google Scholar]
- Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg. 2010;111(6):1400–1410. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf IM, Foley G, Elhilali MM. The effect of dantrium on the canine urethral pressure profile. Invest Urol. 1979;17(3):188–190. [PubMed] [Google Scholar]
- Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53(21):1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Momoi Y, Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J Vet Med Sci. 2007;69(9):887–892. doi: 10.1292/jvms.69.887. [DOI] [PubMed] [Google Scholar]
- Konstandin MH, Sommerer C, Doesch A, Zeier M, Meuer SC, Katus HA, Dengler TJ, Giese T. Pharmacodynamic cyclosporine A-monitoring: relation of gene expression in lymphocytes to cyclosporine blood levels in cardiac allograft recipients. Transpl Int. 2007;20(12):1036–1043. doi: 10.1111/j.1432-2277.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Krach LE. Pharmacotherapy of spasticity: oral medications and intrathecal baclofen. J Child Neurol. 2001;16(1):31–36. doi: 10.1177/088307380101600106. [DOI] [PubMed] [Google Scholar]
- Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59(4):364–373. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Kobayashi T, Katayama A, Nagasaka T, Miwa Y, Uchida K, Nakao A, Yamada K. Evaluation of interleukin-2 mRNA in whole blood as a parameter for monitoring cyclosporine pharmacodynamics. Biol Pharm Bull. 2009;32(4):604–608. doi: 10.1248/bpb.32.604. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McKenzie EC, Valberg SJ, Godden SM, Finno CJ, Murphy MJ. Effect of oral administration of dantrolene sodium on serum creatine kinase activity after exercise in horses with recurrent exertional rhabdomyolysis. Am Jour Vet Res. 2004;65(1):74–79. doi: 10.2460/ajvr.2004.65.74. [DOI] [PubMed] [Google Scholar]
- Meyler WJ, Bakker H, Kok JJ, Agoston S, Wesseling H. The effect of dantrolene sodium in relation to blood levels in spastic patients after prolonged administration. J Neurol Neurosurg Psychiatry. 1981;44(4):334–339. doi: 10.1136/jnnp.44.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE. Malignant hyperthermia in dogs. J Am Vet Med Assoc. 1991;198(6):989–994. [PubMed] [Google Scholar]
- Nemeth ZH, Hasko G, Szabo C, Salzman AL, Vizi ES. Calcium channel blockers and dantrolene differentially regulate the production of interleukin-12 and interferon-gamma in endotoxemic mice. Brain Res Bull. 1998;46(3):257–261. doi: 10.1016/s0361-9230(98)00005-7. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ, Cribb PH, White RJ, Olfert ED, Steiss JE. Canine malignant hyperthermia: diagnosis of susceptibility in a breeding colony. Can Vet J. 1983;24(6):172–177. [PMC free article] [PubMed] [Google Scholar]
- Riggs C, Archer TA, Bulla C, Fellman C, Mackin A. Effect of storage conditions on canine T-cell cytokine production [abstract]. Proceedings of the 63rd Annual Meeting of the American College of Veterinary Pathologists; Seattle, WA. 2012. Abstract nr 41. [Google Scholar]
- Waldron-Mease E, Klein LV, Rosenberg H, Leitch M. Malignant hyperthermia in a halothane-anesthetized horse. J Am Vet Med Assoc. 1981;179(9):896–898. [PubMed] [Google Scholar]
- Ward A, Chaffman MO, Sorkin EM. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs. 1986;32(2):130–168. doi: 10.2165/00003495-198632020-00003. [DOI] [PubMed] [Google Scholar]
- Wells RJ, Sedacca CD, Aman AM, Hackett TB, Twedt DC, Shelton GD. Successful management of a dog that had severe rhabdomyolysis with myocardial and respiratory failure. J Am Vet Med Assoc. 2009;234(8):1049–1054. doi: 10.2460/javma.234.8.1049. [DOI] [PubMed] [Google Scholar]
- Wuis EW, Vree TB, Van Der Kleijn E. Pharmacokinetics of intravenously administered dantrolene and its 5-hydroxy metabolite in dogs. Int J Clin Pharmacol Res. 1990;10(4):203–210. [PubMed] [Google Scholar]