Abstract
Background
Drug eluting beads (DEB) are relatively new embolic agents that allow sustained release of chemotherapeutic agents in a localized fashion to the tumor. This technique is associated with reduced systemic side effects relative to systemic chemotherapy and an increase in the dose of antineoplastic agent delivered to the lesion. The meta-analysis was undertaken to assess the effectiveness of DEB-transcatheter arterial chemoembolization (TACE) in the management of hepatocellular cancer.
Methods
We searched the Web of Science, PubMed, EBSCO, EMBASE, the Wiley Library and Google Scholar for studies on DEB-TACE in the management of hepatocellular cancer from 1979 to April 2013. The risk of bias was assessed using RevMan 5·1. Random and fixed-effects meta-analytical models were used where indicated, and between-study heterogeneity was assessed. Disease control, complications and severe complications were recorded.
Results
Five studies met the selection criteria, three RCTs and two case-control studies, published from 2010 to 2012, included 217 patients in the DEB-TACE group and 237 in the conventional-TACE group. There was no significance over disease control (OR 2.27, 95% CI 0.78–6.63) with moderate between-study heterogeneity (χ2 = 6.83, degrees of freedom [df] = 3; p<0.08; I2 = 56%). Complications in both groups were assessed and no significant difference was observed (χ2 = 6.34, degrees of freedom [df] = 4; p<0.18; I2 = 37%). Severe complications were also assessed and no significant difference was observed (χ2 = 6.47, degrees of freedom [df] = 4; p<0.17; I2 = 38%). No publication bias relating to the above outcomes was detected by funnel plot. DEB-TACE benefited disease control without an increase in complications and severe complications.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy world-wide, with half a million new cases reported every year. HBV, HCV and alcoholic liver disease are the major risk factors in the etiology of HCC, and Southeast Asia and sub-Saharan Africa are the most affected regions. Over the past two decades, the incidence rate of HCC has tripled and the 5-year survival rate has increased from more than 1% to nearly 12% in the USA [1]–[3]. Although the early detection of small HCC has received much attention through recommended surveillance strategies in order to obtain a better response to curative treatments such as liver resection, liver transplantation and locoregional procedures, most patients with HCC are not candidates for curative therapies even at time of diagnosis due to poor liver function or tumor characteristics such as large or multifocal lesions. Survival rates for intermediate stage patients at 1, 3 and 5-years are 80%, 65% and 50%, and for patients with advanced disease are 29%, 16% and 8%, respectively [4].
Since the first report on transcatheter arterial chemoembolization (TACE) in the 1970s, TACE using Lipiodol mixed with antineoplastic emulsion and gelatin particles, now referred to as conventional TACE (cTACE), has been widely performed for unresectable HCC and other cancers as a palliative therapy [5]. Although the effectiveness of this technique remains controversial, a meta-analysis which included five randomized controlled trials (RCT) showed that TACE significantly reduced the overall 2-year mortality rate compared with nonactive treatment [6]. The drug eluting bead (DEB) is a new embolic agent as well as an antineoplastic agent carrier [7]. The use of DEBs which release the antineoplastic gent in the lesion in a controlled fashion is a new technique and has been shown to be associated with a reduction in systemic side effects and an increase in dose of the antineoplastic agent in the local lesion [8]–[16]. Pooled data from six clinical trials showed high local response rates ranging from 52% to 81% [17]–[24]. However, there is no strong evidence to show whether the cTACE is better than DEB-TACE in any terms of effectiveness. We performed a meta-analysis to determine whether DEB-TACE was more effective than cTACE in patients with HCC.
Method
Search
A computerized bibliographic search from 1979 to April 2013 was conducted on Web of Science (including MEDLINE), PubMed, EBSCO, EMBASE, the Wiley Library and Google Scholar, using the following terms: DEB, drug eluting bead, drug eluting microsphere and TACE. The related-articles function was allowed to expand the search findings and all abstracts, studies and citations were reviewed irrespective of language. We also searched the related study on www.clinicaltrials.gov in an attempt to find unpublished studies. The updated search date was April 1st, 2013.
Study selection
Studies were excluded by reading the titles or abstracts, and all studies based on animal or in vitro experiments, single-arm studies, case reports, reviews and studies on metastatic liver lesions were excluded. Following careful reading of abstracts and full-texts, studies which examined DEB-TACE versus cTACE were included if the study complied with the following requirements: clinically diagnosed HCC, Child-Pugh A or B, case-controlled trial or RCT outcomes assessed by modified Response Evaluation Criteria in Solid Tumor (RECIST) or the Europe Association for the Study of Liver Disease (EASL) measurement, and complications were recorded. For repeat studies conducted by the same author, the most recent and informative study was adopted if there were overlapping data.
Assessment of risk of bias in eligible studies
Two review authors (SH, LZ) independently assessed the risk of bias in each included study using Revman 5·1. Agreements were reached by discussion between the two review authors if there were disagreements on specific items in the studies.
Data extraction
All the articles searched were managed by Endnote X5. One reviewer (SH) retrieved articles that potentially met the inclusion criteria. The full text was requested from the authors if the study was included but not readily available on the database. From each included study, we extracted data on patient characteristics, demographics, country, study design, sample size, etiology of HCC, Child-Pugh score, Barcelona Clinic Liver Cancer (BCLC) classification, antineoplastic dose, DEB dose, tumor response, complications and severe complications from both the DEB-TACE group and the cTACE group. The primary endpoint of this meta-analysis was tumor response assessed by the EASL criteria or modified RECIST which focused on viable tumor on imaging evidence and included partial response (PR), complete response (CR), stable disease (SD), and progressive disease (PD) [25]. For converting continuous to dichotomous, we reclassified the PR, CR and SD in raw data as disease control (DC), which means disappearance of all detectable tumor, or a decrease of more than 50% or 30%, or an increase of <20% or 25%, without the appearance of new lesions based on CT or MRI The minimum medical imaging follow-up time was 4 weeks after the procedure in all the included studies. Complications, namely post-TACE complications, were acute liver impairment, encephalopathy, ascites, epigastric pain and fever. Severe complications included increased hospital stay, injury or death.
Statistical analysis
This meta-analysis was carried out in compliance with the recommendations of the Cochrane Reviewers' handbook 4.2.2 [26]. The odds ratio (OR) was used as the summary statistic for statistical analysis of dichotomous variables and represented the odds of a favorable event occurring in the DEB-TACE group compared to the cTACE group and indicated the relative risk. An OR of more than 1 favored the DEB-TACE group. The point estimate of the OR was considered to be statistically significant or a p-value <0.05 was significant if the 95% CI did not include the value 1.
A fixed-effect or a random-effect model was used for the meta-analysis where indicated. Heterogeneity between the groups was evaluated by the χ2 and I2 statistic, and higher χ2 and I2 statistic values indicated greater heterogeneity between the groups [27]. The hypothesis of homogeneity between the groups was thought to be invalid if the p-value was <0.1 and the random-effect model was adopted and the cause of heterogeneity was investigated. The fixed-effect model was then considered. Publication bias was analyzed using a funnel plot. A two-tailed p-value <0.05 was considered statistically significant. Analysis was performed using Review Manager version 5.1.5 for Windows.
Results
In total, 135 potentially relevant articles were identified using the search strategy (Figure 1). One hundred and six articles were excluded after reading the title and abstract, which included 27 animal or in vitro experiments, 48 single arm studies, 18 studies involved patients with liver metastasis, 18 review articles, nine comparative studies which either had no full texts or were ineligible, four case reports and two official files. Following a further review of the remaining nine articles, four were excluded due to overlapping data, not enough information or were ineligible, of which Frenette, who conducted an article involving 274 patients, provided the baseline characteristics of patients and are working on the outcomes.
Figure 1. Search strategy.
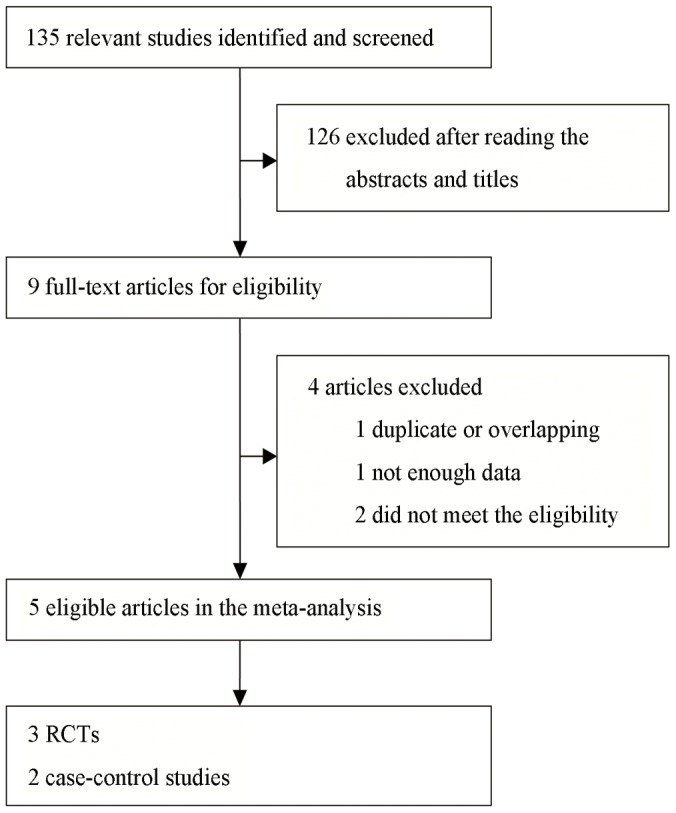
Therefore five studies published between 2010 and 2012 met the selection criteria and were included in this meta-analysis [23], [ 28]–[31]. The references in these studies did not provide further studies for review. The analysis was performed on 217 patients in the DEB-TACE group and 237 in the cTACE group. Of these five studies, three were RCTs and two were case-control studies, of which four were from Europe and one from Asia.
All three RCTs were considered to be of high methodological quality. One of the RCTs conducted by Lammer and colleagues was an international, multicenter, prospective and single-blind study which included 189 patients and constituted the major part (41.6%) of this meta-analysis. The other two RCTs were single center and randomized studies, but failed to provide the specific randomization method. Two case-control studies focused on a definite issue with appropriate method, but failed to provide the exposure factor. Song and colleagues concluded that DEB-TACE resulted in a better treatment response than cTACE, while Wiggermann concluded that there was no significant increase in terms of disease control between the treatment groups. The risk of bias for each study is shown in Figure 2 and Figure 3. The study characteristics and patient demographics are shown in Table 1. Two studies described treatment cycles patients received. In Lammer's study, 82% patients received two cycles of treatment in each group, and 61%, 57% patients received three cycles in DEB-TACE and cTACE group, respectively. Whereas, in Sacco's study, 22.4% received two cycles of treatment in each group and 4.5%, 5.5% received three cycles in DEB-TACE and cTACE group. However, no significant difference was found in both studies.
Figure 2. Risk of bias summary: review of authors' judgments on the risk of bias of each item in each included study.
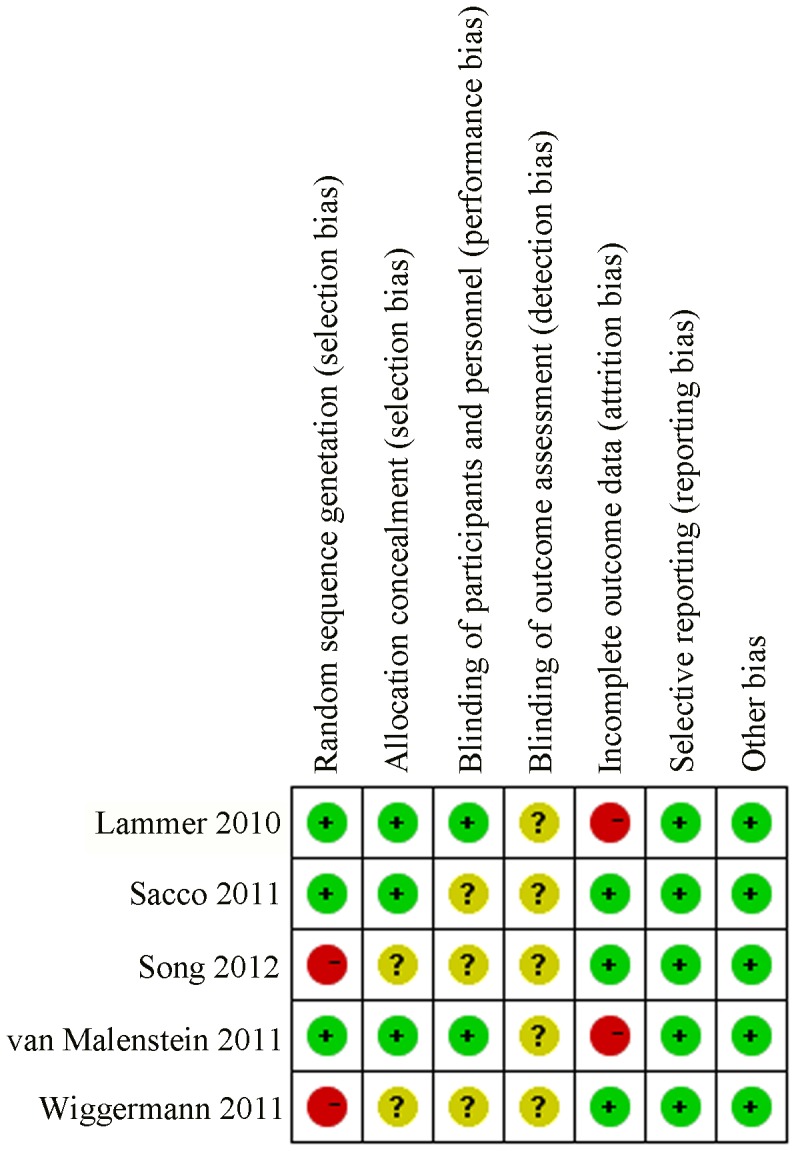
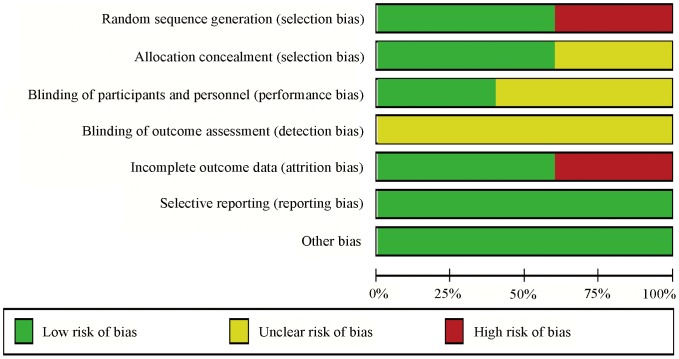
All the blinding method of these articles are unknown but reported the low selective reporting and other bias, which prove the reliability of these studies.
Figure 3. Risk of bias graph: review of authors' judgments on the risk of bias in each item presented as percentages in all included studies, as described in Figure 2.
Table 1. Demographic characteristics of the included studies.
| Year | Article Type | Bead Type | Patient | Mean age | Etiology | Child-Pugh | BCLC | Drop out | Median Follow-up | |
| D/C | D/C | HCV/HBV/AC/O | A/B | A/B/C | ||||||
| Lammer | 2010 | RCT | DC Bead | 93/108 | 67·3/67·4 | 40/34/100/46 | 166/35 | 53/148/0 | 12 | 6mo |
| Sacco | 2011 | RCT | DC Bead | 33/34 | 71·3/68·7 | 47/8/0/12 | 54/13 | 44/23/0 | 0 | 1mo |
| Song | 2012 | Retrospective | DC Bead | 60/69 | 61·7/59·4 | 16/90/16/7 | 119/10 | 55/74/0 | 0 | 18mo |
| van Malenstein | 2011 | RCT | SAP | 16/14 | 67·3/56·6 | 8/4/14/4 | 28/2 | 3/19/8 | 5 | 6w |
| Wiggermann | 2011 | Retrospective | DC Bead | 22/22 | 70·3/67·7 | 9/0/9/26 | 44/0 | 5/32/5 | 0 | 8mo |
Child-Pugh = Child-Pugh classification. BCLC = Barcelona Clinic Liver Cancer tumor staging. D = DEB-TACE group. C = cTACE group. O = others. RCT = randomized controlled trial.
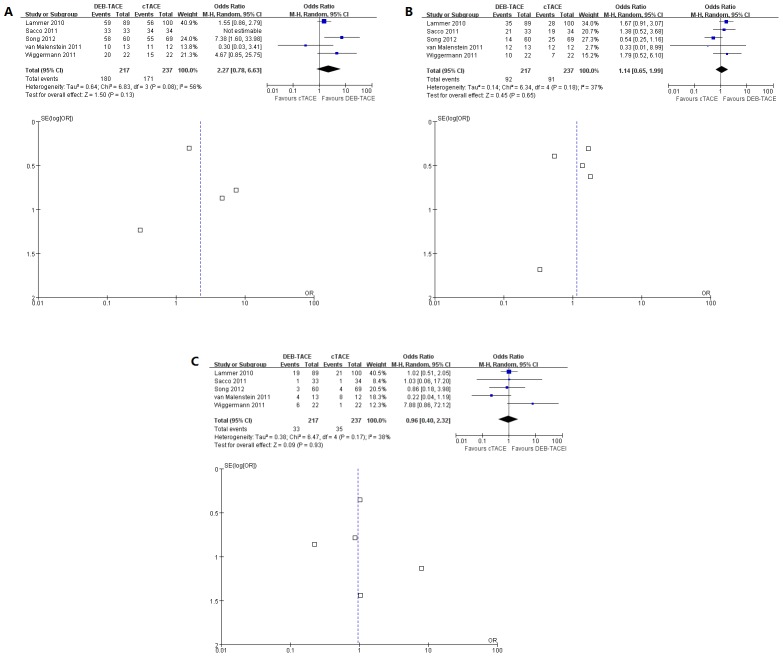
Pooled data from the included studies which assessed disease control showed no significance between two groups (OR 2.27, 95% CI 0.78–6.63) with moderate between-study heterogeneity (χ2 = 6.83, degrees of freedom [df] = 3; p<0.08; I2 = 56%). No publication bias was detected by the funnel plot (Figure 4A).
Figure 4.
A. Forest plot comparison of DEB-TACE vs. cTACE group in terms of disease control shows no significance of odds ratio. The Lammer's study takes greater weight against other studies. No publication bias in the funnel plot. B. Forest plot comparison of DEB-TACE vs. cTACE group in terms of complications show there is no significance of odds ratio and no publication bias. C. Forest plot comparison of severe complications show there is no significance of odds ratio between these two groups and report no publication bias.
Data on complications in both groups were assessed and no significant difference between the two groups was observed (χ2 = 6.34, degrees of freedom [df] = 4; p<0.18; I2 = 37%). No publication bias was detected by the funnel plot (Figure 4B).
Data on severe complications in the studies were assessed and no significant difference between the two groups was observed (χ2 = 6.47, degrees of freedom [df] = 4; p<0.17; I2 = 38%). No publication bias was detected by the funnel plot (Figure 4C).
Discussion
This meta-analysis examined the efficacy of DEB-TACE versus cTACE in patients with HCC. Though the analytical results showed no statistical significance, but the odds ratio is high in terms of disease control and there is no increase in complications or severe complications. There are currently two types of DEB on the market: polyvinyl alcohol-based microspheres (DC Bead, BioCompatibles Ltd., Farnham, UK) and superabsorbent polymer microspheres (HepaSphere, Biosphere Medical, Rockland, MA, USA) [22]. The initial report on the DEB was by Lewis and colleagues in 2006 which presented detailed in vitro characterization of the DEB (DC Bead) [7]. The study showed that modeling of the kinetics of drug elution from the beads in vitro at a loading dose of 25 mg/ml yielded calculated half-lives of 150 hours for the 100–300 µm size range to a maximum of 1,730 hours for the 700–900 µm size range, which was dependent on the ionic strength of the elution medium in comparison with an unstable Lipiodol emulsion that showed a rapid loss of drug with a half-life of approximately 1 hour. Therefore, long-term interaction with the tumor and the high concentration of antineoplastic agent in the lesion were the main advantages over the conventional drug carrier and embolic agent. The largest study as well as RCT, conducted by Lammer, did not show significance in terms of disease control, but the subgroup analyses showed that in 67% of patients with more advanced disease (Child-pugh B, ECOG 1, bilobar or recurrent disease), the incidence of overall survival and disease control were statistically higher (p = 0.038 and p = 0.026, respectively) in DEB-TACE group compared with the cTACE group. The supplementary post hoc analysis indicated that the incidence of severe adverse events within 30 days of a procedure was consistently lower, and AST as well as ALT was significantly less in the DEB-TACE group. The largest retrospective study from Song showed the treatment response in the DEB-TACE group was significantly higher than that in the cTACE group (p<0.001) and the subgroup analysis according to BCLC stage indicated that the treatment responses in intermediate stage was significantly better in DEB-TACE group than cTACE group as well (p<0.001). We may therefore surmise that DEB-TACE could improve the clinical effectiveness in patients with more advanced HCC.
Limitations
There are limitations in this meta-analysis. First, there are limited numbers of articles focusing on DEB-TACE, and even less on the comparison of cTACE and DEB-TACE. The ongoing clinical trials on DEB-TACE are mostly single-arm study. Second, only five studies which included 454 patients were eligible for the inclusion criteria, and four of the included studies have small sample sizes. Therefore, a sensitivity analysis to gain further knowledge on the factors affecting tumor response and other outcomes was not applied. Third, cTACE performed in different medical centers can vary either due to the different embolic agents used or different levels of manual expertise. In terms of the antineoplastics used, four studies used adriamycin and one used cisplatin in the cTACE group. Forth, medical imaging follow-up varied greatly from 6 weeks to 18 months and is quite short, which may have affected the primary endpoint of the meta-analysis. Fifth, survival time was not described in the meta-analysis although three studies recorded survival time in different ways. Finally, we noted that the primary endpoint of the largest RCT by Lammer and colleagues played an important role in this meta-analysis (weighted, 40.9%) and the initial in vitro study was sponsored by DC Bead, BioCompatibles Ltd.
A recent systematic review which focused on the safety and efficacy of DEB-TACE in HCC patients showed that there was sufficient evidence to support the use of the DC Bead as a safe and effective embolic treatment, however, there was overlapping information in this meta-analysis [5]. In our meta-analysis, no severe adverse events related to the safety of DEB were reported. Complications and severe complications between the two groups were not significantly different. With the exception of the RCTs and case-control studies, almost 40 single-arm studies included information on the efficacy of DEB since 2006 which showed the promising application of this treatment in clinical practice [32]–[55]. Based on this meta-analysis, further high quality randomized clinical trials with longer follow-up may focus on patients with more advanced stage, who may be of more benefit with DEB-TACE.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
This work was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2012BAI15B06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 2. Han KH, Kudo M, Ye SL, Choi JY, Poon RT, et al. (2011) Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology 81 Suppl 1158–164. [DOI] [PubMed] [Google Scholar]
- 3. Walzer N, Kulik LM (2008) Hepatocellular carcinoma: latest developments. Curr Opin Gastroenterol 24: 312–319. [DOI] [PubMed] [Google Scholar]
- 4. Martin R, Geller D, Espat J, Kooby D, Sellars M, et al. (2012) Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology 59: 255–260. [DOI] [PubMed] [Google Scholar]
- 5. Takayasu K (2012) Transarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspective. Jpn J Clin Oncol 42: 247–255. [DOI] [PubMed] [Google Scholar]
- 6. Camma C, Schepis F, Orlando A, Albanese M, Shahied L, et al. (2002) Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 224: 47–54. [DOI] [PubMed] [Google Scholar]
- 7. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, et al. (2006) New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 12: 2563–2567. [DOI] [PubMed] [Google Scholar]
- 8. Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, et al. (2006) DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol 17: 335–342. [DOI] [PubMed] [Google Scholar]
- 9. Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, et al. (2006) Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol 17: 1335–1343. [DOI] [PubMed] [Google Scholar]
- 10. Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL (2007) Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci 30: 7–14. [DOI] [PubMed] [Google Scholar]
- 11. Eyol E, Boleij A, Taylor RR, Lewis AL, Berger MR (2008) Chemoembolisation of rat colorectal liver metastases with drug eluting beads loaded with irinotecan or doxorubicin. Clin Exp Metastasis 25: 273–282. [DOI] [PubMed] [Google Scholar]
- 12. Jordan O, Denys A, De Baere T, Boulens N, Doelker E (2010) Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol 21: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 13. Maeda N, Osuga K, Higashihara H, Mikami K, Tomoda K, et al. (2010) In vitro characterization of cisplatin-loaded superabsorbent polymer microspheres designed for chemoembolization. J Vasc Interv Radiol 21: 877–881. [DOI] [PubMed] [Google Scholar]
- 14. Gupta S, Wright KC, Ensor J, Van Pelt CS, Dixon KA, et al. (2011) Hepatic arterial embolization with doxorubicin-loaded superabsorbent polymer microspheres in a rabbit liver tumor model. Cardiovasc Intervent Radiol 34: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weng L, Le HC, Lin J, Golzarian J (2011) Doxorubicin loading and eluting characteristics of bioresorbable hydrogel microspheres: in vitro study. Int J Pharm 409: 185–193. [DOI] [PubMed] [Google Scholar]
- 16. Liu DM, Kos S, Buczkowski A, Kee S, Munk PL, et al. (2012) Optimization of doxorubicin loading for superabsorbent polymer microspheres: in vitro analysis. Cardiovasc Intervent Radiol 35: 391–398. [DOI] [PubMed] [Google Scholar]
- 17. Osuga K, Maeda N, Higashihara H, Hori S, Nakazawa T, et al. (2012) Current status of embolic agents for liver tumor embolization. Int J Clin Oncol 17: 306–315. [DOI] [PubMed] [Google Scholar]
- 18. Tam KY, Leung KC, Wang YX (2011) Chemoembolization agents for cancer treatment. Eur J Pharm Sci 44: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Varela M, Real MI, Burrel M, Forner A, Sala M, et al. (2007) Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 46: 474–481. [DOI] [PubMed] [Google Scholar]
- 20. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, et al. (2007) A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 5: 1100–1108. [DOI] [PubMed] [Google Scholar]
- 21. Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, et al. (2008) Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 31: 269–280. [DOI] [PubMed] [Google Scholar]
- 22. Reyes DK, Vossen JA, Kamel IR, Azad NS, Wahlin TA, et al. (2009) Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J 15: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, et al. (2010) Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 33: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, et al. (2010) Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 33: 541–551. [DOI] [PubMed] [Google Scholar]
- 25. Prajapati HJ, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, et al. (2013) mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol 24: 965–973. [DOI] [PubMed] [Google Scholar]
- 26. Clarke M, Horton R (2001) Bringing it all together: Lancet-Cochrane collaborate on systematic reviews. Lancet 357: 1728. [DOI] [PubMed] [Google Scholar]
- 27. Doi SA, Barendregt JJ, Mozurkewich EL (2011) Meta-analysis of heterogeneous clinical trials: an empirical example. Contemp Clin Trials 32: 288–298. [DOI] [PubMed] [Google Scholar]
- 28. Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, et al. (2011) Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 22: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 29. van Malenstein H, Maleux G, Vandecaveye V, Heye S, Laleman W, et al. (2011) A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie 34: 368–376. [DOI] [PubMed] [Google Scholar]
- 30. Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, et al. (2012) Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 57: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 31. Wiggermann P, Sieron D, Brosche C, Brauer T, Scheer F, et al. (2011) Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE). Med Sci Monit 17: CR189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grosso M, Vignali C, Quaretti P, Nicolini A, Melchiorre F, et al. (2008) Transarterial Chemoembolization for Hepatocellular Carcinoma with Drug-Eluting Microspheres: Preliminary Results from an Italian Multicentre Study. Cardiovascular and Interventional Radiology 31: 1141–1149. [DOI] [PubMed] [Google Scholar]
- 33. Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, et al. (2008) Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: Results of an open-label study of 62 patients. Cardiovascular and Interventional Radiology 31: 269–280. [DOI] [PubMed] [Google Scholar]
- 34. Reyes DK, Vossen JA, Kamel IR, Azad NS, Wahlin TA, et al. (2009) Single-Center Phase II Trial of Transarterial Chemoembolization With Drug-Eluting Beads for Patients With Unresectable Hepatocellular Carcinoma Initial Experience in the United States. Cancer Journal 15: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith BW, Jeffrey GP, Macquillan GC, Tibballs J, Ratman L, et al. (2009) Transarterial chemoembolisation using drug eluting beads for hepatocellular carcinoma. Journal of Gastroenterology & Hepatology 24 Sup: A307–A308.
- 36. Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, et al. (2010) Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. Hpb 12: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia-Hidalgo Alonso M, Lanciego Perez C, De La Cruz Perez G, Velasco Gomez J, Pinto Varela JM, et al. (2010) [Treatment of hepatocellular carcinoma using precision transcatheter arterial chemoembolization (TACE): results of two years' experience in a general hospital]. Quimioembolizacion de hepatocarcinoma mediante TACE-precision: resultados al 2.degrees ano en un hospital general. Radiologia 52: 425–431. [DOI] [PubMed] [Google Scholar]
- 38. Martin RCG, Howard J, Tomalty D, Robbins K, Padr R, et al. (2010) Toxicity of Irinotecan-Eluting Beads in the Treatment of Hepatic Malignancies: Results of a Multi-Institutional Registry. Cardiovascular and Interventional Radiology 33: 960–966. [DOI] [PubMed] [Google Scholar]
- 39. Moschouris H, Malagari K, Papadaki MG, Kornezos I, Matsaidonis D (2010) Contrast-Enhanced Ultrasonography of Hepatocellular Carcinoma After Chemoembolisation Using Drug-Eluting Beads: A Pilot Study Focused on Sustained Tumor Necrosis. Cardiovascular and Interventional Radiology 33: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 40. Sadick M, Haas S, Loehr M, Elshwi M, Singer MV, et al. (2010) Application of DC beads in hepatocellular carcinoma: clinical and radiological results of a drug delivery device for transcatheter superselective arterial embolization. Onkologie 33: 31–37. [DOI] [PubMed] [Google Scholar]
- 41. Malagari K, Pomoni M, Spyridopoulos TN, Moschouris H, Kelekis A, et al. (2011) Safety Profile of Sequential Transcatheter Chemoembolization with DC Bead (TM): Results of 237 Hepatocellular Carcinoma (HCC) Patients. Cardiovascular and Interventional Radiology 34: 774–785. [DOI] [PubMed] [Google Scholar]
- 42. Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, et al. (2011) Embolization of hepatocellular carcinoma with drug-eluting beads: Doxorubicin tissue concentration and distribution in patient liver explants. Journal of Hepatology 55: 1332–1338. [DOI] [PubMed] [Google Scholar]
- 43. Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, et al. (2011) Phase II Trial of Sorafenib Combined With Concurrent Transarterial Chemoembolization With Drug-Eluting Beads for Hepatocellular Carcinoma. Journal of Clinical Oncology 29: 3960–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seki A, Hori S, Kobayashi K, Narumiya S (2011) Transcatheter Arterial Chemoembolization with Epirubicin-Loaded Superabsorbent Polymer Microspheres for 135 Hepatocellular Carcinoma Patients: Single-Center Experience. Cardiovascular and Interventional Radiology 34: 557–565. [DOI] [PubMed] [Google Scholar]
- 45. Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, et al. (2011) Liver, Gastrointestinal, and Cardiac Toxicity in Intermediate Hepatocellular Carcinoma Treated With PRECISION TACE With Drug-Eluting Beads: Results From the PRECISION V Randomized Trial. American Journal of Roentgenology 197: W562–W570. [DOI] [PubMed] [Google Scholar]
- 46. Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, et al. (2012) Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. Journal of Hepatology 56: 1330–1335. [DOI] [PubMed] [Google Scholar]
- 47. Cannon RM, Urbano J, Kralj I, Bosnjakovic P, Martin RCG 2nd (2012) Management of diffuse hepatocellular carcinoma (≧ 10 Lesions) with doxorubicin-loaded DC beads is a safe and effective treatment option. Onkologie 35: 184–188. [DOI] [PubMed] [Google Scholar]
- 48. Chung WS, Lee KH, Park MS, Lee YJ, Kwon J, et al. (2012) Enhancement Patterns of Hepatocellular Carcinoma After Transarterial Chemoembolization Using Drug-Eluting Beads on Arterial Phase CT Images: A Pilot Retrospective Study. American Journal of Roentgenology 199: 349–359. [DOI] [PubMed] [Google Scholar]
- 49. Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, et al. (2012) Chemoembolization With Doxorubicin-Eluting Beads for Unresectable Hepatocellular Carcinoma: Five-Year Survival Analysis. Cardiovascular and Interventional Radiology 35: 1119–1128. [DOI] [PubMed] [Google Scholar]
- 50. Pomoni M, Malagari K, Moschouris H, Spyridopoulos TN, Dourakis S, et al. (2012) Post Embolization Syndrome in Doxorubicin Eluting Chemoembolization with DC Bead. Hepato-Gastroenterology 59: 820–825. [DOI] [PubMed] [Google Scholar]
- 51. Prajapati HJ, Rafi S, El-Rayes BF, Kauh JS, Kooby DA, et al. (2012) Safety and Feasibility of Same-day Discharge of Patients with Unresectable Hepatocellular Carcinoma Treated with Doxorubicin Drug-eluting Bead Transcatheter Chemoembolization. Journal of Vascular and Interventional Radiology 23: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 52. Seki A, Hori S (2012) Switching the Loaded Agent from Epirubicin to Cisplatin: Salvage Transcatheter Arterial Chemoembolization with Drug-eluting Microspheres for Unresectable Hepatocellular Carcinoma. Cardiovascular and Interventional Radiology 35: 555–562. [DOI] [PubMed] [Google Scholar]
- 53. Skowasch M, Schneider J, Otto G, Weinmann A, Woerns MA, et al. (2012) Midterm follow-up after DC-BEAD-TACE of Hepatocellular Carcinoma (HCC). European Journal of Radiology 81: 3857–3861. [DOI] [PubMed] [Google Scholar]
- 54. Sottani C, Poggi G, Quaretti P, Regazzi M, Montagna B, et al. (2012) Serum Pharmacokinetics in Patients Treated with Transarterial Chemoembolization (TACE) Using Two Types of Epirubicin-loaded Microspheres. Anticancer Research 32: 1769–1774. [PubMed] [Google Scholar]
- 55. Syha R, Ketelsen D, Heller S, Schmehl J, Mangold S, et al. (2012) Hepatocellular carcinoma: initial tumour response after short-term and long-interval chemoembolization with drug-eluting beads using modified RECIST. European Journal of Gastroenterology & Hepatology 24: 1325–1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)